Dear Sir,
Metastasis of tumor from a primary site to distant organs poses a significant medical problem and is the leading cause of human cancer deaths. Metastatic tumors are highly refractory to therapeutic treatments, resulting in poor clinical outcomes. For example, early melanoma patients, AJCC (American Joint Committee on Cancer) Stage I and II, have a 10-yr survival rate of approximately 90%, whereas the 5-yr survival rate for AJCC Stage IV patients is <5% (Balch et al., 2009). Histologic examination of early melanoma lesions is difficult and cannot always predict the aggressiveness of the melanoma (Cook et al., 2002). Breslow thickness and ulceration remain the most powerful prognostic factors for primary melanomas. However, these features are not completely accurate biological indicators of malignant potential, as a subgroup of patients with a thin localized melanoma (<2.0 mm) will eventually develop metastases (Becker et al., 2006). The discovery of reliable biomarkers that could identify melanoma patients with metastasis as early as possible for adjuvant treatment would contribute to increased survival.
Circulating nucleic acid (CNA) consists of extracellular genetic material (DNA and RNA) found freely in human body fluids. These information-rich molecules of tumor-derived CNAs have a great potential as a noninvasive source of candidate biomarkers because they can be used to monitor the status of remote tumors by decoding the contained genetic and epigenetic information, potentially avoiding the need for tissue biopsies (Swarup and Rajeswari, 2007). One of the most important factors determining the potential value of CNA as a predictive biomarker is the molecular information intrinsically encoded in the target CNAs. For example, genetic/epigenetic changes in genes specific for the clinicopatho-logic characteristics of tumor, as well as differential levels of circulating mRNAs from genes that have functional roles in the malignant progression, would be near ideal CNA candidates as a source of predictive biomarkers.
Using a global gene expression profiling technique, we previously discovered a gene signature associated with metastatic phenotype in a panel of melanoma cell lines derived from histopathologically well-characterized lesions of melanomas (Ryu et al., 2007). Dr. Winnepenninckx et al. (2006) also reported a 254-gene signature associated with high-risk of recurrent metastasis and poor clinical outcomes in a study using human tissue samples. A significant portion of these gene signatures overlapped. Common molecular pathways mediated by these signature genes in metastatic melanomas involve the activation of DNA replication origins, DNA repair, and mitogenic proliferation. These findings strongly suggest that one of the distinctive molecular characteristics of metastatic melanoma is the over-expression of the genes associated with these cellular pathways. Therefore, we hypothesized that levels of the metastasis-associated gene transcripts circulating in patient's blood might correlate with the presence of metastasis and could potentially be utilized as a serum marker for the occurrence of metastases.
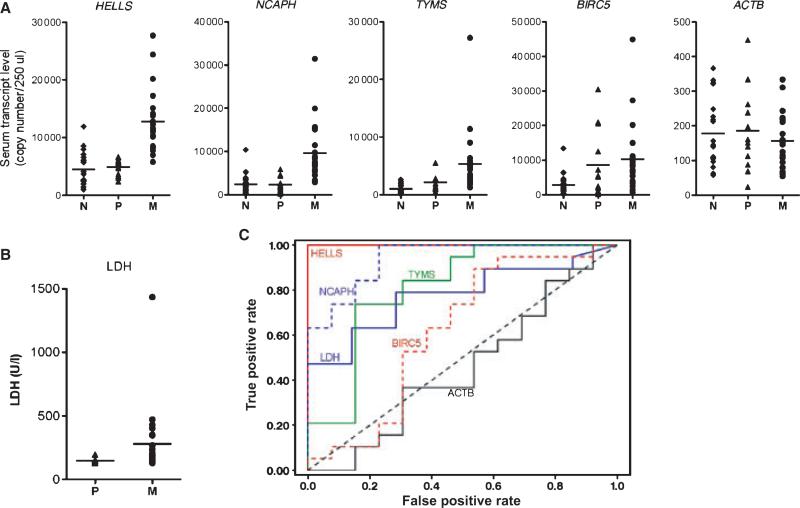
