Abstract
In suspensions of Nafion beads and of cationic gel beads, NMR spectroscopy showed two water–proton resonances, one representing intimate water layers next to the polymer surface, the other corresponding to water lying beyond. Both resonances show notably shorter spin–lattice relaxation times (T1) and smaller self-diffusion coefficients (D) indicating slower dynamics than bulk water. These findings confirm the existence of highly restricted water layers adsorbed onto hydrophilic surfaces and dynamically stable water beyond the first hydration layers. Thus, aqueous regions on the order of micrometers are dynamically different from bulk water.
Water is arguably the most important liquid on earth. Despite its significance and abundance, water remains mysterious in many ways, and many anomalous features remain incompletely understood.1 Among the lingering mysteries are the dynamics at interfaces. Interfacial water is of critical importance to biology, chemistry, and geo-climatic and atmospheric processes, as many catalytic reactions occur at interfaces.2,3 Yet the properties of interfacial water are poorly understood.
It is commonly accepted that in the vicinity of hydrophilic interfaces water organizes into ice-like water, which project from the surface by a few nanometers.4–6 Partly due to the rapid development of surface-characterization techniques, most recent work has focused almost exclusively on near-surface dynamics, i.e., dynamics of the first few molecular layers. Consequently, possible macroscopic effects have not been widely studied.
On the other hand, decades of early work has demonstrated that water can exhibit physical properties quite different than those of bulk water, out to distances on the order of micrometers.7 Further, such an extensive surface water zone near many biological surfaces, known as the unstirred layer, has been observed to significantly impact the rate of colloidal diffusion across cell membranes.8,9
Work begun several years ago in our group indicates that the interfacial water zone may extend unexpectedly far from hydrophilic surfaces.10 Those surfaces include a wide range of materials such as ion-exchange resins, polymers, hydrogels, functionalized monolayers, biological tissues and metal oxides. The near-surface aqueous zones exhibit a number of unique properties, the most notable being that suspended particles (e.g., monodisperse colloids) are excluded from the surface to distances of several hundred micrometers; thus, these particle-free zones have been termed exclusion zones (EZs). EZs exhibit a variety of physical properties differing from those of bulk water. They bear negative charge, as much as 150–200mV near the nucleating surface, they exhibit increased dynamic viscosity, and they show diminished infrared emissivity and retarded T2 relaxation times.11,12 These properties imply that the water within EZs may be more ordered than bulk water.
Nuclear magnetic resonance (NMR) spectroscopy is a well-established technique for determining the chemical structure and environment in aqueous systems.13 NMR chemical shift, relaxation, and self-diffusion measurements have proved especially successful for probing interfacial water structure and dynamics near various biological and polymeric surfaces.14–16 In the present study, high-resolution NMR spectroscopy was employed to measure the chemical shift, spin–lattice (T1) relaxation, and self-diffusion coefficient of interfacial water in order to obtain a more detailed physical picture of the molecular environment within the EZ.
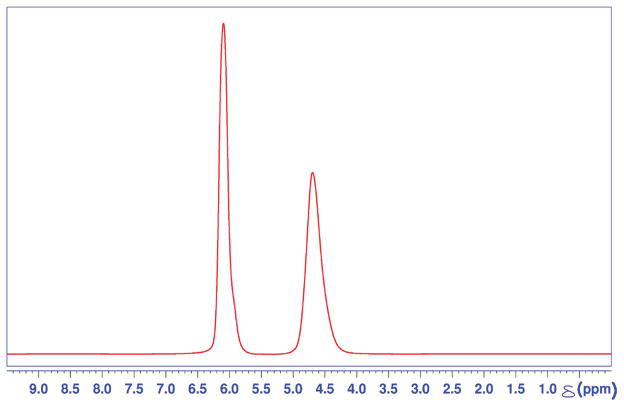
Figure 1 is a representative 1H NMR spectrum of cationic resin/water system at 298° K in thermal equilibrium. Two water–proton resonances are evident, at 4.63 ppm and 6.00 ppm, indicating two distinct and magnetically unique water species, as observed within the NMR time scale. The standard chemical shift value of 1H resonance at 298 K for the HOD peak in an H2O:D2O mixture is 4.8 ppm.17 Since the frequency axis was referenced to an external standard, i.e., 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS), it is reasonable to identify the upfield resonance at 4.6 ppm as that of less restricted water in the interstitial region between the beads. The downfield shift to the resonance at 6.00 ppm is indicative of a more “deshielded” environment of the water, and therefore corresponds to highly restricted water layers adsorbed onto polymer surfaces inside and around the resin. Integrating the fully relaxed 1H spectrum shown in Figure 1 yielded a 1:1 ratio of area under the peaks corresponding to internal water and the interstitial water, respectively. Full width at half-maximum (fwhm) line widths of the downfield-shifted peak are approximately 80% of the peak for interstitial water at 4.63 ppm.
