Review on consequences of TLR2 signaling the activation of Ca2+-dependent proteases (calpains) and link to junctional proteins to facilitate PMN migration into the airway lumen.
Abstract
In airway cells, TLR2 stimulation by bacterial products activates Ca2+ fluxes that signal leukocyte recruitment to the lung and facilitates transepithelial migration into the airway lumen. TLR2 is apically displayed on airway cells, where it senses bacterial stimuli. Biochemical and genetic approaches demonstrate that TLR2 ligands stimulate release of Ca2+ from intracellular stores by activating TLR2 phosphorylation by c-Src and recruiting PI3K and PLCγ to affect Ca2+ release through IP3Rs. This Ca2+ release plays a pivotal role in signaling TLR2-dependent NF-κB activation and chemokine expression to recruit PMNs to the lung. In addition, TLR2-initiated Ca2+ release activates Ca2+-dependent proteases, calpains, which cleave the transmembrane proteins occludin and E-cadherin to promote PMN transmigration. This review highlights recent findings that demonstrate a central role for Ca2+ signaling in airway epithelial cells to induce proinflammatory gene transcription and to initiate junctional changes that accommodate transmigration of recruited PMNs.
Introduction
The airways are exposed continuously to microorganisms; however, healthy individuals maintain a pathogen-free environment in their lower respiratory tract. This is a testament to the efficient host defense system at the mucosal surface of the lung. Airway epithelial cells are positioned strategically to play a key role in host response by providing a physical and an immunological barrier to inhaled pathogens [1, 2]. The physical barrier mediated by epithelial cell-to-cell junctions prevents pathogens from invading, and the immunological barrier is achieved through epithelial cell detection of bacteria and rapid signaling to recruit phagocytes and clear the infection.
A central component in regulating the immunological and physical barriers of the airway epithelium is Ca2+. The participation of Ca2+ as a second messenger is vital to numerous physiological processes [3,4,5]. Cytosolic Ca2+ fluxes are involved in mediating responses to extracellular stimuli, such as the activation of G-protein-coupled receptors [3], TCRs [4, 6, 7], and BCRs [8]. The common respiratory bacterial pathogens, Pseudomonas aeruginosa and Staphylococcus aureus, activate 100 nM Ca2+ fluxes immediately upon contact with airway epithelial cells, and this Ca2+ signal initiates the activation of proinflammatory signaling events [9].
In this overview, we describe a central role for intracellular Ca2+ fluxes in controlling the physical and immunological barriers of the respiratory epithelium during bacterial infection. In airway cells, Ca2+ fluxes mediate the expression of proinflammatory cytokines and chemokines necessary to recruit leukocytes to the lung and also initiate modifications to the epithelial junctions to facilitate leukocyte transmigration into the airway lumen.
HOST—PATHOGEN INTERACTIONS IN THE AIRWAY
Airway epithelial cells contribute to the immunological barrier by detecting the presence of pathogens and secreting chemokines and cytokines (IL-8, IL-6, GM-CSF) to recruit and activate phagocytic cells to clear the invading organism [2]. Airway epithelial cells are protected by mucin and cilia and infrequently come in contact with intact bacteria. Instead, they detect bacterial components or PAMPs through PRRs that detect molecules broadly shared by pathogens [2]. Recognition of extracellular or endosomal PAMPs is mediated predominantly by the TLR, a family of type-I transmembrane proteins, which detects PAMPs through extracellular leucine-rich repeat motifs and mediates signaling through an intracellular TIR domain [10]. Each given tissue or cell expresses multiple TLRs [10]; in human airway epithelial cells, TLRs 1–10 are expressed [11]. Activation of signaling through TLRs results in recruitment of cytoplasmic adaptor molecules such as MyD88, TIR domain-containing adaptor protein (also called Mal), Trif, and Trif-related adaptor molecule. The adaptors activate downstream molecules including protein kinases (IRAK1, IRAK4, TRAF family member-associated NF-κB activator-binding kinase 1, and IKK) that amplify the signal and activate proinflammatory transcription factors, which induce the expression of genes involved in the inflammatory response. In airway cells, activation of NF-κB, a major proinflammatory transcription factor, rapidly signals the expression of IL-8, a chemokine that recruits PMNs [12], which are the first immune cells recruited in response to infection [13, 14]. Interestingly, IL-8 was shown to recruit PMNs from the bloodstream to the basolateral surface of airway epithelial cells; however, it was not involved in mediating PMN transmigration further into the airway lumen [15]. In this regard, the secretion of an arachidonic acid metabolite, hepoxilin A3, by airway epithelial cells has been proposed to direct PMN migration across airway epithelial cells [15]. PMNs are critical in eradicating respiratory pathogens; however, excessive numbers of PMNs in the airway can lead to tissue damage and compromised lung function [2, 16].
In addition to signaling the presence of bacteria to immune cells, epithelial cells form a physical barrier through their cell-to-cell junctions, which prevents pathogen invasion. This barrier is established by the apical junctional complex composed of the tight and adherens junctions. As the epithelial tight junctions mainly function to create a barrier to maintain sterility in the lung, they are more substantial and are 10 times less leaky than endothelial junctions [17]. In the respiratory tract, PMN migration across endothelial cells has been studied in some detail [18, 19]. However, unlike crossing the endothelium, where movement is in the apical-to-basal direction, PMNs migrate in a basal-to-apical direction as they cross the epithelium. This is particularly important in understanding the molecules that may be involved in adhesion and migration. For example, ICAM-1 is responsible for allowing PMNs to adhere to endothelial cells and initiate the steps in transmigration [20]. ICAM-1 is not expressed at the basolateral surface of epithelial cells and is expressed rather abundantly at the apical surface [20]. Therefore, it is unlikely that ICAM-1 plays a role in PMN transepithelial migration but rather, may be involved in retaining PMNs at specific locations in the airway lumen. PMN transepithelial migration occurs independent of a number of molecules, CDlla/CD18, selectins, CD31, and ICAM-1, which are required for transendothelial migration [21].
Much of what is known about neutrophil transepithelial migration comes from in vitro studies using intestinal epithelial monolayers [21]. PMN infiltration into the intestinal mucosa is a central feature of active inflammatory bowel diseases, such as ulcerative colitis and Crohn’s disease [22]. Direct targeting of junctional proteins is one mechanism to facilitate PMN migration. Biopsies of colonic mucosa from patients with ulcerative colitis have dramatic down-regulation of the tight junction protein occludin in regions of actively transmigrating PMNs [23]. Paracellular permeability across epithelial and endothelial cells is also enhanced by disassembly of the junctions upon contraction of the perijunctional actin-myosin ring induced by myosin light chain phosphorylation, which by MLCK or Rho kinase, has been demonstrated in cells exposed to enteropathogenic Escherichia coli [24] or stimulated by basolateral contact with PMNs [21] to facilitate transmigration of bacteria and PMNs.
PMNs must migrate across a complex network of tight and adherens junction proteins to clear the infection in the airway lumen [18, 19, 23]. However, disruption of the junctions in the presence of pathogens in the airway lumen could facilitate bacterial invasion and disseminated infection. Exactly how PMNs are able to migrate between respiratory epithelial cells without breaching the barrier provided by tight junctions is not well understood. We reasoned that signaling through receptors that detect bacteria may induce chemokine expression to recruit PMNs and changes to the epithelial barrier to facilitate PMN paracellular migration.
TLR2-MEDIATED Ca2+ FLUXES SIGNAL PROINFLAMMATORY RESPONSES
There is substantial literature demonstrating that various bacterial products induce Ca2+ fluxes and proinflammatory gene expression in mucosal cells [25]. Several different mechanisms have been implicated, including direct effects of bacterial toxins [26] or activation of purinergic signaling by bacterial adhesins that bind asialoGM1 [27]. The PRR TLR2 initiates the rapid release of intracellular Ca2+ in airway cells [28]. TLR2 is well positioned for initiating signaling, as these receptors are mobilized to the surface of airway cells to rapidly recognize PAMPs in the airway lumen [29, 30]. TLR2 recognizes a variety of microbial components including lipoproteins/lipopeptides, lipoteicoic acid, lipoarabinomannan, and zymosans [29, 31]. Broad ligand specificity of TLR2 is attributed to its ability to dimerize with TLR1, TLR6, or asialoGM1, a glycolipid receptor [29, 32]. At the apical surface of airway cells, TLR2 is associated with necessary downstream signaling molecules including MyD88, IRAK, TNFR-associated factor 6, and c-Src in caveolin-1-containing lipid rafts called caveolae [30, 33]. Efficient downstream signaling can be achieved when receptors and their signaling molecules are in close proximity to each other within these signaling domains.
The canonical Ca2+ release pathway involves PLCγ hydrolysis of phosphatidylinositol 4,5 bisphosphate to IP3 and DAG. IP3 binds to IP3Rs expressed on the ER and signals the release of Ca2+ from the ER, and DAG regulates the activation of members of the PKC family [34].
