Abstract
Background
Evidence on economically efficient strategies to lower blood pressure (BP) from low- and middle-income countries remains scarce. The Control of Blood Pressure and Risk Attenuation (COBRA) trial randomized 1341 hypertensive subjects in 12 randomly selected communities in Karachi, Pakistan, to three intervention programs: combined home health education (HHE) plus trained general practitioner (GP); 2) HHE only; 3) trained GP only. The comparator was no intervention (or usual care). The reduction in BP was most pronounced in the combined group. The present study examined the cost-effectiveness of these strategies.
Methods and Results
Total costs were assessed at baseline and 2 years to estimate incremental cost effectiveness ratios (ICER) based on (a) intervention cost; b) cost of physician consultation, medications and diagnostics, changes in lifestyle, and productivity loss and (c) change in systolic BP. Precision of the ICER estimates was assessed by 1000 bootstrapping replications. Bayesian probabilistic sensitivity analysis was also performed.
The annual per participant cost associated with the combined HHE plus trained GP, HHE alone, and trained GP alone were $3.99, $3.34, and $0.65, respectively.
HHE plus trained GP was the most cost effective intervention with an ICER of $ 23 (6 to 99) per mm Hg reduction in systolic BP compared to usual care and remained so in 97.7% of 1000 bootstrapped replications.
Conclusions
The combined intervention of HHE plus trained GP is potentially affordable and more cost effective for BP control than usual care or either strategy alone in some communities in Pakistan, and possibly other countries in Indo-China with similar healthcare infrastructure.
Keywords: blood pressure, health care costs, health services research, cost effectiveness
Introduction
Cardiovascular diseases (CVD) have become the leading cause of death worldwide, and will particularly affect younger adults in developing countries, with major adverse consequences for national economies. Hypertension confers the highest attributable risk of death from CVD. 1
The burden of hypertension has reached epidemic proportions in Indo-Asian countries, affecting a third of adults in Pakistan. 2 However, public spending on health, especially chronic disease prevention, remains abysmally low in the face of competing priorities. 3 Private providers are the front line care givers and are visited frequently. However, serious deficiencies have been identified in their knowledge and practices with regards to the management of hypertension. 4 Furthermore, there are no national strategies for the control of hypertension or similar efforts to enhance care by private providers. As highlighted in the recent Institute of Medicine Report on Cardiovascular Diseases in low and middle income countries, advocating both public and private sector changes requires locally obtained relevant empirical evidence on sustainable, cost effective and affordable population-based strategies for the prevention and control of hypertension, which is scarce. 5Recently, we reported the results of large cluster randomized factorial design trial on community based strategies of home health education (HHE) and training of general practitioners (GPs) to control high blood pressure (BP). 6 We found that BP declined in all groups, but the reduction was most pronounced in those randomized to the combined group of both strategies than either or no intervention groups.
However, neither the cost nor the cost effectiveness of the interventions was reported. 5 This information is essential for policymakers and health planners as clinically effective interventions may not necessarily represent value for money compared to the alternatives studied in this resource-poor country. 7
We therefore conducted these analyses to determine 1) the cost of the interventions from the affordability perspective of the policymaker, 2) the incremental cost effectiveness of the total health care costs related to the interventions compared to usual care in terms of per mm Hg reduction in BP from the societal perspective. An intervention cost from a policymaker’s perspective within 10% of the existing national healthcare (government spending) budget was considered affordable. 8 The lowest incremental cost effectiveness ratio (most BP reduction at least total health care cost relative to usual care) that was also significantly different compared to usual care, ie bootstrapped 95% CIs also consistent with the mean value in direction and magnitude, was considered most cost-effective. We hypothesized that the combined HHE plus GP intervention would be more cost-effective than single interventions or usual care.
Settings and Patients
The Control of Blood Pressure and Risk Attenuation (COBRA) trial was a cluster randomized controlled trial with a 2×2 factorial design to determine the impact of family based HHE and/or special training of GPs on the BP levels of adults aged 40 years or above with hypertension (registration number NCT00327574, ClinicalTrials.gov). 6 The sampling frame and study design details have been described previously. 6 Briefly, multistage cluster sampling techniques were used to randomly select 12 communities (with about 250 households each) from middle to low income areas in Karachi. Individuals aged 40 years or over residing in the 12 clusters with known hypertension or with consistently elevated BP on two separate visits (mean of last two of three measurements of systolic pressure ≥140 mm Hg or mean diastolic pressure ≥90 mm Hg) were eligible for inclusion in the study. The 12 clusters were then randomly assigned to four groups of 3 clusters each: HHE, trained GP, HHE plus trained GP, and no intervention.
