Abstract
Aeromonas hydrophila leads to both intestinal and extraintestinal infections in animals and humans, and the underlying mechanisms leading to mortality are largely unknown. By using a septicemic mouse model of infection, we showed that animals challenged with A. hydrophila die because of kidney and liver damage, hypoglycemia, and thrombocytopenia. Pretreatment of animals with quorum-sensing-associated signaling molecules N-acyl homoserine lactones (AHLs), such as butanoyl and hexanoyl homoserine lactones (C4- and C6-HSLs), as well as N-3-oxododecanoyl (3-oxo-C12)-HSL, prevented clinical sequelae, resulting in increased survivability of mice. Since little is known as to how different AHLs modulate the immune response during infection, we treated mice with the above AHLs prior to lethal A. hydrophila infection. When we compared results in such animals to those in controls, the treated animals exhibited a significantly reduced bacterial load in the blood and other mouse organs, as well as various levels of cytokines/chemokines. Importantly, neutrophil numbers were significantly elevated in the blood of C6-HSL-treated mice compared to those in animals given phosphate-buffered saline and then infected with the bacteria. These findings coincided with the fact that neutropenic animals were more susceptible to A. hydrophila infection than normal mice. Our data suggested that neutrophils quickly cleared bacteria by either phagocytosis or possibly another mechanism(s) during infection. In a parallel study, we indeed showed that other predominant immune cells inflicted during A. hydrophila infections, such as murine macrophages, when they were pretreated with AHLs, rapidly phagocytosed bacteria, whereas untreated cells phagocytosed fewer bacteria. This study is the first to report that AHL pretreatment modulates the innate immune response in mice and enhances their survivability during A. hydrophila infection.
INTRODUCTION
Many bacterial species use chemicals, termed autoinducers, to signal each other and to coordinate their activities in response to an external stimulus as a function of population density, a phenomenon known as quorum sensing (QS). Several different types of autoinducers have been described; perhaps the best-studied autoinducer molecules for QS are N-acyl homoserine lactones (AHLs), which are produced by a large number of Gram-negative bacteria. AHLs are composed of a homoserine lactone ring (HSL) with an acyl side chain which varies in length from C4 to C18 (26, 30). In some AHL molecules, this acyl chain can be modified by a 3-oxo, a 3-hydroxy, or a terminal methyl branch with various degrees of unsaturation (46). The acyl side chain length and the substitutions on the side chain provide signal specificity (32). AHLs are synthesized by AHL synthases, the LuxI protein family, and once they are produced, they diffuse in and out of the cell by passive as well as active transport mechanisms (21, 34). The concentrations of AHLs eventually reach a critical threshold, and then they are recognized by their cognate receptors, the LuxR protein family, which represents the second component of the system (6). Binding of AHLs to LuxR regulates expression of the luxI gene as well as that of genes that are involved in the virulence mechanisms of many pathogens, including Aeromonas hydrophila, which leads to various clinical manifestations in humans of all age groups (9, 16, 18, 23, 33).
AHLs, such as the N-3-oxododecanoyl homoserine lactone (3-oxo-C12-HSL), not only are important in the regulation of bacterial virulence genes but also interact with various eukaryotic cells to modulate immune responses (38, 40–42, 44, 51). An earlier study has shown that the in vitro effects of 3-oxo-C12-HSL on lymphocytes are immunosuppressive/anti-inflammatory at a low concentration (below 10 μM) and proinflammatory at a high concentration (20 μM and above) (7). Likewise, Telford et al. reported that 3-oxo-C12-HSL inhibited the lipopolysaccharide (LPS)-dependent activation of tumor necrosis factor alpha (TNF-α) and interleukin-12 (IL-12) in murine peritoneal macrophages (45). Later studies further confirmed that 3-oxo-C12-HSL inhibited the proliferation and function (cytokine production) of both mitogen- and antigen-stimulated (4, 36) T lymphocytes. Contrary to these studies, Smith et al. reported that 3-oxo-C12-HSL stimulated the production of neutrophil-attracting chemokines, e.g., IL-8, cyclooxigenase-2 (Cox-2), and prostaglandin E2 (PGE2) synthase, in human lung fibroblasts and epithelial cells (40). However, Kravchenko et al. did not observe such an induction of cytokine or chemokine production in primary respiratory epithelial cells (25).
A chemotactic role of 3-oxo-C12-HSL for neutrophils has been shown in vitro (54), and apart from IL-8 production and neutrophil attraction, other investigators provided evidence that 3-oxo-C12-HSL promoted apoptosis in various cell types, including neutrophils, monocytes, fibroblasts, and breast carcinoma cells (29, 38, 44). Induction of apoptosis in various immune cells by AHLs might decrease inflammation by reducing the number of effective phagocytes and the mediators that they produce (7). This apoptotic induction of host cells mediated by 3-oxo-C12-HSL occurred through a calcium-dependent signaling pathway, while the proinflammatory effect of 3-oxo-C12-HSL resulted through a calcium-independent and peroxisome proliferator-activated receptor (PPAR)-mediated pathway (20). The Toll-like receptor (TLR) pathways were not believed to be involved in AHL signaling (25).
A. hydrophila is an emerging human pathogen that causes both intestinal and extraintestinal infections, including diarrhea, septicemia, cellulitis, and wound and soft tissue infections (13, 17, 49). Association of this pathogen with hemolytic-uremic syndrome (HUS) and necrotizing fasciitis in immunocompromised children was also reported (1, 12). The ability of A. hydrophila to resist the effects of multiple antibiotics poses a potential threat in choosing therapeutic modalities.
A. hydrophila produces two types of AHLs, namely, N-3-butanoyl-dl-homoserine lactone (C4-HSL) and N-3-hexanoyl-dl-homoserine lactone (C6-HSL), of which C4-HSL is the predominant type (43). In our recent study, we examined AHL production from a large number of clinical and water Aeromonas isolates and found that the former isolates produced more AHLs than the latter isolates, which may indicate that AHLs play an important role during human infections (22). Further, we identified AhyRI (LuxRI homologs) in clinical isolate SSU of A. hydrophila and showed that disruption of the ahyRI genes reduced metalloprotease production; biofilm formation; secretion of the type 6 secretion system (T6SS) effectors, e.g., hemolysin-coregulated protein (Hcp) and valine-glycine repeat G (VgrG) family of proteins; and mortality in a septicemic mouse model of infection (23).
As mentioned earlier, several in vitro studies reported varied effects of 3-oxo-C12-HSL on different mammalian cells; however, there is limited information available on the effects of different types of AHLs in vivo. For example, Smith et al. showed that dermal injection of 3-oxo-C12-HSL in mice induced the production of inflammatory mediators, such as IL-1α, IL-6, macrophage inflammatory protein 2 (MIP-2), monocyte chemotactic protein 1 (MCP-1), MIP-1β, and inducible protein 10 (IP-10) (41). On the contrary, Kravchenko et al. reported that injection of 3-oxo-C12-HSL in the peritoneum of mice suppressed an LPS-induced immune response (24). Currently, it is not known how these immunomodulatory effects of different AHL signaling molecules influence the host immune response during bacterial infection.
Consequently, we investigated the immunomodulatory role of three AHLs, namely, C4-HSL, C6-HSL, and 3-oxo-C12-HSL, on the innate immune response to A. hydrophila infection in a septicemic mouse model. Our study is the first to show that pretreatment of mice with AHLs prevents clinical sequelae to enhance survivability of mice after A. hydrophila infection and that they trigger an innate immune response in mice to clear bacteria from the blood and different mouse tissues.
MATERIALS AND METHODS
Bacterial strain and chemicals.
The clinical strain A. hydrophila SSU, isolated from a diarrheal patient, was used in this study. All of the N-acyl homoserine lactones (C4-, C6-, and 3-oxo-C12-HSLs) used in this study were purchased commercially (Sigma, St. Louis, MO) and were of the highest-quality grade (98 to 100% pure), based on high-performance liquid chromatography (HPLC) analysis. Stock solutions (50 mM) of AHLs were prepared by dissolving them in acetonitrile (for C4-HSL and C6-HSL) and dimethyl sulfoxide (DMSO; for 3-oxo-C12-HSL), and stocks were stored at −20°C. Before injection in mice or treatment of RAW 264.7 murine macrophages, appropriate concentrations of AHLs were prepared by dilution in phosphate-buffered saline (PBS) or DMSO. We used 200 μM and 250 μM each AHL for in vitro and in vivo studies, respectively, unless otherwise stated. Cyclophosphamide was purchased from Sigma and was dissolved in water.
