Abstract
The spontaneously hypertensive rat (SHR) is a widely accepted rodent model of Attention Deficit/Hyperactivity Disorder (ADHD), and methylphenidate (MP) is a central nervous system stimulant that has been shown to have a dose-related positive effect on attention task performance in humans with ADHD. The current study was undertaken to compare SHR to its typical control strain, Wistar–Kyoto (WKY) rats, on the performance of a Visual Stimulus Position Discrimination Task (VSPDT) as well as of the responsiveness of the two rat strains to MP treatment. The rats were initially trained on the VSPDT, in which a light cue was presented randomly at three different cue-light intervals (1 s, 300 ms and 100 ms) over one of two levers, and presses on the lever corresponding to the light cue were reinforced with a food pellet. Once rats reached stable performance, the treatment phase of the study began, during which they received daily intraperitoneal (IP) injections of saline, 2 mg/kg, 5 mg/kg, and 10 mg/kg of MP in a randomized order immediately prior to being tested on the VSPDT. Baseline performance accuracy on the VSPDT did not differ between the groups. Furthermore, a striking strain dissociation was evident in the response of the two strains to treatment; VSPDT performance was substantially disrupted by the 5 and 10 mg/kg dose in the WKY rats but only mildly in the SHR rats. Response omissions were also increased only in WKY rats. Finally, both strains had increased locomotor activity in the operant chamber following MP treatment. These findings point to an important difference in response tendency to MP in the two strains that supports a view that a critical difference between these strains may suggest neurochemical and neuroadaptive differences associated with the behavioral impairments of ADHD.
Keywords: Attention deficit, Hyperactivity, Impulsive, Psychostimulant, Learning
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a frequently occurring neuropsychiatric disorder characterized by hyperactivity, impulsivity and inattention (Mannuzza et al., 1993), which begins in early childhood and, in many cases, persists into adolescence and adulthood (Kessler et al., 2006; Mannuzza et al., 1993). Epidemiologic data using comparable assessment instruments and definitions of the disorder indicate the prevalence to be approximately 5–8% worldwide (Kessler et al., 2006; Polanczyk et al., 2007; Resnick, 2005; Schneider and Eisenberg, 2006; Wilens et al., 2002).
Several rodent models of ADHD have been proposed (Sagvolden et al., 1992, 1993), using either different strains of animals selected from the general population (Puumala et al., 1996), animals reared in social isolation (Jones et al., 1991), or those exposed to a variety of environmental manipulations (Collins et al., 2004; Dell’Anna et al., 1993; Diaz-Granados et al., 1994; Holene et al., 1998). There are also different genetically-derived models (Mook et al., 1993; Myers et al., 1982; Sagvolden et al., 1992). In general, a valid animal model for ADHD should approximate the fundamental behavioral characteristics of ADHD (face validity), conform to a theoretical rationale for ADHD (construct validity), and predict aspects of ADHD genetics, neurobiology and therapeutic interventions (both behavioral and pharmacological), not previously examined in human populations (predictive validity) (Sagvolden et al., 2005).
One genetically-derived model, the spontaneous hypertensive rat (SHR) (Mook et al., 1993; Myers et al., 1982; Sagvolden et al., 1992) capitalizes on behaviors exhibited by a strain with a particular genetic mutation (i.e., SHR), which is then contrasted with the progenitor wild-type strain, the Wistar–Kyoto (WKY), as controls. SHR are more active than their WKY counterparts (Berger and Sagvolden, 1998; Hard et al., 1985; Hendley et al., 1985; Mook and Neuringer, 1994; Wultz et al., 1990), and tend to prefer immediate smaller rewards rather than delayed larger rewards (Mill et al., 2005). In addition, they display little reactivity to novel environments (Delini-Stula and Hunn, 1985; Hard et al., 1985; Sutterer et al., 1988) and have specific learning impairments such as that SHR require a longer time interval to learn a new task (Low et al., 1984) or tend to repeat particular sequences of ineffectual responses (Mook et al., 1993).
Despite the considerable appeal of this model, some concern has been raised that these apparent broad ranges of deficits may result from differences in overall response rates in operant tasks, specifically that the Wistar–Kyoto animals often used as the control subjects are hypoactive (Alsop, 2007). Furthermore, few studies have directly examined the SHR rat as a model for deficits in sustained attention in ADHD, leaving this aspect of the model underevaluated. The studies that report deficits in sustained attention, in some cases remediated with psychostimulants (e.g. Sagvolden and Xu, 2008), employed free-operant schedules of reinforcement that are highly influenced by changes in response tendency, especially during signaled extinction components which could readily be interpreted as impairments due to impulsivity rather than inattention (see Sagvolden, 2000). Only one report in the literature employed a sustained attention task that employed a visual signal detection procedure (the five-choice serial reaction time task, 5CSRTT) where response accuracy could be assessed independently of response tendency (van den Bergh et al., 2006). These authors reported no strain differences in response accuracy between SHR and WKY rats in any measures of baseline performance or in strain responsiveness to 0.1–10 mg/kg of MP. If this finding was found to generalize beyond one set of testing procedures, the apparent good construct and face validity of the SHR model as a model of the attention deficits in ADHD would be seriously undermined. Because of the importance of this issue for the SHR rat model of ADHD, the present work was conducted as an important systematic test of the replicability of these results in the 5CSRTT, to produce more confidence in the certainty of that finding.
