Abstract
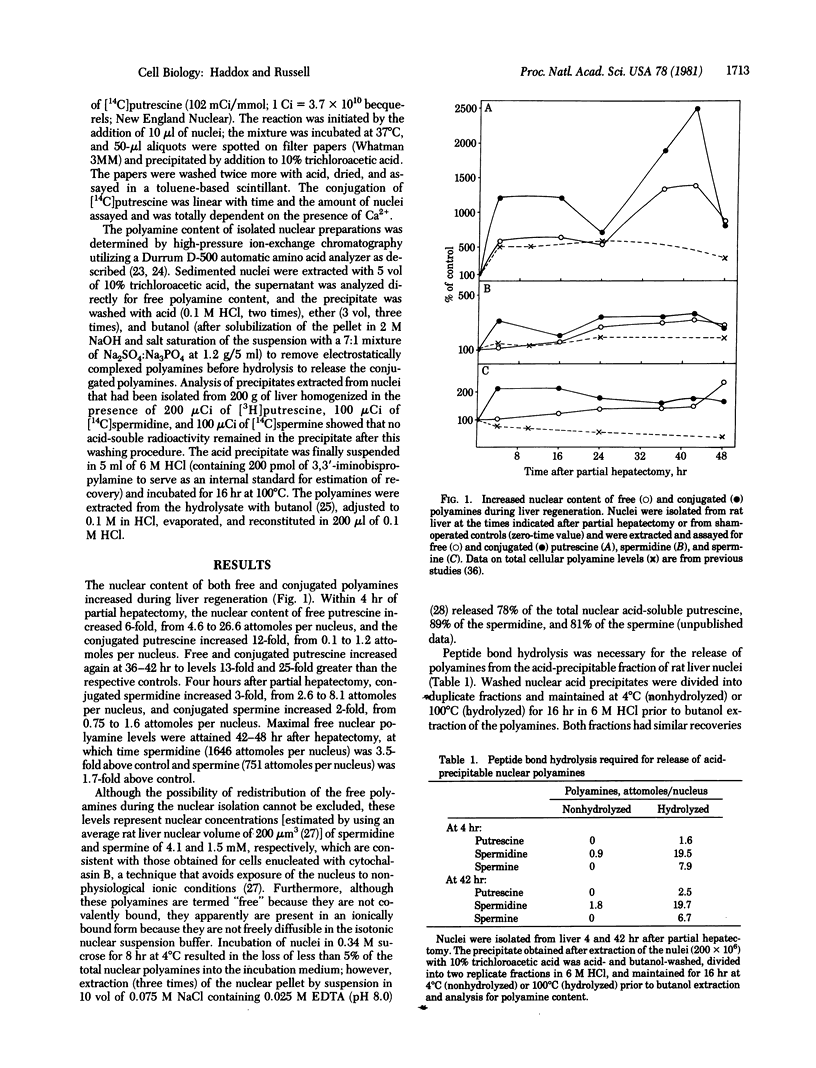
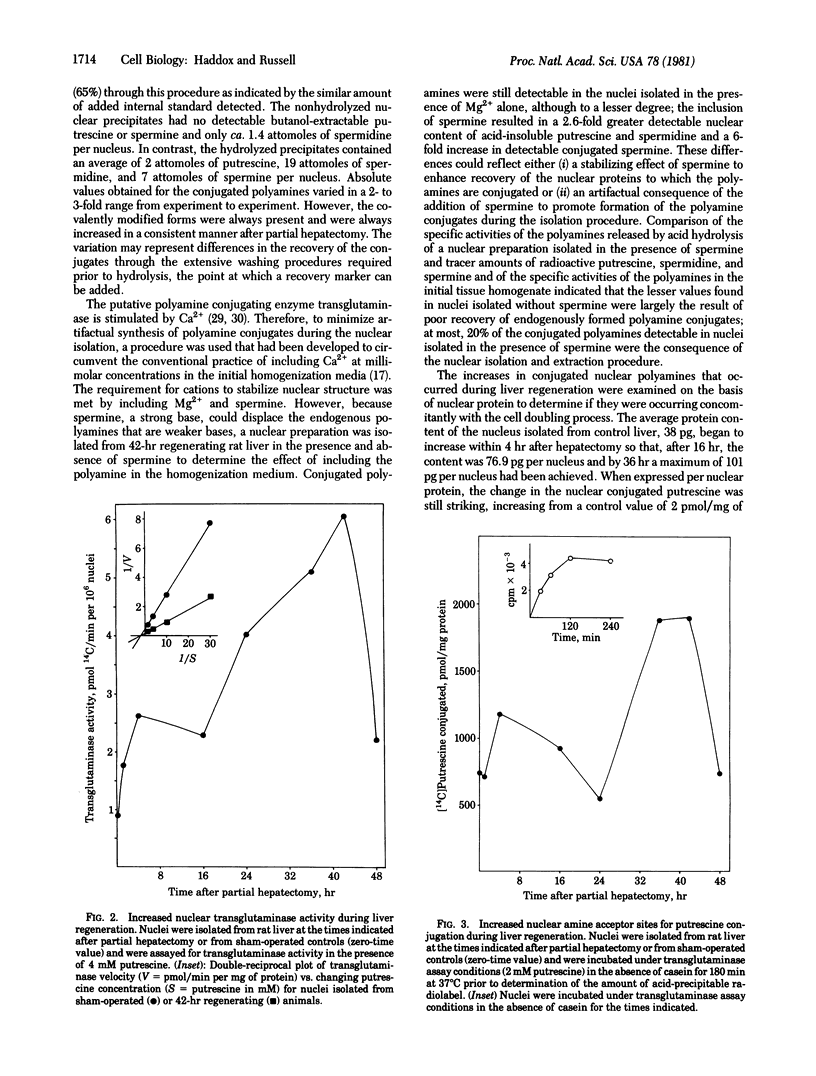
The nuclear content of conjugated polyamines increased during rat liver regeneration. Conjugated polyamines isolated from the acid-precipitable fraction of nuclei required peptide bond hydrolysis for release of the parent compounds. The most striking change occurred in conjugated putrescine which fluctuated in a biphasic manner; maximal nuclear levels 12-fold and 25-fold above those of sham-operated controls were achieved at 4 and 42 hr after hepatectomy, respectively. Conjugated spermidine and spermine increased 3- and 2-fold respectively within 4 hr and remained high throughout the 48 hr studied. When expressed on the basis of mg of nuclear protein, the maximal conjugated putrescine increased 19-fold, conjugated spermidine increased 2-fold, and conjugated spermine decreased by 50%. Therefore, the spermidine and spermine conjugates may be of a more constitutive nature whereas the large changes in the nuclear conjugation of putrescine associated with the onset of growth may play a regulatory role. The nucleus also contained transglutaminase (R-glutaminyl-peptide:amine gamma-glutamyl-yltransferase, EC 2.3.2.13), an enzyme shown in vitro to conjugate polyamines covalently to proteins. The specific activity of the nuclear enzyme increased rapidly after partial hepatectomy to a level 3-fold above control at 4 hr and 7-fold above control at 42 hr. The increased conjugating activity resulted from an increase in detectable maximal velocity and not a change in affinity of the enzyme for putrescine (Km congruent to 0.4 mM). There was also a 3-fold increase at 42 hr in the number of nuclear amine acceptor sites present to which radiolabeled putrescine could be conjugated by endogenous enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHER N. L. REGENERATION OF MAMMALIAN LIVER. Int Rev Cytol. 1963;15:245–300. doi: 10.1016/s0074-7696(08)61119-5. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Orr G. R., Conway E., Patterson M. K., Jr Transglutaminase activity in normal and transformed cells. Cancer Res. 1977 May;37(5):1340–1344. [PubMed] [Google Scholar]
- Birckbichler P. J., Orr G. R., Patterson M. K., Jr Differential transglutaminase distribution in normal rat liver and rat hepatoma. Cancer Res. 1976 Aug;36(8):2911–2914. [PubMed] [Google Scholar]