Mice survivability.
Female 5- to 6-week-old Swiss-Webster mice (average weight, approximately 20 g) were purchased from Charles River Laboratories (Wilmington, MA). Mice were treated intraperitoneally (i.p.) with 250 μM per 100-μl dose each of C4-HSL, C6-HSL, or 3-oxo-C12-HSL; a combination of C4-HSL and C6-HSL; and/or PBS (vehicle control) for 12 h and 6 h before A. hydrophila challenge (i.p.) with either 3 × 107 or 3.5 × 107 CFU. In some experiments, we also injected into mice smaller amounts of C4- and C6-HSLs (25 and 100 μM per 100 μl dose of each) prior to A. hydrophila infection. Deaths were recorded for 16 days postinfection (p.i.). All animal experiments were performed by using protocols approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee.
Blood chemistry.
To determine changes in the blood chemistry, we pretreated mice with various AHLs or vehicle for 12 h and 6 h before challenge with A. hydrophila at a dose of 3 × 107 CFU. A group of mice (n = 5, each group) was treated only with either AHLs or vehicle without A. hydrophila infection. At 4 h (postinfection or posttreatment), animals were euthanized by using isoflurane, and blood was immediately collected via cardiac puncture in blood collection tubes with lithium heparin (Becton Dickinson, Cockeysville, MD). To evaluate changes in the blood chemistry at a later stage in A. hydrophila infection, blood was collected from different groups of mice (n = 5, each time point) at 0, 12, and 24 h postinfection with A. hydrophila at a dose of 3 × 107 CFU. From each blood tube, 100 μl of heparinized whole blood was carefully dispensed into a Vetscan comprehensive diagnostic profile reagent rotor, and blood chemistry profiles were analyzed by using an automated Vetscan chemistry analyzer (Abaxis, Union City, CA). The Vetscan rotor used in this study provided in vitro determination of blood alanine aminotransferase (ALT), albumin (ALB), alkaline phosphatase (ALP), amylase (AMY), total calcium (CA), creatinine (CRE), globulin (GLOB), glucose (GLU), phosphorus (PHOS), potassium (K), sodium (Na), total bilirubin (TBIL), total protein (TP), and urea nitrogen (BUN) levels. The data from all animals in each group were averaged, and the standard deviations were calculated.
Determination of bacterial burden.
Mice were pretreated with various AHLs as described above prior to infection with A. hydrophila at a dose of 3.5 × 107 CFU. To determine bacterial load in the blood, at 2 h and 4 h p.i., mice were euthanized by using isoflurane and the blood was immediately collected via cardiac puncture in blood collection tubes containing potassium EDTA (Becton Dickinson). The animals were then humanely killed by CO2 and cervical dislocation. To examine bacterial dissemination in different tissues, at 48 h p.i., the lungs, liver, and spleen were removed, weighed, and homogenized by using tissue grinders (Kendall, Mansfield, MA) in 1 ml of PBS. The blood and tissue homogenates were serially diluted and cultured on Luria-Bertani (LB) agar plates supplemented with rifampin (200 μg/ml; we used a spontaneous mutant strain of SSU resistant to this antibiotic). After incubation of the plates at 37°C for 12 to 18 h, bacterial colonies were counted, and the numbers of CFU per gram of tissue or per ml of blood were calculated.
In vivo cytokine/chemokine analysis.
To determine cytokine/chemokine production in different tissues, mice were pretreated with various AHLs prior to infection with 3.5 × 107 CFU of A. hydrophila. As controls, mice were treated only with various AHLs without any bacterial challenge but given PBS. At 48 h postinfection or posttreatment with AHLs, the lungs, liver, and spleen were removed, weighed, and homogenized in 1 ml of PBS. The tissue homogenates were centrifuged at 13,000 rpm for 10 min, and the supernatants were collected in 1.5-ml microcentrifuge tubes. The levels of cytokines/chemokines were measured by using a multiplex assay (Bio-Rad, Hercules, CA) as described previously (37).
Blood cell population counts.
Mice were pretreated with various AHLs and then challenged with A. hydrophila as described above. We determined different cell populations, including neutrophils and platelets, in the blood of the above-mentioned animals, as well as those mice that were treated only with AHLs or vehicle control without bacterial infection to serve as a control. At 4 h postinfection or posttreatment with AHLs/vehicle, mice were euthanized and blood was immediately collected in blood collection tubes containing lithium heparin (Becton Dickinson). The blood cell counts were analyzed immediately by using a Hemavet 950 hematology system (Drew Scientific, Inc., Dallas, TX). The numbers of neutrophils and platelets from all animals were averaged, and the data are presented as means ± standard deviations.
In vivo neutrophil depletion.
To further characterize the role of neutrophils in A. hydrophila infection, mice were depleted of neutrophils before A. hydrophila challenge. Animals were rendered neutropenic either by i.p. injection of 250 mg of cyclophosphamide/kg body weight (two doses, one on day 0 at 150 mg/kg body weight and the second on day 4 at 100 mg/kg weight) or by injection of 250 μg of purified anti-Gr-1 monoclonal antibody (no azide/low endotoxin [NA/LE], RB6-8C5; BD Pharmingen) 1 day prior to bacterial challenge. The control mice were given either PBS or an equivalent amount of purified rat immunoglobulin G (rIgG; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Both of these methods have been successfully used by others to generate neutropenic conditions in mice (8, 19, 28, 47).
To confirm neutrophil depletion, the blood was collected by retro-orbital bleeding after mice were anesthetized by using a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg). In the case of animals depleted for neutrophils by using a cyclophosphamide procedure, we challenged normal and neutropenic mice with three different doses of A. hydrophila: 1 × 106, 5 × 106, and 1.5 × 107 CFU (n = 5, each group). Since RB6-8C5 antibodies are expensive, we challenged normal and neutropenic mice with only 1.5 × 107 CFU of A. hydrophila (n = 5, each group). Mice were observed for survivability for up to 16 days postinfection.
Bacterial phagocytosis by murine macrophages.
RAW 264.7 murine macrophages were seeded in six-well plates and incubated until they reached 60 to 70% confluence (approximately 3 × 106 cells/well). Macrophages were treated with either 200 μM C4-HSL, C6-HSL, C4-HSL and C6-HSL together, or vehicle for 1 h, and then the host cells were infected with A. hydrophila at a multiplicity of infection (MOI) of 10. Phagocytosis assay was performed according to the procedure described in our previous study (37). Briefly, after infection, the plates were centrifuged at 1,500 rpm for 10 min to facilitate binding of the bacteria to the host cells. After 30 min of incubation, extracellular bacteria were killed by gentamicin treatment (150 μg/ml) for 1 h, and then the macrophages were washed and lysed with 300 μl of 0.1% Triton X-100 solution. Suspensions of lysed macrophages were serially diluted, and bacterial colonies were counted after incubation at 37°C for 12 to 18 h.
Statistics.
Wherever applicable, at least three independent experiments were performed, and the data were analyzed by using the Student t test or one-way analysis of variance (ANOVA), with P values of ≤0.05 considered statistically significant. The animal data were analyzed by using the Kaplan-Meier survivability test, with P values of ≤0.05 considered statistically significant.
RESULTS
AHL pretreatment provides protection to mice against infection.
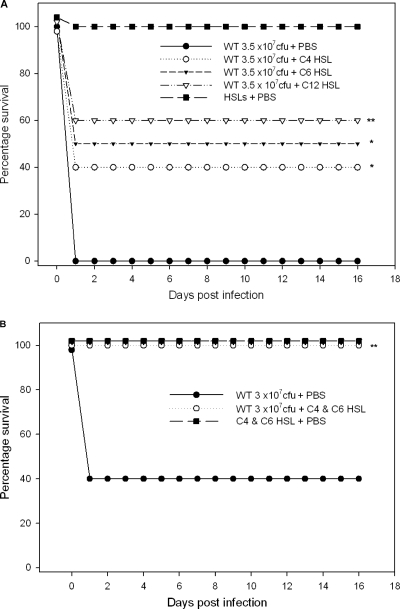
Animals that were pretreated with 250 μM C4-HSL, C6-HSL, or 3-oxo-C12-HSL at 12 h and 6 h before A. hydrophila challenge with 3.5 × 107 CFU were provided protection at rates of 40% (P = 0.03), 50% (P = 0.01), and 60% (P = 0.004), respectively, compared to the rate for control mice (100% death rate) that were treated with PBS and then infected with the same dose of bacteria (Fig. 1 A). Importantly, 100% (P = 0.004) of the mice pretreated with a combination of 250 μM (each) C4-HSL and C6-HSL at 12 h and 6 h before A. hydrophila challenge with 3 × 107 CFU were protected, whereas 60% of the control animals that were pretreated with PBS died at this dose (Fig. 1B). Importantly, similar protection of mice was noted when they were pretreated with smaller tested amounts of HSLs (C4-HSL and C6-HSL combined, 100 and 25 μM each) (data not shown). For most of our subsequent studies, we opted to use larger amounts of HSLs, i.e., 250 μM.
