Abstract
Objective To examine the risk of neurological and autoimmune disorders of special interest in people vaccinated against pandemic influenza A (H1N1) with Pandemrix (GlaxoSmithKline, Middlesex, UK) compared with unvaccinated people over 8-10 months.
Design Retrospective cohort study linking individualised data on pandemic vaccinations to an inpatient and specialist database on healthcare utilisation in Stockholm county for follow-up during and after the pandemic period.
Setting Stockholm county, Sweden.
Population All people registered in Stockholm county on 1 October 2009 and who had lived in this region since 1 January 1998; 1 024 019 were vaccinated against H1N1 and 921 005 remained unvaccinated.
Main outcome measures Neurological and autoimmune diagnoses according to the European Medicines Agency strategy for monitoring of adverse events of special interest defined using ICD-10 codes for Guillain-Barré syndrome, Bell’s palsy, multiple sclerosis, polyneuropathy, anaesthesia or hypoaesthesia, paraesthesia, narcolepsy (added), and autoimmune conditions such as rheumatoid arthritis, inflammatory bowel disease, and type 1 diabetes; and short term mortality according to vaccination status.
Results Excess risks among vaccinated compared with unvaccinated people were of low magnitude for Bell’s palsy (hazard ratio 1.25, 95% confidence interval 1.06 to 1.48) and paraesthesia (1.11, 1.00 to 1.23) after adjustment for age, sex, socioeconomic status, and healthcare utilisation. Risks for Guillain-Barré syndrome, multiple sclerosis, type 1 diabetes, and rheumatoid arthritis remained unchanged. The risks of paraesthesia and inflammatory bowel disease among those vaccinated in the early phase (within 45 days from 1 October 2009) of the vaccination campaign were significantly increased; the risk being increased within the first six weeks after vaccination. Those vaccinated in the early phase were at a slightly reduced risk of death than those who were unvaccinated (0.94, 0.91 to 0.98), whereas those vaccinated in the late phase had an overall reduced mortality (0.68, 0.64 to 0.71). These associations could be real or explained, partly or entirely, by residual confounding.
Conclusions Results for the safety of Pandemrix over 8-10 months of follow-up were reassuring —notably, no change in the risk for Guillain-Barré syndrome, multiple sclerosis, type 1 diabetes, or rheumatoid arthritis. Relative risks were significantly increased for Bell’s palsy, paraesthesia, and inflammatory bowel disease after vaccination, predominantly in the early phase of the vaccination campaign. Small numbers of children and adolescents with narcolepsy precluded any meaningful conclusions.
Introduction
In June 2009 the World Health Organization declared the new influenza of swine origin, A (H1N1), a pandemic.1 In September 2009 the European Medicines Agency authorised three vaccines2 through an expeditious procedure adapted for a pandemic situation. Owing to the need for large quantities of vaccine, WHO had encouraged the development of vaccines with adjuvants.3 Evidence from the development of H5N1 vaccines indicated that adjuvants could reduce the amount of antigen needed to provide an adequate immunological response and reinforce the ability to provide longlasting protection.4 5 Two of the A (H1N1) pandemic vaccines, Pandemrix (GlaxoSmithKline, Middlesex, UK) and Focetria (Novartis, Basel, Switzerland), are based on novel adjuvants AS03 and MF59, containing squalene.
One safety consideration is the suggested autoimmunostimulating potential of such adjuvants.6 7 Another is the increased risk of neurological adverse events, such as the Guillain-Barré syndrome. Previous studies have shown an increased risk of Guillain-Barré syndrome after influenza vaccination, with relative risks ranging from more than 7 in a study from 1957 to about 1.5 in studies from 1976 and 1992-4.8 9 10 Other neuroimmunological events such as Bell’s palsy have also been linked to previous vaccination against influenza.11 12
So far no formal studies have been published on adverse events in people undergoing H1N1 vaccination with any of the three vaccines used in the European Union. Available data are, with one exception,13 limited to case series or highly selected populations with short follow-up or no control group.14 15 16 Increased risks of narcolepsy in children and adolescents have been recently reported in epidemiological studies carried out by the Swedish Medical Products Agency and the Finnish Institute of Health and Welfare.17 18 19
A population based pandemic vaccination programme with Pandemrix was carried out from October 2009 to March 2010 in Sweden (population 9.3 million). The national overall coverage was about 60%. In the Stockholm population of some two million inhabitants, data on exposure to Pandemrix vaccination were linked through the personal identity number20 to data on inpatient and specialist care to ascertain outcomes of special interest according to the European Medicines Agency strategy for monitoring pandemic vaccines.21
Over a period of 8-10 months we examined the risk of neurological and autoimmune disorders of special interest in people vaccinated against pandemic H1N1 with Pandemrix compared with those who remained unvaccinated.
Methods
The study population consisted of all people (vaccinated and unvaccinated) registered in Stockholm county on 1 October 2009 and who had lived in the region since 1 January 1998 (to enable characterisation of cohorts from data tracked in the healthcare database before the pandemic period). The study population comprised 1.98 million people, of whom 52.6% (1 024 019) had been vaccinated.
Exposure to Pandemrix
Before the pandemic vaccination campaign a web based vaccination register, the Vaccinera, was established in Stockholm county. Vaccinated people were registered continually online. Data from Vaccinera included information on the dates for a first and second dose of vaccine, batch number, medical contraindications against vaccination (such as allergies and bleeding disorders), and chronic conditions defining high risk patients. Healthcare institutions that participated in the vaccination campaign were required to enter data in the Vaccinera register, which also allowed the county administration to follow vaccine coverage. To ensure that all vaccinations were registered in Vaccinera, reimbursements for vaccinations were processed only when a record was completed and submitted online.
In Sweden, H1N1 vaccinations were initially (from the start of the campaign in mid-October 2009 and during the subsequent one and half months) targeted at healthcare workers and groups considered to be at high risk of complications from influenza—that is, children with multifunctional disorders; pregnant women; patients with chronic heart or lung disease, diabetes mellitus, chronic liver failure, chronic renal failure, or immunosuppression; people with extreme obesity (body mass index >40); and patients with neuromuscular disease affecting breathing capacity. Data on diagnoses from primary care and hospitals showed that an estimated 10% of the population had a chronic condition putting them at risk.22
We considered people who received at least one dose of vaccine. A potential effect of a second dose was not evaluated.
Outcomes and definitions
For the purposes of this study we defined the pandemic period as starting on 1 October 2009. The vaccination campaign with Pandemrix started in mid-October.