Figure 1.
A fully relaxed one-dimensional (1D) 1H NMR spectrum of a cationic resin/water system. Two water proton resonances are observed at 6.0 ppm and 4.63 ppm, each corresponding to internal and interstitial water, respectively.
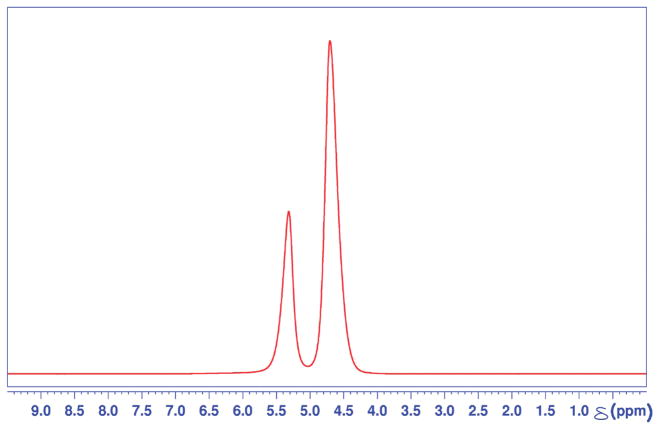
Figure 2 shows a representative 1H NMR spectrum of a Nafion bead/water system at 298° K in thermal equilibrium. As in the cationic resin/water system, two chemical shifts of water protons are observed. The upfield resonance at 4.61 ppm is assigned as interstitial water lying between the Nafion beads, while the downfield resonance at 5.35 is assigned to adsorbed water inside and around Nafion beads. The integrated area under each resonance peak shows an intensity ratio of 7:3, interstitial-to-internal water, respectively. The fwhm line widths of the two resonances are roughly the same.
Figure 2.
A fully relaxed 1D 1HNMRspectra of Nafion bead/water system. Two water proton resonances are observed at 5.35 ppm and 4.61 ppm, each corresponding to internal and interstitial water, respectively.
To further characterize the observed water species in each system, longitudinal relaxation (T1) and self-diffusion coefficients were measured for the respective chemical shifts. Five samples were used for T1 relaxation and self-diffusion measurements to verify the robustness and consistency of the results.
T1 relaxation and diffusion coefficients of the two water proton resonances in cationic resin/water system are shown in Table 1. T1 relaxation for interstitial water identified by the peak at 4.63 ppm was measured to be 1.82 s, which is significantly shorter than the T1 relaxation time of bulk water at 298 K.18 The corresponding self-diffusion coefficient of interstitial water, 14.91 × 10−10 m2/s, was measured to be about 60% of the known diffusion coefficient of bulk water at 298 K, namely, 23.00 × 10−10 m2/s.19
Table 1.
Longitudvinal Relaxation (T1) Times and Self-Diffusion Coefficients of Water Species in a Cationic Exchange Bead/Water System
| δHOD (ppm) | T1 (s)
|
DHOD (1 × 10−10 m2/s) | |
|---|---|---|---|
| component 1 | component 2 | ||
| 6.00 | 0.532 | 1.511 | 6.47 |
| 4.63 | 1.82 | 14.91 | |
Corresponding results for the Nafion resin/water system are shown in Table 2. T1 relaxation of upfield peak at 4.61 ppm corresponding to interstitial water was 1.34 s at 298 K, indicating faster longitudinal relaxation than bulk water as in the case of the cationic resin/water system. The diffusion coefficient of interstitial water in the Nafion resin/water system was determined to be 16.91 × 10−10 m2/s, about 25% smaller than that of bulk water. In order to rule out radiation damping as the cause of the observed shorter T1 values, the same measurements were made with 300 and 500 MHz spectrometers, and relaxation times on the same order of magnitude were confirmed.