Fig. 1.
Survival of Swiss-Webster mice that were pretreated with AHLs by the i.p. route prior to A. hydrophila challenge. Percentages of surviving mice over time are shown, and the data were analyzed by using the Kaplan-Meier survival estimates. (A) Mice (n = 10, each group) were treated with 250 μM per 100-μl dose each of C4-HSL, C6-HSL, 3-oxo-C12-HSL, or PBS (control) at 12 h and 6 h before A. hydrophila challenge by the i.p. route with 3.5 × 107 CFU. Mice (n = 5, each group) were also treated with 250 μM (each) C4-HSL, C6-HSL, and 3-oxo-C12-HSL without bacterial infection and served as controls for determining toxicity of HSL molecules. The survival percentages were statistically significant between control and C4-HSL-treated (*, P = 0.03), C6-HSL-treated (*, P = 0.01), and 3-oxo-C12-HSL-treated (**, P = 0.004) mice after A. hydrophila infection. (B) Mice were treated with combined doses of C4-HSL and C6-HSL (250 μM each) or PBS (n = 10, each group) 12 h and 6 h before A. hydrophila challenge with 3 × 107 CFU. Mice were observed for deaths for 16 days postinfection. Mice (n = 5) were also treated with combined doses of C4-HSL and C6-HSL (250 μM each) without bacterial infection as a toxicity control. **, statistically significant difference in the survival rate of mice between control and combined treatment with C4-HSL and C6-HSL (P = 0.004) after bacterial infection.
In addition, to further dissect the significance of HSLs in A. hydrophila infection, we pretreated mice with C4- and C6-HSLs (250 μM each) in combination at 12 h and 6 h prior to challenge with the ahyRI double-knockout QS mutant of A. hydrophila SSU that is unable to produce its HSLs and also does not respond to exogenous HSLs (23). Mice that were given PBS instead of HSLs but challenged with the mutant were used as a control. We found that HSL pretreatment (C4-HSL plus C6-HSL) also protected mice (75%) from subsequent infection with the QS ahyRI mutant (data not shown). These data suggested that immunomodulation by HSLs protected mice irrespective of whether or not A. hydrophila produced its own QS signaling molecules.
Further, we performed experiments in which HSLs were given to mice at the same time as the bacterial challenge, and no protection was observed, suggesting that HSLs acted in a prophylactic manner (data not shown). We injected 250 μM (each) HSL or 500 μM C4- and C6-HSLs in combination into control mice without infection, and there were no signs or symptoms of discomfort in animals during the duration of the experiment (16 days), and none of the mice died, indicating that the HSLs themselves were not toxic to the animals (Fig. 1A and B). Although 3-oxo-C12-HSL is mainly produced by Pseudomonas aeruginosa and not A. hydrophila, we also examined the protective and immunomodulatory role of this lactone in our study to compare the efficiency and the mechanism of protection of animals by different AHL QS signaling molecules during A. hydrophila infection.
Changes in blood chemistry following A. hydrophila infection of mice with and without AHL pretreatment.
To study systemic effects in mice at different time points following A. hydrophila infection and to evaluate the influence of AHL pretreatment on the blood chemistry, we analyzed blood samples at 0, 4, 12, and 24 h postinfection. As mentioned in the Materials and Methods section, animals were pretreated by the i.p. route with either C4-HSL and C6-HSL in combination or vehicle control at 12 h and 6 h prior to challenge with 3 × 107 CFU of A. hydrophila.
During A. hydrophila infection of mice without AHL pretreatment, the level of ALT was increased from 51 U/liter at 0 h to 76.6 U/liter at 4 h (Table 1), 127.4 U/liter at 12 h, and 159.25 U/liter at 24 h (data not shown) postinfection. Likewise, the level of AMY was increased from 1,106 U/liter at 0 h to 1,543.6 U/liter at 4 h (Table 1) and reached 1,930.5 U/liter at 24 h (data not shown). The phosphorus level increased from 12.14 mg/dl at 0 h to 16.06 mg/dl at 4 h and remained at a similar level or was slightly decreased at 12 h and 24 h postinfection. On the other hand, the GLU level decreased over the course of A. hydrophila infection; its level was 226.4 mg/dl at 4 h postinfection (Table 1); this level dropped to 87.2 mg/dl at 12 h and dipped further to 70.5 mg/dl at 24 h postinfection (data not shown). The levels of ALB and ALP also decreased over the time course of infection (data not shown).
Table 1.
Alteration in blood chemistry during Aeromonas hydrophila infection
| Blood component (unit) | Mean concn ± SDd |
|||
|---|---|---|---|---|
| Vehiclea | AHLsa | Vehicle + A. hydrophilab | AHLs + A. hydrophilac | |
| ALB (g/dl) | 3.82 ± 0.2 | 3.78 ± 0.2 | 3.46 ± 0.3 | 2.94 ± 0.6 |
| ALP (U/liter) | 142.8 ± 36.8 | 136.4 ± 30.1 | 89.4 ± 13.9 | 77 ± 26.8 |
| ALT (U/liter) | 51 ± 11.3 | 42 ± 7.3 | 76.6 ± 18.4 | 48.8 ± 12.8 |
| AMY (U/liter) | 1,106 ± 372.9 | 1,149 ± 230.5 | 1,543.6 ± 486.8 | 1,092.6 ± 173.1 |
| TBIL (mg/dl) | 0.2 ± 0.0 | 0.22 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| BUN (mg/dl) | 14.2 ± 1.8 | 17.4 ± 6.0 | 14.2 ± 3.6 | 10.6 ± 2.8 |
| CA (mg/dl) | 12.3 ± 0.7 | 12.88 ± 0.6 | 12.04 ± 0.1 | 11.36 ± 0.2 |
| PHOS (mg/dl) | 12.14 ± 0.6 | 12.56 ± 1.0 | 16.06 ± 1.6 | 13.84 ± 1.5 |
| CRE (mg/dl) | 0.26 ± 0.1 | 0.22 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| GLU (mg/dl) | 390.2 ± 44.0 | 375.6 ± 102.6 | 226.4 ± 21.7 | 389.4 ± 122.9 |
| Na+ (mmol/liter) | 154 ± 1.2 | 154.2 ± 3.0 | 155.2 ± 1.6 | 152.8 ± 4.1 |
| K+ (mmol/liter) | 7.6 ± 1.0 | 7.68 ± 1.0 | 8.14 ± 0.4 | 7.6 ± 1.5 |
| TP (g/dl) | 5.2 ± 0.3 | 5.44 ± 0.3 | 4.78 ± 0.2 | 4.36 ± 0.8 |
| GLOB (g/dl) | 1.36 ± 0.2 | 1.7 ± 0.1 | 1.34 ± 0.1 | 1.4 ± 0.2 |
Blood chemistry was analyzed for mice that were treated only with vehicle or AHLs at 12 h and 6 h without A. hydrophila infection.
Blood chemistry was analyzed for mice that were treated with vehicle at 12 h and 6 h before challenge with A. hydrophila (3 × 107 CFU). Blood was collected at 4 h postinfection.
Blood chemistry was analyzed for mice that were treated with C4-HSL and C6-HSL in combination at 12 h and 6 h before challenge with A. hydrophila (3 × 107 CFU). Blood was collected at 4 h postinfection.
Data are shown as average mean values of five mice, and standard deviations (SDs) were determined.