We linked individualised data on vaccination to data on utilisation of inpatient and specialist healthcare in the common healthcare registers for Stockholm County Council (the GVR) from 1 January 1998 to 31 August 2010. This database contains information on all admissions to hospital and visits to specialist care in the county, such as dates, diagnoses (international classification of diseases), responsible medical departments, and length of hospital stay. Data in the healthcare database are continually transferred to the healthcare administration database, where a patient’s identity number is replaced by a code number.23 We used the same key for coding for the Vaccinera database to be able to link the two systems on an individual basis without revealing the patient’s identity.
Neurological and autoimmune diagnoses
We selected neurological and autoimmune diagnoses for follow-up in line with the European Medicines Agency strategy for monitoring the safety of pandemic vaccines,21 defined according to ICD-10 (international classification of diseases, 10th revision) codes for hospital admissions and visits to specialist care (see web extra appendix). Neurological outcomes of special interest included Guillain-Barré syndrome, Bell’s palsy, multiple sclerosis, polyneuropathy, anaesthesia or hypoaesthesia, paraesthesia, narcolepsy, and autoimmune conditions such as rheumatoid arthritis, inflammatory bowel disease (ulcerative colitis, Crohn’s disease), and type 1 diabetes. Entering diagnoses into the county healthcare database is part of the doctor’s routine diagnostic work and therefore depends on patients seeking healthcare. We did not actively search for adverse events during the study period.
We examined the risk of narcolepsy in the subgroup of people born in 1990 or later, as case reports have suggested a link between the condition and H1N1 vaccination in that age group. Furthermore, we examined the association between H1N1 vaccination and insulin dependent diabetes in people born from 1990 onwards (onset at age ≤20 years) as a proxy for type 1 diabetes.
Prevalent and incident disease
Prevalent disease was defined as having the selected ICD-10 diagnosis (see web extra appendix) registered in the healthcare administration database from 1 January 1998 to 30 September 2009 (before the pandemic period).
Incident disease was defined as having the selected ICD-10 diagnosis included for the first time from 1 October 2009 to 31 August 2010 (during or after the pandemic period for unvaccinated people and after a first vaccination for vaccinated people).
Socioeconomic classification
We used the Stockholm mosaic classification as a proxy for socioeconomic status.24 This classification is based on 11 exclusive categories of living conditions and economic situations—for example, being affluent and living in the inner Stockholm city or living in a small house in the suburbs or countryside.
Statistical analyses
All statistical analyses were carried out using SAS 9.2. We carried out analyses in two parts. Firstly, we analysed prevalent disease at the start of the follow-up as a predictor for the vaccination. Secondly, we analysed incident disease in relation to history of the vaccination. In all analyses of incident disease we excluded prevalent cases of the respective analysed disease.
Disease history as a predictor for vaccination
At the start of the study we used conditional logistic regression to analyse prevalent disease, with history of disease as the outcome and vaccination status as a predictor. We divided the vaccinated group into two categories of the vaccination campaign: the early phase, defined by vaccination within 45 days of the 1 October 2009, and the late phase, defined by vaccination after that period. The results are presented as prevalence odds ratios with 95% confidence intervals, with 0.05 considered as the level of statistical significance. We used conditioning to adjust estimates for sex, age (five year birth cohort), and socioeconomic status.
Risk of subsequent neurological and autoimmune diseases
We had no formal statistical analysis plan for the risk analyses, beyond the a priori chosen outcomes, choice of time scale, and use of Cox’s regression analysis to assess the overall effects of vaccination. The initial analyses comprised the overall association without adjustment for healthcare utilisation, where four of the selected outcomes showed a significant association with vaccination. Owing to the a priori knowledge that risk groups were prioritised for vaccination, we stratified the risk estimates for vaccination in both the early and the late phases of the campaign. We chose the cut-off point of 45 days to include the first month of the campaign in the early phase. After this stratification we found that the significant associations were confined to those vaccinated in the early phase. Because risk groups can be assumed to have easier access to hospital care, which we used for assessing the outcomes, we further adjusted the risk estimates for utilisation of healthcare. We chose calendar time from 1 October 2009 as the time scale and we considered vaccination as a time varying covariate. Calendar time was chosen as the time scale for analysis to control for potential seasonal effects in incidences of the outcomes under study. In a second model we conditioned further on the number of in-hospital admissions and visits to specialist care one year before vaccination. The results are presented as hazard ratios with 95% confidence intervals, with 0.05 considered as the level of statistical significance. We estimated the number of cases attributable to vaccination in the vaccinated group as the number of observed cases in the vaccinated group multiplied by 1−1/hazard ratio. We estimated the absolute excess risk among vaccinated people as the number of cases attributable to vaccination divided by the number of vaccinated people.
The hazard ratios related to vaccination were stratified on time since vaccination and calendar time of vaccination. We used six weeks as a cut-off point for stratification according to time since vaccination. The early phase of the vaccination campaign was defined by vaccination within 45 days of 1 October 2009 and the late phase by vaccination after that period. The vaccinations started on 13 October (except for 10 people who had been vaccinated earlier); the cut-off point used includes the first 32 days of the vaccination campaign (43.6% of all vaccinated people) in the early phase. To investigate whether there was an acute effect of the vaccination (that is, non-proportional hazards on the time scale time since vaccination), we analysed estimates stratified according to both times (see table 4). We used likelihood ratio tests to determine the interactions between calendar time of vaccination and time since vaccination.
Post hoc analyses
In an attempt to further estimate the influence of underlying comorbidity on health outcomes in patients undergoing H1N1 vaccination, we also estimated short term mortality according to vaccination status.