Table 2.
Longitudinal Relaxation (T1) Times and Self-Diffusion Coefficients of Water Species in a Nafion Bead/Water System
| δHOD (ppm) | T1 (s)
|
DHOD (1 × 10−10 m2/s) | |
|---|---|---|---|
| component 1 | component 2 | ||
| 5.35 | 0.190 | 2.053 | 5.39 |
| 4.61 | 1.34 | 16.91 | |
In both systems, the T1 curve-fitting procedure for the downfield water peak yielded rather poor results if only a single exponential recovery curve was employed, indicating that a single T1 component cannot explain the data accurately. In contrast, when a two-component fit was used, the same set of experimental data gave excellent fit statistics without the necessity to remove even a single data point. This indicates that there are two distinct relaxation times. Thus, both tables show a faster and a slower T1 component for the downfield peaks, which correspond to highly restricted water adsorbed on the hydrophilic sulfonate-group sites within the polymer matrix.
The self-diffusion coefficient of water inside the cationic resin matrix at 298 K was 6.47 × 10−10 m2/s. The self-diffusion coefficient of water inside the Nafion resin matrix at 298 K was slightly smaller, albeit on the same order of magnitude, 5.39 × 10−10 m2/s. The fact that both D values of internal water were significantly lower than that of bulk water is self-consistent with the observed shorter T1 values compared to that of bulk. At room temperature, T1 relaxation is mainly mediated by translational and rotational diffusion in a solution, and the relaxation efficiency is inversely proportional to the rate of diffusion. The shorter diffusion constants of both internal and interstitial waters are indicative of the restricted environments that the water molecules experience. Fundamental NMR relaxation theory of liquids indeed stipulates that the T1 relaxation times are correspondingly shorter than that of water in bulk in slow-diffusing environments.
The most notable finding in this study is that there is no bulk water present in both bead suspensions, as indicated by T1 relaxation and self-diffusion measurements. The adsorbed water in and around both Nafion and ion-exchange beads is shown to be anisotropic due to confined geometry within polymer matrix,20–22 and therefore it is expected that internal water has much slower self-diffusion and shorter T1 relaxation than bulk, indicating highly restricted molecular environment.
Interestingly, our experimental data show that the interstitial water as well has notably shorter T1 relaxation and smaller self-diffusion coefficient than bulk water. This was true in both experimental systems. In the case of the cationic resin system, maximal packing of 180 μm diameter spherical beads in face center cubic lattice-like arrangement would leave the interstitial region to be on the order of tens of micrometers in size. Assuming that the packing was not ideal and taking into account the variability in ion-exchange resin size, one can expect even larger dimensions of interstitial water region. For the Nafion system, this interstitial region is expected to be even larger due to the beads’ irregular shape and larger dimensions, and this is demonstrated by larger interstitial to internal water peak area ratio shown in Figure 2 versus Figure 1.
While such extensive effects of the surface on nearby water dynamics may seem counterintuitive, previously observed characteristics of the EZ near hydrophilic surfaces indicates that nearby water can take on a more stable form out to distances of tens of micrometers.11 Further, convergence of EZs extending from two neighboring nucleating surfaces within 100 μm, as in this case, has been observed. It is then reasonable to assume the interstitial water in both systems to be composed of mostly EZ water, and thus, the restricted water dynamics within the interstitial region observed here complements other known EZ characteristics.