Therefore, we employed another procedure for the examination of performance differences in a signal detection procedure (the Visual Stimulus Position Discrimination Task, VSPDT) in which the detectability of the visual stimulus varied within-session (Presburger and Robinson, 1999), and detection, motivation and motor influences could be dissociated. This procedure has a long history of use as a behavioral model of visual signal detection [(Blough, 1967; Bushnell, 1998; Nevin, 1964) for evaluation of various rodent models of attention] but also requires some degree of sustained attention across the session. One hypothesis that follows from the observation that humans with ADHD show enhanced distractability would be that (1) SHR would be less accurate and omit more detection responses than WKY rats in baseline signal detection and (2) that MP pre-treatment should demonstrate dose-dependent improvements in these aspects of performance. However, an alternative hypothesis, based on a previous report (van den Bergh et al., 2006), would be that the SHR and WKY strains would differ most in baseline performance on measures sensitive to differences in general activity. In this scenario, MP treatment may also fail to be restorative. While this alternative hypothesis would undermine the case for the SHR to serve as a comprehensive simulation of ADHD, it would also be useful in articulating and challenging some of the assumptions of the often made SHR vs. WKY comparison.
2. Methods
2.1. Subjects
Male adolescent SHR [n=9] and WKY [n=9] rats, approximately 5–6 weeks of age and 70–100 g in weight, were obtained from Charles River Laboratories (Wilmington, MA) for use in this study. Once the rats arrived they were individually housed for the remainder of the study in clear acrylic cages with wire covers under standard laboratory conditions (22±2 °C, 50±10% relative humidity). A 12 h/12 h light/dark cycle was used, with lights on at 0900 and off at 2100. All rodents received 20 g of food per day (Rodent Diet 5001, PMI, Richmond, Indiana, USA) and water was available ad-libitum. Experiments were conducted in conformity with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals (NRC, 1996) and Brookhaven National Laboratory Institutional Animal Care and Use Committee protocols.
2.2. Drugs
MP hydrochloride, racemic mixture; (Sigma, St. Louis, MO) was dissolved in physiological saline and injected intraperitonealy (IP) at the following doses: 2 mg/kg, 5 mg/kg, and 10 mg/kg.
2.3. Apparatus
Six identical clear acrylic operant test chambers were used — each measuring 32×25×33 cm and enclosed in a sound-attenuation chamber (Coulbourn Instruments, Allentown, PA, USA). Cage floors were constructed of stainless steel horizontal bars spaced 1.2 cm apart. Test cages were equipped with a food trough located between two response levers (both active) with lights above each lever. Locomotor activity was assessed using an infrared monitor affixed to the ceiling of each cage as well as through direct observation by way of pin-hole video cameras connected to each testing cage. All test data were collected from the test chamber and recorded using Graphic State software. Lever presses, number of pellets dispensed, and locomotor counts were all recorded in each session. Each rat was run for one 30 minute session/day starting at 10:30am.
2.4. Procedures
2.4.1. The VSPDT
Rats were food-deprived to 85% of free-feeding body weight over a period of 7–10 days then trained to press the lever situated directly below a cue light after the light turned on in return for a 45 mg food pellet (Bioserv, NJ, USA). The light stayed on until rats pressed the correct lever appropriately. Rats were trained until they consistently made at least 80% correct lever presses within a 30 minute session, a threshold which typically took 2–3 sessions to achieve. After rats were trained they began a different protocol, in which they could again obtain the food pellet only by pressing the correct lever (i.e., when signaled by the cue light), but this time the light stayed on for only 1 s (Fig. 1a). When the animals reached greater than 80% accuracy in correct lever presses they were again moved to a new protocol, in which the light above one of the two levers would light up for different durations of time: 1 s, 300 ms, and 100 ms. The sequence of stimulus duration was randomized within-session. The accuracy criterion for 1 s cue responses was set at 80%, an intermediate value capable of optimally detecting enhancement of impairment of performance for 3 consecutive days before the rats could begin to receive drug [and the intertrial interval (ITI) was fixed at 10 s].
Fig. 1.