TLR2 phosphorylation by c-Src
The mechanism of TLR2-induced Ca2+ fluxes involves TLR2 phosphorylation by c-Src [28], which signals recruitment and activation of PI3K and PLCγ to affect Ca2+ release through IP3Rs [28] (Fig. 1). Phosphorylation of tyrosine 616 and 761 on the cytoplasmic tail of TLR2 was observed initially in cells treated with S. aureus [35]. The involvement of Src family kinases in the phosphorylation of TLR2 is consistent with several published studies demonstrating the requirement for Src activity in cellular responses to bacterial ligands [36, 37], for cyclooxygenase-2 activation by Helicobacter pylori [38], and for MUC-2 activation [36] and IL-8 expression in response to P. aeruginosa ligands [30]. Biochemical inhibitors, PP1 or PP2 for Src family kinases, are often used to demonstrate a role for Src in TLR2 phosphorylation. A specific role for c-Src and not other Src family kinases was demonstrated in more recent work using c-Src siRNA to inhibit TLR2-dependent signaling [39, 40]. c-Src is the likely TLR2 kinase, as it was also shown to coimmunoprecipitate with TLR2 in airway cells following bacterial stimulation [30]. c-Src and its family members are ordinarily maintained in equilibrium between inactive and primed states by a balance of negative regulatory kinase C-Src tyrosine kinase and its counteracting tyrosine phosphatase(s), both of which act on a regulatory C-terminal tyrosine [41]. We speculate that ligand-binding promotes conformational changes to TLR2 that promote phosphorylation by the available active c-Src.
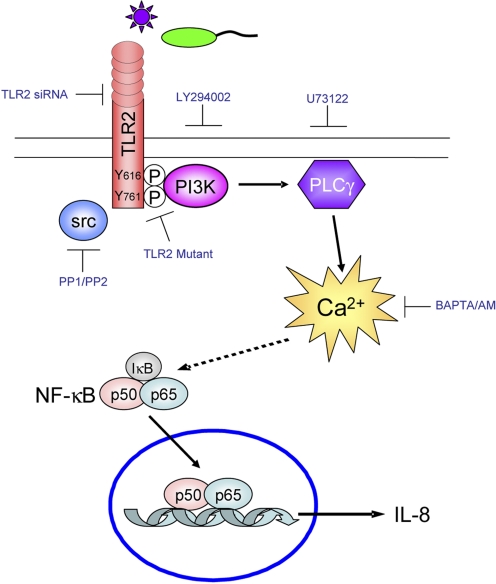
Figure 1.
TLR2-mediated Ca2+ fluxes signal proinflammatory responses in airway epithelial cells. TLR2 ligands stimulate release of Ca2+ by signaling the phosphorylation of TLR2 by c-Src, recruiting PI3K and activating PLCγ. TLR2 or thapsigargin-mediated Ca2+ fluxes signal NF-κB activation and IL-8 expression. Silencing TLR2 by siRNA, blocking TLR2 by overexpression of a TLR2 tyrosine mutant, or using biochemical inhibitors of Src (PP1, PP2), PI3K (LY294002), or PLCγ (U73122) blocks intracellular Ca2+ release, NF-κB activation, and IL-8 expression. Chelating intracellular Ca2+ using BAPTA/AM also blocks NF-κB activation and IL-8 expression.
TLR2 signals PI3K and PLCγ activation
In addition to or in concert with the known TLR pathway involving MyD88, TLR2 directs the activation of class 1A PI3K, which contributes to NF-κB signaling [35, 42]. In response to S. aureus or P. aeruginosa, PI3K is recruited to phosphorylated tyrosines 616 and 761 on the cytoplasmic tail of TLR2 [28, 35]. PI3K catalyzes the phosphorylation of phosphotidylinositides in the plasma membrane, which then become docking sites for PH domain-containing proteins including PLCγ and Akt [43]. The importance of PI3K and PLCγ in immune signaling is well established [44]. Ca2+-dependent responses to E. coli and Salmonella typhimurium also implicate PI3K or PLCγ, as might be expected [45, 46]. PI3Ks are involved in mediating signals generated by bacterial recognition of various TLRs besides TLR2 and are involved in pro- and anti-inflammatory pathways [47,48,49]. As the link between PI3K and specific TLRs is suggested to be tissue-specific, our studies have focused on TLR2 expression in airway cells. However, murine peritoneal macrophages similarly generate Ca2+ fluxes following stimulation with TLR2 ligands [28]. Therefore, the recruitment of PI3K and PLCγ to TLR2 to induce intracellular Ca2+ release may be a generally conserved signaling mechanism.
TLR2 signals activation of NF-κB and expression of IL-8
It is well established that Ca2+ transients regulate gene transcription and expression [5]. The generation of Ca2+ fluxes in airway cells by intact bacteria and flagella stimulates transcription of NF-κB-dependent genes, MUC-2 and IL-8 [30, 50]. Increases in cytosolic Ca2+ induced by thapsigargin, a Sarco/ER calcium ATPase pump inhibitor, are sufficient to activate NF-κB and induce IL-8 expression in airway cells [28]. NF-κB transcription factors are dimers of two out of five subunits—p65 (RelA), c-Rel, RelB, p50 (NF-κB1), and p52. In the absence of stimuli, most NF-κB dimers remain in the cytosol bound to IκBs, which are specific inhibitory proteins. Proinflammatory stimulation downstream of TLR signaling can activate NF-κB through IKK-dependent phosphorylation and degradation of IκB [51]. The exact Ca2+-dependent component of TLR2-mediated NF-κB activation and IL-8 expression is still unknown. However, conventional PKCs (PKCα, PKCβ, and PKCγ) and calmodulin-dependent kinases II are likely candidates, as they are activated by Ca2+ and have been shown to be involved in NF-κB activation [52, 53]. Recent studies have revealed that NF-κB activity is additionally regulated by Ser 276 phosphorylation of the p65 subunit by a variety of kinases [35, 54,55,56]. Most notably, PI3K-mediated activation of Akt, a PH domain containing kinase, was shown to phosphorylate the p65 subunit and optimize NF-κB activation in monocytes stimulated with S. aureus [35].
IL-8 is a CXC chemokine, structurally classified based on the NH2-proximal cysteines, which are separated by an amino acid (X) [57,58,59]. Endothelial cells as well as airway epithelial cells rapidly induce IL-8 expression after bacterial stimulation [2, 60, 61]. IL-8 is expressed in response to microbial components IL-1β and TNF-α [62]. The anti-inflammatory cytokine IL-10 is a potent inhibitor of IL-8 synthesis [63]. The regulation of IL-8 expression by NF-κB is well documented [62, 64]. Nucleotides –1 to –133 within the 5′-flanking region of the IL-8 gene are essential and sufficient for transcriptional regulation of the gene and contain a NF-κB element that is required for activation in all cell types studied [12, 62, 64]. The core IL-8 promoter also contains AP-1- and C/EBP-binding sites [62]. The latter two sites are dispensable for transcriptional activation in some cells but contribute to activation in others. Thus, unlike the NF-κB site, the AP-1 and C/EBP sites are not essential for induction but are required for maximal gene expression [62]. Once secreted, IL-8 binds to two PMN receptors, CXCR1 and CXCR2, which are 7-transmembrane G-protein-coupled receptors that signal PMN chemotaxis and firm adhesion to the endothelium [59, 61]. Other CXCL chemokines, such as growth-related gene product-α and -β (CXCL1 and -2), epithelial cell-derived and neutrophil-activating 78 aa peptide (CXCL5), and granulocyte chemotactic protein-2 (CXCL6), are controlled by NF-κB activation and are chemotactic for PMNs, but their induction has not yet been tested in TLR2-stimulated airway epithelial cells.
Rodents do not express IL-8 and instead, produce CINCs. KC (CINC-1) and MIP-2 (CINC-3) are critical to inflammatory responses in mice and rats [65]. KC and MIP-2 are involved in pulmonary PMN responses during bacterial pneumonia, ozone, and silica dust inhalation and immune-complex deposition [66,67,68,69,70,71].
TLR2-mediated Ca2+ fluxes are communicated from cell to cell
Ca2+ fluxes coordinate signaling in conductive tissues of the muscular and nervous system [72, 73]. In airway epithelial cells, Ca2+ fluxes travel from cell to cell and can also serve to amplify Ca2+-activated proinflammatory signals. In human airway cells, gap junction channels were found to provide a conduit for the movement of Ca2+ from cell to cell. Gap junctions are comprised of Cx monomers, which oligomerize and associate with adjacent oligomers to form a lateral pore between cells. Primary human airway epithelial cells express nine Cx isoforms: Cx26, Cx30, Cx30.3, Cx31, Cx31.1, Cx32, Cx37, Cx40, and Cx43, which exhibit unique conductive and regulatory properties [74, 75]. In response to TLR2 stimulation, gap junctions function to transiently amplify proinflammatory signaling by communicating Ca2+ fluxes from stimulated to adjacent, nonstimulated cells, thus increasing epithelial IL-8 production [76]. Cx43 heterozygous mice displayed reduced PMN influx in response to respiratory LPS exposure [77], further demonstrating the importance of this mechanism of communication in inflammation. However, as unregulated signaling would result in excessive inflammation, the subsequent closing of Cx43 channels is initiated by c-Src-mediated phosphorylation [76]. Similarly, airway epithelial cells treated with TNF-α signaled c-Src-dependent closure of Cx43 gap junction channels [78]. A defect in this closing of Cx43 was observed in cystic fibrosis airway epithelial cells, which have exaggerated inflammatory responses [78].