- Borowski E., Chmara H. The antibiotic edeine. 8. The mode of action of edeine A and B. Acta Microbiol Pol. 1968;17(3):241–248. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Busch H., Narayan K. S., Hamilton J. Isolation of nucleoli in a medium containing spermine and magnesium acetate. Exp Cell Res. 1967 Aug;47(1):329–336. doi: 10.1016/0014-4827(67)90235-2. [DOI] [PubMed] [Google Scholar]
- Cohen S. S. What do the polyamines do? Nature. 1978 Jul 20;274(5668):209–210. doi: 10.1038/274209a0. [DOI] [PubMed] [Google Scholar]
- Dunzendorfer U., Russell D. H. Altered polyamine profiles in prostatic hyperplasia and in kidney tumors. Cancer Res. 1978 Aug;38(8):2321–2324. [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Guarino L. A., Cohen S. S. Mechanism of toxicity of putrescine in Anacystis nidulans. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3660–3664. doi: 10.1073/pnas.76.8.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Cohen S. S. Uptake and accumulation of putrescine and its lethality in Anacystis nidulans. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3184–3188. doi: 10.1073/pnas.76.7.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HECHT L. I., POTTER V. R. Nucleic acid metabolism in regenerating rat liver. I. The rate of deoxyribonucleic acid synthesis in vivo. Cancer Res. 1956 Nov;16(10 Pt 1):988–993. [PubMed] [Google Scholar]
- HIEROWSKI M., KURYLO-BOROWSKA Z. ON THE MODE OF ACTION OF EDEINE. I. EFFECT OF EDEINE ON THE SYNTHESIS OF POLYPHENYLALANINE IN A CELL-FREE SYSTEM. Biochim Biophys Acta. 1965 Apr 19;95:578–589. [PubMed] [Google Scholar]
- Hettinger T. P., Craig L. C. Edeine. II. The composition of the antibiotic peptide edeine A. Biochemistry. 1968 Dec;7(12):4147–4153. doi: 10.1021/bi00852a001. [DOI] [PubMed] [Google Scholar]
- Hettinger T. P., Craig L. C. Edeine. IV. Structures of the antibiotic peptides edeines A1 and B1. Biochemistry. 1970 Mar 3;9(5):1224–1232. doi: 10.1021/bi00807a025. [DOI] [PubMed] [Google Scholar]
- Higashinakagawa T., Muramatsu M., Sugano H. Isolation of nucleoli from rat liver in the presence of magnesium ions. Exp Cell Res. 1972 Mar;71(1):65–74. doi: 10.1016/0014-4827(72)90264-9. [DOI] [PubMed] [Google Scholar]
- KURYLO-BOROWSKA Z. ON THE MODE OF ACTION OF EDEINE. EFFECT OF EDEINE ON THE BACTERIAL DNA. Biochim Biophys Acta. 1964 Jun 22;87:305–313. doi: 10.1016/0926-6550(64)90226-9. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L. Fibrinoligase: the fibrin-stabilizing factor system of blood plasma. Ann N Y Acad Sci. 1972 Dec 8;202:6–30. doi: 10.1111/j.1749-6632.1972.tb16319.x. [DOI] [PubMed] [Google Scholar]
