Abstract
The Salmonella effector protein SopB has previously been shown to induce activation of Akt and protect epithelial cells from apoptosis in vitro. To characterize the role of Akt2 in host defense against Salmonella enterica serovar Typhimurium infection, wild-type (WT) mice and mice lacking Akt2 (Akt2 knockout [KO] mice) were infected using a Salmonella acute gastroenteritis model. Infected Akt2 KO mice showed a more pronounced morbidity and mortality associated with higher bacterial loads in the intestines and elevated levels of proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and MCP-1, in the colons at 1 day postinfection compared to those shown in WT mice. Histopathological assessment and immunohistochemical analysis of cecal sections at 1 day postinfection revealed more severe inflammation and higher levels of neutrophil infiltration in the ceca of Akt2 KO mice. Flow cytometry analysis further confirmed an increase in the recruitment of Gr-1+ CD11b+ neutrophils and F4/80+ CD11b+ macrophages in the intestines of infected Akt2 KO mice. Additionally, enhanced levels of annexin V+ and terminal transferase dUTP nick end labeling-positive (TUNEL+) apoptotic cells in the intestines of infected Akt2 KO mice were also observed, indicating that Akt2 plays an essential role in protection against apoptosis. Finally, the differences in bacterial loads and cecal inflammation in WT and Akt2 KO mice infected with WT Salmonella were abolished when these mice were infected with the sopB deletion mutant, indicating that SopB may play a role in protecting the mice from Salmonella infection through the activation of Akt2. These data demonstrate a definitive phenotypic abnormality in the innate response in mice lacking Akt2, underscoring the important protective role of Akt2 in Salmonella infection.
INTRODUCTION
Salmonella enterica is a Gram-negative facultative intracellular bacterial pathogen capable of infecting a number of hosts and causing significant morbidity and mortality globally (12). S. enterica serovar Typhimurium infection in humans is typically acquired by ingestion of contaminated food or water, leading to acute gastroenteritis with clinical manifestations of diarrhea, abdominal pain, nausea, and vomiting (12, 49). After ingestion, Salmonella reaches the small intestine, where it invades the mucosa by penetrating the epithelial barrier through microfold (M) cells, which subsequently transport it to lymphoid cells in the underlying Peyer's patches (29, 34), where the bacteria multiply and disseminate throughout the body. Such bacterial cell-epithelial cell interactions result in the secretion of chemoattractant molecules such as cytokines and chemokines that recruit neutrophils, monocytes, dendritic cells, and lymphocytes from the circulation to the site of infection (53). While the recruited phagocytes engulf and destroy the invading bacteria to help control the infection (40), infiltration of polymorphonuclear lymphocytes (PMNs) also causes the erosion of the intestinal mucosa, giving rise to the histopathological characteristics of intestinal inflammation (12, 16). Studies of the cellular and molecular mechanisms involved in Salmonella-induced intestinal inflammation have been greatly facilitated by the development of an acute gastroenteritis model in mice in response to oral S. Typhimurium infection (2). In this model, following oral administration of streptomycin, mice infected orally with S. Typhimurium develop acute colitis, showing signs of intestinal inflammation which manifests most prominently in the cecum and shares many of the pathological features of Salmonella enterocolitis in humans (5, 41).
Salmonella invasion of host cells requires bacterial proteins encoded in the chromosomal locus Salmonella pathogenicity island 1 (SPI1) (22). One SPI1 translocated effector protein, SopB, has previously been shown to be required for the activation of Akt in infected epithelial cells (54). The serine/threonine kinase Akt is expressed in 3 distinctly coded isoforms, namely Akt1, Akt2, and Akt3 (48). All 3 proteins share similar functions and structures (61) and are known to play a key role in cell survival and proliferation (37). Despite the high level of homology between these isoforms, Akt isoforms were found to distribute differently and may have different functions. Akt1 is expressed in most tissues and promotes cell survival by inhibiting apoptosis (7). Akt1 also induces protein synthesis and is critical to growth and development (9, 47). Akt2 has been shown to be present in intestinal cells (35), although it is expressed mainly in insulin-responsive organs, including liver, skeletal muscle, and adipose tissue, and functions primarily in insulin signaling (8, 11, 18, 23). Akt3 is expressed abundantly in the brain and testis, and mice lacking Akt3 have smaller brains (19, 57). While Akt activation is involved in the regulation of apoptosis in normal intestinal epithelial cells (65), the Salmonella-induced activation of Akt has been shown to protect epithelial cells from apoptosis (32).
Apoptosis, or programmed cell death, is a highly conserved mechanism for intracellular disassembly without changing membrane integrity (20, 32, 62). This cellular process can be characterized by the externalization of phosphatidylserine to the cell surface, DNA fragmentation, chromatin condensation, and release of apoptotic bodies (32, 62). Bacterial and viral pathogens can manipulate the host cell suicide mechanisms to enhance its pathogenicity (20, 32). Depending on the host cell type and stage of infection, Salmonella can induce different apoptotic responses (31, 32). Various mechanisms of these Salmonella-induced apoptotic events in different cell types have been identified, but their role in pathogenesis remains unclear (32). Previously, the sustained activation of Akt has been identified as an important prerequisite for the SopB-dependent antiapoptotic pathway in Salmonella-infected epithelial cells in vitro (32). It is believed that SopB is involved in the delay of apoptosis, although the role of apoptosis in gastroenteritis is still unclear (32).
Although the role of the prosurvival molecule Akt in infectious colitis has not yet been explored, Akt2 has been shown to play a role in enterocyte differentiation (35). In the present study, we used a Salmonella-induced intestinal inflammation model to examine the role of Akt2 in host defense against Salmonella infection. During Salmonella infection, we found that in mice lacking the functional protein kinase Akt2, host cells were more prone to apoptosis and more susceptible to infection.
MATERIALS AND METHODS
Bacterial culture.
S. enterica serovar Typhimurium SL1344 (26) and the sopB deletion mutant (ΔsopB mutant) (30, 54) were grown overnight with shaking (200 rpm) in Luria-Bertani (LB) broth supplemented with 50 μg/ml streptomycin at 37°C for 18 h.
Mice.
Wild-type (WT) 129S and homozygous 129S Akt2-deficient (Akt2 knockout [KO]) mice, originally from the Wellcome Trust Sanger Institute (Hinxton, Cambridge, United Kingdom), were bred at the Vaccine and Infectious Disease Organization, University of Saskatchewan. Adult mice (8 to 10 weeks) were transported and maintained under specific-pathogen-free conditions at the animal facility, University of British Columbia. All animal experiments were done according to institutional guidelines and were approved by the Animal Care Committee of the University of British Columbia.
Mouse model of Salmonella-induced intestinal inflammation.
The protocol for S. Typhimurium-induced enterocolitis was used as described previously (2, 13). Briefly, mice aged 8 to 10 weeks were given 20 mg of streptomycin by oral gavage with a 21-gauge feeding needle. Twenty-four hours later, 3 × 106 S. Typhimurium bacteria were administered by oral gavage. Mice were assigned randomly to survival groups to monitor for survival and to organ harvest groups to assess for intestinal inflammation. To allow a humane endpoint, mice in the survival groups were monitored twice daily for 18 days after bacterial inoculation for signs of morbidity (reduced level of motion, piloerection, labored breathing, weight loss). Mice that showed distress of these signs or became moribund were euthanized with CO2 and considered nonsurvival. For other experiments, mice were euthanized at designated time points postinfection. Colons and ceca were harvested aseptically for bacterial enumeration, and ceca were collected for histopathology.