Home Health Education
Six community health workers (CHW) (one for each cluster) trained over six weeks provided advice at 3-monthly intervals on the importance of engaging in moderate physical activity; maintaining normal body weight; reducing salt intake; maintaining an adequate intake of potassium; and consuming a diet rich in fruit, vegetables, and low-fat dairy products and low in saturated and total fat (including sample recipes for culturally acceptable and economically feasible food products), and smoking cessation. 9 This intervention was modelled on the existing Lady Health Workers program in Pakistan. The details of training have been published previously. 6
General Practitioner Training
All GPs in the six study areas assigned to this intervention were invited for training, with the realistic aim to train at least two-thirds of all GPs from each area. The annual training was a one day session focused on standard treatment for the management of hypertension, based on the seventh report of the Joint National Committee (JNC 7) and the Fourth Working Party of the British Hypertension Society guidelines modified for the Indo-Asian population. 10,11
All study participants aged 40 years or above with hypertension were advised to consult a local GP. Those in the clusters randomized to the trained GP arm were given a list of trained GPs within their cluster. However, GP selection remained the choice of the participants. There was no provision for supplying medications, or reimbursing fee-for-healthcare services in the study. The participation by subjects and GPs was voluntary.
Screening and Recruitment
All households in each cluster were visited, and informed consent was obtained for screening from all adults aged 40 and above, who then underwent measurement of BP 3 times with a calibrated automated device (Omron HEM-737 IntelliSense; Omron Healthcare Inc., Vernon Hills, Illinois, US) in the sitting position after 5 minutes of rest. Those with an elevated BP and not on antihypertensive medication were visited again for re-measurement of BP 1–4 weeks after the initial visit. If mean BP remained elevated, these individuals were invited to participate. In addition, those with known hypertension were also invited to participate, irrespective of measured BP.
Hypertensive adults were evaluated by trained field staff, masked to randomization status, at baseline and 4-monthly intervals when three consecutive BP readings, were taken. Information on diet (food frequency and expenditure on fruits and vegetables), physical activity (international physical activity questionnaire), current antihypertensive medications, frequency of visits to health providers in the last two weeks, and expenditure on healthcare costs (provider fee, medications and laboratory costs), and any hospitalizations were collected. Information on days of work lost due to illness during this period was recorded. BP was measured as described above. The planned median duration of follow-up was 2 years.
Costs
We estimated the cumulative intervention costs over 2 years. These costs included personnel, equipment, and training material and supplies for development of curricula, and transport and other operational expenses over 2 years (Table 2). Personnel costs for the interventions included salaries and fringe benefits of faculty, a nutritionist (for HHE), and community health workers (seven full time for HHE and six part-time field workers for the trained GP group). To account for office rent and maintenance, an administrative cost of 30% was applied to intervention costs. Since one of the goals was to assess the affordability from policymaker’s perspective, and implications on public funds, only the intervention cost was used for this purpose The cost effectiveness analyses were performed from a societal perspective, so needed to account for total health care costs. These costs included the cost of intervention, patient costs on health care and related life style (diet), and productivity losses. The patient costs for health care included payments made by patients to the health care provider, the cost of purchasing medications and expenditure on diagnostics. Patient data on costs were collected from participants at baseline and final visit by questionnaire. The questionnaire was administered in the homes of respondents by trained interviewers. Participants (from all four groups) were asked to provide detailed information on any medical consultations, medications purchased, medical tests conducted and periods of hospitalization during the previous 2 weeks. These cost data were annualised to determine the direct annual healthcare cost for all four groups at baseline and final follow-up visit. (Table 3a) This rationale was consistent with our analysis of the primary effectiveness outcome of change in BP, as reported previously. 6 For the cost effectiveness analysis, the difference in annualised cost from baseline to follow-up for each group was projected over two years, with adjustment for inflation.