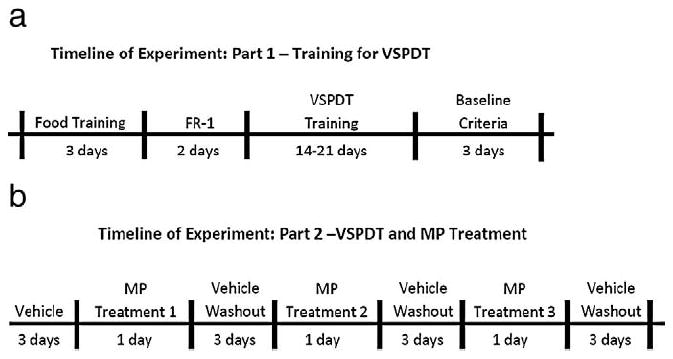
Timeline of experiment. The duration of the food and task training including the days when animals’ baseline performance was recorded are shown on the upper panel. The duration of the treatment phases for the low, medium and high doses are shown on the lower panel.
2.4.2. MP treatment and the VSPDT
Once the performance criteria were reached, animals received a saline treatment for 3 consecutive days to evaluate performance in a drug-free state to overcome potential performance disruptions due to injection treatment. In the treatment phase the animals were treated with three different concentrations of MP for 1 day separated by 3 days of saline washout. The treatment order was randomly assigned using a Latin square design (Fig. 1b).
2.5. Measures of behavioral performance and statistics
We examined the following behavioral measures: i) Response accuracy: Response accuracy has been shown to be a function of two factors (Echevarria et al., 2005). The first is a stimulus property, discriminability, which is demonstrated by decreased baseline accuracy as the stimulus duration is decreased. The second property of the response accuracy measure is in detecting subtle changes in motivational state, in that it has been shown that reward devaluation procedures reduce accuracy at the short stimulus durations (100 and 300 ms). ii) Response Omissions: Baseline response omissions increase as stimulus duration is decreased, indicating some sensitivity to stimulus discriminability. However, the experimental devaluation of the reinforcer results in a strong increase in omissions at all stimulus durations, indicating that this measure is primarily sensitive to the motivational aspect of sustained performance, including direct (e.g. change in deprivation conditions) and indirect (e.g. enhanced motility) effects on reward value. iii) Impulsive responding unrelated to task performance: This measured intertrial interval (ITI) responses and is indicative of failure of non-consequential response suppression during signaled non-reinforcement periods. iv) Locomotor activity (i.e., total number of beam breaks detected during a session): Given the familiarity of the environment for the animals after the extensive pretraining, this measure is probably best indicating general arousal level of the animals during the session.
Measures were initially examined using three-way repeated measures ANOVA with treatment condition, stimulus duration, and strain as independent variables. For brevity, the results are reported only for significant main effects and interactions of theoretical interest. The Huynh–Feldt reduction in degrees of freedom was applied to adjust for violation of assumptions in the repeated measures ANOVA. Matched sample t-tests were used to follow up on significant ANOVA results of interest, and where significant are reported in the figures. All data were analyzed using SPSS software (SPSS Inc. Chicago, IL, USA). Specific planned t-tests of each measure were also performed comparing strains for baseline levels.
3. Results
3.1. Signal detection accuracy
The strains did not differ significantly [F(1,16)=2.607, p>.05] when compared in the saline treatment only, but there was a significant difference in cue interval [F(2,32)=89.437, p<.0005; Fig. 2.1], reflecting more correct responses in longer intervals. There was also a significant interaction [F(2,32)=6.944, p=.01] reflecting stronger cue interval differences in SHR [F(2, 16)=53.951, p<.0005] than WKY [F(2,16)=36.314, p<.0005]. Within-session performance accuracy did not differ between the two strains (Fig. 2.2).
Fig. 2.
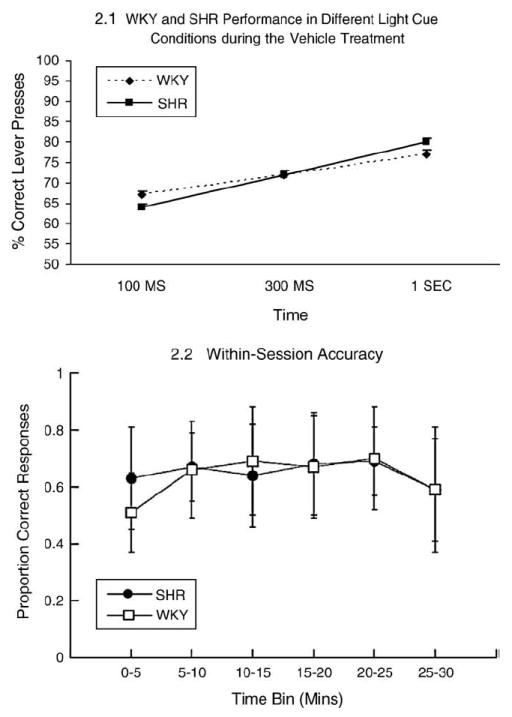
(2.1)WKY and SHR performance in different light cue conditions during the vehicle treatment. The three light cues (100 ms, 300 ms, 1 s) are shown on the X axis and the percent correct responses are shown on the Y axis. The two line graphs represent the choice accuracy for SHR and WKY rats as a function of stimulus cue duration. No difference was evident between the groups in the baseline condition. (2.2) Within-session data comparing SHR and WKY rats at the 100 ms signal for the saline treatment session prior to drug treatment. A slight warm-up effect and end-of-session drop off effect is revealed, but no differential accuracy between the two groups at the final session blocks, as might be predicted if the two groups differed in vigilance or fatigue.