We conducted an exploratory study to test this hypothesis. Four genes (HELLS, TYMS, NCAPH, and BIRC5) representing the metastasis-associated gene signature were selected. Serum levels of the gene transcripts circulating in patient blood were measured using duplex real-time q-PCR technique (see Appendix S1 for detailed experimental methods). Serum levels of the selected gene transcripts were compared in two groups of melanoma patients; group 1, patients with localized melanomas (AJCC stage 0/1) and group 2, patients with distant organ metastasis (AJCC Stage IV). To establish base levels of the circulating gene transcripts in a normal control population, serum samples from sex- and age-matched healthy controls with no history of illness (average age, 57.8 yr) were included. Key clinical and histopathologic characteristics of the melanoma patients included in this study are summarized in Supporting Information Table S1. As shown in Figure 1A, there was a clear difference in the levels of circulating transcript of three of the selected genes (HELLS, NCAPH, and TYMS) in patients with early stage primary melanomas and patients with distant organ metastasis, but this difference was not seen for BIRC5. Serum levels of ACTB (β-actin) gene transcript served as a negative control and showed no differences between groups 1 and 2 (Figure 1A). Statistical calculations using analysis of variance (anova) demonstrated that the differences were significant for HELLS (P-value <0.001), NCAPH (P-value <0.001), and TYMS (P-value = 0.004), but not for BIRC5 (P-value = 0.633) or ACTB (P-value = 0.918) (Supporting Information Table S2).
Figure 1.
Distribution patterns of circulating gene transcripts in patient sera and area under the receiver operating characteristic (AUC-ROC) curves. (A) Comparison of quantitative mRNA copy number for the genes HELLS, NCAPH, TYMS, BIRC5, and ACTB in patient sera among the sample groups (N: normal control, P: patients with Stage 0/1 primary melanomas, M: patients with Stage IV metastatic melanomas). ACTB served as a negative control. (B) Comparison of serum LDH concentrations in the patients with primary and metastatic melanomas. (C) AUC-ROC curves for each individual analyte are shown. Analyte HELLS shows the highest value.
To calculate the predictive value of the serum levels of the gene transcripts for metastasis presence, a logistic regression analysis was performed. The probability of metastasis was increased with increasing serum levels of HELLS and NCAPH but not TYMS or BIRC5. The odds ratio of HELLS (94.2) was greater than that of NCAPH (10.5) (Table 1). HELLS and NCAPH were included in a multiple logistic regression analysis, and backward elimination was used to determine independent predictors for metastasis. Results showed that only HELLS was independently predictive of metastasis. Serum lactate dehydrogenase (LDH) is a strong prognostic factor in the AJCC staging system for metastatic melanoma, and may influence treatment outcome (Balch et al., 2009; Deichmann et al., 1999). We compared the probability that the serum levels of the four gene transcripts were metastasis-predicting indicators with the probability of serum LDH level as an indicator by calculating receiver operating characteristic – area under curve (ROC-AUC) values (Figure 1B, C). The AUC area was greater for HELLS (1.0) and NCAPH (0.939) than for LDH (0.778). The serum transcript level of HELLS had the highest ROC-AUC value (Figure 1C). These findings clearly indicate that measurement of the target gene transcript levels in patient serum had a better discriminatory potential than LDH for detection of metastatic melanoma.
Table 1.
Logistic regression predicting probability of each gene transcript for the presence of metastatic melanomas
| Biomarkers (candidate genes) | ||||||
|---|---|---|---|---|---|---|
| Statistic | LDH | HELLS | NCAPH | TYMS | BIRC5 | ACTB |
| Logistic Regression P-value | 0.203 | 0.005b | 0.016b | 0.108b | 0.999b | 0.939 |
| Odds Ratioa | NR | 94.2 | 10.5 | NR | NR | NR |
Odds ratios associated with log10 increase reported for each statistically significant biomarker.