Results
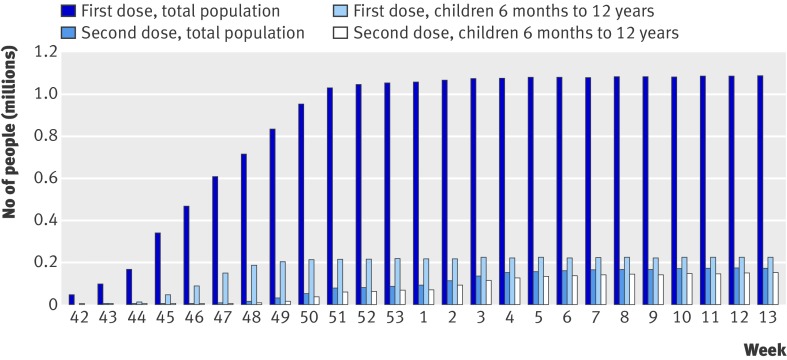
By 31 March 2010 virtually all vaccination activity had been completed, with a cumulative 1 024 019 people vaccinated (52.6% of the study population; figure). In all, 222 388 people, of whom 66.4% received a second dose, were vaccinated between the ages of six months and 12 years. Those belonging to a high risk group were largely over-represented compared with the total population during the first six weeks of the campaign (mid-October to November 2009); 74% of all those vaccinated in the first week and 30% in the sixth week. In a second phase (from the beginning of December) the remainder of the population was offered vaccination.22
Cumulative numbers of people vaccinated against A (H1N1) influenza in Stockholm county, Sweden, October 2009 (week 42) to March 2010 (week 13)
Table 1 shows vaccine coverage by sex, socioeconomic status, and birth cohort. More women than men were vaccinated (55.9% v 49.3%). Children and middle aged and older people were more often vaccinated than younger adults. Vaccine coverage was greater in those of a higher socioeconomic status.
Table 1.
Numbers and percentage proportions of vaccine coverage by sex, socioeconomic status, birth cohorts, and healthcare utilisation one year before pandemic period, in Stockholm county, Sweden
| Category | Vaccination status | Coverage (%) | |
|---|---|---|---|
| Unvaccinated | Vaccinated | ||
| All | 921 005 | 1 024 019 | 52.6 |
| Women | 434 727 | 550 856 | 55.9 |
| Men | 486 278 | 473 163 | 49.3 |
| Socioeconomic status*: | |||
| Affluent, inner city | 90 819 | 109 179 | 54.6 |
| Inner city | 86 379 | 92 568 | 51.7 |
| Mixed, near city | 124 323 | 128 330 | 50.8 |
| Young, near suburb | 23 684 | 18 852 | 44.3 |
| Older, near suburb | 120 958 | 108 221 | 47.2 |
| Poor, suburb, apartment | 52 074 | 51 208 | 49.6 |
| Multicultural suburb | 149 956 | 98 832 | 39.7 |
| Affluent, near suburb | 140 457 | 247 888 | 63.8 |
| Own house, suburb | 47 963 | 70 022 | 59.3 |
| Small house, suburb | 36 520 | 48 666 | 57.1 |
| Countryside | 47 872 | 50 253 | 51.2 |
| Birth year cohort: | |||
| 1900-9 | 240 | 174 | 42.0 |
| 1910-19 | 7075 | 8249 | 53.8 |
| 1920-39 | 73 596 | 110 031 | 59.9 |
| 1940-9 | 93 806 | 120 071 | 56.1 |
| 1950-79 | 421 872 | 392 329 | 48.2 |
| 1980-4 | 86 234 | 47 332 | 35.4 |
| 1985-9 | 83 127 | 31 871 | 27.7 |
| 1990-4 | 59 117 | 64 814 | 52.3 |
| 1995-9 | 22 074 | 81 822 | 78.8 |
| 2000-4 | 24 984 | 89 239 | 78.1 |
| 2005-1 Oct 2009 | 48 879 | 78 087 | 61.5 |
| No of hospital admissions: | |||
| None | 854 064 | 928 972 | 52.1 |
| 1 | 47 199 | 66 601 | 58.5 |
| 2 | 11 239 | 15 878 | 58.5 |
| ≥3 | 8503 | 12 568 | 59.6 |
| No of ambulatory care visits to hospital | |||
| None | 599 097 | 596 869 | 49.9 |
| 1 | 124 429 | 154 379 | 55.4 |
| 2 | 62 181 | 81 793 | 56.8 |
| 3 | 37 035 | 50 119 | 57.5 |
| 4 | 23 659 | 33 038 | 58.3 |
| ≥5 | 74 605 | 107 821 | 59.1 |
*Stockholm mosaic classification.24
Prevalent disease at vaccination
Neurological and autoimmune disorders were more prevalent in those vaccinated in the early phase of the campaign (first 45 days) than in the unvaccinated cohort (table 2). No such differences were seen for those vaccinated in the late phase (>45 days) compared with the unvaccinated cohort, except for inflammatory bowel disease (prevalence odds ratio 1.17, 95% confidence interval 1.12 to 1.22). Those vaccinated in the late phase had a lower prevalence of Guillain-Barré syndrome (0.79, 0.67 to 0.95) and type 1 diabetes (0.77, 0.64 to 0.92, for those born in 1990 and later). This pattern of morbidity is consistent with the Swedish strategy to prioritise high risk groups in the early phase of the campaign.
Table 2.
Associations of defined prevalent diseases with vaccination status (vaccinated versus unvaccinated), in subcohorts vaccinated in early and late phases of H1N1 vaccination campaign in Stockholm county, Sweden
| Diseases and vaccination status | No | Prevalence odds ratios (95% CI) |
|---|---|---|
| Neurological diseases | ||
| Bell’s palsy | ||
| Unvaccinated | 3236 | 1.00 (reference) |
| ≤45 days | 1998 | 1.26 (1.19 to 1.34) |
| >45 days | 1816 | 1.01 (0.95 to 1.08) |
| Guillain-Barré syndrome: | ||
| Unvaccinated | 408 | 1.00 (reference) |
| ≤45 days | 322 | 1.29 (1.11 to 1.50) |
| >45 days | 188 | 0.79 (0.67 to 0.95) |
| Multiple sclerosis: | ||
| Unvaccinated | 1275 | 1.00 (reference) |
| ≤45 days | 1866 | 2.97 (2.76 to 3.20) |
| >45 days | 654 | 0.92 (0.83 to 1.01) |
| Anaesthesia or hypoaesthesia: | ||
| Unvaccinated | 991 | 1.00 (reference) |
| ≤45 days | 671 | 1.40 (1.26 to 1.55) |
| >45 days | 504 | 0.92 (0.82 to 1.02) |
| Paraesthesia: | ||
| Unvaccinated | 5436 | 1.00 (reference) |
| ≤45 days | 3605 | 1.36 (1.30 to 1.42) |
| >45 days | 3005 | 1.01 (0.97 to 1.06) |
| Polyneuropathy: | ||
| Unvaccinated | 1559 | 1.00 (reference) |
| ≤45 days | 1556 | 1.46 (1.36 to 1.57) |
| >45 days | 833 | 0.95 (0.88 to 1.04) |
| Narcolepsy: | ||
| Unvaccinated | 86 | 1.00 (reference) |
| ≤45 days | 61 | 1.35 (0.96 to 1.90) |
| >45 days | 57 | 1.19 (0.84 to 1.68) |
| Narcolepsy (aged ≤20 years): | ||
| Unvaccinated | 1 | 1.00 (reference) |
| ≤45 days | 3 | 3.53 (0.31 to 39.8) |
| >45 days | 3 | 2.69 (0.25 to 28.5) |
| Autoimmune diseases | ||
| Rheumatoid arthritis: | ||
| Unvaccinated | 4861 | 1.00 (reference) |
| ≤45 days | 5894 | 2.03 (1.95 to 2.11) |
| >45 days | 2695 | 0.94 (0.89 to 0.98) |
| Inflammatory bowel disease: | ||
| Unvaccinated | 6058 | 1.00 (reference) |
| ≤45 days | 4946 | 1.79 (1.72 to 1.86) |
| >45 days | 3838 | 1.17 (1.12 to 1.22) |
| Type 1 diabetes*: | ||
| Unvaccinated | 268 | 1.00 (reference) |
| ≤45 days | 1086 | 6.17 (5.36 to 7.10) |
| >45 days | 219 | 0.77 (0.64 to 0.92) |
*Population born 1990 or later.