Bacterial enumeration.
Colons and ceca were harvested at designated time points postinfection with S. Typhimurium or the ΔsopB mutant and weighed, and each sample was collected into 1 ml of sterile phosphate-buffered saline (PBS) and homogenized with a Mixer Mill 301 (Retsch, Newtown, PA). Serial dilutions of the resulting homogenates were plated on LB agar plates containing 100 μg/ml streptomycin. Plates were incubated at 37°C for 24 h. Colony counts were expressed as the numbers of CFU per ml.
Histopathology.
Ceca of experimental animals were fixed in 10% formalin for 18 h, followed by 18 h in 70% ethanol prior to being embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) by Wax-it Histology Services (Vancouver, Canada). Stained sections were examined in a blinded fashion for signs of inflammation, PMN infiltration, edema, crypt abscess formation, regenerative changes, and necrosis. Pathological scores were determined by grading the histopathologic findings from 0 (none) to 1+ (mild), 2+ (moderate), and 3+ (severe) and averaging six fields/sample, according to a scoring system previously described (13).
Colonic cytokine measurements.
Colon samples collected at 1 day postinfection were assayed for the presence of cytokines. Colon homogenates collected as described above were spun twice at 13,200 rpm in an Eppendorf benchtop centrifuge (5415R) for 30 min each at 4°C to remove insoluble matters. Colon supernatants were frozen at −80°C until assayed for cytokines using the mouse inflammation cytometric bead array (CBA) assay kit (BD Biosciences), according to the manufacturer's instructions.
MPO staining.
For myeloperoxidase (MPO) immunofluorescent staining for the detection of neutrophils in the ceca of naïve and infected mice, paraffin-embedded tissues were deparaffinized in xylene (twice for 5 min each); rehydrated in 100%, 95%, and 70% ethanol (5 min each); and blocked with 2% normal goat serum in TPBS-BSA (PBS containing 0.05% Tween 20 and 0.1% bovine serum albumin; BSA was obtained from Sigma) for 40 min at room temperature. They were then incubated with the primary antibody, a polyclonal rabbit anti-MPO antibody (Thermo Scientific) diluted 1:200, overnight at 4°C. The sections were washed with TPBS-BSA and then incubated for 1 h at room temperature with Alexa 488-conjugated goat anti-rabbit IgG (diluted 1:1,000) as the secondary antibody. Subsequently, after being rinsed with TPBS-BSA, coverslips were mounted using Prolong Gold antifade reagent containing 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Digital images were captured and processed using a Zeiss Axiophot epifluorescence microscope.
Flow cytometry analysis.
Single-cell suspensions of colonic and cecal lamina propria cells were prepared by following a standard protocol (63). Briefly, colons and ceca from naïve or infected mice were rinsed in Hank's buffered saline solution (HBSS) to remove fecal contents. Epithelial cells were removed by shaking for 30 min at 37°C in HBSS containing 5 mM EDTA (Sigma), 5% fetal calf serum (HyClone), and 1 mM dithiothreitol (Sigma). The tissues were washed several times with HBSS, cut into small pieces, and digested with a mixture of collagenase-dispase (Roche) for 1 h at 37°C. The digested fragments were passed through a 70-mm nylon mesh. Supernatant containing endothelial cells was collected, and the pellet was further purified by centrifugation over a discontinuous Percoll gradient (40%/70%) for 20 min at 2,000 rpm to obtain lamina propria leukocytes. Purified cells from the colonic and cecal lamina propria were surface stained with fluorochrome-conjugated antibodies against CD11b (clone M1/70; BD Biosciences), Gr-1 (clone RB6-8C5; BD Biosciences), F4/80 (clone BM8; eBioscience), and annexin V (recombinant protein from Escherichia coli; eBioscience) before being subjected to flow cytometry analysis with FACSCalibur (Becton Dickinson, San Jose, CA) using CellQuest software. 7-Aminoactinomycin D (7-AAD; Sigma) was included in all staining to define viable cells.
TUNEL staining.
Ceca of experimental animals were fixed in 4% formaldehyde for 3 h at room temperature, followed by being washed with PBS. The tissues were then frozen and sectioned (10 μm). Cecal sections were then assessed for apoptotic features of damaged cells by terminal transferase dUTP nick end labeling (TUNEL) staining using the Apo-BrdU in situ DNA fragmentation assay kit (BioVision, Inc., Mountain View, CA), according to the manufacturer's instructions. Transferred bromolated dUTP nucleotides (Br-dUTP) to the free 3′-OH of cleaved DNA by terminal deoxynucleotide transferase (TdT) were detected by the anti-BrdU-fluorescein isothiocyanate (FITC) antibody using FITC and rhodamine filters on an Olympus 10i confocal microscope. Propidium iodide was used for counterstaining.
Statistical analysis.
The survival curves of infected mice were compared using Kaplan-Meier analysis followed by the log rank test. Bacterial loads, total pathological scores of infected ceca, and CBA assays for cytokines in the colons of infected mice were compared using the two-tailed, unpaired t test. All analyses were performed with a 95% confidence interval using GraphPad Prism version 4.0.
RESULTS
Akt2 is critical for host survival in S. Typhimurium infections.
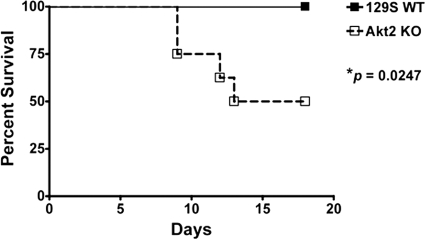
Although Salmonella-induced activation of Akt has been shown to protect epithelial cells from apoptosis in vitro (32), the role of Akt in Salmonella-induced gastroenteritis remains unclear. We hypothesized that deficiency of Akt would impair the host's defense mechanisms against Salmonella infection, leading to increased morbidity and mortality. To address this, we orally infected mice deficient in the antiapoptotic kinase Akt2 with S. Typhimurium. A significantly higher mortality rate (P = 0.0247) in Akt2 KO mice than that in 129S WT mice was noted throughout the period of observation (Fig. 1), indicating that Akt2 may play an important role in host survival during Salmonella infection.
Fig. 1.
Akt2 activation controls host survival to S. Typhimurium infection. WT mice and mice lacking Akt2 were treated with streptomycin prior to infection with S. Typhimurium and monitored for signs of morbidity and mortality. The percent survival in Akt2 KO mice and WT mice is shown. P values for survival curves of KO mice compared to those of WT mice were determined using the log rank test. Data are representative of two independent experiments with a total of at least 15 mice from each group.
S. Typhimurium colonization in the colons and ceca in mice with Akt2 deficiency is enhanced.
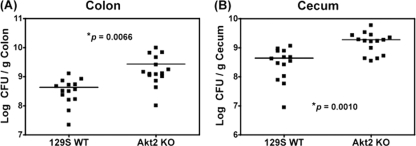
To determine the role of Akt2 in Salmonella colonization in the colon and cecum, we orally infected mice deficient in Akt2 and WT mice with S. Typhimurium. On day 1 postinfection, significantly higher bacterial loads were harvested from the colons (P = 0.0066) (Fig. 2A) and ceca (P = 0.0010) (Fig. 2B) of Akt2 KO mice than those harvested from WT mice. At 4 days postinfection, similarly high levels of bacterial loads were recovered from the colons and ceca of both Akt2 KO and WT mice (data not shown). These data indicate that loss of Akt2 is associated with the early enhanced Salmonella colonization of the colon and cecum.