Ca2+ FLUXES ALTER THE APICAL JUNCTIONAL COMPLEX TO FACILITATE PMN MIGRATION
As a major function of TLR2-dependent Ca2+ signaling in airway cells is stimulating chemokine expression and the resultant PMN recruitment, we postulated that the same signaling event would modify the cell junctions to facilitate migration of PMNs across the epithelial barrier. Following bacterial exposure, PMNs accumulate rapidly in the airway lumen [13, 14], suggesting that changes in the permeability characteristics of the paracellular junctions are an immediate consequence of the epithelial proinflammatory signaling cascade. PMNs have been implicated in the pathogenesis of many inflammatory lung diseases, including cystic fibrosis, acute respiratory distress syndrome, chronic obstructive pulmonary disease, and asthma. To access the airway lumen, PMNs must pass through distinct tissue compartments. PMNs exit the bloodstream by migrating across the endothelial cells. Once in the interstitial space, PMNs travel through the extracellular matrix and interact with the basolateral surface of the epithelium. Finally, they migrate between the epithelial cells to reach the airway lumen [13, 17]. Endothelial Ca2+ fluxes promote transendothelial migration of PMNs by opening their intercellular junctions [79]. Ca2+ flux is not required for PMN adhesion but is required for PMN migration across HUVEC monolayers [79]. In endothelial cells, extracellular Ca2+ entry has also been implicated in modifying cell-to-cell junctions. Ca2+ release from TRPCs was shown to alter permeability across endothelial junctions by signaling cytoskeletal reorganization through the activation of Ca2+-dependent MLCK [80, 81]. Ca2+entry through TRPC1 was shown to promote rearrangement of the actin cytoskeleton and thus increase endothelial permeability [80]. In addition, endothelial cells from TRPC4 knockout mice demonstrated a significantly less dramatic decrease in transendothelial resistance compared with wild-type cells in response to thrombin [82]. Whether TRPCs are activated in response to TLR signaling is unknown. However, in airway epithelial cells, inhibiting Ca2+ entry through L-type Ca2+ channels or chelating extracellular Ca2+ did not affect TLR2 signaling [9, 28], suggesting that intracellular Ca2+ release is responsible for TLR2-dependent responses.
In airway epithelial cells, TLR2 stimulation transiently raised levels of cytosolic Ca2+ [83] and increased the accessibility of biotin at the cell-to-cell junctions [84]. Consistent with the increase in biotin at the cell-to-cell junctions, the expected “chicken wire” distribution of transmembrane junction proteins, occludin and E-cadherin, was altered substantially following 4 h exposure of airway cells to P. aeruginosa or the TLR2 agonist P3C [84]. Yet, despite loss of occludin and E-cadherin at the cell borders, there was no concomitant decrease in the transepithelial resistance measured across the monolayers over this time period nor was there an increase in permeability to 10,000 MW fluorescent dextran or to bacteria across the paracellular space, thereby indicating that the barrier function of the monolayer remained intact [84]. Therefore, we propose that TLR2-dependent intracellular Ca2+ fluxes cause a subtle change in the cell-to-cell junctions to facilitate PMN migration without disrupting the barrier function of the epithelium. Recent work by Chin et al. [85] demonstrates more dramatic junctional changes after direct contact of the PMN with the basolateral surface of intestinal epithelial cells. Serine proteases released by PMNs activated epithelial protease-activated receptors to signal myosin light chain phosphorylation, decrease transepithelial resistance, and increase epithelial permeability to facilitate PMN transepithelial migration [85]. Taken together, TLR2-mediated signaling recruits PMNs to the site of infection and initiates changes in the junctions that can be altered further by direct contact of the epithelial cells with the PMNs.
PROTEASES TARGET MEMBERS OF THE APICAL JUNCTIONAL COMPLEX
The movement of fluid, ions, macromolecules, and inflammatory cells across airway epithelium depends on the integrity of its apical junctional complex composed of the tight and adherens junctions. Previous studies suggest involvement of occludin, a component of the tight junction, and E-cadherin, a component of the adherens junction, in immune cell diapedesis across a monolayer of cells [23, 86, 87]. The importance of proteases, especially the MMP, in inflammatory processes is well established [88]. MMP7 targets several epithelial components [89], including E-cadherin, and contributes to the shedding of its ectodomain and endocytosis [90]. MMP9 facilitates translocation of PMNs from endovascular spaces causing MMP9−/− mice to have a defect in PMN transepithelial migration in response to infection [91]. Occludin proteolysis is also reported to be a component of PMN-dependent inflammation [92]. However, the observation that occludin proteolysis remains unaltered in the MMP9−/− mice [91] indicated that additional protease(s) must target this tight junction protein. The failure of a general MMP inhibitor to block occludin or E-cadherin cleavage in airway cells, as we demonstrate [84], also suggested the involvement of a different protease. As TLR2 signaling initiates Ca2+ fluxes, the Ca2+-dependent calpain proteases were likely candidates to target epithelial junction proteins. TLR2 stimulation of airway epithelial cells signals a rapid increase in calpain activation consistent with the rapid Ca2+ fluxes observed [28, 84]. This response was blocked in cells treated with a calpain inhibitor calpeptin or cells expressing TLR2 siRNA oligonucleotides [84] (see Fig. 3).
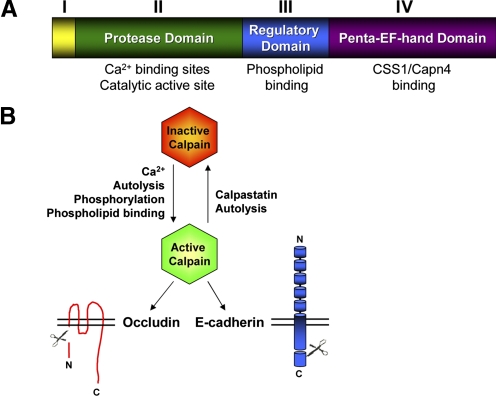
Figure 2.
Calpains cleave occludin and E-cadherin. (A) A schematic representation of the 80-kD large subunit of the classical calpains is shown. The N-terminal Domain I undergoes intermolecular autolysis upon calpain activation. Ca2+-binding sites and catalytic activity are contained within Domain II. Domain III comprises a C2-like domain that contains sites for phospholipid binding. Five consecutive EF-hand motifs make up Domain IV and contribute to Ca2+ binding of the large subunits and dimerization with the small regulatory subunit, CSS1/Capn4. (B) Calpains are positively regulated by Ca2+-binding, Domain I autolysis, phosphorylation, and phospholipid binding and inhibited by calpastatin and autolysis. The N-terminus (N) of occludin and the C-terminus (C) of E-cadherin are targets for calpain cleavage.
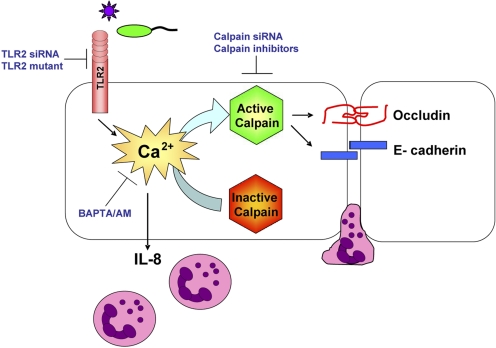
Figure 3.
TLR2-mediated Ca2+ fluxes activate calpains, which cleave junctional proteins and facilitate PMN transmigration. Bacterial detection by TLR2 mediates a Ca2+ release that signals IL-8 expression to recruit PMNs and also initiates modifications to the epithelial junctions to facilitate PMN transmigration. TLR2-mediated Ca2+ fluxes activate calpains that target the transmembrane junctional proteins, occludin and E-cadherin. Calpain activation is inhibited in TLR2 siRNA-expressing cells. Occludin and E-cadherin cleavage is blocked in airway cells expressing a TLR2 tyrosine mutant or calpain siRNA oligonucleotides. Occludin and E-cadherin cleavage and PMN migration across airway cells are blocked by calpain inhibitors.
Calpains were found throughout the cytoplasm of unstimulated airway epithelial cells, whereas the transmembrane junctional proteins, occludin and E-cadherin, were concentrated at the plasma membrane [84]. By 1 h after bacterial or P3C exposure, calpains assumed a peripheral distribution colocalizing with membrane-associated occludin and E-cadherin [84]. This membrane localization of calpain allows it to be in close proximity to its junctional substrates and is also consistent with the role of membrane phospholipid-binding in regulating calpain activation [93,94,95].