Risk of selected, incident, neurological and autoimmune diseases
Compared with the unvaccinated cohort the vaccinated cohort showed positive associations with Bell’s palsy (hazard ratio 1.25, 95% confidence interval 1.06 to 1.48) and paraesthesia (1.11, 1.00 to 1.23), after adjustment for age, sex, socioeconomic status, and utilisation of healthcare (table 3). This corresponds to absolute excess risks in the vaccinated population of 8.4 cases per 100 000 vaccinated person years for Bell’s palsy (95% confidence interval 2.3 to 13.4) and 9.2 cases per 100 000 person years for paraesthesia (0 to 17.5). The small number of cases of narcolepsy observed among people aged 20 years and younger (six in the vaccinated cohort and two in the unvaccinated cohort) preclude any meaningful interpretation.
Table 3.
Risk of selected neurological and autoimmune diseases in vaccinated versus unvaccinated cohort and in subcohorts vaccinated in early and late phases of H1N1 vaccination campaign in Stockholm county, Sweden
| Diseases and vaccination status | Hazard ratio (95% CI)* | |
|---|---|---|
| Model 1 | Model 2 | |
| Neurological diseases | ||
| Bell’s palsy: | ||
| All vaccinated | 1.35 (1.14 to 1.59) | 1.25 (1.06 to 1.48) |
| ≤45 days | 1.49 (1.23 to 1.81) | 1.34 (1.11 to 1.64) |
| >45 days | 1.19 (0.97 to 1.47) | 1.16 (0.94 to 1.43) |
| Guillain-Barré syndrome: | ||
| All vaccinated | 1.27 (0.79 to 2.06) | 1.07 (0.66 to 1.74) |
| ≤45 days | 1.40 (0.81 to 2.42) | 1.08 (0.62 to 1.87) |
| >45 days | 1.13 (0.61 to 2.09) | 1.07 (0.58 to 1.99) |
| Multiple sclerosis: | ||
| All vaccinated | 0.99 (0.73 to 1.35) | 0.93 (0.68 to 1.26) |
| ≤45 days | 1.17 (0.82 to 1.66) | 1.04 (0.72 to 1.48) |
| >45 days | 0.81 (0.54 to 1.21) | 0.80 (0.54 to 1.20) |
| Anaesthesia or hypoaesthesia: | ||
| All vaccinated | 1.18 (0.87 to 1.61) | 1.05 (0.77 to 1.43) |
| ≤45 days | 1.50 (1.06 to 2.12) | 1.23 (0.86 to 1.74) |
| >45 days | 0.86 (0.57 to 1.30) | 0.84 (0.56 to 1.27) |
| Paraesthesia: | ||
| All vaccinated | 1.20 (1.08 to 1.33) | 1.11 (1.00 to 1.23) |
| ≤45 days | 1.43 (1.27 to 1.61) | 1.25 (1.10 to 1.41) |
| >45 days | 0.96 (0.83 to 1.10) | 0.94 (0.82 to 1.09) |
| Polyneuropathy: | ||
| All vaccinated | 1.33 (1.11 to 1.59) | 1.11 (0.92 to 1.32) |
| ≤45 days | 1.40 (1.15 to 1.71) | 1.09 (0.89 to 1.33) |
| >45 days | 1.22 (0.97 to 1.53) | 1.14 (0.90 to 1.44) |
| Narcolepsy: | ||
| All vaccinated | 1.45 (0.56 to 3.75) | 1.25 (0.47 to 3.30) |
| ≤45 days | 1.82 (0.63 to 5.21) | 1.38 (0.46 to 4.17) |
| >45 days | 1.10 (0.34 to 3.59) | 1.12 (0.34 to 3.66) |
| Narcolepsy (aged ≤20 years): | ||
| All vaccinated | 1.54 (0.30 to 7.89) | 1.54 (0.30 to 8.01) |
| ≤45 days | 0.67 (0.06 to 7.70) | 0.58 (0.05 to 6.69) |
| >45 days | 2.06 (0.38 to 11.0) | 2.21 (0.41 to 11.8) |
| Autoimmune diseases | ||
| Rheumatoid arthritis: | ||
| All vaccinated | 1.03 (0.89 to 1.19) | 0.94 (0.81 to 1.09) |
| ≤45 days | 1.17 (0.99 to 1.38) | 1.01 (0.86 to 1.20) |
| >45 days | 0.86 (0.71 to 1.04) | 0.84 (0.69 to 1.02) |
| Inflammatory bowel disease: | ||
| All vaccinated | 1.22 (1.04 to 1.42) | 1.13 (0.97 to 1.32) |
| ≤45 days | 1.43 (1.20 to 1.71) | 1.25 (1.04 to 1.50) |
| >45 days | 1.01 (0.83 to 1.23) | 1.00 (0.82 to 1.22) |
| Type 1 diabetes†: | ||
| All vaccinated | 1.03 (0.70 to 1.53) | 0.99 (0.67 to 1.47) |
| ≤45 days | 1.21 (0.77 to 1.89) | 1.13 (0.72 to 1.78) |
| >45 days | 0.91 (0.59 to 1.41) | 0.88 (0.57 to 1.38) |
Both models were adjusted for age, sex, and socioeconomic status. Model 2 was further adjusted for healthcare consumption (number of hospital admissions and visits to specialist care one year before pandemic period).