Table 2. Per participant Annual Intervention Cost.
| Characteristics | HHE and Trained GP | HHE Only | Trained GP Only |
No Intervention |
|---|---|---|---|---|
| Personnel* | 2.77 | 2.46 | 0.31 | - |
| Equipment† | 0.14 | 0.14 | - | - |
| Training supplies‡ |
0.22 | 0.14 | 0.08 | - |
| Transport | 0.38 | 0.37 | 0.01 | - |
| Indirect Cost§ | 0.47 | 0.23 | 0.24 | - |
| Total | 3.99 | 3.34 | 0.65 | - |
Three Assistant Professors at $1336 per person for 1 month, one nutrition consultant at 5% effort for $70 per month for 24 months; one nutrition trainer at 50% effort for $185 per month for 24 months; Seven community health workers at $ 123 per person per month over 2 years. In addition, eight workers for part-time work at $48.4 per person for one month. Two part-time chaperons at $34.6 per month.
Three computers for total $2594, one DVD player for $ 112; CDs for $10; for all three interventions
Training manuals and charts printing for $ 750 in groups receiving HHE and $1190 in those with trained GP, Office stationary and photocopies for $1743 in HHE and $333 in trained GP
Office Rent at $4358 (trained GP and HHE groups), Indirect Cost is 30% of all direct costs
All expenses are expressed in US $, valued of exchanges rate based on July 2007 (1 USD=Pak Rs 61)
Table 3a. Patient Level Cost for two years in US $.
| Characteristic | HHE and Trained GP (n = 332) |
HHE Only (n = 348) |
Trained GP Only (n = 335) |
No Intervention (n = 326) |
*p-value |
|---|---|---|---|---|---|
| On GP Consultations | |||||
| At baseline | 61.9 | 44.3 | 33.3 | 45.6 | 0.182 |
| At final visit | 16.3 | 12.0 | 19.4 | 17.7 | 0.202 |
| On Medication | |||||
| At baseline | 58.6 | 46.4 | 70.2 | 67.1 | 0.234 |
| At final visit | 162.2 | 107.4 | 133.7 | 117.5 | 0.021 |
| On Laboratory | |||||
| At baseline | 13.2 | 15.3 | 15.4 | 6.1 | 0.862 |
| At final visit | 40.7 | 9.0 | 37.1 | 45.6 | 0.071 |
| Total Expenses on Health Care | |||||
| At baseline | 133.7 | 106.0 | 118.8 | 118.8 | 0.303 |
| At final visit | 219.2 | 128.3 | 190.2 | 180.9 | 0.021 |
| On Fruits & Vegetables | |||||
| At baseline | 105.6 | 101.0 | 114.3 | 94.9 | 0.161 |
| At final visit | 81.8 | 82.2 | 98.0 | 86.7 | 0.132 |
| Total Patient Costs on Health included main analysis† | |||||
| At baseline | 241.2 | 196.3 | 229.1 | 206.2 | 0.295 |
| At final visit | 306.0 | 206.0 | 283.4 | 258.8 | 0.020 |
| Meat Consumption cost | |||||
| At baseline | 9.1 | 8.8 | 10.1 | 8.5 | 0.632 |
| At final visit | 6.7 | 7.8 | 10.0 | 8.2 | <0.001 |
| Smoking Cost | |||||
| At baseline | 0.5 | 1.0 | 1.0 | 1.0 | 0.201 |
| At final visit | 0.6 | 0.9 | 0.8 | 0.8 | 0.543 |
p-values are calculated based on raw data
Total patient cost s on health included main analysis comprised of GP consultation, medication, laboratory, fruit and vegetable expenses.
All expenses are expressed in US $, valued of exchanges rate based on July 2007 (1 USD=Pak Rs 61)
The cost of fruit and vegetables was also included in the societal aspect of life style modification. Information on fruit and vegetables, and meat purchased by the household during the previous 2 weeks was collected, and since interventions were delivered at the community level, the denominator for per-patient calculation was all subjects aged 5 years and above.
Following the recommendation of Gold et al on the importance of accounting for patient and caregiver’s time, values for productivity losses were imputed for the opportunity costs to participants and caregivers; irrespective of whether or not they were in paid employment. 12 We used the average market wage rate for men and women in urban Sindh (Government of Pakistan, 2009) as the value of time lost. 12,13 Productivity losses resulting from illness and treatment were imputed for participants and projected over 2 years.