T1 relaxation time and the self-diffusion coefficient of solutions can be profoundly affected by the solute that is dispersed in the medium, and the presence of trace impurities could in theory account for observed changes in T1 relaxation and diffusion coefficient of water. However, the 1H chemical shift spectrum of a given compound is a robust fingerprint of what is present in a solution. One may then state with reasonable certainty that there are no impurities of unknown origin in our experimental system, evidenced by the absence of any anomalous peaks present other than water proton peaks.
Invoking impurities as the cause of change in water dynamics also lacks theoretical rationale. No well-established theory of chemical shifts in solution NMR can account for the observed “multiple resonance” spectra of water found in two distinct sample preparations studied here, as due to the effect of “trace impurities” of unknown origin. Indeed, to our knowledge, there are no aqueous NMR spectra that exhibit anything other than a single bulk water NMR peak in and around 4.8 ppm at 298 K. This is true, independent of the kind of solute that is dissolved in water, ranging from small organic molecules to large biomolecules such as proteins or nucleic acids.
The only reports found in literature that had recorded such multiple resonances of water are associated with biological tissues or polymer systems that are quite similar to the ion exchange resins or Nafion that were used in this study.23–26 These studies, however, did not address the dynamics of water lying outside the polymers or tissues.
Thus, the unique dynamic properties of water presented in this study are evidently not due to the presence of impurities, but rather caused by the presence of hydrophilic surfaces. While chemical shift provides unique structural identification of a molecule, it does not report the possible existence of intermolecular interactions and the resulting long-range ordering of a solution system. In contrast, T1 relaxation and self-diffusion measurements do provide this crucial information, as shown here. In fact, although the chemical shift of EZ water is quite similar to that of bulk water, albeit the small difference of 0.2 ppm, the T1 and D values turn out to be clear identifiers of the more restricted and long-range coupled nature of EZ water.
Hence, the most interesting implications of this study may be that, in the presence of Nafion, resin beads, or other hydrophilic surfaces, water is readily organizing and changing its otherwise bulk dynamic properties extensively. The results of this study therefore underscore the organizational and dynamic complexity of water juxtaposed next to certain classes of surface.
EXPERIMENTAL SECTION
For all experiments, deionized water (Type I HPLC Grade (18.2 MΩ·cm)) was collected from a Barnstead D3750 Nanopure Diamond purification system. A 5% D2O/H2O (v/v) solution was used in all experiments, and was prepared by diluting 99.9% deuterium oxide (Cambridge Isotopes DLM-4-100) with deionized water.
Two types of polymer beads were used as EZ-nucleating surfaces. One was analytical-grade cationic ion-exchange resin, 180–425 μm diameter, in H-form (AG 50W-X8, Bio-Rad, CA) with cross-linking of 8% divinylbenzene (DVB). The other was Nafion, a sulfonated tetrafluoroethylene based fluoropolymer, with dimensions on the order of 300–500 μm in H-form (495786, Sigma-Aldrich, MO). Both types of polymer have abundant sulfonic acid sites, providing highly hydrophilic environments.
Beads were first hydrated in experimental solvent for 1 h to ensure sufficient level of hydration. Once hydrated, the beads were placed in a standard 5 mm OD NMR tube (WG-1241-7, Wilmad Glass, NJ) and left to settle in 100 mL of 5% D2O/H2O (v/v). For both types of bead systems, the final height of tightly packed beads was 3 cm as measured from the bottom of the tube.
The 1H NMR spectra were recorded on a 750 MHz Bruker AVANCE II NMR spectrometer equipped with a 1H {C,N} high-resolution triple resonance probe with Z-gradient. A spectroscopic standard sample of 1% sucrose in 10% H2O/D2O (v/v) solvent mixture was used to shim the magnet to optimal B0 field homogeneity prior to recording the spectra from the resin beads and Nafion beads. Raw data were collected with 8K time-domain points with a spectral width of 10 ppm centered at 5.0 ppm, recycle delay of 1 s, and acquisition time of 0.55 s. These were then Fourier transformed without zero filling.
Proper referencing of spectra was ensured by adding a trace quantity of DSS as external reference for one sample and the ensuing spectrum reference frequency was used for calibrating the 0 ppm in subsequent sample preparations. Five identical NMR sample tubes were prepared for repeating the measurements and establishing consistency of the results. The sample temperature was set to 298 K ± 0.1 K and the accuracy of this value at the sample was confirmed by measuring the chemical shift difference of the doublet of neat methanol and cross checking against published values.