These changes in blood chemistry indicated kidney and liver damage, hypoglycemia, and a nutritional disorder in mice due to A. hydrophila infection, leading to their demise. Interestingly, blood chemistry results revealed that prior treatment of animals with C4-HSL and C6-HSL combined rescued them from these devastating systemic effects of A. hydrophila infection. For instance, the ALT level remained normal at 48.8 U/liter at 4 h postinfection when mice were pretreated with AHLs, while this value was higher (76.6 U/liter) in mice infected with A. hydrophila without AHL pretreatment (Table 1). In addition, AHL pretreatment helped mice to retain glucose, amylase, and phosphorus levels at normal levels after 4 h of A. hydrophila infection. These data indicated that AHL pretreatment was beneficial to mice when they were subsequently infected with A. hydrophila (Table 1). Treatment of mice only with AHLs at 12 h and 6 h without the A. hydrophila infection did not result in any alterations in blood chemistry (Table 1).
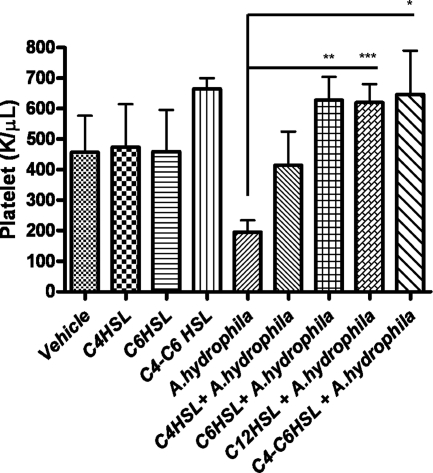
AHL pretreatment rescues mice from thrombocytopenia evoked by A. hydrophila.
The number of platelets dropped significantly in animals challenged with A. hydrophila without AHL pretreatment compared with that in vehicle control- and AHL-treated control mice without the infection, indicating that A. hydrophila induced thrombocytopenia in the animals (Fig. 2). Thrombocytopenia is a key feature of HUS (14, 39), which could be one of the major causes of animal death after A. hydrophila infection. Pretreatment of mice with C4-HSL, C6 HSL, 3-oxo-C12-HSL, or a combination of C4- and C6-HSL increased platelet counts of animals to a level within the normal range (Fig. 2), which further supported our notion that AHL pretreatment improved mouse survivability during A. hydrophila infection.
Fig. 2.
Prior AHL treatment rescued mice from thrombocytopenia induced by A. hydrophila. Platelets were counted in blood collected from mice (n = 5, each treatment group) that were pretreated (i.p.) with C4-HSL, C6-HSL, a combination of C4-HSL and C6-HSL, or 3-oxo-C12-HSL and/or vehicle for 12 h and 6 h with and/or without A. hydrophila infection. AHL pretreatment without A. hydrophila infection did not influence platelet numbers. During infection without AHL treatment, the platelets were significantly decreased (thrombocytopenia), while AHL pretreatment before infection (**, P = 0.001 for C6-HSL; ***, P = 0.0004 for 3-oxo-C12-HSL; *, P = 0.016 for C4- and C6-HSLs) normalized the platelet numbers to the normal range. Asterisks denote statistically significant platelet count differences between controls (without the AHL treatment) and with AHL pretreatment followed by the infection. K, thousands of cells.
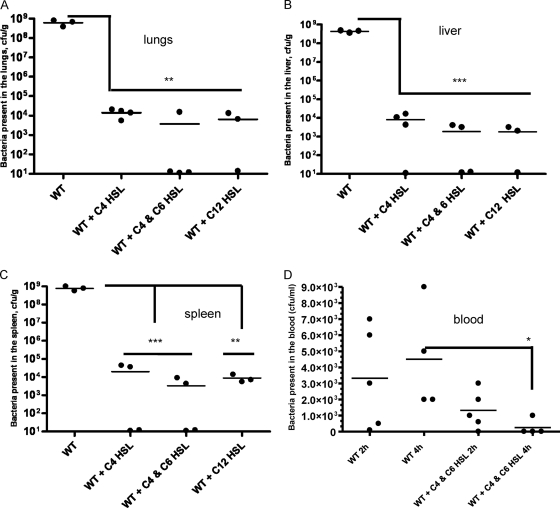
AHL pretreatment enhances bacterial clearance from mice.
To examine whether the enhanced survivability of AHL-pretreated animals following A. hydrophila infection was due to rapid clearance of bacteria from different tissues (lungs, liver, and spleen), we determined bacterial loads in these organs. Briefly, we pretreated mice with 250 μM C4-HSL, the combination of C4-HSL and C6-HSL, and/or 3-oxo-C12-HSL at 12 h and 6 h before A. hydrophila challenge with 3.5 × 107 CFU. All of the mice that were infected only with bacteria without the HSL treatment developed acute infection. At 48 h postinfection, significantly reduced numbers of bacteria were found in the lungs (Fig. 3 A), liver (Fig. 3B), and spleen (Fig. 3C) of mice pretreated with C4-HSL, C4-HSL and C6-HSL in combination, or 3-oxo-C12-HSL compared to those in animals given PBS and then infected with the bacteria.
Fig. 3.
AHL pretreatment enhanced bacterial clearance from different mouse tissues and blood. To measure bacterial burden, we randomly obtained tissues from 7 out of 10 animals (with n = 4 at 24 h p.i. [data not shown] and n = 3 at 48 h p.i.) that were infected only with A. hydrophila without HSL pretreatment. We randomly obtained tissues from 3 to 4 animals at 48 h p.i. that responded to different HSL pretreatments. As evident from Fig. 1, some mice did not respond to HSL pretreatment and died after A. hydrophila infection. Data for these animals were excluded when the data were plotted. Mice were pretreated (i.p.) with C4-HSL, C4-HSL and C6-HSL combined, 3-oxo-C12-HSL, or PBS at 12 h and 6 h before A. hydrophila challenge (i.p.) with 3.5 × 107 CFU. At 48 h p.i., bacterial loads were determined in the lungs (A), liver (B), and spleen (C) after culturing on the LB agar plates. (D) Mice were pretreated (i.p.) with the combined dose of C4-HSL and C6-HSL or PBS (control) at 12 h and 6 h before A. hydrophila challenge (i.p.) with 3.5 × 107 CFU. At 2 h (n = 5, each group) and 4 h (n = 4, each group) p.i., the bacterial load was determined in the blood. Statistical analysis was performed between AHL- and PBS-treated infected animals using the Student t test, and P values of ≤0.05 were considered significant. WT, wild type (A. hydrophila SSU); *, P = 0.04; **, P = 0.001; ***, P = 0.0007.
The average number of bacteria in the tissues (lungs, liver, and spleen) of control mice at 24 h postinfection (n = 4) was approximately 5 × 107 CFU per gram of tissue (data not shown), and this number was as high as 1 × 109 CFU per gram of tissue (n = 3) at 48 h postinfection (Fig. 3A to C). On the contrary, on average, there were approximately 1 × 104 CFU in AHL-pretreated mice (n = 3 to 4) at 48 h postinfection (Fig. 3A to C). Since A. hydrophila infection leads to bacteremia, we also investigated the bacterial load in the blood at the early time points of 2 h and 4 h. For these experiments, mice were pretreated with 250 μM (each) C4-HSL and C6-HSL in combination prior to A. hydrophila challenge. At 2 h and 4 h postinfection, the bacterial load was determined in AHL- and PBS-treated mice. The bacterial counts in control mice were higher, i.e., 3.3 × 103 to 4.6 × 103 CFU/ml of blood after 2 to 4 h of infection, than those in the AHL-pretreated mice (Fig. 3D). The average bacterial count in the AHL-pretreated mice at 2 h was approximately 1.3 × 103 CFU/ml, and this number was significantly reduced at 4 h (5 × 102 CFU/ml) (Fig. 3D).