When all treatments were considered (Fig. 3), there were significant main effects of strain [F(1, 16) =60.183, p<.0005], reflecting more correct responses in SHR than WKY, stimulus duration [F(1.367, 21.872) =103.600, p<.0005], reflecting more correct responses in longer intervals, and treatment condition [F(2.636, 42.176)=40.011, p<.0005], reflecting fewer correct responses with higher MP doses. There were significant interactions for cue interval X strain [F(1.367, 21.872)=4.811, p<.034] and treatment X strain [F(2.636, 42.176)=26.780, p<.0005], reflecting stronger differences in both for WKY than SHR. There was a non-significant trend for the interaction of strain X cue interval X treatment [F(4.795, 76.728)=1.974, p=.095], reflecting a significant cue interval X treatment interaction for WKY [F(2.78, 22.26)=37.257, p<.0005] but not for SHR.
Fig. 3.
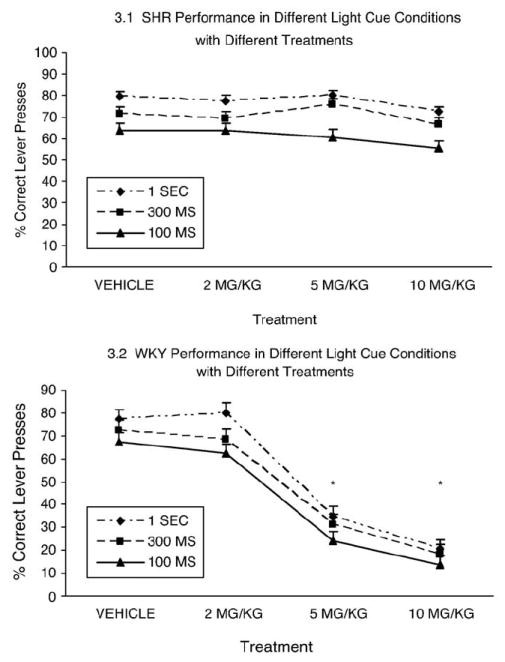
WKY and SHR correct lever presses in different light cue conditions with different treatments. The four dose treatments (vehicle, 2 mg/kg, 5 mg/kg, 10 mg/kg) are shown on the X axis and the percent correct responses are shown on the Y axis. The line graphs represent the correct lever presses during the three light cues (100 ms, 300 ms, 1 s) for SHR (3.1) and WKY (3.2) rats. MP treatments with 5 mg/kg and 10 mg/kg produced pronounced choice accuracy deficits at all stimulus duration for the WKY rats (lower panel) but only mild impairment for the SHR rats (top panel).
* Annotates significant change from performance in vehicle condition
3.2. Response omissions
There were significant main effects (Fig. 4.1) of strain [F(1, 16)= 82.310, p<.0005], stimulus duration [F(1.709, 27.351)=4.937, p<.019] and treatment [F(2.076, 33.210)=19.379, p<.0005]. The two way interaction was significant for treatment X strain [F(2.076, 33.210)= 18.650, p<.0005]. No significant strain X cue interval, cue interval X treatment, or strain X cue interval X treatment interactions were detected. Separate analyses by strain also revealed a significant main effect of treatment, but only for WKY rats [F(2.194, 17.550)=19.339, p<.005]. There were no significant main effects of cue interval for either strain, and no significant cue interval by treatment interaction.
Fig. 4.