Bonferroni adjusted p-values for evaluating four test genes.
The serum markers S100B and melanoma-inhibiting activity (MIA) have been proposed as prognostic markers for melanoma progression because they have a strong association with overall survival. However, these markers do not have an independent prognostic capability when compared to LDH serum concentration (Deichmann et al., 1999). Therefore, LDH is currently the only serum marker integrated in the AJCC staging system (Balch et al., 2009). Our study clearly demonstrates that measurement of metastasis-associated gene transcript levels in patient serum is superior to serum LDH concentration in discriminating patients with distant organ metastasis (Figure 1C). This preliminary finding warrants a larger-scale prospective study to validate circulating metastasis-associated gene transcripts, especially HELLS gene transcript, as a serum prognostic marker for the risk of metastasis.
HELLS gene encodes a lymphoid-specific helicase, HELLS. HELLS is a member of an SNF2 subfamily of helicases which is crucial for regulation of chromatin remodeling, DNA replication, repair, recombination, methylation, and transcription (Lee et al., 2000). The molecular function of HELLS is to facilitate de novo DNA methylation, which appears to play an important role in embryonic stem cell differentiation by silencing stemness genes (Xi et al., 2009). However, functional roles of the protein in tumor initiation and metastasis development are unknown. Higher levels of circulating HELLS mRNA may be a reflection of higher numbers of circulating tumor cells in metastatic patient blood, suggesting that HELLS may regulate tumor cell migration and invasion, as metastasis-competent cells are most likely invasive and migratory, enabling access to a tumor vascular system. Although HELLS mRNA expression levels are very low in normal peripheral blood lymphocytes, they increase significantly when the cells are activated and stimulated to proliferate (Lee et al., 2000). This implies that the status of immunologic responses of patients may affect outcome, and this should be carefully considered in future prospective validation studies.
Quantitative detection of cell-free CNAs in patient blood may be a desirable approach to developing diagnostic, prognostic, and therapy response-predicting markers (Swarup and Rajeswari, 2007). Since the seminal study demonstrating the feasibility of RT-PCR for detection of melanoma in peripheral blood (Smith et al., 1991), several reports have suggested that RT-PCR detection of circulating melanoma cells may have prognostic significance. The majority of these studies used RT-PCR detection of melanocytic differentiation antigens including tyrosinase, MART-1, and MAGE3 gene transcripts (Quaglino et al., 2004). Notably, Scoggins et al. (2006) reported that RT-PCR detection of tyrosinase and other markers in peripheral blood showed significant differences in prognoses (Scoggins et al., 2006). In this paper, we analyzed a novel class of genes that appear to be functionally associated with the development of melanoma metastasis and demonstrated that quantitative measurement of these gene transcripts may have potential value as a prognostic marker for melanoma metastasis. Given the functional association of this new class of candidate genes with tumor cell proliferation and metastasis, rather than melanin biosynthesis, RT-PCR detection of these novel markers can be used for assessment of prognostic risk in patients with amelanotic melanomas as well.
In conclusion, circulating mRNA levels of HELLS and NCAPH transcripts in melanoma patient blood were found to be significantly higher in patients with distant organ metastasis than in those with localized tumors. The area under ROC curves of HELLS and NCAPH were greater compared to serum LDH, which at present is one of the most useful serum prognostic indicators of metastatic melanoma. Multiple logistic regression analysis identified HELLS as a statistically significant independent marker of the presence of distant metastasis. This study shows that elevated serum levels of HELLS gene transcripts may have a better potential as serum biomarkers than LDH.
Supplementary Material
Table S1. Comparison of melanoma patient characteristics between AJCC stage 0/I and stage IV groups used in the supervised analysis serum samples.
Table S2. anova results comparing individual gene transcript between the local and metastatic groups.