*Cox’s regression analyses.
†Population born 1990 or later.
The risks of neurological and autoimmune diseases after vaccination were further examined in relation to the early and late phases of the vaccination campaign (table 4). In the analyses without adjustment for healthcare utilisation, the difference in risk between those vaccinated in the early and late phases was significant for paraesthesia, inflammatory bowel disease, rheumatoid arthritis, anaesthesia or hypoaesthesia, and Bell’s palsy. However, after adjustment for healthcare utilisation this difference between the two phases remained significant only for paraesthesia (P=0.003) and inflammatory bowel disease (P=0.04).
Early vaccinations (≤45 days) were associated with a significantly increased risk for Bell’s palsy (hazard ratio 1.34, 95% confidence interval 1.11 to 1.64), paraesthesia (1.25, 1.10 to 1.41), and inflammatory bowel disease (1.25, 1.04 to 1.50; table 3) after adjustment for healthcare utilisation in addition to age, sex, and socioeconomic status. These risk estimates became lower after adjustment for healthcare utilisation before the pandemic period, reflecting outcomes in people within risk groups. In this subcohort the increased risk was not evident for Guillain-Barré syndrome, multiple sclerosis, type 1 diabetes, or rheumatoid arthritis. In the subcohort of people vaccinated in the late phase of the campaign, none of the risk estimates was statistically significantly increased for the vaccinated cohort compared with unvaccinated cohort.
In analyses stratified according to time since first vaccination (table 4) the excess incidences of Bell’s palsy and paraesthesia were most pronounced among those vaccinated in the early phase (1.74, 1.16 to 2.59) and during the six weeks after vaccination (1.60, 1.25 to 2.05). These hazard ratios correspond to absolute excess risks of 30 (95% confidence interval 10 to 44) and 65 (1.5 to 89) cases per 100 000 vaccinated person years, respectively. Significant but smaller excess risks of Bell’s palsy and paraesthesia more than six weeks after vaccination were also observed among those vaccinated in the early phase. The excess risk of inflammatory bowel disease among those vaccinated in the early phase was only observed more than six weeks after vaccination. The overall absence of an excess risk for type 1 diabetes among people aged less than 20 years was consistent in both risk windows (within and more than six weeks from vaccination) and among people vaccinated in the early and late phases. However, formal tests to determine whether risks further differed between within and more than six weeks from vaccination were only statistically significant for paraesthesia (P=0.005).
Table 4.
Number and risk of neurological or autoimmune diseases among vaccinated cohort, stratified according to time since vaccination (≤6 weeks and >6 weeks) and vaccination in early and late phases of H1N1 vaccination campaign in Stockholm county, Sweden
| Outcome | ≤6 weeks after vaccination | >6 weeks after vaccination | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Early phase | Late phase | Early phase | Late phase | ||||||
| No of events | Hazard ratio* (95% CI) | No of events | Hazard ratio* (95% CI) | No of events | Hazard ratio* (95% CI) | No of events | Hazard ratio* (95% CI) | ||
| Neurological diseases: | |||||||||
| Bell’s palsy | 37 | 1.74 (1.16 to 2.59) | 21 | 1.02 (0.64 to 1.63) | 145 | 1.26 (1.01 to 1.57) | 130 | 1.16 (0.92 to 1.45) | |
| Guillain-Barré syndrome | 3 | 0.72 (0.19 to 2.66) | 3 | 1.92 (0.68 to 5.40) | 20 | 1.18 (0.64 to 2.17) | 13 | 0.92 (0.45 to 1.89) | |
| Multiple sclerosis | 12 | 1.35 (0.68 to 2.67) | 8 | 1.17 (0.53 to 2.57) | 36 | 0.95 (0.63 to 1.44) | 26 | 0.71 (0.45 to 1.12) | |
| Anaesthesia or hypoaesthesia | 8 | 1.61 (0.77 to 3.39) | 7 | 1.04 (0.43 to 2.55) | 50 | 1.15 (0.78 to 1.69) | 29 | 0.79 (0.50 to 1.24) | |
| Paraesthesia | 90 | 1.60 (1.25 to 2.05) | 55 | 1.23 (0.91 to 1.65) | 360 | 1.17 (1.02 to 1.34) | 245 | 0.87 (0.75 to 1.02) | |
| Polyneuropathy | 33 | 1.13 (0.75 to 1.70) | 28 | 1.79 (1.16 to 2.77) | 156 | 1.07 (0.86 to 1.34) | 90 | 1.01 (0.77 to 1.31) | |
| Narcolepsy | 1 | 0.98 (0.10 to 9.67) | 0 | — | 6 | 1.57 (0.45 to 5.50) | 5 | 1.42 (0.40 to 5.08) | |
| Narcolepsy (age ≤20 years) | 0 | — | 0 | — | 1 | 0.58 (0.05 to 6.72) | 5 | 2.25 (0.42 to 12.1) | |
| Autoimmune diseases: | |||||||||
| Rheumatoid arthritis | 40 | 1.08 (0.74 to 1.57) | 22 | 0.82 (0.52 to 1.28) | 212 | 1.00 (0.83 to 1.20) | 142 | 0.84 (0.68 to 1.04) | |
| Inflammatory bowel disease | 35 | 1.10 (0.74 to 1.63) | 27 | 1.00 (0.65 to 1.54) | 168 | 1.29 (1.06 to 1.58) | 130 | 1.01 (0.82 to 1.25) | |
| Type 1 diabetes† | 6 | 0.89 (0.31 to 2.52) | 7 | 1.18 (0.47 to 2.96) | 37 | 1.18 (0.72 to 1.94) | 39 | 0.85 (0.52 to 1.38) | |
*Adjusted for age, sex, socioeconomic status, and healthcare utilisation one year before start of follow-up.
†Population born 1990 or later.
In a post hoc analysis of the risk of death (table 5), those who were vaccinated in the early phase were at a slightly reduced risk of death compared with those who remained unvaccinated after adjustment for earlier healthcare utilisation in addition to age, sex, and socioeconomic status (hazard ratio 0.94, 95% confidence interval 0.91 to 0.98). In contrast, people undergoing vaccination more than 45 days after study entry had a lower overall mortality than those who remained unvaccinated, also after adjustment for previous healthcare utilisation in addition to age, sex, and socioeconomic status (0.68, 0.64 to 0.71).
Table 5.