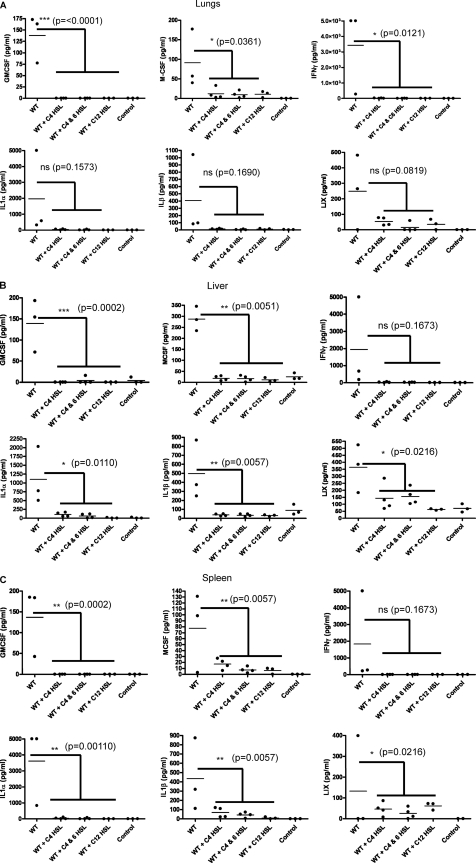
AHL pretreatment of mice reduces cytokine/chemokine levels in different tissues induced by bacterial infection.
Increased levels of proinflammatory cytokines/chemokines may cause detrimental effects in the host, and thus, we measured and compared their levels in different tissues between control and AHL-pretreated mice to determine the extent of inflammation by using a BioPlex assay. Mice were pretreated with C4-HSL, a combination of C4-HSL and C6-HSL, and/or 3-oxo-C12-HSL for 12 h and 6 h before challenge with 3.5 × 107 CFU of A. hydrophila. The levels of 23 cytokines/chemokines were determined in the lungs, liver, and spleen of control and AHL-pretreated mice. Irrespective of the types of AHLs used for the pretreatment or the tissues examined, in general, when mice were pretreated with AHLs, most of the cytokine/chemokine levels were reduced compared to those in PBS-treated and infected control mice at 48 h postinfection.
Further, our data indicated that in the lungs, the levels of 3 cytokines/chemokines, i.e., granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), and gamma interferon (IFN-γ), were significantly reduced in mice when they were pretreated with C4-HSL, C4-HSL and C6-HSL combined, and/or 3-oxo-C12-HSL compared to the findings in control animals at 48 postinfection with A. hydrophila (Fig. 4A). Although IL-1α, IL-1β, and lipopolysaccharide-inducible CXC chemokine (LIX) levels were also reduced in the HSL-pretreated and infected mice compared to control animals, the results were not statistically significant (Fig. 4A). Likewise, in liver and spleen, while the levels of five cytokines/chemokines, i.e., GM-CSF, M-SCF, IL-1α, IL-1β, and LIX, were significantly reduced in HSL-pretreated mice compared to those in control mice at 48 h postinfection, the IFN-γ level was also reduced in these tissues, but the data were not statistically significant (Fig. 4B and C). In addition, the levels of cytokines and chemokines such as GM-CSF, M-CSF, and LIX in these tissues of control mice (infected but not AHL pretreated) were similar at 24 h and 48 h postinfection; however, the levels of IFN-γ, IL-1α, and IL-1β were 3 to 5 times lower at 24 h than at 48 h postinfection (data not shown). Importantly, there were no significant changes in the cytokine/chemokine levels in these tissues from mice that were treated with AHLs alone compared to those in the PBS-treated control animals without the A. hydrophila challenge (see Fig. S1A to C in the supplemental material).
Fig. 4.
AHL pretreatment reduced cytokine/chemokine levels in different mouse tissues. To measure cytokine and chemokine levels, we randomly obtained tissues from 7 out of 10 animals (with n = 4 at 24 h p.i. [data not shown] and n = 3 at 48 h p.i.) that were infected only with A. hydrophila without HSL pretreatment. We randomly obtained tissues from 3 to 4 animals at 48 h p.i. that responded to different HSL pretreatments. As is evident from Fig. 1, some mice did not respond to HSL pretreatment and died after A. hydrophila infection. Data for these animals were excluded when the data were plotted. Mice were pretreated (i.p.) with C4-HSL, C4-HSL and C6-HSL combined, 3-oxo-C12-HSL, or PBS at 12 h and 6 h before A. hydrophila challenge (i.p.) with 3.5 × 107 CFU. Cytokines/chemokines were measured after 48 h p.i. by using the BioPlex assay. The data are shown for the level of six different cytokines/chemokines, i.e., GM-CSF, M-CSF, IFN-γ, IL-1-α, IL-1-β, and LIX, from the lungs (A), liver (B), and spleen (C) of AHL-pretreated and PBS-treated infected mice. The basal levels of cytokines/chemokines from noninfected mice that were treated only with PBS (control) were also measured. Statistical analysis between AHL- and PBS-treated infected animals was performed by using one-way ANOVA, and P values of ≤0.05 were considered significant. WT, wild type (A. hydrophila SSU); ns, not significant.
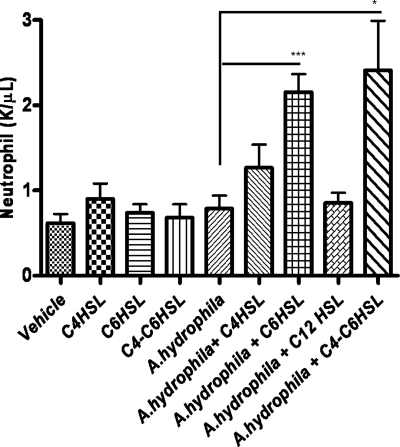
AHL pretreatment induces neutrophil recruitment in the blood of infected mice.
To determine the specific mechanism(s) as to how AHL-pretreated mice were clearing the bacteria, we evaluated the counts of various cells in blood collected from AHL-pretreated and control (vehicle-treated) mice with and without A. hydrophila infection by using an automated hematology system. After 4 h of bacterial challenge, the number of neutrophils in the blood collected from C6-HSL-pretreated mice was significantly higher than that in the blood of the control animals that were given only vehicle (Fig. 5). Pretreatment with C4-HSL slightly increased neutrophil counts compared to those in controls, but the data were not statistically significant, whereas prior 3-oxo-C12-HSL treatment did not induce neutrophil recruitment in the blood of mice when they were challenged with A. hydrophila. Without the bacterial challenge, the number of neutrophils in blood from AHL- and vehicle-treated mice was similar (Fig. 5), which suggested to us that neutrophil recruitment in the blood required both the AHL treatment and exposure to different antigens of A. hydrophila. The other cell types, such as monocytes, eosinophils, and basophils, in the blood were not affected by the AHL pretreatment and infection (data not shown).
Fig. 5.
Prior C4-HSL and C6-HSL treatment enhanced neutrophil recruitment in the blood. Neutrophils were counted in the blood collected from mice (n = 5, each treatment group) pretreated (i.p.) with C4-HSL, C6-HSL, C4-HSL and C6-HSL combined, or 3-oxo-C12-HSL and/or vehicle for 12 h and 6 h with and/or without A. hydrophila infection. AHL pretreatment without A. hydrophila infection did not increase neutrophils, while AHL pretreatment, specifically in C6-HSL-treated and infected animals, significantly increased neutrophil numbers in the blood. Statistical analysis between AHL- and vehicle-treated infected animals was performed by using the Student t test, and P values of ≤0.05 were considered significant. Increased neutrophil recruitment in the blood with treatment with C6-HSL (***, P = 0.0008) and C4-HSL and C6 HSL in combination (*, P = 0.0273) was statistically significant compared to that for the vehicle-treated and infected control mice. K, thousands of cells.
Neutropenic mice are more susceptible to A. hydrophila infection.
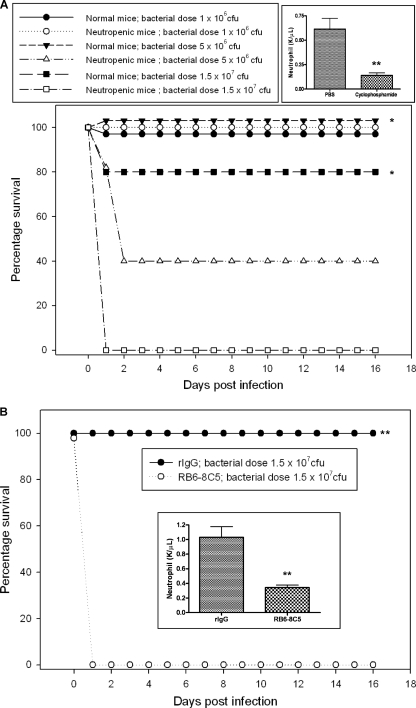
To further investigate the role of neutrophils in A. hydrophila infection, we depleted this cell population from animals by either cyclophosphamide or anti-Gr-1 monoclonal antibody treatment. Both of these treatments specifically depleted the neutrophil population from mice (Fig. 6 A and B).