Appendix S1
Materials and Methods
Patient Populations. Melanoma patients with available fresh frozen serum samples in the Nevada Cancer Institute (NVCI) tissue bank linked with characterization of patient's clinical stage and risk factors were identified from the files of the Laboratory Core. Serum samples from 32 cutaneous melanoma patients evaluated at NVCI between May 2006 and December 2009 were used. These samples included 13 samples from Stage 0/I patients with localized primary melanoma and 19 samples from Stage IV patients with distant organ melanoma. Blood samples were obtained at the time of initial diagnosis (Stage 0/I) and detection of metastasis (stage IV). Twenty serum samples from sex- and age-matched healthy controls with no history of illness (average age, 57.8 yr) were used as a normal control. Key clinical and histopathologic characteristics of the melanoma patients included in this study are summarized in Table S1. This analysis was approved by the University Medical Center IRB, Las Vegas, Nevada.
RNA Extraction and Reverse Transcription. In vitro transcribed luciferase mRNA (10 ng) was spiked into 250 μl serum samples to monitor quality of subsequent RNA extraction and reverse transcription steps. Total RNA was isolated from each serum sample using a mirvanaPARIS Kit (Applied Biosystems/Ambion, Austin, TX) according to the manufacturer's instruction. First-strand cDNA was generated using High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA) using random hexamers according to the manufacturer's protocol.
Duplex Quantitative Real-time PCR. Primers and TaqMan Probes for corresponding genes were selected from the mRNA sequences obtained from NCBI's reference sequence (http://www.ncbi.nlm.nih.gov/refseq/) database using Primer Expression Software 3.0 (Applied Biosystems, Foster City, CA). The primer sets and TaqMan probes for duplex quantitative real-time PCR were follows: HELLS, 5’-CCCTCCTTTCTTCTAGTAATGCAGTT-3’ (forward), 5’-CCCAATCTCTCCCCATGAAAA-3’ (reverse), and 6FAM-ATGGGCTTTAGGTACTTC-BHQ-1 (probe); TYMS, 5’-TTTTGGACAGCCTGGGATTC-3’ (forward), 5’-GCCATAAACTGGGCCCAAGT-3’ (reverse), and 6FAM-CCACCAGAGAAGAAG-BHQ-1 (probe); NCAPH, 5’-CTGGATTACAGGCTGCTGAC-3’ (forward), 5’-GGTCAGAGTTCCCAACAGGT-3’ (reverse), and 6FAM-CACAAATAAGTCATCCAAAT-BHQ-1(probe); BIRC5, 5’-CGCTTTCCTTTCTGTCAAGA-3’ (forward), 5’-CTTGGCTCTTTCTCTGTCCA-3’ (reverse), and 6FAM-AGTTTGAAGAATTAACCCTT-BHQ-1 (probe); ACTB, 5’-CTGGAACGGTGAAGGTGACA-3’ (forward), 5’-CGGCCACATTGTGAACTTTG-3’ (reverse), and 6FAM-TCGGTTGGAGCGAGC-BHQ-1 (probe); luciferase, 5’-CGTACGTGATGTTCACCTCGAT-3’ (forward), 5’-CGCCCTGGTTCCTGGAA-3’ (reverse), and VIC-TGCATCTGTAAAAGCA-BHQ-1 (probe).
Two different types of fluorescent dye labeled probes were utilized for the duplex quantitative real-time PCR assay. This allows detection and quantitation of levels of two gene transcripts in a single reaction mixture. PCR amplications were performed using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) and were performed in a reaction volume of 20 μl containing 1 × TaqMan Universal PCR Master Mix II, 2 μl of template cDNA, paired primers and TaqMan MGB probes for targeting gene of interest and exogenously added Luciferase RNA, which served as loading control. The PCR amplifications were initiated with an initial denaturing step at 95°C for 5 min followed by 60 cycles of alternating annealing and elongation at 60°C for 1 minute each, followed by denaturation at 95°C for 15 seconds. Each reaction was run in duplicate. To normalize the circulating target gene transcript levels from sample to sample, we amplified the exogenously added internal control gene, Luciferase. It should be noted that luciferase gene detection remained proportional to total RNA in each sample. The ratio of the target gene and luciferase was calculated to normalized transcript levels of each target gene. The serum levels of target gene transcripts were determined from standard curves. The results were expressed as copy numbers per 250 μl serum.