Since BP screening by community health workers was common to all four randomized groups, the cost of screening was not considered in the comparative analysis. However, it was included in estimating the impact of up-scaling strategies on the national health budget, and for this it was assumed that the BP monitors would have a useful life of 5 years with no resale value after that.
All costs are reported in US $ at the 2007 rate. To control for inflation, all costs were inflated to prices in 2007, using the gross domestic product deflator of 7.7% for Pakistan. 14 The mean exchange rate for the year was used to convert costs from Pakistan rupees into US dollars. To adjust for time preference all costs and effects were discounted; following the International Society for Pharmacoeconomics and Outcomes Research ISPOR guidelines an annual discount rate of 5% over 2 years was used. 15,16
Effectiveness
The primary outcome measure for the study was the change in systolic BP from baseline to the last follow-up visit. The secondary outcome measure was the proportion of participants with controlled BP (<140 mm Hg for systolic BP and <90 mm Hg for diastolic BP) at the last follow-up visit.
Statistical Analysis
We compared the costs of each randomized intervention to the no intervention group on an intention- to- treat principle.
Incremental cost-effectiveness ratios were computed to compare the additional costs and effects of each intervention over the other 12 Uncertainty in the point estimate of the ICERs was investigated using a non-parametric bootstrap with 1000 replications varying the BP effects, while costs remained the same. 8 The bootstrap was used to estimate 95% confidence intervals (CI) around differences in ICERs between randomized groups and to produce scatter plots for the interventions on a cost-effectiveness plane. (Figure 1) 17,18
Figure 1.
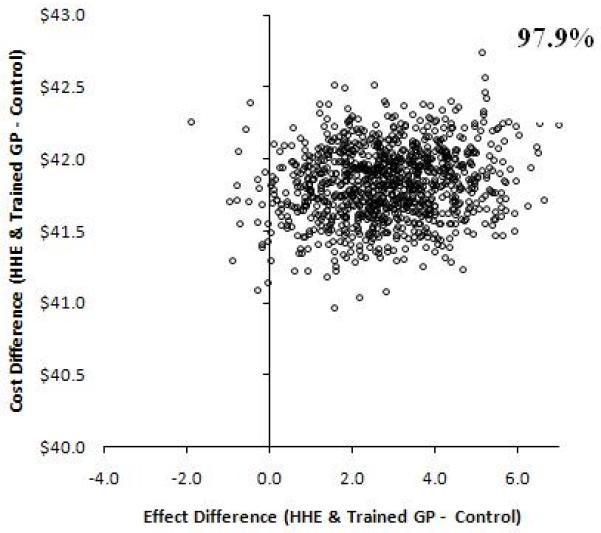
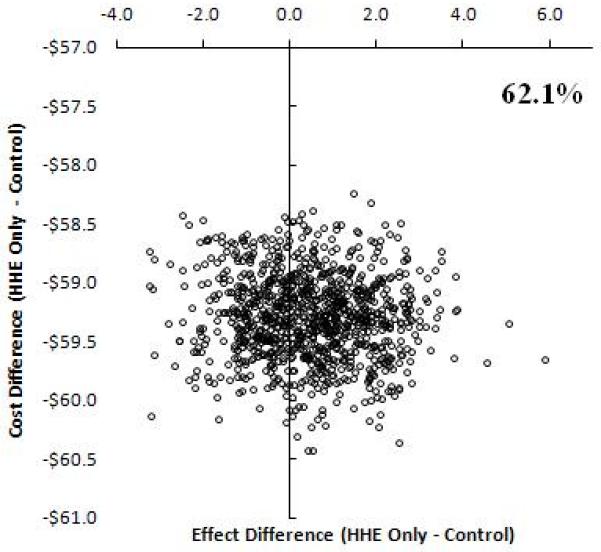
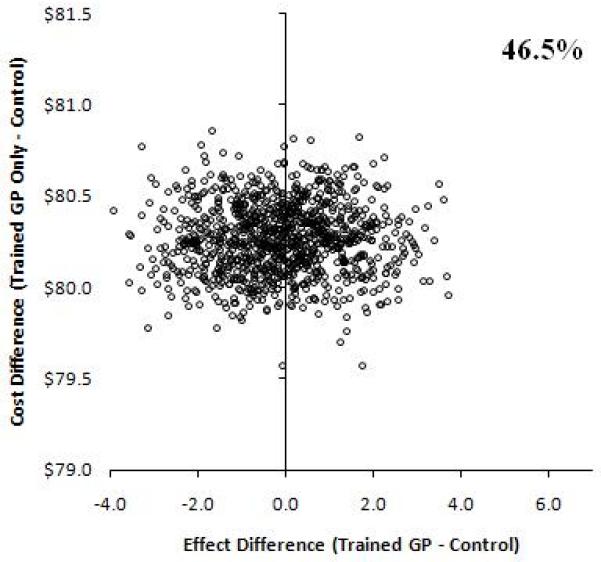
Incremental Cost-effectiveness plane at 5% discounting. Bootstrap replications showing the effect differences in change in blood pressure between randomized treatment programs versus usual care on the x-axis. An effect of larger than zero means greater reduction in BP in the treatment group. The proportions on the graphs indicate the percent of values out of the1000 replications for which effect difference was greater than 0. The y-axis shows the cost difference between intervention versus usual care. Positive values suggest the intervention is more expensive compared to usual care and vice versa.