Fig. 6.
Effect of inducing neutropenia by using cyclophosphamide or anti-Gr-1 monoclonal antibody (RB6-8C5) on the survival rates of mice after challenge with A. hydrophila. (A) Neutrophils were depleted by the i.p. injection of 250 mg of cyclophosphamide/kg body weight, and control mice were pretreated with PBS (n = 5, each group). Neutrophil depletion was confirmed and was statistically significant (**) by counting neutrophil numbers in the blood after treatment with cyclophosphamide. Normal and neutropenic mice were challenged with three different doses of A. hydrophila, 1 × 106, 5 × 106, and 1.5 × 107 CFU (n = 5, each group), and deaths were observed for 16 days postinfection. Percentages of surviving mice over time are shown, and the data were analyzed by using the Kaplan-Meier survival estimates. *, statistically significant differences in the percentage of neutropenic and normal mice surviving following challenge with 5 × 106 (P = 0.05) and 1.5 × 107 (P = 0.014) CFU of A. hydrophila. (B) Neutrophils were depleted by the i.p. injection of 250 μg of RB6-8C5, and control mice were given equivalent amounts of purified rIgG (n = 5, each group). (Inset) Neutrophil depletion was confirmed and was statistically significant (**) by counting neutrophil numbers in the blood obtained by retro-orbital bleeding of normal and neutropenic mice prior to challenge with A. hydrophila at a dose of 1.5 × 107 CFU (n = 5, each group), and deaths were observed for 16 days postinfection. Percentages of surviving mice over time are shown, and the data were analyzed by using Kaplan-Meier survival estimates. **, statistically significant difference in survival rate between neutropenic and normal mice (P = 0.003).
To determine susceptibility to A. hydrophila infection, normal and neutropenic mice (generated by cyclophosphamide treatment) were challenged with three different doses of bacteria: 1 × 106, 5 × 106, and 1.5 × 107 CFU. Interestingly, 60% of the neutropenic mice died at 48 h postinfection and the remaining 40% survived 16 days of the observation period when they were challenged with 5 × 106 CFU of A. hydrophila. All of the normal mice were healthy and survived the duration of the experiment at this dose. This difference in survival rate between normal and neutropenic mice was statistically significant (P = 0.05) (Fig. 6A). At the dose of 1.5 × 107 CFU, all of the neutropenic mice died at 48 h postinfection, while 80% of the normal mice survived (Fig. 6A), and these data were statistically significant (P = 0.014). However, none of the neutropenic or normal mice died when they were challenged with 1 × 106 CFU of A. hydrophila. Since by using cyclophosphamide we also reduced the number of other immune cells, such as monocytes (data not shown), in the blood, we specifically depleted the neutrophil population in the blood by using anti-Gr-1 monoclonal antibody RB6-8C5. When mice rendered neutropenic by this antibody method were challenged with 1.5 × 107 CFU of A. hydrophila, all of the neutropenic mice died within 24 h postinfection, whereas all the normal mice survived (P = 0.003) and remained healthy for the duration of the experiment at this dose (Fig. 6B).
AHL pretreatment induces bacterial phagocytosis by murine macrophages in vitro.
Our data provided evidence that at earlier time points, AHL pretreatment stimulated neutrophil recruitment in the blood. Since bacterial dissemination to different tissues (lungs, liver, and spleen) also decreased in mice pretreated with AHLs, we hypothesized that at the later time course of A. hydrophila infection, AHL pretreatment might trigger other immune cells, such as monocytes/macrophages, in different tissues to clear bacteria. To address this scenario, we performed an in vitro phagocytosis assay by using RAW 264.7 murine macrophages after treatment with C4-HSL, C6-HSL, C4-HSL and C6-HSL combined, or vehicle only for 1 h. Interestingly, we observed that treatment of either type of AHL or AHLs in combination increased the phagocytosis of A. hydrophila by murine macrophages (Fig. 7). For example, compared with the findings in PBS-treated control macrophages, the number of phagocytosed bacteria was approximately double when macrophages were treated with C4-HSL, this number was approximately 4 times higher in the case of treatment with C6-HSL, and the number was 6 times higher when macrophages were treated with C4-HSL and C6-HSL in combination (Fig. 7).
Fig. 7.
Prior AHL treatment of murine macrophages induced bacterial phagocytosis. RAW 264.7 murine macrophages were treated with 200 μM C4-HSL, C6-HSL, C4-HSL and C6-HSL together, or vehicle for 1 h. Macrophages were infected with A. hydrophila at an MOI of 10, and after extracellular bacteria were killed by gentamicin treatment, host cells were lysed and the intracellular bacteria on the LB agar plates were counted. Data from at least three independent experiments (each treatment in triplicate) were averaged, and the standard deviations are shown. Asterisks describe statistically significant differences in phagocytosis between AHL-treated and vehicle-treated control murine macrophages.
DISCUSSION
Communication through the QS systems facilitates bacterial population coordination of activities in the environment as well as in the host. QS represents a global regulator in bacteria, because it controls many important biological activities, including motility, virulence factor production, and biofilm formation (9–11, 16, 18, 27). Recent studies also unveiled the fact that QS signaling molecules, such as AHLs, not only are the regulators in bacteria but also modulate different host cells (38, 40–42, 44, 51). However, the mechanisms of this modulation of host cells by AHLs are largely unknown.
It was recently shown that immunization of mice with 3-oxo-C12-HSL–bovine serum albumin conjugate induced antibody responses which protected animals from lethal P. aeruginosa lung infection (31). However, currently, it is not known whether AHL QS signaling molecules induce protective innate immune responses against bacterial infection in an animal model. Consequently, the present study examined the immunomodulatory role of different types of AHLs in a septicemic mouse model of A. hydrophila infection. The novel findings of our study are that pretreatment of mice with AHLs (C4-HSL, C6-HSL, or 3-oxo-C12-HSL) prevents clinical sequelae during A. hydrophila infection and thus provides mice protection against mortality and that AHLs modulate the innate immune response of animals to clear bacterial infection in a prophylactic manner.
Commercially available HSLs, such as C4-HSL and C6-HSL, were purified from Chromobacterium violaceum, and 3-oxo-C12-HSL was purified from P. aeruginosa. These HSL molecules and their receptor protein LuxR are well conserved in different Gram-negative bacteria. In our recent study, we showed that exogenous addition of both of the C4- and C6-HSLs to an autoinducer synthase (ahyI; ahyI is a homolog of luxI) mutant of A. hydrophila SSU restored phenotypic properties (such as biofilm formation, metalloprotease production, and secretion of type 6 secretion effectors) that are controlled by the AhyRI QS system to the levels in wild-type bacteria (23). These data clearly suggested that the C4- and C6-HSLs produced by A. hydrophila SSU are functionally similar to those produced by other bacteria.
We used 250 μM amounts of HSLs in our animal studies, as P. aeruginosa can produce as much as 600 μM 3-oxo-C12-HSLs in biofilms (3). Although in vivo concentrations of HSLs are noted to be in the nanomolar to low-micromolar range, it has been reported that physiological pH and temperature conditions can reduce detectable levels of HSLs, and thus, the actual concentrations of HSLs in the tissues appear to be much lower (52). Importantly, however, we also demonstrated that smaller amounts of HSLs (25 μM [each] C4-HSL and C6-HSL in combination) provided a similar level of protection in mice as the 250 μM dose.
In our study, treatment of RAW 264.7 cells with as much as 400 μM C4-HSL and C6-HSL in combination for 2 to 4 h did not induce any cell cytotoxicity. Therefore, the use of HSLs at concentrations of 250 μM in vivo and 200 μM in vitro in the present study was relevant to the use of HSLs as an option for treatment. Further, these doses were comparable to those in other earlier studies, in which similar concentrations of HSLs were employed (20, 38).
A. hydrophila induced thrombocytopenia in mice (Fig. 2), which is a major feature of HUS caused by E. coli. However, the mechanism by which the platelets are depleted by A. hydrophila is not known. A recent study showed that the downregulation of platelet surface CD47 expression was associated with Shiga toxin-producing Escherichia coli, which could possibly represent the mechanism of HUS associated with such E. coli strains (14). Strikingly, our data showed that AHL pretreatment of mice normalized systemic effects, including platelet numbers, and improved animal survival rates following A. hydrophila infection.