Standard Curves. To generate the standard curves, we followed a quantitation protocol for real-time PCR provided by ABI (Please refer to the Absolute Quantification Getting Started Guide, Applied Biosystems, Foster City, CA). In brief, plasmid vectors harboring RT-PCR product from total RNA isolated 1205Lu melanoma cells, were prepared by cloning into pCR8/GW/TOPO vector. The copy numbers were calculated from the following formula. m = [n][1.096e-21 g/bp], where: n = plasmid size (bp), m = mass. The mass of plasmid DNA needed for given copy number was obtained by multiplying the mass of single plasmid with the copy number of interest. The prepared standard solutions made into small aliquots and stored at -20°C and thawed only once before use. The standard curves for each gene of interest were obtained using the plasmids containing target genes ranging from 30 to 300,000 copies and primers and probes in duplex with luciferase for internal control. Each sample was run in duplicate. The curves generated for each gene of interest showed a linear relationship between copy numbers and the CT values of real-time PCR for both target genes and luciferase gene.
Statistical Analysis. The probability of metastatic disease versus local disease was modeled as functions of log10 transformations of the individual biomarkers (serum circulating gene transcript levels and serum LDH) using logistic regression (based on weighted least squares estimation). Each biomarker was tested at the alpha = 0.05 significance level. For each analyte found to be statistically significant, the odds ratio was estimated indicating the increase in the odds of metastatic disease associated with a log10 increase in the biomarker. The ROC curve (and corresponding AUC) was calculated for each analyte. As a supplemental analysis, each analyte was compared between the metastatic and local disease groups using ANOVA techniques (p-values were calculated based on the log10 transformed data). To preserve a family-wise Type I error rate of 0.05 in the logistic regression and ANOVA analyses of the four test genes (HELLS, TYMS, NCAPH, BIRC5), Bonferroni-adjusted p-values were calculated.
Acknowledgements
Authors thank Dr. Wolfram Samlowski for comments on the manuscript. This work was supported by NIH K01 CA113779 and Nevada Cancer Institute (NVCI) Startup Grant for Dr. Ryu and DOE DEFG02-08ER64608 grant for Bio-repository program at NVCI.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Materials and Methods.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Mihm MC, Hewitt SM, Sondak VK, Fountain JW, Thurin M. Markers and tissue resources for melanoma: meeting report. Cancer Res. 2006;66:10652–10657. doi: 10.1158/0008-5472.CAN-06-0921. [DOI] [PubMed] [Google Scholar]
- Cook MG, Spatz A, Brocker EB, Ruiter DJ. Identification of histological features associated with metastatic potential in thin (<1.0 mm) cutaneous melanoma with metastases. A study on behalf of the EORTC Melanoma Group. J. Pathol. 2002;197:188–193. doi: 10.1002/path.1093. [DOI] [PubMed] [Google Scholar]
- Deichmann M, Benner A, Bock M, Jackel A, Uhl K, Waldmann V, Naher H. S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on cancer stage IV melanoma. J. Clin. Oncol. 1999;17:1891–1896. doi: 10.1200/JCO.1999.17.6.1891. [DOI] [PubMed] [Google Scholar]