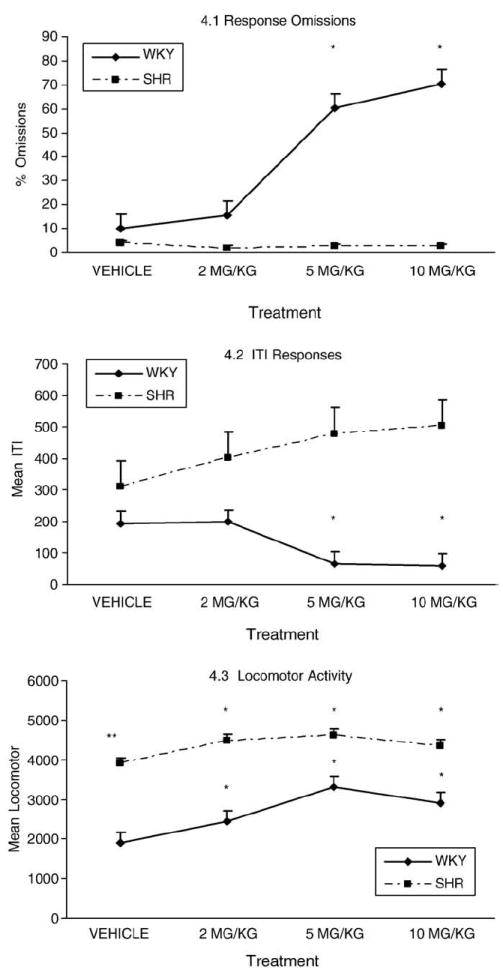
Behavioral measures for WKY and SHR with different treatments. 4.1. Response omissions. The four dose treatments (vehicle, 2 mg/kg, 5 mg/kg, 10,g/kg) are shown on the X axis. The percent omissions are shown on the Y axis. MP treatments with 5 mg/kg and 10 mg/kg produced marked increase in response omissions for the WKY rats but not for the SHR rats. Due to the fact that the omission curves for each strain were very similar across light cue conditions, the four treatments were collapsed in a single line graph for each strain. 4.2. ITI responses. The four dose treatments (vehicle, 2 mg/kg, 5 mg/kg, 10 mg/kg) are shown on the X axis. The ITI responses are shown on the Y axis. The MP treatments with 5 mg/kg and 10 mg/kg produced significant decreases in non-reinforced ITI responses for the WKY rats and non-significant increase for the SHR rats. 4.3. Locomotor activity. The four dose treatments (vehicle, 2 mg/kg, 5 mg/kg, 10,g/kg of MP) are shown on the X axis. The locomotion measures are shown on the Y axis. MP treatments magnified the baseline activity difference between the two stains represented by the line graphs for the WKY and the SHR rats.
* Annotates significant change from performance in vehicle condition
** Annotates significant difference between locomotor activity between WKY and SHR vehicle condition
The significant findings from between-treatment comparisons are summarized in Fig. 4.1, and show that in all three stimulus durations the treatment effect for WKY was larger than for SHR (larger increase of number of omissions with higher dose treatments).
3.3. Intertrial interval responses
There was a significant main effect (Fig. 4.2) for strain [F(1, 16)= 12.173, p<.003] but not treatment. There was also a significant interaction for strain X treatment [F(2.482, 39.718)=8.529, p<.005]. Within-strain analyses revealed that the effect of treatment was significant for WKY [F(3, 24)=13.600, p<.005] but not SHR rats. Similarly, pair-wise comparisons in SHR rats showed no significant difference in ITI by treatment condition. In contrast, in WKY the higher dose treatments (5 and 10 mg/kg) significantly decreased the intertrial interval responses compared to both vehicle and the 2 mg/kg treatment. However, significant differences in WKY performance should be interpreted with caution since high dose treatments also produced an overall decrease in absolute number of responses to all light cues (i.e. response omissions).
3.4. Locomotor activity during VSPDT
There were significant main effects (Fig. 4.3) for both strain [F(1, 16) = 42.967, p<.0005] and treatment [F(2.593, 16)=14.727, p<.0005], but there were no significant strain X treatment interactions. Overall, SHR rats exhibited higher mean locomotor activity than WKY rats at baseline (t=6.238, df=16, p<.0001). Within-strain analyses revealed significant main effects of treatment for both SHR [F(2.182, 17.452)=8.446, p<.002] and WKY [F(2.481, 19.844)=8.769, p<.002] rats. Pair-wise comparisons within the SHR group found a significant increase in locomotor activity with the 2, 5 and 10 mg/kg doses compared to saline. For WKY, all treatments also increased locomotor activity compared to saline. The data also indicates that treatment condition produced similar patterns of change for both strains (see Fig. 4.3). All three treatments increased locomotor activity for both strains compared to vehicle, but not in a dose-linear fashion; the 5.0 mg/kg dose produced a higher magnitude of increase than both the 2 and 10 mg/kg dose treatments.
4. Discussion
The present data are strongly consistent with an interpretation of the baseline differences between the SHR and WKY as being primarily limited to motor parameters but not signal detection accuracy/sustained attention. Furthermore, they reveal a strong differential sensitivity of the WKY rats to MP treatment in motor and sustained attention parameters of the VSPDT task. These are discussed in detail below.
4.1. Baseline performance of SHR and WKY
Baseline performance (e.g. vehicle condition) on the VSPDT showed no significant differences on response accuracy in the most demanding (and thereby revealing) 300 and 100 ms light stimulus durations, and on intertrial interval responses at all three cue durations (Fig. 2.1) as well as within-session accuracy patterns (Fig. 2.2). These findings do not support strain-specific differences between SHR and WKY rats on sustained attention and are consistent with those reported previously using the five-choice serial reaction time task (van den Bergh et al., 2006) and a simple visual discrimination procedure (Sagvolden and Xu, 2008). However, this later report, that also used a sustained attention test that required detection (and remembering) the position of an unsignaled concurrent random interval 180 s-extinction schedule, showed poorer performance in SHR than WKY rats. The extending periods on non-reinforcement of this test may have been a critical difference. Taken together, these findings suggest that frequent cues and serve to reduce the potential for distraction of the SHR rats, and that it is under conditions of extended periods where attention is required but little change in environmental conditions occur that their deficits are best revealed (Alsop, 2007).