Fig. 2.
S. Typhimurium colonization in the colons and ceca of Akt2-deficient mice is enhanced at 1 day postinfection. Mice deficient in Akt2 and WT mice were treated with streptomycin prior to infection with S. Typhimurium and sacrificed at 1 day postinfection. Bacterial loads harvested from the colons (A) and ceca (B) of infected mice are shown. P values for the differences in bacterial loads between the Akt2 KO mice and the WT mice were determined by two-tailed, unpaired t test. Three independent experiments were performed, and bars indicate the geometric means from each group of 15 mice.
S. Typhimurium-induced cecal inflammation is more severe in mice deficient in Akt2.
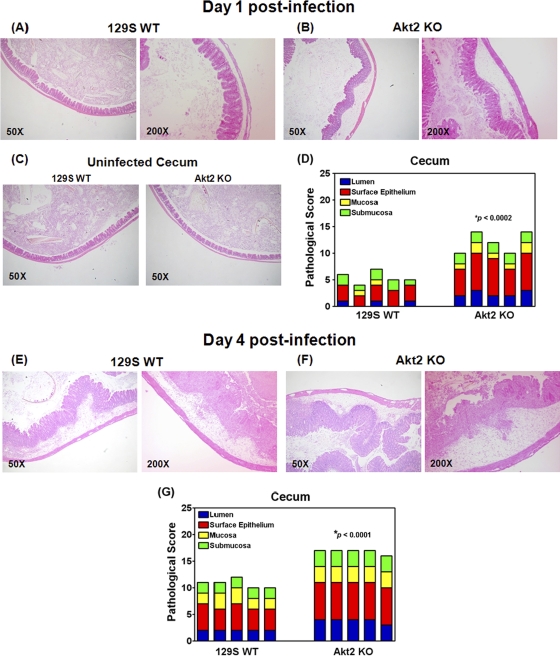
To assess the role of Akt2 in S. Typhimurium-induced cecal inflammation, we compared the cecal pathology of infected Akt2 KO mice with that of the WT mice. Histopathological analysis of H&E-stained tissue sections revealed extensive pathological changes of the ceca from infected Akt2 KO mice (Fig. 3B) at 1 day postinfection. In the ceca of these mice, we observed marked edema and infiltration of PMNs in the submucosa, formation of crypt abscesses and mucinous plugs in the mucosa, marked regenerative changes, and desquamation in the surface epithelium layer, as well as the presence of necrotic epithelial cells and infiltration of neutrophils into the lumen. In contrast, only mild inflammation in the ceca of infected WT mice at 1 day postinfection was observed (Fig. 3A). These pathological features of inflamed ceca were absent from uninfected mice among both groups (Fig. 3C). Using the pathological scoring scheme, we found that at 1 day postinfection with S. Typhimurium, cecal inflammation was significantly more severe in Akt2 KO mice than in WT mice (P < 0.0002) (Fig. 3D).
Fig. 3.
Histology of ceca from S. Typhimurium-infected mice showing severe inflammation in Akt2-deficient mice at 1 day and 4 days postinfection. Mice defective in Akt2 and WT mice were treated with streptomycin prior to infection with S. Typhimurium and sacrificed at 1 day and 4 days postinfection. Representative H&E staining of the ceca of one mouse from each group of at least 4 mice is shown. At 1 day postinfection, severe inflammation in the lumina of the ceca of infected Akt2 KO mice was observed (B), while only mild inflammation in the WT mice was observed (A). At 4 days postinfection, more severe cecal inflammatory features in Akt2 KO mice were detected (F), and to a lesser extent, cecal inflammation in infected WT mice was also observed (E). (C) These pathological features of inflamed ceca were absent from uninfected mice for both groups. Images are shown at magnifications of ×50 and ×200. Pathological scoring for the cecal samples harvested from Akt2 KO mice and WT mice at 1 day postinfection (D) and at 4 days postinfection (G) is shown. P values for the differences in pathological scoring of the ceca between the Akt2 KO mice and the WT mice were determined by two-tailed, unpaired t test. Data are representative of three independent experiments.
At 4 days postinfection, more severe cecal inflammatory features, including edema and the presence of PMN aggregates in the submucosa, crypt destruction and formation of mucinous plugs in the mucosa, profound regenerative changes, and desquamation in the surface epithelium, as well as the infiltration of neutrophils in the epithelium and lumen, were observed in Akt 2 KO mice (Fig. 3F). Cecal inflammation in infected WT control mice was also observed but to a lesser extent (Fig. 3E). Pathological scoring also indicated that cecal inflammation was significantly more severe in Akt2 KO mice than in the WT control mice (P < 0.0001) (Fig. 3G) at 4 days postinfection.
S. Typhimurium induces increased levels of proinflammatory cytokines in the colons of Akt2-deficient mice.
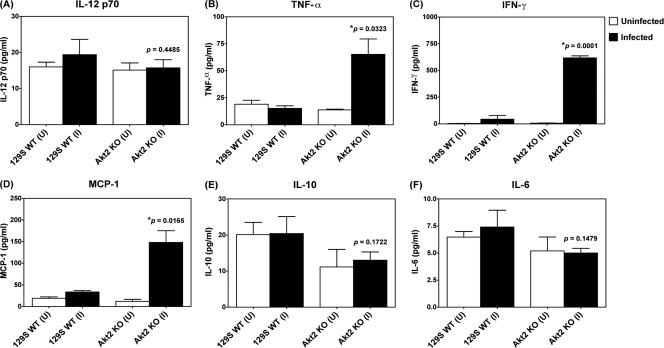
Consistent with Salmonella colonization of the colon, we found that at 1 day postinfection, there was a significant increase in proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α; P = 0.0323) (Fig. 4B), gamma interferon (IFN-γ; P = 0.0001) (Fig. 4C), and MCP-1 (P = 0.0165; Fig. 4D), in the colons of infected Akt2 KO mice compared to those in the colons of infected WT mice, whereas the levels of interleukin-12 (IL-12) p70 (Fig. 4A), IL-10 (Fig. 4E), and IL-6 (Fig. 4F) present in the colons of infected Akt2 KO mice were comparable to those in the colons of WT mice. Enhanced levels of the proinflammatory cytokines and chemokines, including TNF-α, IFN-γ, and MCP-1, in Akt2 KO mice at 1 day postinfection may contribute to the increased cecal inflammation in mice with Akt2 function deficiencies.
Fig. 4.
S. Typhimurium induced enhanced levels of TNF-α, IFN-γ, and MCP-1 in the colons of Akt2-deficient mice. Mice lacking Akt2 and WT mice were treated with streptomycin prior to infection with S. Typhimurium and sacrificed at 1 day postinfection. Cytokines present in the colon extracts were determined by CBA assay. P values for the differences in colonic cytokines between the infected Akt2 KO mice and the infected WT mice were determined by two-tailed Student's t test. (U) = colon cytokines from uninfected mice; (I) = colon cytokines from infected mice. Results (means ± standard errors of the means [SEM]) were obtained from 5 mice from each group. Data are representative of three independent experiments with a total of at least 15 animals from each group.