A. hydrophila produces an array of virulence factors, and the ability of this pathogen to disseminate to any organ via blood contributes to its pathogenesis (5). Therefore, induction of an innate immune response and the clearance of bacteria from the blood and other organs would be crucial as the first line of host defense against Aeromonas infection. Our data provided evidence that the AHL pretreatment of mice increased bacterial clearance from the blood, as well as other tissues (Fig. 3), which correlated with enhanced mouse survivability (Fig. 1A and B).
In general, the levels of various cytokines/chemokines were very high in the lungs, liver, and spleen when mice were challenged with A. hydrophila without AHL pretreatment, suggesting that animals would die because of host tissue damage as a result of a proinflammatory cytokine storm (Fig. 4A to C). Interestingly, the level of these cytokines/chemokines was decreased in tissues of mice pretreated with AHL, resulting in a reduced inflammatory response and improved survivability of mice. Previous studies also reported that treatment with AHLs, specifically, 3-oxo-C12-HSL, reduced proinflammatory cytokine production in various host cells in vitro (45), as well as inhibited LPS-induced proinflammatory cytokine production in a mouse model by disrupting NF-κB signaling (24).
On the contrary, Smith et al. showed that dermal injection of 3-oxo-C12-HSL in mice induced the production of various proinflammatory cytokines (41). These discrepancies in results could be attributed to differences in the concentrations of AHLs that were used, differences in the host cell types and mouse strains employed, and/or the different routes by which AHL treatment was administered to animals. We noted that only AHL treatment in mice without A. hydrophila infection did not influence cytokine production, suggesting that other bacterial components might be necessary in modulating this inhibitory effect of AHLs. Earlier studies reported that small acyl chains, such as C4-HSL, did not show any immunomodulatory activity in vitro (44, 54); however, in contrast, our in vivo data indicated that all three AHLs, namely, C4-HSL, C6-HSL, and 3-oxo-C12-HSL, had immunomodulatory activity (Fig. 1 to 4).
Since the intensity of an inflammatory response can be directly related to the number of organisms present in various tissues of an infected animal, it is possible that the reduction in cytokine/chemokine levels that we noted in different organs of infected mice after AHL pretreatment could be due to the reduction of bacterial load. However, a direct role of these HSLs on cytokine/chemokine production in infected animals cannot be ruled out.
Neutrophils are the first line of host defense cells which are recruited from the blood to infection sites (2, 15). Several studies pointed out the crucial role that neutrophils play in the innate immune response in clearing invading pathogens (28, 47); however, the role of neutrophils in Aeromonas infection has not been studied. Intrigued by our findings of rapid clearance of A. hydrophila from the blood and other mouse tissues, we were prompted to examine the recruitment of various host defense cells in the blood after intraperitoneal treatment of mice with different types of AHLs. Importantly, C6-HSL pretreatment significantly increased neutrophil numbers at 4 h postinfection with A. hydrophila, while C4-HSL pretreatment marginally increased neutrophil numbers in the blood (Fig. 5). However, 3-oxo-C12-HSL pretreatment did not induce neutrophil recruitment in the blood at all. These data indicate that different AHLs might operate via different mechanisms.
These results are in contrast to those of studies in which 3-oxo-C12-HSL was shown to induce directed migration (chemotaxis) of human polymorphonuclear neutrophils (PMNs) in vitro (54), and it also upregulated the expression of receptors known to be involved in host defense, such as adhesion proteins CD11b/CD18 and the immunoglobulin receptors CD16 and CD64 (50). These data further reflect that immunomodulation by different AHLs may vary depending on in vitro and in vivo conditions. In addition to neutrophil recruitment in the blood, we speculate that AHL treatment might also stimulate various antimicrobial functions of neutrophils to clear bacteria from animals.
In our future studies, we plan to investigate the influence of AHL treatment on bacterial killing mechanisms of neutrophils, such as production of antimicrobial enzymes (e.g., myeloperoxidase and elastase) and antimicrobial peptides (e.g., defensins and cathelicidins), as well as neutrophil extracellular trap (NET) formation. In addition, it can be speculated from our data that different AHLs might activate different immune cells to clear bacteria from the host. Therefore, it would be interesting to investigate in the future whether C4-HSL, C6-HSL, or 3-oxo-C12-HSL also activates other immune cells, such as natural killer (NK) cells and/or dendritic cells (DCs), in vitro as well as in vivo.
The acyl side chain length as well as the substitutions on the side chain of HSLs provides signal specificity, and several studies showed that immunomodulation by different HSLs varies depending on the length of the side chain (44, 54). Likewise, in our study, we observed that the degree of neutrophil recruitment in the blood varied when mice were treated with different lactones (Fig. 5), and the survivability of mice also varied with different HSL pretreatments (Fig. 1). Therefore, it is plausible that different lactones are recognized by different pattern recognition receptors of the host cells.
Our results showed that the number of neutrophils was increased in the blood of infected mice that were treated with C4- and C6-HSLs but not with 3-oxo-C12-HSL, although a reduction in the number of bacteria was seen with all of the AHLs (Fig. 3). We believe that the protective effect of HSLs seen in mice is a result of a combination of several factors that culminated in reduction of the number of bacteria and cytokine and chemokines levels in different tissues (Fig. 4). Since by using the neutropenic mouse model we observed that neutrophils played an important role in the host defense against A. hydrophila infection, recruitment of these cells to the blood by C4- and C6-HSLs could be one of the several mechanisms as to how mice were clearing bacteria from the blood during the earlier course of infection.
In the phagocytosis assay (Fig. 7), the uptake rate of bacteria by macrophages was 2 times higher in the case of C6-HSL pretreatment than C4-HSL pretreatment, which matched our in vivo neutrophil data, whereby C6-HSL pretreatment recruited more neutrophils in the blood than did C4-HSL treatment (Fig. 5). Interestingly, we also observed that C6-HSL pretreatment provided better protection than C4-HSL in terms of mouse survivability (Fig. 1A). From these data, we conclude that C6-HSL is a more effective immunomodulator than C4-HSL in terms of inducing a protective immune response against A. hydrophila infection. We were not able to perform a phagocytosis assay after treatment of macrophages with 3-oxo-C12-HSL, as it induced cell lysis (data not shown), which was in accordance with findings from a previous study by Tateda et al. (44), in which they showed that 3-oxo-C12-HSL treatment accelerated apoptosis of macrophages and neutrophils isolated from mice.
On the contrary, other investigators reported that 3-oxo-C12-HSL treatment stimulated the phagocytic activity of human monocyte-derived macrophages (48) and human PMNs (50). Our in vitro phagocytosis results by using murine macrophages warrant further investigation through the evaluation of phagocytosis of bacteria by monocytes/macrophages in mice during infection (35) following pretreatment of animals with AHLs.
In conclusion, our study reveals that QS signaling molecules, such as AHLs, can stimulate protective innate immune responses in mice infected with A. hydrophila, which underscores the necessity of examining the immunotherapeutic potency of AHL molecules against other bacterial pathogens.
From an evolutionary standpoint, it is intriguing why bacteria would produce molecules that can be used in QS but that also result in their own killing. When bacteria infect a host, microbes express their virulence machineries only when they sense favorable conditions in terms of the bacterial population governed by a threshold level of lactones. By doing so, pathogens overcome host defenses to cause disease. On the other hand, pretreatment of a host (in our case, mice) with different HSLs helps the host to prepare against bacterial infections by activating different host defense/immune cells (e.g., neutrophils and macrophages) to efficiently clear bacteria from different tissues. This dual role of HSLs is not surprising, as many antibiotics produced by microbes under natural environmental conditions function as cell signaling molecules, including QS (53), in addition to their therapeutic function.
Supplementary Material
ACKNOWLEDGMENTS
Bijay K. Khajanchi is a recipient of the J. W. McLaughlin Predoctoral Fellowship, UTMB. The funding obtained from EPA and NIH grants (UC7 and AI041611) is duly acknowledged.
We thank Mardelle Susman for editing the manuscript and Giovanni Suarez for his technical assistance in performing the BioPlex assay.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 2 May 2011.