4.2. MP effect on SHR and WKY performance on VSPDT
In contrast to the limited baseline differences, MP treatment revealed striking dissociations of effects on several measures. Overall, the SHR rats were much less sensitive to MP treatment than WKY rats in the choice accuracy and response omissions measures. SHR rats were only mildly impaired in response accuracy compared to the WKY rats, which showed pronounced impairments at both 5.0 and 10.0 mg/kg MP doses at all retention intervals. It certainly could be argued that if one assumed that baseline choice accuracy performance and response omissions were at a performance ceiling at the time of drug treatment, then this lack of a MP-induced impairment in choice accuracy and response omissions represents a positive validation of the effectiveness of MP on SHR. While this argument may apply to the omissions measure, Sprague–Dawley rats given a more extended period of pretraining in this exact procedure achieve choice accuracy levels at least 10–15% higher than the present animals who were deliberately trained only to a more modest performance criterion prior to treatment. Therefore, it seems reasonable that at least an improvement in choice accuracy could have been detected. This argument suggests that it would be worthwhile to further evaluate the SHR vs. WKY strains in behavior paradigms that place high demands for sustained cognitive load, studies that are not present in the literature.
Both strains showed modestly enhanced locomotion, but this only added to the baseline difference between the strains and did not reveal an interaction. MP treatment enhanced the difference between the two strains in non-functional ITI responses. The direction of this effect was interesting, as it was the WKY rats that emitted fewer ITI responses. Taken together with the pronounced increase in omissions and decreased accuracy, these data suggest that the overall effect of MP treatment on the WKY was to reduce their engagement (or alternatively, enhance their distractability) in all aspects of the task. Since their overall activity remained constant under MP treatment, it suggests that this loss of engagement was fundamentally the result of a MP-induced loss of reinforcer efficacy (i.e. motivational rather than motor).
4.3. Limitations
A few significant points to be considered in this study as limitations and opportunity of further study include the following. First, the rats were socially isolated for this study from the start as well as restricted to 20 g of food per day. Since these are stressors the results presented here between strains could also be related to differences in sensitivity of each strain to such stress. Second, while operant paradigms such as this are unlikely to invoke anxiety, given the length of pretraining, it is possible some of the differences in activity and drug sensitivity are the result of differences in levels of anxiety-like behavior between the two strains. SHR behaviors possibly related to lower levels of anxiety have been mostly assessed through open field paradigms that measure the level of exploration and activity (Howells et al., 2009). The one measure from our experiment that may be considered an indicator for different levels of anxiety is the measure of locomotion. However, since the graphs that depict locomotion for the 2 strains change in parallel (Fig. 4.3), suggesting that any baseline differences in anxiety-like behavior are not modulated by the drug treatment. Third, the rats were treated using a Latin square design which while having many advantages in that each animal serves as its own control there is also a limitation that must be acknowledged. While rats were treated with MP and separated by 3 days of vehicle washout there may have been some sensitization taking place (even though the MP doses were presented in random order) and SHR and WKY rats may be differently sensitive to this phenomenon. Fourthly, since the WKY rats are themselves a strain derived from a wild-type progenitor, some limitation exists in the ability to generalize our results to other more commonly employed strains such as Sprague–Dawley and Long–Evans. Finally, it is also worth noting that it is possible that the mild performance deficit of the SHR rats compared with WKY rats at the 100 ms stimulus duration (Fig. 2) may reflect a small decline in sustained attention over the course of the session.
4.4. General discussion
Overall, our findings clearly indicate that the SHR strain, when evaluated on a task that measures multiple aspects of sustained operant signal detection performance, show strikingly different behavior patterns as compared to a background control strain in both baseline and drug responses. The first theoretical context in which to consider these findings is one presented by Sagvolden, Russell and colleagues (e.g. Russell et al., 1995, 2000; Sagvolden and Sergeant, 1998). These authors argue that hypoactive mesolimbocor-tical dopamine in the SHR rat models a similar deficit in ADHD, which is ameliorated by MP treatment. In contrast, in WKY rats, MP, by producing enhanced synaptic dopamine in meso-striatal dopamine projections, may have disturbed natural (food) reward related neurotransmission resulting in the decreased reward sensitivity we observed. This dissociation provides a parsimonious mediator of this pronounced dissociation of response.