- Lee DW, Zhang K, Ning ZQ, Raabe EH, Tintner S, Wieland R, Wilkins BJ, Kim JM, Blough RI, Arceci RJ. Proliferation-associated SNF2-like gene (PASG): a SNF2 family member altered in leukemia. Cancer Res. 2000;60:3612–3622. [PubMed] [Google Scholar]
- Quaglino P, Savoia P, Osella-Abate S, Bernengo MG. RT-PCR tyrosinase expression in the peripheral blood of melanoma patients. Expert Rev. Mol. Diagn. 2004;4:727–741. doi: 10.1586/14737159.4.5.727. [DOI] [PubMed] [Google Scholar]
- Ryu B, Kim DS, Deluca AM, Alani RM. Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS ONE. 2007;2:e594. doi: 10.1371/journal.pone.0000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoggins CR, Ross MI, Reintgen DS, et al. Prospective multi-institutional study of reverse transcriptase polymerase chain reaction for molecular staging of melanoma. J. Clin. Oncol. 2006;24:2849–2857. doi: 10.1200/JCO.2005.03.2342. [DOI] [PubMed] [Google Scholar]
- Smith B, Selby P, Southgate J, Pittman K, Bradley C, Blair GE. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991;338:1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- Swarup V, Rajeswari MR. Circulating (cell-free) nucleic acids – a promising, non-invasive tool for early detection of several human diseases. FEBS Lett. 2007;581:795–799. doi: 10.1016/j.febslet.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Winnepenninckx V, Lazar V, Michiels S, et al. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J. Natl Cancer Inst. 2006;98:472–482. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- Xi S, Geiman TM, Briones V, Guang Tao Y, Xu H, Muegge K. Lsh participates in DNA methylation and silencing of stem cell genes. Stem Cells. 2009;27:2691–2702. doi: 10.1002/stem.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of melanoma patient characteristics between AJCC stage 0/I and stage IV groups used in the supervised analysis serum samples.
Table S2. anova results comparing individual gene transcript between the local and metastatic groups.
Appendix S1
Materials and Methods
Patient Populations. Melanoma patients with available fresh frozen serum samples in the Nevada Cancer Institute (NVCI) tissue bank linked with characterization of patient's clinical stage and risk factors were identified from the files of the Laboratory Core. Serum samples from 32 cutaneous melanoma patients evaluated at NVCI between May 2006 and December 2009 were used. These samples included 13 samples from Stage 0/I patients with localized primary melanoma and 19 samples from Stage IV patients with distant organ melanoma. Blood samples were obtained at the time of initial diagnosis (Stage 0/I) and detection of metastasis (stage IV). Twenty serum samples from sex- and age-matched healthy controls with no history of illness (average age, 57.8 yr) were used as a normal control. Key clinical and histopathologic characteristics of the melanoma patients included in this study are summarized in Table S1. This analysis was approved by the University Medical Center IRB, Las Vegas, Nevada.
RNA Extraction and Reverse Transcription. In vitro transcribed luciferase mRNA (10 ng) was spiked into 250 μl serum samples to monitor quality of subsequent RNA extraction and reverse transcription steps. Total RNA was isolated from each serum sample using a mirvanaPARIS Kit (Applied Biosystems/Ambion, Austin, TX) according to the manufacturer's instruction. First-strand cDNA was generated using High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA) using random hexamers according to the manufacturer's protocol.