S. Typhimurium-induced neutrophil recruitment is more pronounced in the ceca of Akt2-deficient mice.
Histopathological analysis of the H&E-stained sections revealed more pronounced neutrophil infiltration in the ceca of S. Typhimurium-infected Akt2 KO mice than that in the ceca of WT mice at 1 day postinfection. To confirm this, we stained tissue sections of infected ceca harvested at 1 day postinfection with antibodies against MPO, which present most abundantly in neutrophils. As shown in Fig. 5, infiltration of MPO-positively stained neutrophils was observed in the submucosa of the ceca of infected Akt2 KO mice (Fig. 5D) but was absent in the ceca of infected WT mice (Fig. 5C) and the uninfected mice among both groups (Fig. 5A and B).
Fig. 5.
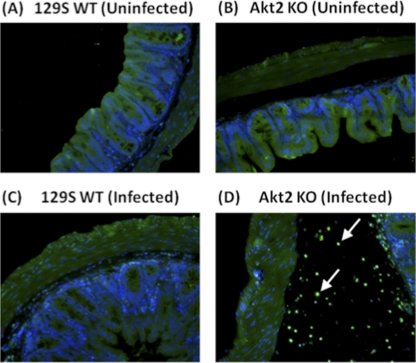
S. Typhimurium induced increased neutrophil recruitment in the ceca of mice lacking Akt2. Mice deficient in Akt2 and WT mice were treated with streptomycin prior to infection with S. Typhimurium and sacrificed at 1 day postinfection. Ceca from uninfected WT (A) and Akt2 KO (B) mice and infected WT (C) and Akt2 KO (D) mice were stained with MPO immunofluorescence for neutrophils. DAPI staining is indicated in blue in the submucosa, and anti-MPO immunostaining for neutrophils is indicated in green. Arrows show the presence of MPO-positively stained neutrophils in the submucosal area. Pictures are representative of three independent experiments with a total of at least 15 mice from each group.
In addition to the microscopic staining of neutrophils on cross sections of fixed cecal tissues using anti-MPO antibody, we also performed fluorescence-activated cell sorter (FACS) staining to stain for live cells prepared from the entire intestinal tract using other markers to further characterize the phenotype of the neutrophil populations. Lamina propria leukocytes were harvested from the colons and ceca of naïve and infected Akt2 KO and WT mice and analyzed for the expression of several surface markers for neutrophils (Gr-1 and CD11b) and macrophages (F4/80 and CD11b) using flow cytometry.
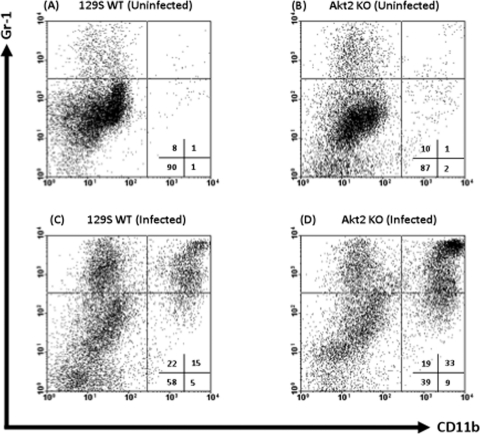
Figure 6A to D illustrate representative FACS plots comparing the expression levels of CD11b and Gr-1 on neutrophils harvested from naïve and infected colons and ceca of Akt2 KO and WT mice. Only 1% of cells from uninfected mice were mature neutrophils expressing CD11b+ Gr-1+ (Fig. 6A and B, upper right quadrants). At 1 day postinfection with Salmonella, the percentage of CD11b+ Gr-1+ cells from the colons and ceca of infected Akt2 KO mice (Fig. 6D) increased to 33%, while the percentage of CD11b+ Gr-1+ cells from the colons and ceca of infected WT mice (Fig. 6C) only increased to 15%. The percentage of immature neutrophils expressing CD11b− Gr-1+ (upper left quadrants) from the colons and ceca of infected Akt2 KO mice and WT mice were similar (19% and 22%, respectively).
Fig. 6.
S. Typhimurium infection is associated with an elevated influx of Gr-1+ CD11b+ and Gr-1+ CD11b− cells in the colons and ceca of mice lacking Akt2. Mice deficient in Akt2 and WT mice were treated with streptomycin prior to infection with S. Typhimurium and sacrificed at 1 day postinfection. Single-cell suspensions from the colons and ceca of uninfected WT (A) and Akt2 KO (B) mice and infected WT (C) and Akt2 KO (D) mice were subjected to FACS staining. Representative FACS plots comparing the expression levels of CD11b and GR-1 neutrophils harvested from the colons and ceca of each group are shown. FACS plots were gated on live cells, with 7-AAD-positive dead cells excluded from analysis. Numbers indicate the percentage of cells in each quadrant. FACS plots are representative of four independent experiments with a total of 12 mice from each group.
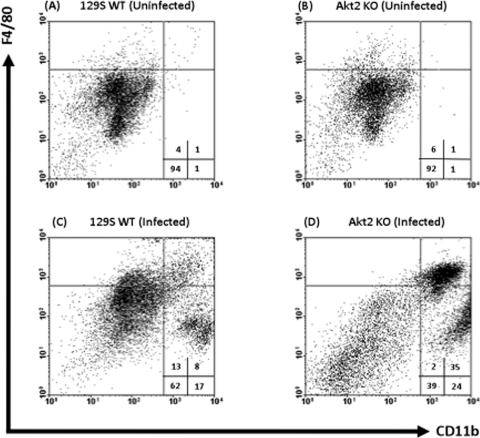
Figure 7A to D show representative FACS plots comparing the expression levels of CD11b and F4/80 on macrophages harvested from naïve and infected colons and ceca of Akt2 KO and WT mice. Only 1% of cells from uninfected mice were macrophages expressing CD11b+ F4/80+ (Fig. 7A and B, upper right quadrants). At 1 day following infection with Salmonella, the percentage of CD11b+ F4/80+ cells from infected Akt2 KO mice (Fig. 7D) increased to 35%, while the percentage of CD11b+ F4/80+ cells from the colons and ceca of infected WT mice (Fig. 7C) increased to 8%.
Fig. 7.
S. Typhimurium infection is associated with an elevated influx of CD11b+ F4/80+ macrophages in the colons and ceca of mice lacking Akt2. Mice deficient in Akt2 and WT mice were treated with streptomycin prior to infection with S. Typhimurium and sacrificed at 1 day postinfection. Single-cell suspensions from the colons and ceca of uninfected WT (A) and Akt2 KO (B) mice and infected WT (C) and Akt2 KO (D) mice were subjected to FACS staining. Representative FACS plots comparing the expression levels of CD11b and F4/80 macrophages harvested from the colons and ceca of each group are shown. FACS plots were gated on live cells, with 7-AAD-positive dead cells excluded from analysis. Numbers indicate the percentage of cells in each quadrant. FACS plots are representative of four independent experiments with a total of 12 mice from each group.
These data indicate that loss of Akt2 facilitates transmigration of neutrophils and macrophages to the sites of infection. The excessive accumulation of granulocytes and the subsequent release of enzymes and oxygen radicals from these cells in the colons and ceca of infected Akt2 KO mice may result in the enhanced mucosal inflammatory reaction.