Calpains
Calpains are Ca2+-dependent cysteine proteases that are known to target junctional proteins [96, 97]. They are linked to numerous cellular processes including motility, apoptosis, and inflammation [98, 99]. Strategies to target calpain activity in inflammatory diseases such as arthritis have been successful in mice [100]. The calpain family of proteases contains 16 genes [98]. Of these, 14 encode for a large 80-kD subunit that contains a catalytic domain; the other two encode smaller 28 kD regulatory proteins that associate with the larger subunit to form a heterodimeric protease [98]. Although most calpains are expressed in specific tissues, the typical or conventional calpains, μ-calpain (calpain 1) and m-calpain (calpain 2), along with their regulatory subunit CSS1 (calpain 4), are ubiquitously expressed and abundant in the airway [101]. The involvement of conventional calpains in regulating the cytoskeleton of the lung was reported over a decade ago in response to phorbol esters [102].
The crystal structure of the conventional calpain molecules reveals four distinct domains [103,104,105]. Domain I contains a 19-residue N-terminal domain that is cleaved intermolecularly upon activation. Domain II comprises the catalytic active site and also the Ca2+-binding sites that act as the Ca2+ switch to align the catalytic triad Cys 105, His 262, and Asn286 [105, 106]. Domain III is a regulatory domain, which contains phosphorylation sites and a C2 domain first identified in PKC as a region that binds phospholipids in a Ca2+-dependent manner [93]. Domain IV contains five EF-hand Ca2+-binding domains, important in forming the heterodimer with the regulatory subunit CSS1/Capn4 [98] (Fig. 2A).
μ-Calpain (calpain 1) and m-calpain (calpain 2) were named for the micromolar and millimolar concentrations of Ca2+ required to activate them in vitro, respectively [98]. However, such high levels of Ca2+ do not exist in living cells, and additional regulatory mechanisms to eliminate or lower the Ca2+ requirement have been identified. Phosphorylation at several sites activates calpains in the absence of increased Ca2+ [107]. Autolysis of the N-terminal fragment in Domain I and membrane phospholipid binding to Domain III were shown to lower the requirement for Ca2+ [108]. As excessive protease activity could be detrimental to cells, calpain activity is negatively regulated by rapid autolysis or binding to calpastatin, an endogenous inhibitor that associates with activated Ca2+-bound calpain [109, 110] (Fig. 2B).
In general, calpains cleave proteins at a limited number of sites and produce large polypeptide fragments rather than small peptides or amino acids [98]. This would suggest that calpain cleavage regulates the function of its targets as opposed to degrading them. Calpain 1 and calpain 2 appear to have similar, if not identical, substrate specificity [98]. There is no putative amino acid sequence that defines a calpain cleavage site, but rather, subsite specificity is recognized by conformation of the polypeptide chain [98]. Calpains targeted and cleaved occludin and E-cadherin directly but did not target all transmembrane junctional proteins, as claudin-1 and junctional adherence molecule 1 were not cleaved by calpains [84]. The involvement of calpain-dependent cleavage in vivo was verified by identifying occludin and E-cadherin cleavage products in whole lung lysates of infected wild-type mice but not in uninfected controls and substantially decreased cleavage products in the calpeptin-treated animals [84].
Occludin
The tight junction is the most apical component of the junctional complex and functions as the major paracellular barrier and “fence” separating apical from basolateral domains. These functions of tight junctions are critical for epithelial and endothelial cells to establish distinct tissue compartments and maintain homeostasis [111,112,113]. Occludin, the first tight junction protein identified, is a tetraspan membrane protein with two extracellular loops: a short intracellular turn and N- and C-terminal cytoplasmic domains. The extracellular loops are thought to associate with corresponding occludin loops on adjacent cells to regulate paracellular permeability and cell adhesion [114]. Overexpression experiments using full-length and mutated occludin in MDCK cells or Xenopus cells, as well as a study using synthetic peptides corresponding to the extracellular loops of occludin, suggest a role of occludin in the barrier and fence function of tight junctions [115,116,117]. However, occludin null mice showed no abnormalities in the structure of epithelial tight junctions, although they did display inflammation and hyperplasia of the gastric mucosa [118, 119]. Other tight junction proteins, most notably, members of the claudin family, have been implicated in maintaining the barrier properties of the junctions [120]. Although occludin does not appear to be essential in maintaining the tight junctions, it is thought to have a critical regulatory function through its interaction with proteins that form the junctional plaque [114, 121]. Occludin is linked to the actin cytoskeleton at the tight junctions through its C-terminal interaction with ZO-1, a major scaffolding molecule of the tight and adherens junctions [122]. Dissociation of ZO-1 and occludin disassembles the tight junction complex [123, 124].
Proteolytic cleavage of occludin disrupts barrier properties of the epithelial junctions. Occludin is a 60-kD protein, which is cleaved to products that range from 20 kD to 55 kD. During apoptosis, caspase 3 specifically cleaves occludin C-terminal to Asp320, generating a 31-kD fragment [125]. Several studies demonstrate proteolytic cleavage of occludin in epithelial cells exposed to bacteria (e.g., Vibrio cholerae, H. pylori, and Burkholderia cepacia), dust mite allergen (Der p 1) and ATP [114, 126, 127]. A role for calpains in occludin cleavage was demonstrated in cervical epithelial cells, where a biochemical inhibitor of calpains, N-acetyl-leu-leu-Nle-CHO, blocked ATP or diacylglyceride-induced occludin cleavage [126].
In airway epithelial cells, calpain cleavage of occludin occurs on the N-terminal tail of occludin to generate a 45-kD cleavage fragment [84] (Fig. 2B). This is consistent with previous studies, which have shown that expressing occludin mutants with modified N-terminal cytoplasmic domains up-regulated PMN migration, whereas deletion of the C-terminal cytoplasmic domain did not have an effect [86]. In addition, expression of an occludin N-terminal truncation mutant decreases barrier function in murine epithelial cells [128], consistent with our mapping data. TLR2-dependent occludin cleavage was blocked in cells expressing TLR2 siRNA and cells treated with the intracellular Ca2+ chelator, BAPTA/AM, or the calpain inhibitor, calpeptin [84]. To further confirm the role of calpains in occludin cleavage, calpain 1 and 2 expression was silenced by siRNA. The occludin cleavage product was detected in cells expressing scrambled oligos in response to P3C stimulation but not in cells expressing calpain 1 and 2 siRNA [84]. Knockdown of calpain 1 or calpain 2 individually was not sufficient to block occludin cleavage in response to P3C stimulation [84].
E-cadherin
Adherens junctions link membrane and cytoskeletal components at discrete contact sites and are required for basic cell–cell adhesion. Adhesive binding is mediated by cadherins, a family of type I single transmembrane-spanning glycoproteins that dimerize and form extracellular Ca2+-dependent interactions with identical molecules on the surfaces of adjacent cells [129]. Mutant vascular endothelial-cadherin lacking the extracellular domain results in impaired PMN migration in response to chemotactic stimuli [130]. The cadherin cytoplasmic domain controls structural and signaling activities required for adhesion through its association with three distinct members of the catenin family: β-catenin, α-catenin, and p120ctn [113, 129, 131]. These catenins work together to protect cadherins from proteolytic degradation and link the cadherin cytoplasmic domain to actin and myosin filaments [113, 129, 132]. Interactions between cadherins and the small GTPases that regulate actin polymerization affect paracellular permeability in endothelial cells [133].
E-cadherin, the best-characterized cadherin, is expressed specifically in epithelial cells [113, 129]. E-cadherin null mouse embryos do not survive past the blastocyte stage, demonstrating the importance of E-cadherin in early development [134,135,136]. Keratinocyte-specific deletion of E-cadherin in mice results in hyperproliferation of epidermal basal cells and impaired terminal differentiation, consistent with the observed decrease in E-cadherin in many human cancers [135]. In response to various stimuli, E-cadherin undergoes endocytosis as part of the dynamic process of membrane homeostasis [129, 137]. E-cadherin trafficking has been studied intensively and plays a central role in cellular growth and development, maintenance of epithelial polarity, and Wnt signaling [129]. E-cadherin is bound indirectly to the cytoskeleton through a linkage of its intracellular C-terminus to β-catenin, which in turn, is linked to α-catenin, binding to the actin cytoskeleton [129]. E-cadherin is involved in the Wnt signaling pathway through its association with β-catenin, a key component of this pathway [138].
E-cadherin cleavage can also signal the dissociation of β-catenin and E-cadherin and lead to accumulation of β-catenin in the cytoplasm to promote Wnt signaling and subsequent transcription of target genes [139]. E-cadherin is a target of proteases, such as MMP7 [140], TNF-α-converting enzyme (ADAM 17) [141], and ADAM 10 [142], which mediates the shedding of the extracellular domain of E-cadherin in damaged and apoptotic cells.