Risk of death from any cause in vaccinated versus unvaccinated individuals in subcohorts vaccinated in early and late phases of H1N1 vaccination campaign in Stockholm county, Sweden
| Vaccination status | No of people | No of deaths | Hazard ratio (95% CI)* | |
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| All vaccinated | 1 024 019 | 6605 | 0.95 (0.92 to 0.99) | 0.85 (0.82 to 0.89) |
| ≤45 days | 446 770 | 4881 | 1.12 (1.08 to 1.16) | 0.94 (0.91 to 0.98) |
| >45 days | 577 249 | 1724 | 0.67 (0.63 to 0.70) | 0.68 (0.64 to 0.71) |
Both models were adjusted for age, sex, and socioeconomic status. Model 2 was further adjusted for healthcare consumption (number of hospital admissions and visits to specialist care one year before pandemic period).
*Cox’s regression analysis.
Discussion
Among the more than one million people vaccinated with the squalene adjuvanted Pandemrix vaccine in Stockholm county (the only vaccine used in Sweden against pandemic H1N1), the risks of Bell’s palsy and paraesthesia were increased. Excess risks for Bell’s palsy, paraesthesia, and inflammatory bowel disease were, however, observed only among those vaccinated in the early phase of the vaccination campaign (≤45 days), when high risk groups predominated. In contrast, among people vaccinated after the first 45 days of the campaign, representing more closely the general population, we found no statistically significant associations between vaccination and autoimmune or neurological diseases. Change in the risks for Guillain-Barré syndrome, multiple sclerosis, diabetes, or rheumatoid arthritis was not evident in any of the analyses. As to the risk of narcolepsy in adolescents and children, small numbers precluded any meaningful conclusions.
People vaccinated in the first 45 days consistently more often had a previous diagnosis of neurological or autoimmune disease than those who remained unvaccinated. Earlier neurological and autoimmune disease was therefore a strong predictor for vaccination in the first 45 days—for example, those with type 1 diabetes were at a sixfold increased risk of being vaccinated early. Together with the high proportion reported to have a high risk condition according to the Vaccinera database, this suggests that the national recommendation to vaccinate high risk groups first was followed. Earlier comorbidity could explain some of the excess risks seen in those vaccinated early. Even if hazard ratios decreased for almost all outcomes (for example, Bell’s palsy from 1.49 to 1.34; inflammatory bowel disease from 1.43 to 1.25, and paraesthesia from 1.43 to 1.25) after adjustment for the number of hospital admissions and visits to specialist care, some residual confounding may still exist. These small excess risks may be partly or entirely explained by other factors that were not captured by a crude measure of healthcare utilisation. Nevertheless, if true, these hazard ratios would translate into low absolute risks.
In contrast, those vaccinated in the later part of the campaign had a similar distribution of earlier neurological and autoimmune disease (with the exception of a small increase in previous inflammatory bowel disease) as unvaccinated people. Furthermore, those in this subcohort were at no statistically increased risk of any of our analysed outcomes, suggesting that in a general population vaccination with Pandemrix is unlikely to lead to an important effect on the risk for neurological or autoimmune diseases (not accounting for risk of narcolepsy). However, mortality, even after adjustment for previous healthcare utilisation in addition to age, sex, and socioeconomic status, was lower in this vaccinated subcohort than in the unvaccinated cohort. This may reflect a healthy selection of people for those vaccinated and that our risk estimates for neurological and autoimmune outcomes may be underestimates.
Comparisons with previous studies and findings
Most available data on the safety of A (H1N1) pandemic vaccines—with Pandemrix being the most commonly used vaccine in the European Union (estimated use in some 30 million)—are based on reports of spontaneous adverse drug events to national regulatory agencies. Such data have been generally reassuring during and after the pandemic period. However, an increased risk of narcolepsy in children and adolescents, with increased relative risks ranging from fourfold to ninefold, have been recently reported from authorities in Sweden and Finland,17 18 19 leading to regulatory action by the European drug regulatory body, the European Medicines Agency, in July 2011 to restrict the use of Pandemrix vaccinations.25 Regarding other outcomes, some pertinent conclusions can be drawn from published studies on the safety of influenza vaccinations in general.
Although previous studies have produced conflicting results on an association between Guillain-Barré syndrome and influenza vaccination,8 9 10 26 two larger studies reported no positive association.27 28 In our study we found no association between vaccination with an adjuvanted H1N1 vaccine and Guillain-Barré syndrome. Our findings are consistent with a Chinese study of a non-adjuvanted pandemic vaccine14 and add to that study because we were able to estimate hazard ratios and our study population was not restricted to previously healthy people.
Although a large number of studies have examined the association between various types of vaccinations and type 1 diabetes,29 30 31 32 none has shown an association.33 To our knowledge no studies have been carried out on influenza vaccinations (for example, using squalene adjuvanted vaccines) and risk of type 1 diabetes. In our study we found no association between H1N1 vaccination and type 1 diabetes in the age group where most cases of the disease occur (those born in 1990 and later).
The excess risk for paraesthesia may constitute a local symptom (for example, pain, redness, swelling, tingling) at the injection site from the H1N1 vaccination. The excess risk of paraesthesia was only of borderline significance (95% confidence interval 1.00 to 1.23) and absent in patients undergoing vaccination in the late phase.
We cannot explain the small increase in risk for Bell’s palsy seen in this study. Potential causes include viral infection and pregnancy, neither of which could be dealt with using the data in our analyses. The absolute risk of Bell’s palsy was low, 6.4 cases per 100 000 vaccinated population.
Safety considerations of European Medicines Agency for adjuvanted pandemic A (H1N1) vaccines
The pandemic vaccines were developed by using a prototype vaccine that contained the H5N1 antigen and an adjuvant—for Pandemrix, squalene combined with DL-α-tocopherol. This mock-up vaccine was recommended for approval in 2008 by the European Medicines Agency, based on data on efficacy (antibody response) and safety in some 5000 people aged 18-65 years. After approval of the final vaccine in September 2009, a trial was carried out in 300 children aged 3-12 years. Thus at the time of release on to the market in October 2009 the safety experience of Pandemrix was deemed to be limited. The European Medicines Agency encouraged a strategy for enhanced pharmacovigilance, implying stimulated reporting of spontaneous adverse drug reactions and the start of epidemiological studies. During and after the pandemic vaccination period in Sweden, reports on adverse drug reactions for Pandemrix were generally reassuring but produced a new signal for allergic reactions. In the autumn of 2010 an unexpectedly large number of reports on narcolepsy in adolescents and children was noted by the Medical Products Agency in Sweden (as in Finland).18 Subsequent epidemiological studies in Sweden and Finland reported several-fold increased risks of narcolepsy in children and adolescents.17 18 19 Our trial is the first data based study on an array of neurological and autoimmune safety outcomes for one of the pandemic vaccines used in the European Union.