S. Typhimurium induces enhanced levels of annexin V+ and TUNEL+ apoptotic cells in the colons and ceca of Akt2-deficient mice.
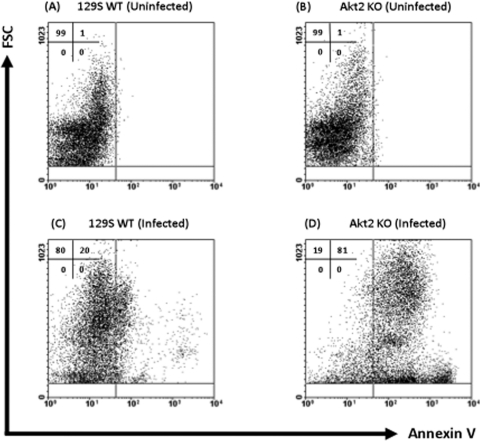
Our data mentioned above suggest that Akt2 KO mice are more susceptible to Salmonella infection. It has been shown that Salmonella protects epithelial cells from camptothecin-induced apoptosis in vitro by activation of the Akt pathway (32). We next determined if mice lacking Akt2 are unable to inhibit apoptosis of intestinal epithelial cells and thus contribute to mucosal inflammation and mortality during Salmonella infection. Intestinal epithelial cells and lamina propria leukocytes were harvested from the colons and ceca of naïve and infected Akt2 KO and WT mice and analyzed for expression of annexin V, which binds to the externalized phosphatidylserine of apoptotic cells, using flow cytometry. Figure 8A to D illustrate representative FACS plots comparing expression levels of annexin V on the total population of cells harvested from naïve and infected colons and ceca of Akt2 KO mice and WT mice. Only 1% of cells from the colons and ceca of uninfected mice were annexin V+ (Fig. 8A and B, upper right quadrants). At 1 day following infection with S. Typhimurium, the percentage of annexin V+ apoptotic cells from the colons and ceca of infected Akt2 KO mice increased to 81% (Fig. 8D), while annexin V+ apoptotic cells from the colons and ceca of infected WT mice increased only to 20% (Fig. 8C).
Fig. 8.
S. Typhimurium induced enhanced levels of annexin V+ apoptotic cells in the colons and ceca of Akt2-deficient mice. Mice deficient in Akt2 and WT mice were treated with streptomycin prior to infection with S. Typhimurium and sacrificed at 1 day postinfection. Single-cell suspensions from the colons and ceca of uninfected WT (A) and Akt2 KO (B) mice and infected WT (C) and Akt2 KO (D) mice were subjected to FACS staining. Representative FACS plots comparing the expression levels of annexin V apoptotic cells on the total population of cells harvested from the colons and ceca of each group are shown. FACS plots were gated on total cells, with 7-AAD-positive dead cells excluded from analysis. Numbers indicate the percentage of cells in each quadrant. FACS plots are representative of three independent experiments with a total of 9 mice from each group. FSC, forward scatter.
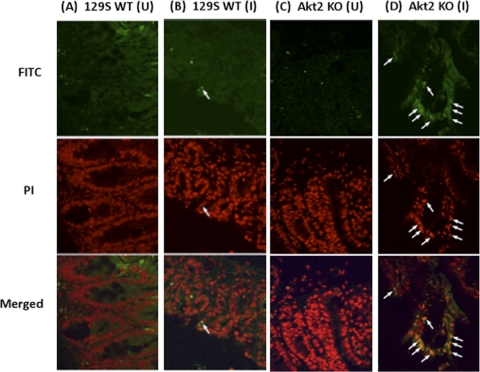
Cecal sections of naïve and infected Akt2 KO and WT mice were further assessed for apoptosis using TUNEL staining to identify DNA fragmentation of apoptotic cells. TUNEL-positive apoptotic cells exhibit green fluorescence, and cell nuclei counterstained with propidium iodide demonstrate red fluorescence. Merged images for apoptotic cells reveal yellow fluorescence (Fig. 9B and D, white arrows). As shown in Fig. 9A and C, TUNEL+ cells were absent in the ceca of uninfected mice for both groups. At 1 day postinfection with S. Typhimurium, increased levels of TUNEL+ apoptotic cells were observed in the ceca of infected Akt2 KO mice (Fig. 9D, white arrows), while only a few TUNEL+ cells were present in the ceca of infected WT mice (Fig. 9B).
Fig. 9.
S. Typhimurium induced increased levels of TUNEL+ apoptotic cells in the ceca of Akt2-deficient mice. Mice deficient in Akt2 and WT mice were treated with streptomycin prior to infection with S. Typhimurium and sacrificed at 1 day postinfection. Cecal sections of uninfected WT (A) and Akt2 KO (C) mice and infected WT (B) and Akt2 KO (D) mice were assessed for apoptosis using TUNEL staining. TUNEL+ apoptotic cells exhibit green fluorescence (FITC), and cell nuclei counterstained with propidium iodide (PI) demonstrate red fluorescence. Merged images for apoptotic cells reveal yellow fluorescence (white arrows). (U) = cecal sections from uninfected mice; (I) = cecal sections from infected mice.
Collectively, these data suggest that Akt2 may play a critical role in inhibiting apoptosis of intestinal cells and thus protect the host from mucosal inflammation and mortality against Salmonella infection.
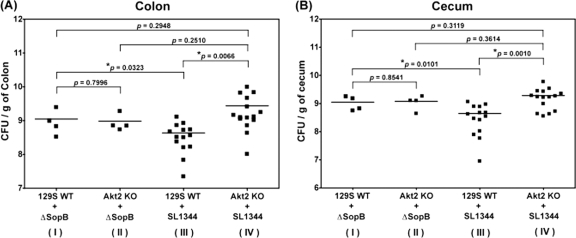
Deletion of sopB abolishes the differences in bacterial colonization and cecal inflammation in Salmonella-infected WT and Akt2 KO mice.
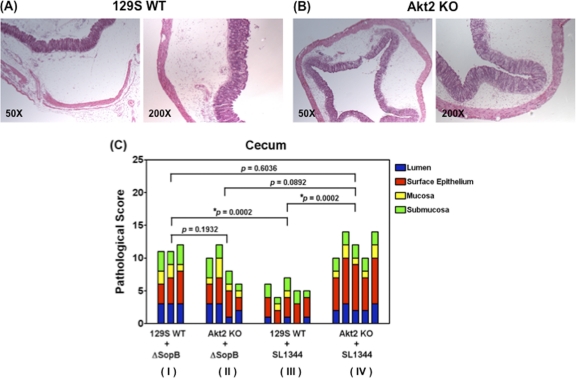
The Salmonella effector protein SopB has been shown to protect epithelial cells from apoptosis in vitro by activation of Akt (32). We next sought to determine if SopB is required for Akt activation and protects the mice from bacterial colonization and cecal inflammation in the intestine. To address this, 129S WT mice and mice deficient in Akt2 were orally infected with the sopB deletion mutant. Data obtained from mice infected with Salmonella enterica serovar Typhimurium WT strain SL1344 were also included in Fig. 10A and B and 11C for comparison. As described earlier, the Salmonella WT strain SL1344 induced significantly higher bacterial loads in the colons (P = 0.0066) (Fig. 10A) and ceca (P = 0.0010) (Fig. 10B) of Akt2 KO mice (group IV) than in those of 129S WT mice (group III). However, such differences in bacterial loads were abolished in the colons (P = 0.7996) (Fig. 10A) and ceca (P = 0.8541) (Fig. 10B) when 129S WT mice (group I) and Akt2 KO mice (group II) were infected with the sopB deletion mutant. Similarly, the Salmonella WT SL1344 strain induced significantly more severe cecal inflammation in the ceca of Akt2 KO mice (group IV) than in those of 129S WT mice (group III) (P = 0.0002) (Fig. 11C), and such differences in cecal inflammation were abolished when 129S WT mice (group I) and Akt2 KO mice (group II) were infected with the sopB deletion mutant (P = 0.1932) (Fig. 11C).