Airway epithelial cells treated with P3C or P. aeruginosa induce calpain cleavage of the intracellular domain of E-cadherin to generate a 100-kD E-cadherin cleavage product, which is inhibited in the presence of calpeptin [84] (Fig. 2B). Treatment of cells with thapsigargin to elevate cytosolic Ca2+ also induced colocalization of calpains with E-cadherin and generated the 100-kD E-cadherin cleavage product [84]. In prostate and mammary epithelial cells, calpain activation efficiently generated the same 100-kD cleavage fragment of E-cadherin, also found in abundance in metastatic prostate cancer [143]. The calpain cleavage site on E-cadherin was mapped to residues 782–787 in the C-terminal domain, directly upstream of β-catenin- and γ-catenin-binding domains [144]. These data suggest that calpains are involved in disassociation of the E-cadherin catenins complex, which signals loss of E-cadherin at the junctions.
CALPAIN ACTIVATION FACILITATES PMN TRANSMIGRATION
A major function of TLR2 signaling is stimulating chemokine expression and signaling PMN recruitment. The Ca2+ fluxes that initiate TLR2-NF-κB-IL-8 signaling also stimulate calpain activity [84] (Fig. 3). In response to P. aeruginosa or a synthetic TLR2 agonist, PMN transepithelial migration was inhibited by the calpain inhibitor calpeptin, which did not inhibit the migration of PMNs directly across a porous transwell in response to bacteria or a chemokine gradient [84]. In resting PMNs, calpains are constitutively active and block the ability of PMNs to migrate, and in fact, calpain inhibition was shown to promote PMN movement by initiating MAPK and Rac GTPase activation [145, 146]. Thus, targeting epithelial, and not PMN, calpain activity is responsible for inhibiting PMN transmigration.
Although TLR2-mediated Ca2+ fluxes and subsequent calpain activation are rapid and observed within seconds or minutes [28, 84], cleavage of junctional proteins and PMN transmigration is detected after 1 or 4 h stimulation [84]. This discrepancy in kinetics may be a result of the cells’ ability to tightly control the levels of cytosolic Ca2+ by uptake into internal stores, extracellular release through efflux pumps, or chelation by Ca2+-buffering molecules [147, 148]. Additionally, activated calpain proteases are autolysed rapidly or inhibited by calpastatin [108] and may require further protein synthesis to achieve enough active protease to detect downstream responses. Furthermore, limitations in the detection of cleavage fragments may also contribute to the discrepancy in kinetics, as the initial generation of these fragments may also be degraded rapidly.
The biological importance of TLR2-induced calpain activity in vivo was tested in neonatal and adult murine models of airway infection. Compared with untreated controls, calpeptin-treated mice had 37% fewer PMNs recruited in the whole lung and 90% fewer PMNs recruited into the airway lumen, suggesting a more pronounced effect on the epithelial junctions [84]. In contrast to in vivo models of intestinal inflammation, which use organisms or chemical agents that induce significant epithelial injury [21], intranasal P. aeruginosa treatment in our murine model of infection is cleared readily with minimal damage to the lungs. The colon, unlike the airway, is colonized with commensal organisms and is hyporesponsive to TLR2 and TLR4 stimulation and requires epithelial damage to facilitate PMN recruitment [52]. In contrast, the normally sterile airway is hyperresponsive to pathogenic insult, and epithelial integrity is maintained, preserving lung function, and specific junctional changes facilitate PMN recruitment [84]. Finally, the rapid junctional changes and subsequent PMN recruitment into the airway in response to bacteria make it a favorable model to study PMN transepithelial migration.
CONCLUDING REMARKS
The participation of Ca2+ as a second messenger is vital to numerous physiological processes including the epithelial response to bacteria [3,4,5]. Ligation of TLR2 stimulates Ca2+ fluxes important in NF-κB activation as well as calpain activity.
TLR2-mediated Ca2+ fluxes signal changes in epithelial junctions to accommodate PMN egress to the airway luman. There is likely TLR2-independent signaling involved in facilitating PMN transmigration as well. TLR5, which activates Ca2+ fluxes in airway cells in response to flagella [9], may also contribute to PMN transmigration.
TLR2 activation in the airway epithelium coordinates the afferent and efferent limbs of the initial inflammatory response by signaling intracellular Ca2+ release. Not only do the airway epithelial cells produce chemokines to direct PMN recruitment, but they also use the same signaling cascade to modulate the cell junctions to accommodate PMN egress without breaching the epithelial barrier. Neither a decrease in transepithelial resistance nor an increase in bacterial invasion across the epithelium was observed in response to this Ca2+-dependent signaling event. Therefore, this pathway may provide a useful pharmacological target in pulmonary infection to selectively limit PMN recruitment into the lung, without entirely compromising host defenses to bacterial infection.
Footnotes
Abbreviations: ADAM 17=a disintegrin and metallopeptidase domain 17, Capn4=calpain 4, CINC=cytokine-induced neutrophil chemoattractant, CSS1=calpainsmall subunit 1, Cx=connexin, DAG=diacylglycerol, EF=helix-loop-helix structural domain, ER=endoplasmic reticulum, IKK=IκB kinse, IP3R=inositol (1,4,5)-trisphosphate receptor, IRAK=IL-1R-associated kinase, KC=keratinocyte-derived chemokine, MLCK=myosin light chain kinase, MMP=matrix metalloproteinase, MUC-2=mucine 2, P3C=Pam 3Cys-SK4, PAMP=pathogen-associated molecular pattern, PH=pleckstrin homology, PKC=protein kinase C, PLCγ=phospholipase C γ, PMN=polymorphonuclear leukocyte, PRR=pattern recognition receptor, siRNA=small interfering RNA, TIR=Toll/IL-1R, Trif=TIR domain-containing adaptor-inducing IFN-β, TRPC=transient receptor potential channel, ZO-1=zona occludens 1
References
- Polito A. J., Proud D. Epithelia cells as regulators of airway inflammation. J Allergy Clin Immunol. 1998;102:714–718. doi: 10.1016/s0091-6749(98)70008-9. [DOI] [PubMed] [Google Scholar]
- Gomez M. I., Prince A. Airway epithelial cell signaling in response to bacterial pathogens. Pediatr Pulmonol. 2008;43:11–19. doi: 10.1002/ppul.20735. [DOI] [PubMed] [Google Scholar]
- Werry T. D., Wilkinson G. F., Willars G. B. Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+ Biochem J. 2003;374:281–296. doi: 10.1042/BJ20030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo E. M., Cante-Barrett K., Crabtree G. R. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- Mellstrom B., Savignac M., Gomez-Villafuertes R., Naranjo J. R. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev. 2008;88:421–449. doi: 10.1152/physrev.00041.2005. [DOI] [PubMed] [Google Scholar]
- Gardner P. Calcium and T lymphocyte activation. Cell. 1989;59:15–20. doi: 10.1016/0092-8674(89)90865-9. [DOI] [PubMed] [Google Scholar]
- Wang C., Mooney J. L., Meza-Romero R., Chou Y. K., Huan J., Vandenbark A. A., Offner H., Burrows G. G. Recombinant TCR ligand induces early TCR signaling and a unique pattern of downstream activation. J Immunol. 2003;171:1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- Scharenberg A. M., Humphries L. A., Rawlings D. J. Calcium signaling and cell-fate choice in B cells. Nat Rev Immunol. 2007;7:778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner A. J., Bryan R., Weber A., Nguyen S., Barnes D., Pitt A., Gelber S., Cheung A., Prince A. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J Biol Chem. 2001;276:19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- Takeda K., Akira S. Toll-like receptors. Curr Protoc Immunol. 2007;May:14.12. doi: 10.1002/0471142735.im1412s77. [DOI] [PubMed] [Google Scholar]
- Muir A., Soong G., Sokol S., Reddy B., Gomez M. I., Van Heeckeren A., Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–783. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- Harada A., Sekido N., Akahoshi T., Wada T., Mukaida N., Matsu-shima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- Wagner J. G., Roth R. A. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- Reutershan J., Basit A., Galkina E. V., Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–L815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- Hurley B. P., Siccardi D., Mrsny R. J., McCormick B. A. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J Immunol. 2004;173:5712–5720. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- Gomez M. I., Prince A. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr Opin Pharmacol. 2007;7:244–251. doi: 10.1016/j.coph.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Burns A. R., Smith C. W., Walker D. C. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- Aghajanian A., Wittchen E. S., Allingham M. J., Garrett T. A., Bur-ridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J Thromb Haemost. 2008;6:1453–1460. doi: 10.1111/j.1538-7836.2008.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor M., Parkos C. A. Disassembly of endothelial and epithelial junctions during leukocyte transmigration. Front Biosci. 2008;13:6638–6652. doi: 10.2741/3178. [DOI] [PubMed] [Google Scholar]