REFERENCES
- 1. Abuhammour W., Hasan R. A., Rogers D. 2006. Necrotizing fasciitis caused by Aeromonas hydrophilia in an immunocompetent child. Pediatr. Emerg. Care 22:48–51 [DOI] [PubMed] [Google Scholar]
- 2. Borregaard N., Cowland J. B. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503–3521 [PubMed] [Google Scholar]
- 3. Charlton T. S., et al. 2000. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ. Microbiol. 2:530–541 [DOI] [PubMed] [Google Scholar]
- 4. Chhabra S. R., et al. 2003. Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-l-homoserine lactone as immune modulators. J. Med. Chem. 46:97–104 [DOI] [PubMed] [Google Scholar]
- 5. Chopra A. K., et al. 2000. The cytotoxic enterotoxin of Aeromonas hydrophila induces proinflammatory cytokine production and activates arachidonic acid metabolism in macrophages. Infect. Immun. 68:2808–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Churchill M. E., Chen L. 2011. Structural basis of acyl-homoserine lactone-dependent signaling. Chem. Rev. 111:68–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooley M., Chhabra S. R., Williams P. 2008. N-Acylhomoserine lactone-mediated quorum sensing: a twist in the tail and a blow for host immunity. Chem. Biol. 15:1141–1147 [DOI] [PubMed] [Google Scholar]
- 8. Cote C. K., Van Rooijen N., Welkos S. L. 2006. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect. Immun. 74:469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies D. G., et al. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298 [DOI] [PubMed] [Google Scholar]
- 10. de Kievit T. R. 2009. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 11:279–288 [DOI] [PubMed] [Google Scholar]
- 11. de Kievit T. R., Iglewski B. H. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Figueras M. J., et al. 2007. Aeromonas hemolytic uremic syndrome. A case and a review of the literature. Diagn. Microbiol. Infect. Dis. 58:231–234 [DOI] [PubMed] [Google Scholar]
- 13. Galindo C. L., Gutierrez C., Jr., Chopra A. K. 2006. Potential involvement of galectin-3 and SNAP23 in Aeromonas hydrophila cytotoxic enterotoxin-induced host cell apoptosis. Microb. Pathog. 40:56–68 [DOI] [PubMed] [Google Scholar]
- 14. Guo Y. L., Liu D. Q., Bian Z., Zhang C. Y., Zen K. 2009. Down-regulation of platelet surface CD47 expression in Escherichia coli O157:H7 infection-induced thrombocytopenia. PLoS One 4:e7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hampton M. B., Kettle A. J., Winterbourn C. C. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007–3017 [PubMed] [Google Scholar]
- 16. Henke J. M., Bassler B. L. 2004. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 186:3794–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmberg S. D., et al. 1986. Aeromonas intestinal infections in the United States. Ann. Intern. Med. 105:683–689 [DOI] [PubMed] [Google Scholar]
- 18. Hussain M. B., et al. 2008. The acyl-homoserine lactone-type quorum-sensing system modulates cell motility and virulence of Erwinia chrysanthemi pv. zeae. J. Bacteriol. 190:1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inatsuka C. S., et al. 2010. Pertactin is required for Bordetella species to resist neutrophil-mediated clearance. Infect. Immun. 78:2901–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jahoor A., et al. 2008. Peroxisome proliferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer. J. Bacteriol. 190:4408–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaplan H. B., Greenberg E. P. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khajanchi B. K., et al. 2010. Distribution of virulence factors and molecular fingerprinting of Aeromonas species isolates from water and clinical samples: suggestive evidence of water-to-human transmission. Appl. Environ. Microbiol. 76:2313–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khajanchi B. K., et al. 2009. N-Acylhomoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology 155:3518–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kravchenko V. V., et al. 2008. Modulation of gene expression via disruption of NF-kappaB signaling by a bacterial small molecule. Science 321:259–263 [DOI] [PubMed] [Google Scholar]
- 25. Kravchenko V. V., et al. 2006. N-(3-Oxo-acyl)homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J. Biol. Chem. 281:28822–28830 [DOI] [PubMed] [Google Scholar]
- 26. Kumari A., Pasini P., Daunert S. 2008. Detection of bacterial quorum sensing N-acyl homoserine lactones in clinical samples. Anal. Bioanal. Chem. 391:1619–1627 [DOI] [PubMed] [Google Scholar]
- 27. Labbate M., et al. 2004. Quorum sensing-controlled biofilm development in Serratia liquefaciens MG1. J. Bacteriol. 186:692–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leendertse M., et al. 2009. Neutrophils are essential for rapid clearance of Enterococcus faecium in mice. Infect. Immun. 77:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li L., Hooi D., Chhabra S. R., Pritchard D., Shaw P. E. 2004. Bacterial N-acylhomoserine lactone-induced apoptosis in breast carcinoma cells correlated with down-modulation of STAT3. Oncogene 23:4894–4902 [DOI] [PubMed] [Google Scholar]
- 30. Marketon M. M., Gronquist M. R., Eberhard A., Gonzalez J. E. 2002. Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-acyl homoserine lactones. J. Bacteriol. 184:5686–5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyairi S., et al. 2006. Immunization with 3-oxododecanoyl-l-homoserine lactone-protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J. Med. Microbiol. 55:1381–1387 [DOI] [PubMed] [Google Scholar]
- 32. Parsek M. R., Greenberg E. P. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. U. S. A. 97:8789–8793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pearson J. P., Feldman M., Iglewski B. H., Prince A. 2000. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 68:4331–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pearson J. P., Van Delden C., Iglewski B. H. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pitz A. M., Perry G. A., Jensen-Smith H. C., Gentry-Nielsen M. J. 2010. A flow cytometric assay to quantify in vivo bacterial uptake by alveolar macrophages. J. Microbiol. Methods 81:194–196 [DOI] [PubMed] [Google Scholar]
- 36. Ritchie A. J., Yam A. O., Tanabe K. M., Rice S. A., Cooley M. A. 2003. Modification of in vivo and in vitro T- and B-cell-mediated immune responses by the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone. Infect. Immun. 71:4421–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sha J., et al. 2008. Braun lipoprotein (Lpp) contributes to virulence of yersiniae: potential role of Lpp in inducing bubonic and pneumonic plague. Infect. Immun. 76:1390–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shiner E. K., et al. 2006. Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signalling. Cell. Microbiol. 8:1601–1610 [DOI] [PubMed] [Google Scholar]
- 39. Siegler R. L. 1995. The hemolytic uremic syndrome. Pediatr. Clin. North Am. 42:1505–1529 [DOI] [PubMed] [Google Scholar]
- 40. Smith R. S., et al. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J. Immunol. 167:366–374 [DOI] [PubMed] [Google Scholar]
- 41. Smith R. S., Harris S. G., Phipps R., Iglewski B. 2002. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 184:1132–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith R. S., Kelly R., Iglewski B. H., Phipps R. P. 2002. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 169:2636–2642 [DOI] [PubMed] [Google Scholar]
- 43. Swift S., et al. 1999. Quorum sensing-dependent regulation and blockade of exoprotease production in Aeromonas hydrophila. Infect. Immun. 67:5192–5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tateda K., et al. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71:5785–5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Telford G., et al. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thiel V., Kunze B., Verma P., Wagner-Dobler I., Schulz S. 2009. New structural variants of homoserine lactones in bacteria. Chembiochem 10:1861–1868 [DOI] [PubMed] [Google Scholar]
- 47. van Faassen H., et al. 2007. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect. Immun. 75:5597–5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vikstrom E., Magnusson K. E., Pivoriunas A. 2005. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone stimulates phagocytic activity in human macrophages through the p38 MAPK pathway. Microbes Infect. 7:1512–1518 [DOI] [PubMed] [Google Scholar]
- 49. Vila J., et al. 2003. Aeromonas spp. and traveler's diarrhea: clinical features and antimicrobial resistance. Emerg. Infect. Dis. 9:552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wagner C., et al. 2007. The quorum-sensing molecule N-3-oxododecanoyl homoserine lactone (3OC12-HSL) enhances the host defence by activating human polymorphonuclear neutrophils (PMN). Anal. Bioanal. Chem. 387:481–487 [DOI] [PubMed] [Google Scholar]
- 51. Williams S. C., et al. 2004. Pseudomonas aeruginosa autoinducer enters and functions in mammalian cells. J. Bacteriol. 186:2281–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yates E. A., et al. 2002. N-Acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 70:5635–5646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yim G., Wang H. H., Davies J. 2007. Antibiotics as signalling molecules. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362:1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zimmermann S., et al. 2006. Induction of neutrophil chemotaxis by the quorum-sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone. Infect. Immun. 74:5687–5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.