Fig. 10.
Deletion of sopB abolishes the differences in bacterial colonization in Salmonella-infected WT and Akt2 KO mice. Mice deficient in Akt2 and WT mice were treated with streptomycin prior to infection with the sopB deletion mutant ΔsopB and sacrificed at 1 day postinfection. Bacterial loads harvested from the colons (A) and ceca (B) of infected mice are shown. Bacterial loads harvested from 129S WT mice and Akt2 KO mice infected with the Salmonella WT SL1344 were also included for comparison. P values for the differences in bacterial loads among the various groups were determined by two-tailed, unpaired t test. Bars indicate the geometric means from each group of at least 4 mice.
Fig. 11.
Deletion of sopB abolishes the differences in cecal inflammation in Salmonella-infected WT and Akt2 KO mice. Mice defective in Akt2 and WT mice were treated with streptomycin prior to infection with the sopB deletion mutant ΔsopB and sacrificed at 1 day postinfection. Representative H&E staining of ceca from one mouse from each group of at least 3 mice is shown. At 1 day postinfection, severe inflammation in the lumen of the ceca of sopB-infected WT mice (A) and Akt2 KO mice (B) was observed. Images are shown at magnifications of ×50 and ×200. (C) Pathological scoring for the cecal samples harvested from WT mice and Akt2 KO mice at 1 day postinfection with the sopB deletion mutant ΔsopB and the Salmonella WT SL1344 is shown. P values for the differences in pathological scoring of the ceca among the various groups were determined by two-tailed, unpaired t test.
Additionally, similar bacterial loads from the colons (P = 0.2510) (Fig. 10A) and ceca (P = 0.3614) (Fig. 10B) as well as comparable cecal pathology (P = 0.0892) (Fig. 11C) were observed in the Akt2 KO mice when these mice were infected with either the Salmonella WT SL1344 (group IV) or the sopB deletion mutant (group II), suggesting that SopB and Akt2 may be involved in the same signaling pathway to protect the mice from Salmonella infection. This is further supported by our finding that in the group of 129S WT mice infected with the sopB deletion mutant (group I) (Fig. 10A and B and 11C), higher bacterial loads in the colons (P = 0.0323) (Fig. 10A) and ceca (P = 0.0101) (Fig. 10B) as well as more severe cecal pathology (P = 0.0002) (Fig. 11C) were observed compared to those in the 129S WT mice infected with the Salmonella WT SL1344 (group III) (Fig. 10A and B and 11C). Furthermore, our data also revealed that similar bacterial loads in the colons (P = 0.2948) (Fig. 10A) and ceca (P = 0.3119) (Fig. 10B) as well as similar cecal pathology (P = 0.6036) (Fig. 11C) were harvested in both the 129S WT mice infected with the sopB deletion mutant (group I) (Fig. 10A and B and 11C) and the Akt2 KO mice infected with the Salmonella WT SL1344 (group IV) (Fig. 10A and B and 11C). These data suggest that SopB may play a role in protecting the mice from Salmonella colonization and cecal inflammation through activation of Akt2.
DISCUSSION
The prosurvival Akt protein kinase is critical in preventing cells from undergoing apoptosis (17). Akt activation has been shown to be essential for SopB-mediated protection against apoptosis in epithelial cells following Salmonella invasion (32, 38). In S. Typhimurium-induced colitis, the role of Akt has not been explored. To address this, we analyzed S. Typhimurium-induced intestinal inflammation in Akt2-deficient mice. An increase in apoptotic cells associated with enhanced mortality and morbidity, including higher bacterial loads and more severe proinflammatory responses in the colons and ceca in mice deficient in Akt2 following infection with S. Typhimurium, indicates functional involvement of Akt2 in this disease model.
Bacterial pathogens can either induce or prevent apoptosis to gain survival advantage in the host and to enhance infection. Many bacterial pathogens that cause apoptosis target immune cells such as macrophages (66) and neutrophils (4) because these cells would otherwise kill the bacteria (24, 33). Alternatively, bacterial pathogens could inhibit apoptosis during infection, which provides a survival benefit for the bacteria to survive and replicate inside host cells (10, 21, 32, 38, 43, 55). In the present study, we have demonstrated that Salmonella-induced apoptosis at the sites of infection in mice lacking Akt2 and Akt2 deficiency renders these mice more susceptible to infection, suggesting that apoptosis may play an important role in the pathogenesis of Salmonella-induced colitis. Although Akt2 has been shown to play an antiapoptotic role (17), the enhanced level of apoptosis observed in Salmonella-infected Akt2 KO mice may also be due to the decrease in glucose availability, as Akt2 modulates glucose homeostasis and downstream apoptotic pathways during development (28) and Akt2 knockout mice have been shown to develop a type 2 diabetic phenotype (8).
It has been suggested that the principal event leading to inflammatory diseases is cell damage as a result of apoptosis or necrosis (36, 52, 56). Increased permeability of the epithelial barrier and increased apoptotic rates of epithelial cells have been implicated to be major factors in the pathogenesis of intestinal inflammation (25, 64). Our observation that cecal inflammation is more pronounced in Akt2 KO mice during Salmonella infection may be associated with the lack of the antiapoptotic molecule Akt2. In the current study, a large number of annexin V+ and TUNEL+ apoptotic cells in the intestines of Salmonella-infected Akt2-deficient mice were detected (Fig. 8D and 9D). The massive cell damage would cause leakage of cellular contents into the adjacent tissues, resulting in the transmigration of granulocytes into the injured tissue of infected Akt2 KO mice (Fig. 5D, 6D, and 7D). Furthermore, the release of Salmonella from infected apoptotic cells into adjacent tissues would contribute to the additional neutrophil infiltration to the sites of infection. Although neutrophils have been shown to contribute to host protection from S. Typhimurium infection since they can target the virulence factors and effectively eliminate the bacteria (60), excessive accumulation of neutrophils and monocytes would result in the release of toxic products such as proteases and reactive oxygen intermediates (45), leading to endothelial damage and thus allowing the bacteria to invade the epithelium and enhance the intestinal inflammatory reaction.