- Burns A. R., Takei F., Doerschuk C. M. Quantitation of ICAM-1 expression in mouse lung during pneumonia. J Immunol. 1994;153:3189–3198. [PubMed] [Google Scholar]
- Chin A. C., Parkos C. A. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol. 2007;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- Chin A. C., Parkos C. A. Neutrophil transepithelial migration and epithelial barrier function in IBD: potential targets for inhibiting neutrophil trafficking. Ann N Y Acad Sci. 2006;1072:276–287. doi: 10.1196/annals.1326.018. [DOI] [PubMed] [Google Scholar]
- Kucharzik T., Walsh S. V., Chen J., Parkos C. A., Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–2009. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savkovic S. D., Ramaswamy A., Koutsouris A., Hecht G. EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am J Physiol Gastrointest Liver Physiol. 2001;281:G890–G898. doi: 10.1152/ajpgi.2001.281.4.G890. [DOI] [PubMed] [Google Scholar]
- TranVan Nhieu G., Clair C., Grompone G., Sansonetti P. Calcium signaling during cell interactions with bacterial pathogens. Biol Cell. 2004;96:93–101. doi: 10.1016/j.biolcel.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Ganguly U., Chaudhury A. G., Basu A., Sen P. C. STa-induced translocation of protein kinase C from cytosol to membrane in rat enterocytes. FEMS Microbiol Lett. 2001;204:65–69. doi: 10.1111/j.1574-6968.2001.tb10864.x. [DOI] [PubMed] [Google Scholar]
- McNamara N., Khong A., McKemy D., Caterina M., Boyer J., Julius D., Basbaum C. ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc Natl Acad Sci USA. 2001;98:9086–9091. doi: 10.1073/pnas.161290898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J., Prince A. Activation of Ca2+-dependent signaling by TLR2. J Immunol. 2006;177:1330–1337. doi: 10.4049/jimmunol.177.2.1330. [DOI] [PubMed] [Google Scholar]
- Adamo R., Sokol S., Soong G., Gomez M. I., Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and Toll-like receptor 2 as well as Toll-like receptor 5. Am J Respir Cell Mol Biol. 2004;30:627–634. doi: 10.1165/rcmb.2003-0260OC. [DOI] [PubMed] [Google Scholar]
- Soong G., Reddy B., Sokol S., Adamo R., Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113:1482–1489. doi: 10.1172/JCI20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschning C. J., Schumann R. R. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr Top Microbiol Immunol. 2002;270:121–144. doi: 10.1007/978-3-642-59430-4_8. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott E. M., O'Neill L. A. The potential of targeting Toll-like receptor 2 in autoimmune and inflammatory diseases. Ir J Med Sci. 2007;176:253–260. doi: 10.1007/s11845-007-0103-1. [DOI] [PubMed] [Google Scholar]
- Triantafilou M., Manukyan M., Mackie A., Morath S., Hartung T., Heine H., Triantafilou K. Lipoteichoic acid and Toll-like receptor 2 internalization and targeting to the Golgi are lipid raft-dependent. J Biol Chem. 2004;279:40882–40889. doi: 10.1074/jbc.M400466200. [DOI] [PubMed] [Google Scholar]
- Marshall A. J., Niiro H., Yun T. J., Clark E. A. Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cγ pathway. Immunol Rev. 2000;176:30–46. doi: 10.1034/j.1600-065x.2000.00611.x. [DOI] [PubMed] [Google Scholar]
- Arbibe L., Mira J. P., Teusch N., Kline L., Guha M., Mackman N., Godowski P. J., Ulevitch R. J., Knaus U. G. Toll-like receptor 2-mediated NF-κ B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- Li J. D., Dohrman A. F., Gallup M., Miyata S., Gum J. R., Kim Y. S., Nadel J. A., Prince A., Basbaum C. B. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA. 1997;94:967–972. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M. D., Cochrane C. L., Duncan S. K., Schrader J. W. Pure lipopolysaccharide or synthetic lipid A induces activation of p21Ras in primary macrophages through a pathway dependent on Src family kinases and PI3K. J Immunol. 2005;175:8236–8241. doi: 10.4049/jimmunol.175.12.8236. [DOI] [PubMed] [Google Scholar]
- Chang Y. J., Wu M. S., Lin J. T., Sheu B. S., Muta T., Inoue H., Chen C. C. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-κB activation. Mol Pharmacol. 2004;66:1465–1477. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- Lee I. T., Wang S. W., Lee C. W., Chang C. C., Lin C. C., Luo S. F., Yang C. M. Lipoteichoic acid induces HO-1 expression via the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. J Immunol. 2008;181:5098–5110. doi: 10.4049/jimmunol.181.7.5098. [DOI] [PubMed] [Google Scholar]
- Manukyan M., Nalbant P., Luxen S., Hahn K. M., Knaus U. G. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-κB. J Immunol. 2009;182:3522–3529. doi: 10.4049/jimmunol.0802280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengifo-Cam W., Konishi A., Morishita N., Matsuoka H., Yamori T., Nada S., Okada M. Csk defines the ability of integrin-mediated cell adhesion and migration in human colon cancer cells: implication for a potential role in cancer metastasis. Oncogene. 2004;23:289–297. doi: 10.1038/sj.onc.1207041. [DOI] [PubMed] [Google Scholar]
- Schmeck B., Huber S., Moog K., Zahlten J., Hocke A. C., Opitz B., Hammerschmidt S., Mitchell T. J., Kracht M., Rosseau S., Suttorp N., Hippenstiel S. Pneumococci induced TLR- and Rac1-dependent NF-κB-recruitment to the IL-8 promoter in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L730–L737. doi: 10.1152/ajplung.00271.2005. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu J., Wang Z. Akt binds to and phosphorylates phospholipase C-γ1 in response to epidermal growth factor. Mol Biol Cell. 2006;17:2267–2277. doi: 10.1091/mbc.E05-10-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4:313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- Sukumaran S. K., McNamara G., Prasadarao N. V. Escherichia coli K-1 interaction with human brain micro-vascular endothelial cells triggers phospholipase C-γ1 activation downstream of phosphatidylinositol 3-kinase. J Biol Chem. 2003;278:45753–45762. doi: 10.1074/jbc.M307374200. [DOI] [PubMed] [Google Scholar]
- Khullar M., Singh R. D., Smriti M., Ganguly N. K. Anaerobiosis-induced virulence of Salmonella enterica subsp. enterica serovar Typhimurium: role of phospholipase Cγ signaling cascade. J Med Microbiol. 2003;52:741–745. doi: 10.1099/jmm.0.05186-0. [DOI] [PubMed] [Google Scholar]
- Fukao T., Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- Strassheim D., Asehnoune K., Park J. S., Kim J. Y., He Q., Richter D., Kuhn K., Mitra S., Abraham E. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol. 2004;172:5727–5733. doi: 10.4049/jimmunol.172.9.5727. [DOI] [PubMed] [Google Scholar]
- Hazeki K., Nigorikawa K., Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30:1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- McNamara N., Gallup M., Khong A., Sucher A., Maltseva I., Fahy J., Basbaum C. Adenosine up-regulation of the mucin gene, MUC2, in asthma. FASEB J. 2004;18:1770–1772. doi: 10.1096/fj.04-1964fje. [DOI] [PubMed] [Google Scholar]
- Park G. Y., Christman J. W. Nuclear factor κ B is a promising therapeutic target in inflammatory lung disease. Curr Drug Targets. 2006;7:661–668. doi: 10.2174/138945006777435317. [DOI] [PubMed] [Google Scholar]
- Liu A. M., Wong Y. H. Activation of nuclear factor κB by somatostatin type 2 receptor in pancreatic acinar AR42J cells involves Gα14 and multiple signaling components: a mechanism requiring protein kinase C, calmodulin-dependent kinase II, ERK, and c-Src. J Biol Chem. 2005;280:34617–34625. doi: 10.1074/jbc.M504264200. [DOI] [PubMed] [Google Scholar]
- Liu A. M., Wong Y. H. G16-mediated activation of nuclear factor κB by the adenosine A1 receptor involves c-Src, protein kinase C, and ERK signaling. J Biol Chem. 2004;279:53196–53204. doi: 10.1074/jbc.M410196200. [DOI] [PubMed] [Google Scholar]
- Yoon C., Korade Z., Carter B. D. Protein kinase A-induced phosphorylation of the p65 subunit of nuclear factor-κB promotes Schwann cell differentiation into a myelinating phenotype. J Neurosci. 2008;28:3738–3746. doi: 10.1523/JNEUROSCI.4439-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak D. E., Tian B., Jamaluddin M., Boldogh I., Vergara L. A., Choudhary S., Brasier A. R. RelA Ser276 phosphorylation is required for activation of a subset of NF-κB-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol Cell Biol. 2008;28:3623–3638. doi: 10.1128/MCB.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Naumann M. Beyond IκBs: alternative regulation of NF-κB activity. FASEB J. 2007;21:2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- Nickel R., Beck L. A., Stellato C., Schleimer R. P. Chemokines and allergic disease. J Allergy Clin Immunol. 1999;104:723–742. doi: 10.1016/s0091-6749(99)70281-2. [DOI] [PubMed] [Google Scholar]