- Marton L. J., Heby O., Wilson C. B., Lee P. L. An automated micromethod for the quantitative analysis of di- and polyamines utilizing a sensitive high pressure liquid chromatographic procedure. FEBS Lett. 1974 Apr 15;41(1):99–103. doi: 10.1016/0014-5793(74)80963-4. [DOI] [PubMed] [Google Scholar]
- McCormick F. Polyamine metabolism in enucleated mouse L-cells. J Cell Physiol. 1977 Nov;93(2):285–292. doi: 10.1002/jcp.1040930214. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Quittner S., Rubin A. L., Stenzel K. H. Transglutaminase activity in human lymphocytes: early activation by phytomitogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1157–1161. doi: 10.1073/pnas.75.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrode J., Folk J. E. Transglutaminase-catalyzed cross-linking through diamines and polyamines. J Biol Chem. 1978 Jul 25;253(14):4837–4840. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. The complete conversion of spermidine to a peptide derivative in Escherichia coli. Biochem Biophys Res Commun. 1970 Oct 9;41(1):232–238. doi: 10.1016/0006-291x(70)90493-6. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Isolation, characterization, and turnover of glutathionylspermidine from Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2648–2654. [PubMed] [Google Scholar]
- Takami H., Busch F. N., Morris H. P., Busch H. Comparison of salt-extractable nuclear proteins of regenerating liver, fetal liver, and Morris hepatomas 9618A and 3924A. Cancer Res. 1979 Jun;39(6 Pt 1):2096–2105. [PubMed] [Google Scholar]
- Taylor C. W., Yeoman L. C., Daskal I., Busch H. Two-dimensional electrophoresis of proteins of citric acid nuclei prepared with aid of a Tissumizer. Exp Cell Res. 1973 Nov;82(1):215–226. doi: 10.1016/0014-4827(73)90264-4. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Polyamines in mammalian biology and medicine. Perspect Biol Med. 1979 Spring;22(3):421–453. doi: 10.1353/pbm.1979.0013. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Wilson J., Beil R. E., Lorand L. Transglutaminase reactions associated with the rat semen clotting system: modulation by macromolecular polyanions. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1192–1198. doi: 10.1016/0006-291x(77)91132-9. [DOI] [PubMed] [Google Scholar]