Apoptosis can be induced by proinflammatory cytokines such as TNF-α (39, 46, 50), IFN-γ (1), and MCP-1 (3). In the present study, significant increases in TNF-α, IFN-γ, and MCP-1 production in the colons of Salmonella-infected mice deficient in Akt2 were detected at 1 day postinfection, in comparison with that in the colons of WT mice. The enhanced levels of these proinflammatory cytokines and chemokines would contribute to the increased levels of apoptotic cells found in the colons and ceca of Salmonella-infected Akt2 KO mice. Furthermore, neutrophils have been shown to produce enhanced levels of proinflammatory cytokines during infectious colitis (44), and the increased levels of TNF-α, IFN-γ, and MCP-1 production observed in the colons of infected Akt2 KO mice may be attributable to the large influx of neutrophils. Intriguingly, MCP-1 is a pivotal chemokine in the recruitment of monocytes (51) and neutrophils (58); the expression of MCP-1 can be induced by a variety of factors, including inflammatory cytokines such as TNF-α (14, 59) and IFN-γ (27). Thus, the increased levels of TNF-α, IFN-γ, and MCP-1 in Akt2 KO mice would contribute to the additional neutrophil infiltration and apoptotic activity.
Host cell apoptosis very often facilitates the bacterial attack of the host and gaining access to the tissue (15). It has been shown previously that caspase-1, which is both proapoptotic and proinflammatory, is essential for S. Typhimurium to efficiently colonize the ceca and Peyer's patches and subsequently cause systemic typhoid-like disease in mice (42). It was proposed that early in infection of the Peyer's patches, the bacteria escape the macrophages by inducing apoptosis. The simultaneous recruitment of PMNs and other cellular elements of the immune system to the site of infection appears to provide a new intracellular niche for the bacteria. We find that mice deficient in the host Akt2 kinase are more susceptible to colonization by S. Typhimurium. Significantly enhanced colonization in the colons and ceca of Akt2 KO mice occurs at 1 day postinfection with Salmonella, compared to that in the colons and ceca off WT mice. Such an increase in Salmonella colonization may be associated with the increase in apoptosis and thus enhanced neutrophil and monocyte recruitment and inflammation in the intestines of infected Akt2 KO mice. The induction of apoptosis and inflammation would be expected to aid in the systemic dissemination of S. Typhimurium as the newly recruited immune cells provide a new niche for the intracellular bacteria. Although a significant increase in Salmonella colonization was not harvested from the intestines of Akt2 KO mice compared with that harvested from WT mice at 4 days postinfection, significantly more severe cecal pathology in Akt2-deficient mice at 1 and 4 days postinfection was observed. These data suggest that the early mortality in Salmonella-infected Akt2 KO mice may be attributable to the drastic tissue damage and massive infiltration of inflammatory cells at the site of infection but not due to bacterial colonization. Our data clearly indicate that Akt2 may play an important protective role in S. Typhimurium-induced gastroenterocolitis. Nonetheless, it has been shown that neutrophils in Akt2 KO mice exhibited decreased cell migration, granule enzyme release, and O2− production (6); thus, the increase in susceptibility in Akt2 KO mice during Salmonella infection may also be attributable to the defective functions of neutrophils in Akt2 KO mice.
It has been shown that the Salmonella effector protein SopB protects epithelial cells from apoptosis in vitro by activation of Akt (32). In the present study, our finding that the differences in bacterial loads (Fig. 10A and B) and cecal pathology (Fig. 11C) harvested from the intestines of 129S WT mice and Akt2 KO mice infected with the Salmonella WT SL1344 (groups III and IV, respectively) were abolished when these mice were infected with the sopB deletion mutant (groups I and II, respectively) indicates that SopB may play a role in protecting the mice from Salmonella colonization through activation of Akt2. This is further supported by our observation that the bacterial loads and cecal pathology in the Akt2 KO mice were similar when infected with either the Salmonella WT SL1344 or the sopB deletion mutant. Furthermore, our findings that the bacterial burden and cecal pathology in the group of 129S WT mice infected with the sopB deletion mutant were higher than those in the group of 129S WT mice infected with the Salmonella WT SL1344 and yet similar to those in the group of Akt2 KO mice infected with the Salmonella WT SL1344 suggest that SopB and Akt2 may participate in the same pathway to protect the mice from Salmonella infection.
Collectively, using the murine S. Typhimurium-induced intestinal inflammation model, our findings suggest that Akt2 signaling plays an important role in host innate immune response and host survival against S. Typhimurium infection. During the early stages of infection, in the absence of Akt2, we detected a proapoptotic effect on the cells isolated from the infected colons and ceca. This might lead to an excessive influx of neutrophils and secretion of proinflammatory cytokines, resulting in an increase in bacterial colonization, intestinal inflammation, and mortality. In view of the high level of structure and function homology shared among the 3 Akt isoforms (61), it would be interesting to investigate if Akt2 could coordinate with other Akt isoforms to mediate protection in mice during Salmonella infection.
ACKNOWLEDGMENTS
We thank W. Deng for critical reading of the manuscript.
This work was supported by the Genome British Columbia and Genome Prairie for the Pathogenomics of Innate Immunity Research Program and the Canadian Institutes for Health Research (CIHR). H.B.Y. is supported by a CIHR fellowship. B.B.F. is the UBC Peter Wall Distinguished Professor.
Footnotes
Published ahead of print on 9 May 2011.
REFERENCES
- 1. Ahn E. Y., Pan G., Vickers S. M., McDonald J. M. 2002. IFN-gamma upregulates apoptosis-related molecules and enhances Fas-mediated apoptosis in human cholangiocarcinoma. Int. J. Cancer 100:445–451 [DOI] [PubMed] [Google Scholar]
- 2. Barthel M., et al. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bidzhekov K., Zernecke A., Weber C. 2006. MCP-1 induces a novel transcription factor with proapoptotic activity. Circ. Res. 98:1107–1109 [DOI] [PubMed] [Google Scholar]
- 4. Blomgran R., Zheng L., Stendahl O. 2004. Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect. Immun. 72:4570–4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyd J. F. 1985. Pathology of the alimentary tract in Salmonella typhimurium food poisoning. Gut 26:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J., Tang H., Hay N., Xu J., Ye R. D. 2010. Akt isoforms differentially regulate neutrophil functions. Blood 115:4237–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen W. S., et al. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cho H., et al. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728–1731 [DOI] [PubMed] [Google Scholar]
- 9. Cho H., Thorvaldsen J. L., Chu Q., Feng F., Birnbaum M. J. 2001. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276:38349–38352 [DOI] [PubMed] [Google Scholar]
- 10. Clark C. S., Maurelli A. T. 2007. Shigella flexneri inhibits staurosporine-induced apoptosis in epithelial cells. Infect. Immun. 75:2531–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cleasby M. E., Reinten T. A., Cooney G. J., James D. E., Kraegen E. W. 2007. Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol. Endocrinol. 21:215–228 [DOI] [PubMed] [Google Scholar]
- 12. Coburn B., Grassl G. A., Finlay B. B. 2007. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 85:112–118 [DOI] [PubMed] [Google Scholar]
- 13. Coburn B., Li Y., Owen D., Vallance B. A., Finlay B. B. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 73:3219–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colotta F., et al. 1992. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J. Immunol. 148:760–765 [PubMed] [Google Scholar]
- 15. Cywes Bentley C., Hakansson A., Christianson J., Wessels M. R. 2005. Extracellular group A Streptococcus induces keratinocyte apoptosis by dysregulating calcium signalling. Cell. Microbiol. 7:945–955 [DOI] [PubMed] [Google Scholar]