- Rollins B. J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Pease J. E., Sabroe I. The role of interleukin-8 and its receptors in inflammatory lung disease: implications for therapy. Am J Respir Med. 2002;1:19–25. doi: 10.1007/BF03257159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khair O. A., Davies R. J., Devalia J. L. Bacterial-induced release of inflammatory mediators by bronchial epithelial cells. Eur Respir J. 1996;9:1913–1922. doi: 10.1183/09031936.96.09091913. [DOI] [PubMed] [Google Scholar]
- Rainger G. E., Fisher A. C., Nash G. B. Endothelial-borne platelet-activating factor and interleukin-8 rapidly immobilize rolling neutrophils. Am J Physiol. 1997;272:H114–H122. doi: 10.1152/ajpheart.1997.272.1.H114. [DOI] [PubMed] [Google Scholar]
- Hoffmann E., Dittrich-Breiholz O., Holtmann H., Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Schottelius A. J., Mayo M. W., Sartor R. B., Baldwin A. S., Jr Interleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- Mukaida N., Okamoto S., Ishikawa Y., Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–558. [PubMed] [Google Scholar]
- Shibata F., Konishi K., Kato H., Komorita N., al-Mokdad M., Fujioka M., Nakagawa H. Recombinant production and biological properties of rat cytokine-induced neutrophil chemoattractants, GRO/CINC-2 α, CINC-2 β and CINC-3. Eur J Biochem. 1995;231:306–311. doi: 10.1111/j.1432-1033.1995.tb20701.x. [DOI] [PubMed] [Google Scholar]
- Huang S., Paulauskis J. D., Godleski J. J., Kobzik L. Expression of macrophage inflammatory protein-2 and KC mRNA in pulmonary inflammation. Am J Pathol. 1992;141:981–988. [PMC free article] [PubMed] [Google Scholar]
- Driscoll K. E., Howard B. W., Carter J. M., Asquith T., Johnston C., Detilleux P., Kunkel S. L., Isfort R. J. α-Quartz-induced chemokine expression by rat lung epithelial cells: effects of in vivo and in vitro particle exposure. Am J Pathol. 1996;149:1627–1637. [PMC free article] [PubMed] [Google Scholar]
- Driscoll K. E., Simpson L., Carter J., Hassenbein D., Leikauf G. D. Ozone inhalation stimulates expression of a neutrophil chemotactic protein, macrophage inflammatory protein 2. Toxicol Appl Pharmacol. 1993;119:306–309. doi: 10.1006/taap.1993.1074. [DOI] [PubMed] [Google Scholar]
- Frevert C. W., Farone A., Danaee H., Paulauskis J. D., Kobzik L. Functional characterization of rat chemokine macrophage inflammatory protein-2. Inflammation. 1995;19:133–142. doi: 10.1007/BF01534386. [DOI] [PubMed] [Google Scholar]
- Frevert C. W., Huang S., Danaee H., Paulauskis J. D., Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154:335–344. [PubMed] [Google Scholar]
- Koto H., Salmon M., Haddad el B., Huang T. J., Zagorski J., Chung K. F. Role of cytokine-induced neutrophil chemoattractant (CINC) in ozone-induced airway inflammation and hyperresponsiveness. Am J Respir Crit Care Med. 1997;156:234–239. doi: 10.1164/ajrccm.156.1.9606095. [DOI] [PubMed] [Google Scholar]
- Rozental R., Carvalho A. C., Spray D. C. Gap junctions in the cardiovascular and immune systems. Braz J Med Biol Res. 2000;33:365–368. doi: 10.1590/s0100-879x2000000400001. [DOI] [PubMed] [Google Scholar]
- Rozental R., Giaume C., Spray D. C. Gap junctions in the nervous system. Brain Res Brain Res Rev. 2000;32:11–15. doi: 10.1016/s0165-0173(99)00095-8. [DOI] [PubMed] [Google Scholar]
- Koval M. Sharing signals: connecting lung epithelial cells with gap junction channels. Am J Physiol Lung Cell Mol Physiol. 2002;283:L875–L893. doi: 10.1152/ajplung.00078.2002. [DOI] [PubMed] [Google Scholar]
- Isakson B. E., Olsen C. E., Boitano S. Laminin-332 alters connexin profile, dye coupling and intercellular Ca2+ waves in ciliated tracheal epithelial cells. Respir Res. 2006;7:105. doi: 10.1186/1465-9921-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. J., Prince A. S. TLR2 regulates gap junction intercellular communication in airway cells. J Immunol. 2008;180:4986–4993. doi: 10.4049/jimmunol.180.7.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarieddine M. Z., Scheckenbach K. L., Foglia B., Maass K., Garcia I., Kwak B. R., Chanson M. Connexin43 modulates neutrophil recruitment to the lung. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2008.00654.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Jornot L., Wiszniewski L., Rochat T., Suter S., Lacroix J. S., Chanson M. Src signaling links mediators of inflammation to Cx43 gap junction channels in primary and transformed CFTR-expressing airway cells. Cell Commun Adhes. 2003;10:279–285. doi: 10.1080/cac.10.4-6.279.285. [DOI] [PubMed] [Google Scholar]
- Huang A. J., Manning J. E., Bandak T. M., Ratau M. C., Hanser K. R., Silverstein S. C. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmmed G. U., Malik A. B. Functional role of TRPC channels in the regulation of endothelial permeability. Pflugers Arch. 2005;451:131–142. doi: 10.1007/s00424-005-1461-z. [DOI] [PubMed] [Google Scholar]
- Goeckeler Z. M., Wysolmerski R. B. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi C., Freichel M., Vogel S. M., Paria B. C., Mehta D., Flockerzi V., Malik A. B. Impairment of store-operated Ca2+ entry in TRPC4−/− mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- Guttman J. A., Samji F. N., Li Y., Vogl A. W., Finlay B. B. Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo. Infect Immun. 2006;74:6075–6084. doi: 10.1128/IAI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J., Prince A. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe. 2009;5:47–58. doi: 10.1016/j.chom.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A. C., Lee W. Y., Nusrat A., Vergnolle N., Parkos C. A. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J Immunol. 2008;181:5702–5710. doi: 10.4049/jimmunol.181.8.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D., Balda M. S., Matter K. Occludin modulates transepithelial migration of neutrophils. J Biol Chem. 2000;275:5773–5778. doi: 10.1074/jbc.275.8.5773. [DOI] [PubMed] [Google Scholar]
- Ginzberg H. H., Cherapanov V., Dong Q., Cantin A., McCulloch C. A., Shannon P. T., Downey G. P. Neutrophil-mediated epithelial injury during transmigration: role of elastase. Am J Physiol Gastrointest Liver Physiol. 2001;281:G705–G717. doi: 10.1152/ajpgi.2001.281.3.G705. [DOI] [PubMed] [Google Scholar]
- Parks W. C., Wilson C. L., Lopez-Boado Y. S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Li Q., Park P. W., Wilson C. L., Parks W. C. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- McGuire J. K., Li Q., Parks W. C. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyasu H., McCormack J. M., McCarthy K. M., Dombkowski D., Preffer F. I., Schneeberger E. E. Matrix metalloproteinase-9-deficient dendritic cells have impaired migration through tracheal epithelial tight junctions. Am J Respir Cell Mol Biol. 2004;30:761–770. doi: 10.1165/rcmb.2003-0370OC. [DOI] [PubMed] [Google Scholar]
- Wachtel M., Frei K., Ehler E., Fontana A., Winterhalter K., Gloor S. M. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J Cell Sci. 1999;112:4347–4356. doi: 10.1242/jcs.112.23.4347. [DOI] [PubMed] [Google Scholar]
- Tompa P., Emori Y., Sorimachi H., Suzuki K., Friedrich P. Domain III of calpain is a Ca2+-regulated phospholipid-binding domain. Biochem Biophys Res Commun. 2001;280:1333–1339. doi: 10.1006/bbrc.2001.4279. [DOI] [PubMed] [Google Scholar]
- Saido T. C., Shibata M., Takenawa T., Murofushi H., Suzuki K. Positive regulation of μ-calpain action by polyphosphoinositides. J Biol Chem. 1992;267:24585–24590. [PubMed] [Google Scholar]
- Shao H., Chou J., Baty C. J., Burke N. A., Watkins S. C., Stolz D. B., Wells A. Spatial localization of m-calpain to the plasma membrane by phosphoinositide biphosphate binding during epidermal growth factor receptor-mediated activation. Mol Cell Biol. 2006;26:5481–5496. doi: 10.1128/MCB.02243-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco S. J., Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- Wells A., Huttenlocher A., Lauffenburger D. A. Calpain proteases in cell adhesion and motility. Int Rev Cytol. 2005;245:1–16. doi: 10.1016/S0074-7696(05)45001-9. [DOI] [PubMed] [Google Scholar]