Strengths and limitations of the study
Through the vaccination register (Vaccinera) our study covered all vaccinated people in Stockholm county. The unique personal identity number enabled us to ascertain data on earlier utilisation of healthcare as well as to adjust for sex, age, and socioeconomic status. We used ICD codes assigned by doctors to identify neurological and autoimmune diseases recorded in the Stockholm healthcare database. Our large number of study participants allowed for precise risk estimates for many of the outcomes. For instance, for a rare disease such as Guillain-Barré syndrome we could rule out a hazard ratio of 1.7 or greater (table 3). Through data on healthcare utilisation before the pandemic period we could also explain at least part of the excess risks seen in those vaccinated early against H1N1.
Our study has some limitations that may have influenced our risk estimates. The neurological and autoimmune diseases studied were diagnosed and entered in the healthcare database as part of the clinical routine in the county and thus depended on patients seeking healthcare because of their greater availability for specialist care. We also lacked detailed data on covariates, with the possibility for residual confounding. For instance, the lower mortality in those vaccinated in the late phase was not explained through adjustment for earlier healthcare utilisation. If people in this subcohort are healthier than the general population our study may have underestimated the risk of adverse effects in those who were vaccinated. Two circumstances argue against our hazard ratios being underestimates. Firstly, neurological and autoimmune diseases were similar in people of this subcohort undergoing late vaccination and in the unvaccinated cohort. Secondly, as cardiovascular disease and cancer are by far the most common causes of death in Sweden, the lower mortality in those vaccinated should be sought in low levels of smoking or a low body mass index. However, neither smoking nor high body mass index is a major risk factor for neurological or autoimmune diseases, and therefore a skewed distribution of these characteristics is unlikely to hide a true association between H1N1 vaccination and our outcomes.
Furthermore, high risk groups were over-represented in the early phase of the vaccination campaign (as shown by a higher prevalence at the start of follow-up for most of the selected outcome diagnoses). This implies that this subgroup is not obviously comparable with the unvaccinated subgroup, thus potentially leading to selection bias. Since we have access only to data on visits to specialist care and hospital admissions, surveillance bias is also a concern—namely, that vaccinated patients may have better access to specialist care than unvaccinated patients, especially those belonging to medical risk groups. To some extent we controlled for both selection bias and surveillance bias by adjustment for healthcare utilisation before the start of follow-up, which generally resulted in reduced risk estimates. Residual confounding is, however, still a possibility.
Overall, 90% of the vaccinated people had a follow-up time ranging from 256 days to 315 days. For certain conditions requiring a long period of investigation or if an adverse effect of the vaccination is delayed, the follow-up time may be too short to reveal the full effect, which would result in an underestimation of the true effect. The influence of chance is a problem when evaluating multiple end points divided according to two temporal aspects. Furthermore, the power of our study to detect change of risk for rare outcomes such as narcolepsy was insufficient.
Conclusions and implications
Based on data from follow-up during 8-10 months among more than one million people vaccinated with Pandemrix and 900 000 unvaccinated people in the entire population of Stockholm county, we found mostly reassuring results, notably for Guillain-Barré syndrome, multiple sclerosis, type 1 diabetes, and rheumatoid arthritis. Although we found small excess risks (ranging from 1.25 to 1.34) for some neurological and autoimmune diseases after vaccination, such as Bell’s palsy, paraesthesia, and inflammatory bowel disease, these were only seen in those among high risk groups targeted for early vaccination and who were likely to have earlier comorbidity, which could partly or entirely have explained the findings. As to the association between vaccination with Pandemrix and narcolepsy in adolescents and children, small numbers precluded meaningful results.
What is already known on this topic
Studies are lacking on adverse events (except for narcolepsy) with any of the three vaccines used in the European Union against H1N1 during the pandemic period
Available data are limited to case series or highly selected populations with short follow-up or no control group
What this study adds
Excess risks for Bell’s palsy, paraesthesia, and inflammatory bowel disease after H1N1 vaccination with adjuvanted Pandemrix in Sweden were small but significant among more than one million vaccinated, but only in high risk groups targeted for early vaccination and who were likely to have earlier comorbidity
The risk of Guillain-Barré syndrome, multiple sclerosis, type 1 diabetes, and rheumatoid arthritis remained unchanged
Small numbers of children and adolescents with narcolepsy precluded any meaningful conclusions
We thank Peter Rönnerfalk, chief medical officer, Stockholm County Council, for supporting this study, to be done within the county without external funding.
Contributors: CB, IP, ÅÖ, FG, and UB conceived and designed the study. FG analysed the data. All authors (CB, IP, ÅÖ, FG, JFL, and UB) interpreted the data, critically revised and prepared the manuscript, and gave final approval of the version to be published. CB is the guarantor. This manuscript represents the views of the authors, not necessarily those of the Swedish drug regulatory agency (Medical Products Agency) where two of the authors are employed (CB, IP)
Funding: Stockholm County Council and the Medical Products Agency.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the regional ethics committee in Stockholm, Sweden (No 2010/773-31/1 and 2010/1772-32).
Data sharing: No additional data available.
Cite this as: BMJ 2011;343:d5956
Web Extra. Extra material supplied by the author
Selected neurological and autoimmune diagnoses and ICD-10 codes included in analyses
References
- 1.World Health Organization. New influenza A (H1N1) virus; global epidemiological situation. Wkly Epidemiol Rec 2009;84:249-57. [PubMed] [Google Scholar]
- 2.Johansen K, Nicoll A, Ciancio BC, Kramarz P. Pandemic influenza A(H1N1) 2009 vaccines in the European Union. Euro Surveill 2009;14:19361. [PubMed] [Google Scholar]
- 3.World Health Organization. Influenza pandemic preparedness and response. Report by the secretariat. WHO, 2005.