Relative to the control group (usual care) at the origin, the incremental costs are divided across the horizontal axis of the plane (higher positive, lower negative) and the incremental effect by the vertical axis (higher to the right, lower to the left). Thus, each quadrant has a different implication for the choice of intervention.
Interventions with ICERs falling in the southeast quadrant would be more effective and less costly, and therefore always cost-effective relative to the comparator. Interventions with ICERs in the northwest quadrant would be less effective and more costly, and not cost-effective.
An intervention with ICERs falling in the northeast quadrant would be more effective but also more costly; consequently, a trade-off between costs and effects would need to be considered, as this would represent a situation where interventions may be cost-effective compared to usual care, depending upon the value at which the ICER is considered good value for investment
HHE=home health education; GP=trained general practitioner group.
Cost Per CVD DALY averted
To facilitate comparison with other programs for competing priorities, the most cost-effective of the alternatives considered was expressed in terms of the cost per disability adjusted life year (DALY) averted. The DALY is a generic measure of the burden of disease that combines healthy life years lost because of premature mortality with those lost as a result of disability. 19 The metric thus enables assessment of burden of disease, and is helpful for policymakers for resource allocation during health planning.
To assess the impact on the national health budget, the per participant cost of intervention was translated into per-capita cost for the population of Pakistan from the policymakers perspective. In addition, the per- capita cost per CVD DALY averted analysis was performed from both the policymakers’ and the societal perspectives.. Since the relationship between the fall in BP and the impact on CVD events is linear and well defined, a reduction in 5 mm Hg in BP was assumed to lead to 20% reduction in CVD DALYs. 20, 21,22The cost of one CVD DALY averted was computed for annual burden of 3,176,000 DALYs from CVD for Pakistan, as observed in GBD Study 2004. 23
Sensitivity Analysis
We undertook a number of sensitivity analyses to assess the impact of uncertainty on the estimates of cost-effectiveness. These included different discount rates of costs and values of SBP effectiveness, exclusion of productivity losses, and additional cost inputs including lifestyle factors such as smoking, expense related to meat consumption, and productivity loss of caregiver’s time while chaperoning GP visits, and exclusion of lifestyle related costs (ie fruit and vegetables). Probabilistic sensitivity analysis using Bayesian principles was conducted to assess simultaneous changes in all patient level variables involved in the costs and main effects. Monte Carlo simulation was performed to determine the mean costs, effects on BP, and ICERs among the treatment groups. We also computed ICERs for the primary outcome incrementally to compare the least costly with the next more costly intervention. 24
From the societal perspective the incremental cost per CVD DALY averted was also computed based on incremental cost estimates from the simulated models. In addition, CVD DALYs averted were computed for declining persistence of effect despite continued intervention assuming a reduction of 1% annually thus ranging from 20% to 10%; or a 50% reduction in benefit.