- 16. Darwin K. H., Miller V. L. 1999. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 12:405–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Datta S. R., Brunet A., Greenberg M. E. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927 [DOI] [PubMed] [Google Scholar]
- 18. Dummler B., et al. 2006. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol. Cell. Biol. 26:8042–8051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Easton R. M., et al. 2005. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol. Cell. Biol. 25:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elbim C., Katsikis P. D., Estaquier J. 2009. Neutrophil apoptosis during viral infections. Open Virol. J. 3:52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fischer S. F., et al. 2004. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J. Exp. Med. 200:905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galan J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53–86 [DOI] [PubMed] [Google Scholar]
- 23. Garofalo R. S., et al. 2003. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J. Clin. Invest. 112:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grassme H., Jendrossek V., Gulbins E. 2001. Molecular mechanisms of bacteria induced apoptosis. Apoptosis 6:441–445 [DOI] [PubMed] [Google Scholar]
- 25. Hagiwara C., Tanaka M., Kudo H. 2002. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. J. Gastroenterol. Hepatol. 17:758–764 [DOI] [PubMed] [Google Scholar]
- 26. Hoiseth S. K., Stocker B. A. 1985. Genes aroA and serC of Salmonella typhimurium constitute an operon. J. Bacteriol. 163:355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ito R., et al. 2006. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin. Exp. Immunol. 146:330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jensen P. J., Gunter L. B., Carayannopoulos M. O. 2010. Akt2 modulates glucose availability and downstream apoptotic pathways during development. J. Biol. Chem. 285:17673–17680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones B. D., Ghori N., Falkow S. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knodler L. A., et al. 2002. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Mol. Microbiol. 43:1089–1103 [DOI] [PubMed] [Google Scholar]
- 31. Knodler L. A., Finlay B. B. 2001. Salmonella and apoptosis: to live or let die? Microbes Infect. 3:1321–1326 [DOI] [PubMed] [Google Scholar]
- 32. Knodler L. A., Finlay B. B., Steele-Mortimer O. 2005. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J. Biol. Chem. 280:9058–9064 [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi S. D., et al. 2003. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. U. S. A. 100:10948–10953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kohbata S., Yokoyama H., Yabuuchi E. 1986. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer's patches in ligated ileal loops: an ultrastructural study. Microbiol. Immunol. 30:1225–1237 [DOI] [PubMed] [Google Scholar]
- 35. Li X., et al. 2004. Akt2, phosphatidylinositol 3-kinase, and PTEN are in lipid rafts of intestinal cells: role in absorption and differentiation. Gastroenterology 126:122–135 [DOI] [PubMed] [Google Scholar]
- 36. Mackay I. R., Leskovsek N. V., Rose N. R. 2008. Cell damage and autoimmunity: a critical appraisal. J. Autoimmun. 30:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manning B. D., Cantley L. C. 2007. AKT/PKB signaling: navigating downstream. Cell 129:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marcus S. L., Wenk M. R., Steele-Mortimer O., Finlay B. B. 2001. A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Lett. 494:201–207 [DOI] [PubMed] [Google Scholar]
- 39. Marini M., et al. 2003. TNF-alpha neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc. Natl. Acad. Sci. U. S. A. 100:8366–8371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mastroeni P. 2002. Immunity to systemic Salmonella infections. Curr. Mol. Med. 2:393–406 [DOI] [PubMed] [Google Scholar]
- 41. McGovern V. J., Slavutin L. J. 1979. Pathology of salmonella colitis. Am. J. Surg. Pathol. 3:483–490 [DOI] [PubMed] [Google Scholar]
- 42. Monack D. M., et al. 2000. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192:249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morales P., et al. 2006. Infection of human fallopian tube epithelial cells with Neisseria gonorrhoeae protects cells from tumor necrosis factor alpha-induced apoptosis. Infect. Immun. 74:3643–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nikolaus S., et al. 1998. Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin 10 during intestinal inflammation. Gut 42:470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parent C., Eichacker P. Q. 1999. Neutrophil and endothelial cell interactions in sepsis. The role of adhesion molecules. Infect. Dis. Clin. North Am. 13:427–447 [DOI] [PubMed] [Google Scholar]
- 46. Park S. W., et al. 2011. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab. Invest. 91:63–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peng X. D., et al. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17:1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Plas D. R., Thompson C. B. 2005. Akt-dependent transformation: there is more to growth than just surviving. Oncogene 24:7435–7442 [DOI] [PubMed] [Google Scholar]
- 49. Rabsch W., Tschape H., Baumler A. J. 2001. Non-typhoidal salmonellosis: emerging problems. Microbes Infect. 3:237–247 [DOI] [PubMed] [Google Scholar]
- 50. Rath P. C., Aggarwal B. B. 1999. TNF-induced signaling in apoptosis. J. Clin. Immunol. 19:350–364 [DOI] [PubMed] [Google Scholar]
- 51. Rollins B. J. 1996. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol. Med. Today 2:198–204 [DOI] [PubMed] [Google Scholar]
- 52. Scaffidi P., Misteli T., Bianchi M. E. 2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195 [DOI] [PubMed] [Google Scholar]
- 53. Srikanth C. V., Cherayil B. J. 2007. Intestinal innate immunity and the pathogenesis of Salmonella enteritis. Immunol. Res. 37:61–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Steele-Mortimer O., et al. 2000. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J. Biol. Chem. 275:37718–37724 [DOI] [PubMed] [Google Scholar]
- 55. Sukumaran S. K., Selvaraj S. K., Prasadarao N. V. 2004. Inhibition of apoptosis by Escherichia coli K1 is accompanied by increased expression of BclXL and blockade of mitochondrial cytochrome c release in macrophages. Infect. Immun. 72:6012–6022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tonetti M. S., Cortellini D., Lang N. P. 1998. In situ detection of apoptosis at sites of chronic bacterially induced inflammation in human gingiva. Infect. Immun. 66:5190–5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tschopp O., et al. 2005. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132:2943–2954 [DOI] [PubMed] [Google Scholar]
- 58. Wan M. X., Wang Y., Liu Q., Schramm R., Thorlacius H. 2003. CC chemokines induce P-selectin-dependent neutrophil rolling and recruitment in vivo: intermediary role of mast cells. Br. J. Pharmacol. 138:698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Watanabe T., et al. 2004. Monocyte chemotactic protein-1 regulates leukocyte recruitment during gastric ulcer recurrence induced by tumor necrosis factor-alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 287:G919–G928 [DOI] [PubMed] [Google Scholar]
- 60. Weinrauch Y., Drujan D., Shapiro S. D., Weiss J., Zychlinsky A. 2002. Neutrophil elastase targets virulence factors of enterobacteria. Nature 417:91–94 [DOI] [PubMed] [Google Scholar]
- 61. Yang Z. Z., et al. 2004. Physiological functions of protein kinase B/Akt. Biochem. Soc. Trans. 32:350–354 [DOI] [PubMed] [Google Scholar]
- 62. Yrlid U., Wick M. J. 2000. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191:613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zaph C., et al. 2007. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature 446:552–556 [DOI] [PubMed] [Google Scholar]
- 64. Zeissig S., et al. 2004. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut 53:1295–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang H. M., et al. 2004. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J. Biol. Chem. 279:22539–22547 [DOI] [PubMed] [Google Scholar]
- 66. Zhang Y., Ting A. T., Marcu K. B., Bliska J. B. 2005. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J. Immunol. 174:7939–7949 [DOI] [PubMed] [Google Scholar]