- 4.Diez-Domingo J, Garcés-Sanchez M, Baldó JM, Planelles MV, Ubeda I, JuBert A, et al. Immunogenecity and safety of H5N1 A/Vietnam/1194/2004 (Clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase ii, randomized, open, controlled study. Pediatr Infect Dis J 2010;29:e35-46. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz TF, Horacek T, Knuf M, Damman HG, Roman F, Dramé M, et al. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine 2009;27:6284-90. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda Y, Nactionales DC, Akaogi J, Reeves WH, Satoh M. Autoimmunity induced by adjuvant hydrocarbon oil components of vaccine. Biomed Pharmacother 2004;58:325-37. [DOI] [PubMed] [Google Scholar]
- 7.Satoh M, Kuroda Y, Yoshida H, Behney KM, Mizutani A, Akaogi J, et al. Induction of lupus autoantibodies by adjuvants. J Autoimmun 2003;21:1-9. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz ES, Schonberger LB, Nelson DB, Holman RC. Guillain-Barré syndrome and the 1978-1979 influenza vaccine. N Engl J Med 1981;304:1557-61. [DOI] [PubMed] [Google Scholar]
- 9.Lasky T, Terracciano GJ, Magder L, Koski CL, Ballesteros M, Nash D, et al. The Guillain-Barré syndrome and the 1992-1993 and 1993-1994 influenza vaccines. N Engl J Med 1998;339:1797-802. [DOI] [PubMed] [Google Scholar]
- 10.Safranek TJ, Lawrence DN, Kurland LT, Culver DH, Wiederholt WC, Hayner NS, et al. Reassessment of the association between Guillain-Barré syndrome and receipt of swine influenza vaccine in 1976-1977: results of a two-state study. Expert Neurology Group. Am J Epidemiol 1991;133:940-51. [DOI] [PubMed] [Google Scholar]
- 11.Zhou W, Pool V, DeStefano F, Iskander JK, Haber P, Chen RT. A potential signal of Bell’s palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS)—United States, 1991-2001. Pharmacoepidemiol Drug Saf 2004;13:505-10. [DOI] [PubMed] [Google Scholar]
- 12.Stowe J, Andrews N, Wise L, Miller E. Bell’s palsy and parenteral inactivated influenza vaccine. Hum Vaccine 2006;2:110-2. [DOI] [PubMed] [Google Scholar]
- 13.Liang XF, Li L, Liu DW, Li KL, Wu WD, Zhu BP, et al. Safety of influenza A (H1N1) vaccine in postmarketing surveillance in China. N Engl J Med 2011;364:638-47. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Xu F, Lu L, Lu M, Miao L, Gao T, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med 2010;363:2416-23. [DOI] [PubMed] [Google Scholar]
- 15.Lu CC, Wang YC, Lai JH, Lee TS, Lin HT, Chang DM. A/H1N1 influenza vaccination in patients with systemic lupus erythematosus: safety and immunity. Vaccine 2011;29:444-50. [DOI] [PubMed] [Google Scholar]
- 16.Park SW, Lee JH, Kim ES, Kwak YG, Moon CS, Yeom JS, et al. Adverse events associated with the 2009 H1N1 influenza vaccination and the vaccination coverage rate in health care workers. Am J Infect Control 2011;39:69-71. [DOI] [PubMed] [Google Scholar]
- 17.Medical Products Agency. A registry based comparative cohort study in four Swedish counties of the risk for narcolepsy after vaccination with Pandemrix—a first and preliminary report. 2011. www.mpa.se.
- 18.National Institute for Health and Welfare (THL). National Narcolepsy Task Force interim report. 2011. www.thl.fi.
- 19.Medical Products Agency. Occurrence of narcolepsy with cataplexy among children and adolescents in relation to the H1N1 pandemic and Pandemrix vaccinations. Results of a case inventory study by the MPA in Sweden during 2009-2010. 2011. www.mpa.se.
- 20.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Medicine Agency. CHMP recommendations for the pharmacovigilance plan as part of the risk management plan to be submitted with the marketing authorisation application for a pandemic influenza vaccine. Report No EMEA/359381/2009 rev. EMA, 2009.
- 22.Örtqvist Å, Berggren I, Insulander M, de Jong B, Svenungsson B. Effectiveness of an adjuvanted mono-valent vaccine against the 2009 pandemic strain of influenza A (H1N1)v, in Stockholm county, Sweden. Clin Infect Dis 2011;52:1203-11. [DOI] [PubMed] [Google Scholar]
- 23.Löfgren S, Ljunggren G, Brommels M. No ticking time bomb: hospital utilisation of 28,528 hip fracture patients in Stockholm during 1998-2007. Scand J Public Health 2010;38:418-25. [DOI] [PubMed] [Google Scholar]
- 24.Szegö J. Bebyggelsens mosaik [The mosaic of the settlement]. Regionplanekontoret, Stockholm report No 7:2009.
- 25.European Medicines Agency recommends restricting use of Pandemrix. Press release 21 July 2011. www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true.
- 26.Haber P, DeStefano F, Angulo FJ, Iskander J, Shadomy SV, Weintraub E. Guillain-Barre syndrome following influenza vaccination. JAMA 2004;292:2478-81. [DOI] [PubMed] [Google Scholar]
- 27.Roscelli JD, Bass JW, Pang L. Guillain-Barre syndrome and influenza vaccination in the US Army, 1980-1988. Am J Epidemiol 1991;133:952-5. [DOI] [PubMed] [Google Scholar]
- 28.Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol 2009;169:382-8. [DOI] [PubMed] [Google Scholar]
- 29.DeStefano F, Mullooly JP, Okoro CA, Chen RT, Marcy SM, Ward JI, et al. Childhood vaccinations, vaccination timing, and risk of type 1 diabetes mellitus. Pediatrics 2001;108:E112. [DOI] [PubMed] [Google Scholar]
- 30.Karvonen M, Cepaitis Z, Tuomilehto J. Association between type 1 diabetes and Haemophilus influenzae type b vaccination: birth cohort study. BMJ 1999;318:1169-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EURODIAB Substudy 2. Infections and vaccinations as risk factors for childhood type I (insulin-dependent) diabetes mellitus: a multicentre case-control investigation. Diabetologia 2000;43:47-53. [DOI] [PubMed] [Google Scholar]
- 32.Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Childhood vaccination and type 1 diabetes. N Engl J Med 2004;350:1398-404. [DOI] [PubMed] [Google Scholar]
- 33.Silfverdal SA, Nilsson L, Blennow M, Carlsson RM, Hanson LÅ, Lindberg A, et al. Vaccination of children summary and conclusions from a systematic review. Acta Paediatr 2010;99:1287-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selected neurological and autoimmune diagnoses and ICD-10 codes included in analyses