Spin–lattice (T1) relaxation of water in the polymer/water system was measured using inversion–recovery (π−τ1−π/2) pulse sequence. Longitudinal magnetization was collected with 10 time delays varying from 1 ms to 25 s. A standard inversion recovery curve with three unknowns was fitted to the integrated volume of each resonance as a function of the delay. When a single T1-component fit exhibited a substantial number of outliers leading to poor χ2 statistics, a two-component fit was employed, leading to excellent fits with no outliers from the recorded set of data points.
Self-diffusion measurements of the two chemical shifts of water in polymer/water system were carried out using the stimulated spin–echo pulse sequence ((π/2) − g(δ) − (π/2) − Δ − (π/2) − g(δ)). The attenuation in spin–echo amplitude was recorded as a function of increasing gradient strength, g. In an isotropic system, where diffusion is unrestricted, the echo attenuation is given by the Stejskal–Tanner equation:
| (1) |
Here γ is the gyromagnetic ratio, δ and g are duration and amplitude of the magnetic-field gradient, respectively, Δ is the duration for phase encoding the diffusing magnetization, and D is the bulk self-diffusion coefficient of the solvent. D was computed from the slope obtained by fitting eq 1 as a function g2 to the echo amplitude decay. Prior to making measurements with the bead systems, the bulk self-diffusion coefficient of deionized water was measured at 298 K and confirmed to agree with literature values.
Acknowledgments
This study was supported by a grant from the NIH (5R01 GM093842).
References
- 1.Finney JL. Water? What’s so Special about It? Philosophical Transactions: Biological Sciences. 2004;359:1145–1165. doi: 10.1098/rstb.2004.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senadheera L, Conradi MS. Rotation and Diffusion of H2 in Hydrogen - Ice Clathrate by 1H NMR. J Phys Chem B. 2007;111:12097–12102. doi: 10.1021/jp074517+. [DOI] [PubMed] [Google Scholar]
- 3.Catalano JG, Fenter P, Park C. Water Ordering and Surface Relaxations at the Hematite (110)–Water Interface. Geochim Cosmochim Acta. 2009;73:2242–2251. [Google Scholar]
- 4.Noguchi H, et al. Interfacial water structure at polymer gel/quartz interfaces investigated by sum frequency generation spectroscopy. Phys Chem Chem Phys. 2008;10:4987–4993. doi: 10.1039/b807297n. [DOI] [PubMed] [Google Scholar]
- 5.Tian C, Ji N, Waychunas GA, Shen YR. Interfacial Structures of Acidic and Basic Aqueous Solutions. J Am Chem Soc. 2008;130:13033–13039. doi: 10.1021/ja8021297. [DOI] [PubMed] [Google Scholar]
- 6.Smith JD, et al. Energetics of Hydrogen Bond Network Rearrangements in Liquid Water. Science. 2004;306:851–853. doi: 10.1126/science.1102560. [DOI] [PubMed] [Google Scholar]
- 7.Henniker JC. The Depth of the Surface Zone of a Liquid. Rev Mod Phys. 1949;21:322–341. [Google Scholar]
- 8.Green K, Otori T. Direct Measurements of Membrane Unstirred Layers. J Physiol (London) 1970;207:93–102. doi: 10.1113/jphysiol.1970.sp009050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson FA, Sallee VL, Dietschy JM. Unstirred Water Layers in Intestine: Rate Determinant of Fatty Acid Absorption from Micellar Solutions. Science. 1971;174:1031–1033. doi: 10.1126/science.174.4013.1031. [DOI] [PubMed] [Google Scholar]