The second context in which to evaluate these findings is in the degree that the SHR can serve as a comprehensive model of ADHD. While clinical studies have shown that treatment with MP decreases hyperactivity and impulsivity and improves measures on attention in adults and children with ADHD (Epstein et al., 2006; McGough et al., 2005; Prince, 2006; Steinhoff, 2004) and that ADHD patients in general seem to exhibit a positive linear correlation between symptom improvement and dose increase (Vitiello et al., 2001), our results are clearly not consistent with this sort of simple rat to human correspondence view. However, to a large extent, this is a “straw man” in that no such claim is actually explicitly made in the literature, nor is necessarily achievable. If viewed in a more limited context, the present data further demonstrate the utility of the SHR vs. WKY comparison in evaluating the potential contributions of a single factor (sensitivity to the reinforcer) in an operant model, and support the more focused claim that the SHR serves as a useful simple system for the examination of the potential role of this one factor in ADHD (Sagvolden, 2000).
Acknowledgments
This work was supported by the NIAAA Intramural Research Program (AA11034, AA07574, and AA07611), and NIDA/AACAP K 23 (PA-00-003).
References
- Alsop B. Problems with spontaneously hypertensive rats (SHR) as a model of attention-deficit/hyperactivity disorder (AD/HD) J Neurosci Methods. 2007;162(1–2):42–8. doi: 10.1016/j.jneumeth.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Berger DF, Sagvolden T. Sex differences in operant discrimination behaviour in an animal model of attention-deficit hyperactivity disorder. Behav Brain Res. 1998;94(1):73–82. doi: 10.1016/s0166-4328(97)00171-x. [DOI] [PubMed] [Google Scholar]
- Blough D. Stimulus generalization as signal detection in pigeons. Science. 1967;158:940–1. doi: 10.1126/science.158.3803.940. [DOI] [PubMed] [Google Scholar]
- Bushnell P. Behavioral approaches to the assessment of attention in animals. Psychopharmacology. 1998;138:231–59. doi: 10.1007/s002130050668. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res. 2004;153(2):175–87. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Delini-Stula A, Hunn C. Neophobia in spontaneous hypertensive (SHR) and normotensive control (WKY) rats. Behav Neural Biol. 1985;43(2):206–11. doi: 10.1016/s0163-1047(85)91377-9. [DOI] [PubMed] [Google Scholar]
- Dell’Anna ME, Luthman J, Lindqvist E, Olson L. Development of monoamine systems after neonatal anoxia in rats. Brain Res Bull. 1993;32(2):159–70. doi: 10.1016/0361-9230(93)90070-r. [DOI] [PubMed] [Google Scholar]
- Diaz-Granados JL, Greene PL, Amsel A. Selective activity enhancement and persistence in weanling rats after hippocampal X-irradiation in infancy: possible relevance for ADHD. Behav Neural Biol. 1994;61(3):251–9. doi: 10.1016/s0163-1047(05)80008-1. [DOI] [PubMed] [Google Scholar]
- Echevarria DJ, Brewer A, Burk J, Manuzon H, Brown S, Robinson JK. Construct validity of an operant signal detection task for rats. Behav Brain Res. 2005;157:283–90. doi: 10.1016/j.bbr.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LE, Abikoff HB, et al. Assessing medication effects in the MTA study using neuropsychological outcomes. J Child Psychol Psychiatry. 2006;47(5):446–56. doi: 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Hard E, Carlsson SG, Jern S, Larsson K, Lindh AS, Svensson L. Behavioral reactivity in spontaneously hypertensive rats. Physiol Behav. 1985;35(4):487–92. doi: 10.1016/0031-9384(85)90128-3. [DOI] [PubMed] [Google Scholar]
- Hendley ED, Wessel DJ, Atwater DG, Gellis J, Whitehorn D, Low WC. Age, sex and strain differences in activity and habituation in SHR and WKY rats. Physiol Behav. 1985;34(3):379–83. doi: 10.1016/0031-9384(85)90199-4. [DOI] [PubMed] [Google Scholar]
- Holene E, Nafstad I, Skaare JU, Sagvolden T. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res. 1998;94(1):213–24. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- Howells FM, Bindewald L, Russell VA. Cross-fostering does not alter the neurochemistry or behavior of spontaneously hypertensive rats. Behav Brain Funct. 2009;5(24) doi: 10.1186/1744-9081-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, Robbins TW. Behavioural rigidity and rule-learning deficits following isolation-rearing in the rat: neurochemical correlates. Behav Brain Res. 1991;43(1):35–50. doi: 10.1016/s0166-4328(05)80050-6. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–23. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low WC, Whitehorn D, Hendley ED. Genetically related rats with differences in hippocampal uptake of norepinephrine and maze performance. Brain Res Bull. 1984;12(6):703–9. doi: 10.1016/0361-9230(84)90151-5. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50(7):565–76. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- McGough JJ, Biederman J, Wigal SB, Lopez FA, McCracken JT, Spencer T, et al. Long-term tolerability and effectiveness of once-daily mixed amphetamine salts (Adderall XR) in children with ADHD. J Am Acad Child Adolesc Psych. 2005;44(6):530–8. doi: 10.1097/01.chi.0000157550.94702.a2. [DOI] [PubMed] [Google Scholar]