Duplex Quantitative Real-time PCR. Primers and TaqMan Probes for corresponding genes were selected from the mRNA sequences obtained from NCBI's reference sequence (http://www.ncbi.nlm.nih.gov/refseq/) database using Primer Expression Software 3.0 (Applied Biosystems, Foster City, CA). The primer sets and TaqMan probes for duplex quantitative real-time PCR were follows: HELLS, 5’-CCCTCCTTTCTTCTAGTAATGCAGTT-3’ (forward), 5’-CCCAATCTCTCCCCATGAAAA-3’ (reverse), and 6FAM-ATGGGCTTTAGGTACTTC-BHQ-1 (probe); TYMS, 5’-TTTTGGACAGCCTGGGATTC-3’ (forward), 5’-GCCATAAACTGGGCCCAAGT-3’ (reverse), and 6FAM-CCACCAGAGAAGAAG-BHQ-1 (probe); NCAPH, 5’-CTGGATTACAGGCTGCTGAC-3’ (forward), 5’-GGTCAGAGTTCCCAACAGGT-3’ (reverse), and 6FAM-CACAAATAAGTCATCCAAAT-BHQ-1(probe); BIRC5, 5’-CGCTTTCCTTTCTGTCAAGA-3’ (forward), 5’-CTTGGCTCTTTCTCTGTCCA-3’ (reverse), and 6FAM-AGTTTGAAGAATTAACCCTT-BHQ-1 (probe); ACTB, 5’-CTGGAACGGTGAAGGTGACA-3’ (forward), 5’-CGGCCACATTGTGAACTTTG-3’ (reverse), and 6FAM-TCGGTTGGAGCGAGC-BHQ-1 (probe); luciferase, 5’-CGTACGTGATGTTCACCTCGAT-3’ (forward), 5’-CGCCCTGGTTCCTGGAA-3’ (reverse), and VIC-TGCATCTGTAAAAGCA-BHQ-1 (probe).
Two different types of fluorescent dye labeled probes were utilized for the duplex quantitative real-time PCR assay. This allows detection and quantitation of levels of two gene transcripts in a single reaction mixture. PCR amplications were performed using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) and were performed in a reaction volume of 20 μl containing 1 × TaqMan Universal PCR Master Mix II, 2 μl of template cDNA, paired primers and TaqMan MGB probes for targeting gene of interest and exogenously added Luciferase RNA, which served as loading control. The PCR amplifications were initiated with an initial denaturing step at 95°C for 5 min followed by 60 cycles of alternating annealing and elongation at 60°C for 1 minute each, followed by denaturation at 95°C for 15 seconds. Each reaction was run in duplicate. To normalize the circulating target gene transcript levels from sample to sample, we amplified the exogenously added internal control gene, Luciferase. It should be noted that luciferase gene detection remained proportional to total RNA in each sample. The ratio of the target gene and luciferase was calculated to normalized transcript levels of each target gene. The serum levels of target gene transcripts were determined from standard curves. The results were expressed as copy numbers per 250 μl serum.
Standard Curves. To generate the standard curves, we followed a quantitation protocol for real-time PCR provided by ABI (Please refer to the Absolute Quantification Getting Started Guide, Applied Biosystems, Foster City, CA). In brief, plasmid vectors harboring RT-PCR product from total RNA isolated 1205Lu melanoma cells, were prepared by cloning into pCR8/GW/TOPO vector. The copy numbers were calculated from the following formula. m = [n][1.096e-21 g/bp], where: n = plasmid size (bp), m = mass. The mass of plasmid DNA needed for given copy number was obtained by multiplying the mass of single plasmid with the copy number of interest. The prepared standard solutions made into small aliquots and stored at -20°C and thawed only once before use. The standard curves for each gene of interest were obtained using the plasmids containing target genes ranging from 30 to 300,000 copies and primers and probes in duplex with luciferase for internal control. Each sample was run in duplicate. The curves generated for each gene of interest showed a linear relationship between copy numbers and the CT values of real-time PCR for both target genes and luciferase gene.
Statistical Analysis. The probability of metastatic disease versus local disease was modeled as functions of log10 transformations of the individual biomarkers (serum circulating gene transcript levels and serum LDH) using logistic regression (based on weighted least squares estimation). Each biomarker was tested at the alpha = 0.05 significance level. For each analyte found to be statistically significant, the odds ratio was estimated indicating the increase in the odds of metastatic disease associated with a log10 increase in the biomarker. The ROC curve (and corresponding AUC) was calculated for each analyte. As a supplemental analysis, each analyte was compared between the metastatic and local disease groups using ANOVA techniques (p-values were calculated based on the log10 transformed data). To preserve a family-wise Type I error rate of 0.05 in the logistic regression and ANOVA analyses of the four test genes (HELLS, TYMS, NCAPH, BIRC5), Bonferroni-adjusted p-values were calculated.