- Goll D. E., Thompson V. F., Li H., Wei W., Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Fettucciari K., Fetriconi I., Mannucci R., Nicoletti I., Bartoli A., Coaccioli S., Marconi P. Group B Streptococcus induces macrophage apoptosis by calpain activation. J Immunol. 2006;176:7542–7556. doi: 10.4049/jimmunol.176.12.7542. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S., McDonald M. C., Mazzon E., Siriwardena D., Serraino I., Dugo L., Britti D., Mazzullo G., Caputi A. P., Thiemermann C. Calpain inhibitor I reduces the development of acute and chronic inflammation. Am J Pathol. 2000;157:2065–2079. doi: 10.1016/S0002-9440(10)64845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Kasai Y., Kawashima S. Preferential localization of calcium-activated neutral protease in epithelial tissues. Biochem Biophys Res Commun. 1987;148:567–574. doi: 10.1016/0006-291x(87)90914-4. [DOI] [PubMed] [Google Scholar]
- Dwyer-Nield L. D., Miller A. C., Neighbors B. W., Dinsdale D., Malkinson A. M. Cytoskeletal architecture in mouse lung epithelial cells is regulated by protein-kinase C-α and calpain II. Am J Physiol. 1996;270:L526–L534. doi: 10.1152/ajplung.1996.270.4.L526. [DOI] [PubMed] [Google Scholar]
- Dutt P., Spriggs C. N., Davies P. L., Jia Z., Elce J. S. Origins of the difference in Ca2+ requirement for activation of μ- and m-calpain. Biochem J. 2002;367:263–269. doi: 10.1042/BJ20020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Hosfield C. M., Davies P. L., Elce J. S. Crystal structure of calpain and insights into Ca2+-dependent activation. Methods Mol Biol. 2002;172:51–67. doi: 10.1385/1-59259-183-3:051. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T., Hosfield C. M., Lim D., Elce J. S., Jia Z., Davies P. L. A Ca2+ switch aligns the active site of calpain. Cell. 2002;108:649–660. doi: 10.1016/s0092-8674(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Hata S., Sorimachi H., Suzuki K. [Structure and function of calpain superfamily] Seikagaku. 2001;73:1129–1140. [PubMed] [Google Scholar]
- Glading A., Bodnar R. J., Reynolds I. J., Shiraha H., Satish L., Potter D. A., Blair H. C., Wells A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elce J. S., Hegadorn C., Arthur J. S. Autolysis, Ca2+ requirement, and heterodimer stability in m-calpain. J Biol Chem. 1997;272:11268–11275. doi: 10.1074/jbc.272.17.11268. [DOI] [PubMed] [Google Scholar]
- Li H., Thompson V. F., Goll D. E. Effects of autolysis on properties of μ- and m-calpain. Biochim Biophys Acta. 2004;1691:91–103. doi: 10.1016/j.bbamcr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Wendt A., Thompson V. F., Goll D. E. Interaction of calpastatin with calpain: a review. Biol Chem. 2004;385:465–472. doi: 10.1515/BC.2004.054. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Contreras R. G., Shoshani L., Flores-Benitez D., Larre I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta. 2008;1778:770–793. doi: 10.1016/j.bbamem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Hartsock A., Nelson W. J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger E. E., Lynch R. D. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Feldman G. J., Mullin J. M., Ryan M. P. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Balda M. S., Flores-Maldonado C., Cereijido M., Matter K. Multiple domains of occludin are involved in the regulation of paracellular permeability. J Cell Biochem. 2000;78:85–96. [PubMed] [Google Scholar]
- Balda M. S., Whitney J. A., Flores C., Gonzalez S., Cereijido M., Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V., Gumbiner B. M. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M., Furuse M., Sasaki H., Schulzke J. D., Fromm M., Takano H., Noda T., Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzke J. D., Gitter A. H., Mankertz J., Spiegel S., Seidler U., Amasheh S., Saitou M., Tsukita S., Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Furuse M., Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Harhaj N. S., Antonetti D. A. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–1237. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Muller S. L., Portwich M., Schmidt A., Utepbergenov D. I., Huber O., Blasig I. E., Krause G. The tight junction protein occludin and the adherens junction protein α-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- Furuse M., Itoh M., Hirase T., Nagafuchi A., Yonemura S., Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. K., Basuroy S., Rao V. U., Karnaky K. J., Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-β-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojarski C., Weiske J., Schoneberg T., Schroder W., Mankertz J., Schulzke J. D., Florian P., Fromm M., Tauber R., Huber O. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117:2097–2107. doi: 10.1242/jcs.01071. [DOI] [PubMed] [Google Scholar]
- Zhu L., Li X., Zeng R., Gorodeski G. I. Changes in tight junctional resistance of the cervical epithelium are associated with modulation of content and phosphorylation of occludin 65-kilodalton and 50-kilodalton forms. Endocrinology. 2006;147:977–989. doi: 10.1210/en.2005-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Winton H. L., Soeller C., Tovey E. R., Gruenert D. C., Thompson P. J., Stewart G. A., Taylor G. W., Garrod D. R., Cannell M. B., Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth S. D., Kniesel U., Wolburg H., Engelhardt B., Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci. 1999;112:1879–1888. doi: 10.1242/jcs.112.12.1879. [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Stow J. L. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Orrington-Myers J., Gao X., Kouklis P., Broman M., Rahman A., Vogel S. M., Malik A. B. Regulation of lung neutrophil recruitment by VE-cadherin. Am J Physiol Lung Cell Mol Physiol. 2006;291:L764–L771. doi: 10.1152/ajplung.00502.2005. [DOI] [PubMed] [Google Scholar]
- Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Davis M. A., Ireton R. C., Reynolds A. B. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk P. Endothelial signaling in leukocyte transmigration. Cell Biochem Biophys. 2003;38:305–322. doi: 10.1385/cbb:38:3:305. [DOI] [PubMed] [Google Scholar]
- Ohsugi M., Larue L., Schwarz H., Kemler R. Cell-junctional and cytoskeletal organization in mouse blastocysts lacking E-cadherin. Dev Biol. 1997;185:261–271. doi: 10.1006/dbio.1997.8560. [DOI] [PubMed] [Google Scholar]
- Tinkle C. L., Lechler T., Pasolli H. A., Fuchs E. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc Natl Acad Sci USA. 2004;101:552–557. doi: 10.1073/pnas.0307437100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L., Ohsugi M., Hirchenhain J., Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege R. M., Gavard J., Lambert M. Regulation of cell–cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Xu W., Kimelman D. Mechanistic insights from structural studies of β-catenin and its binding partners. J Cell Sci. 2007;120:3337–3344. doi: 10.1242/jcs.013771. [DOI] [PubMed] [Google Scholar]
- Parisiadou L., Fassa A., Fotinopoulou A., Bethani I., Efthimiopoulos S. Presenilin 1 and cadherins: stabilization of cell–cell adhesion and proteolysis-dependent regulation of transcription. Neurodegener Dis. 2004;1:184–191. doi: 10.1159/000080984. [DOI] [PubMed] [Google Scholar]
- McGuire J. K., Li Q., Parks W. C. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhusen U., Weiske J., Badock V., Tauber R., Bommert K., Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem. 2001;276:4972–4980. doi: 10.1074/jbc.M006102200. [DOI] [PubMed] [Google Scholar]
- Maretzky T., Reiss K., Ludwig A., Buchholz J., Scholz F., Proksch E., de Strooper B., Hartmann D., Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell–cell adhesion, migration, and β-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Doria J., Day K. C., Kuefer R., Rashid M. G., Chinnaiyan A. M., Rubin M. A., Day M. L. The role of calpain in the proteolytic cleavage of E-cadherin in prostate and mammary epithelial cells. J Biol Chem. 2003;278:1372–1379. doi: 10.1074/jbc.M208772200. [DOI] [PubMed] [Google Scholar]
- Lieberman D. Pseudomonal infections in patients with COPD: epidemiology and management. Am J Respir Med. 2003;2:459–468. doi: 10.1007/BF03256673. [DOI] [PubMed] [Google Scholar]
- Lokuta M. A., Nuzzi P. A., Huttenlocher A. Calpain regulates neutrophil chemotaxis. Proc Natl Acad Sci USA. 2003;100:4006–4011. doi: 10.1073/pnas.0636533100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsube M., Kato T., Kitagawa M., Noma H., Fujita H., Kitagawa S. Calpain-mediated regulation of the distinct signaling pathways and cell migration in human neutrophils. J Leukoc Biol. 2008;84:255–263. doi: 10.1189/jlb.0907664. [DOI] [PubMed] [Google Scholar]
- Thul R., Bellamy T. C., Roderick H. L., Bootman M. D., Coombes S. Calcium oscillations. Adv Exp Med Biol. 2008;641:1–27. doi: 10.1007/978-0-387-09794-7_1. [DOI] [PubMed] [Google Scholar]
- Schwaller B. The continuing disappearance of “pure” Ca2+ buffers. Cell Mol Life Sci. 2009;66:275–300. doi: 10.1007/s00018-008-8564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]