- 10.Zheng JM, Pollack GH. Long-Range Forces Extending from Polymer–Gel Surfaces. Phys Rev E: Stat Nonlinear, Soft Matter Phys. 2003;68:031408. doi: 10.1103/PhysRevE.68.031408. [DOI] [PubMed] [Google Scholar]
- 11.Zheng JM, Chin WC, Khijniak E, Khijniak E, Jr, Pollack GH. Surfaces and Interfacial Water: Evidence That Hydrophilic Surfaces Have Long-Range Impact. Adv Colloid Interface Sci. 2006;127:19–27. doi: 10.1016/j.cis.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Hyok Yoo DRB, Pirie CM, Hovakeemian B, Pollack GH. In: Water: The Forgotten Biological Molecule. Denis Le Bihan HF, editor. Pan Stanford Publishing; Singapore: 2011. [Google Scholar]
- 13.Levitt MH. Spin Dynamics: Basics of Nuclear Magnetic Resonance. Wiley; New York: 2002. [Google Scholar]
- 14.Duval FP, Porion P, Van Damme H. Microscale and Macroscale Diffusion of Water in Colloidal Gels. A Pulsed Field Gradient and NMR Imaging Investigation. J Phys Chem B. 1999;103:5730–5735. [Google Scholar]
- 15.Perrin JC, Lyonnard S, Guillermo A, Levitz P. Water Dynamics in Ionomer Membranes by Field-Cycling NMR Relaxometry. J Phys Chem B. 2006;110:5439–5444. doi: 10.1021/jp057433e. [DOI] [PubMed] [Google Scholar]
- 16.Topgaard D, Söderman O. Self-Diffusion in Two- and Three-Dimensional Powders of Anisotropic Domains: An NMR Study of the Diffusion of Water in Cellulose and Starch. J Phys Chem B. 2002;106:11887–11892. [Google Scholar]
- 17.Gottlieb HE, Kotlyar V, Nudelman A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J Org Chem. 1997;62:7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 18.Simpson JH, Carr HY. Diffusion and Nuclear Spin Relaxation in Water. Phys Rev. 1958;111:1201–1202. [Google Scholar]
- 19.Holz M, Heil SR, Sacco A. Temperature-Dependent Self-Diffusion Coefficients of Water and Six Selected Molecular Liquids for Calibration in Accurate 1H NMR PFG Measurements. Phys Chem Chem Phys. 2000;2:4740–4742. [Google Scholar]
- 20.Webber JBW, Dore JC, Strange JH, Anderson R, Tohidi B. Plastic Ice in Confined Geometry: The Evidence from Neutron Diffraction and NMR Relaxation. J Phys: Condens Matter. 2007:415117. doi: 10.1088/0953-8984/19/41/415117. [DOI] [PubMed] [Google Scholar]
- 21.Webber JBW, Anderson R, Strange JH, Tohidi B. Clathrate Formation and Dissociation in Vapor/Water/Ice/Hydrate Systems in SBA-15, Sol–Gel and CPG Porous Media, As Probed by NMR Relaxation, Novel Protocol NMR Cryoporometry, Neutron Scattering and Ab Initio Quantum-Mechanical Molecular Dynamics Simulation. Magn Reson Imaging. 2007;25:533–536. doi: 10.1016/j.mri.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Liu E, et al. Neutron Diffraction and NMR Relaxation Studies of Structural Variation and Phase Transformations for Water/Ice in SBA-15 Silica: I. The Over-Filled Case. J Phys : Condens Matter. 2006;18:10009–10028. [Google Scholar]
- 23.Gough TE, Sharma HD, Subraman N. Proton Magnetic Resonance Studies of Ionic Solvation in Ion-Exchange Resins 0.1. Sulfonated Cation-Exchange Resins. Can J Chem. 1970;48:917–923. [Google Scholar]
- 24.Lenk R, Bonzon M, Greppin H. Dynamically Oriented Biological Water as Studied by NMR. Chem Phys Lett. 1980;76:175–177. [Google Scholar]
- 25.OConnor PJ, et al. H-1 NMR characterization of swelling in cross-linked polymer systems. Macromolecules. 1996;29:7872–7884. [Google Scholar]
- 26.Howery DG, Shore L, Kohn BH. Proton Magnetic Resonance Studies of the Structure of Water in Dowex 50W. J Phys Chem. 1972;76:578–581. [Google Scholar]