- Mill J, Sagvolden T, Asherson P. Sequence analysis of Drd2, Drd4, and Dat1 in SHR and WKY rat strains. Behav Brain Funct. 2005;1:24. doi: 10.1186/1744-9081-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook DM, Neuringer A. Different effects of amphetamine on reinforced variations versus repetitions in spontaneously hypertensive rats (SHR) Physiol Behav. 1994;56(5):939–44. doi: 10.1016/0031-9384(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Mook DM, Jeffrey J, Neuringer A. Spontaneously hypertensive rats (SHR) readily learn to vary but not repeat instrumental responses. Behav Neural Biol. 1993;59(2):126–35. doi: 10.1016/0163-1047(93)90847-b. [DOI] [PubMed] [Google Scholar]
- Myers MM, Musty RE, Hendley ED. Attenuation of hyperactivity in the spontaneously hypertensive rat by amphetamine. Behav Neural Biol. 1982;34(1):42–54. doi: 10.1016/s0163-1047(82)91397-8. [DOI] [PubMed] [Google Scholar]
- Nevin J. A method for the determination of psychophysical functions in the rat. J Exp Anal Behav. 1964;7:169. [Google Scholar]
- Na NRC. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Presburger GRJ, Robinson J. Spatial signal detection in rats is differentially disrupted by delta-9-tetrahydrocannabinol,scopolamine, and MK-801. Behav Brain Res. 1999;99(1):27–34. doi: 10.1016/s0166-4328(98)00065-5. [DOI] [PubMed] [Google Scholar]
- Prince JB. Pharmacotherapy of attention-deficit hyperactivity disorder in children and adolescents: update on new stimulant preparations, atomoxetine, and novel treatments. Child Adolesc Psychiatr Clin N Am. 2006;15(1):13–50. doi: 10.1016/j.chc.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Puumala T, Ruotsalainen S, Jakala P, Koivisto E, Riekkinen P, Jr, Sirvio J. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiol Learn Mem. 1996;66(2):198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- Resnick RJ. Attention deficit hyperactivity disorder in teens and adults: they don’t all outgrow it. J Clin Psychol. 2005;61(5):529–33. doi: 10.1002/jclp.20117. [DOI] [PubMed] [Google Scholar]
- Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder—the spontaneously hypertensive rat. Brain Res. 1995;676(2):343–51. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- Russell VA, de Villiers AS, Sagvolden T, Lamm MC, Taljaard JJ. Methylphenidate affects striatal dopamine differently in an animal model for attention-deficit/hyperactivity disorder—the spontaneously hypertensive rat. Brain Res Bull. 2000;53(2):187–92. doi: 10.1016/s0361-9230(00)00324-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24(1):31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Xu T. l-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD) Behav Brain Funct. 2008;23(4):3. doi: 10.1186/1744-9081-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder—from brain dysfunctions to behaviour. Behav Brain Res. 1998;94(1):1–10. [PubMed] [Google Scholar]
- Sagvolden T, Metzger MA, Schiorbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58(2):103–12. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Pettersen MB, Larsen MC. Spontaneously hypertensive rats (SHR) as a putative animal model of childhood hyperkinesis: SHR behavior compared to four other rat strains. Physiol Behav. 1993;54(6):1047–55. doi: 10.1016/0031-9384(93)90323-8. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1239–47. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schneider H, Eisenberg D. Who receives a diagnosis of attention-deficit/hyperactivity disorder in the United States elementary school population? Pediatrics. 2006;117(4):e601–9. doi: 10.1542/peds.2005-1308. [DOI] [PubMed] [Google Scholar]
- Steinhoff KW. Attention-deficit/hyperactivity disorder: medication treatment-dosing and duration of action. Am J Manag Care. 2004;10(4 Suppl):S99–S106. [PubMed] [Google Scholar]
- Sutterer JR, McSparren J, Ingerman B. Auditory startle in normotensive and hypertensive rats. Behav Neural Biol. 1988;49(3):310–4. doi: 10.1016/s0163-1047(88)90306-8. [DOI] [PubMed] [Google Scholar]
- van den Bergh FS, Bloemarts E, Chan JS, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83(3):380–90. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Severe JB, Greenhill LL, Arnold LE, Abikoff HB, Bukstein OG, et al. Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. J Am Acad Child Adolesc Psych. 2001;40(2):188–96. doi: 10.1097/00004583-200102000-00013. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med. 2002;53:113–31. doi: 10.1146/annurev.med.53.082901.103945. [DOI] [PubMed] [Google Scholar]
- Wultz B, Sagvolden T, Moser EI, Moser MB. The spontaneously hypertensive rat as an animal model of attention-deficit hyperactivity disorder: effects of methylpheni-date on exploratory behavior. Behav Neural Biol. 1990;53(1):88–102. doi: 10.1016/0163-1047(90)90848-z. [DOI] [PubMed] [Google Scholar]