Finally, we also performed additional sensitivity analysis to estimate the cost effectiveness of the interventions in a high income country like the United States assuming similar effectiveness on blood pressure while applying US cost estimates. The latter was done by 1) converting all costs in Pakistan rupees to US international dollar equivalent corresponding to purchasing power parity for the year 2007 (1 US $=22.7 Pakistan Ruppee); and 2) specific replacement of itemized costs in Pakistan with US estimates related to GP consultation fee, personnel and health workers’ salaries, medications, and diagnostics which take into account the higher healthcare prices in the US. 25 The cost of one CVD DALY averted was based on the ICER for US cost data, and computed for an annual burden of 5,853,000 DALYs from CVD, as observed in the GBD Study 2004.
Results
Overall 1341 study participants were randomised: 332 to HHE plus trained GP, 348 to HHE only, 335 to trained GP only, and 326 to no intervention. The baseline characteristics of hypertensive adults in the randomized groups are shown in Table 1. A total of 1044 subjects (78%) completed 2 years of follow-up.
Table 1. Comparison Baseline Participant Characteristics by Interventions.
| Characteristic | HHE and Trained GP |
HHE Only | Trained GP Only |
No Intervention |
|---|---|---|---|---|
| Cluster characteristics | ||||
| Clusters, n | 3 | 3 | 3 | 3 |
| Households, n | 656 | 673 | 657 | 664 |
| Mean residents per household (SD), n |
6.5 (3.0) | 6.7 (3.0) | 6.6 (3.0) | 6.4 (3.1) |
| Number of GP’s | 61 | 61 | 58 | 69 |
| Patient characteristics | ||||
| Households, n | 278 | 283 | 281 | 275 |
| Hypertension, n | 332 | 348 | 335 | 326 |
| Mean age (SD), y | 54.0 (11.5) | 52.7 (11.4) | 55.3 (11.5) | 53.3 (11.5) |
| Men, n (%) | 112 (33.7) | 133 (38.2) | 138 (41.2) | 118 (36.2) |
| Education level, n (%) | ||||
| Illiterate | 131 (39.5) | 110 (32.8) | 151 (43.4) | 162 (49.7) |
| Primary or higher | 201 (60.5) | 197 (56.6) | 225 (67.1) | 164 (50.3) |
| Current Smoker, n (%) | 23 (37.7) | 36 (48.6) | 46 (54.8) | 37 (56.9) |
| Physical activity MET score ≥840, n (%)* |
96 (28.9) | 116 (33.3) | 96 (28.7) | 148 (45.4) |
| Low socioeconomic status, n (%)† | 232 (69.9) | 246 (70.7) | 188 (56.1) | 266 (81.6) |
| Sought ambulatory care in last two weeks, n (%) |
109 (32.8) | 113 (32.5) | 94 (28.1) | 105 (32.2) |
| Total # of GP visits in last two weeks |
278 | 279 | 201 | 287 |
| Employment Status, n (%) | ||||
| Currently Employed | 99 (29.8) | 107 (30.7) | 109 (32.5) | 92 (28.2) |
| Jobless | 9 (2.7) | 13 (3.9) | 13 (3.9) | 12 (3.7) |
| Housewives | 190 (57.2) | 199 (57.2) | 178 (53.1) | 191 (58.6) |
| Retired | 34 (10.2) | 29 (8.3) | 35 (10.4) | 31 (9.5) |
GP = general practitioner; HHE = home health education; MET = metabolic equivalent.
Calculated as total MET minutes/wk = walk (MET minutes × days) + moderate (MET minutes × days) + vigorous (MET minutes × days).
Monthly household income < $70 as reported by the Federal Bureau of Statistics.
Intervention and resource utilisation costs
The annual per participant intervention costs (i.e. from the perspective of the policymaker) varied substantially for the intervention groups: the most expensive being the joint intervention group, around 4 times higher than the GP intervention only group (Table 2).
The per participant expenditure on direct healthcare including physician consultation, medications and diagnostics at baseline and follow-up are shown in Table 3a. The use and associated cost of antihypertensive medications increased from baseline to follow-up in all groups; this increase was more pronounced in the groups with trained GPs (Table 3b). The decrease in expenditure on fruits and vegetables was most marked in the combined HHE plus trained GP group, probably reflecting a shift to low cost seasonal produce as advised. However, despite emphasis during GP training, that thiazide diuretics be the first-line agent for most hypertensive patients, their use remained low (less than 3% in all groups).
Table 3b. Use of Antihypertensive Medications by Intervention*.
| HHE and Trained GP (n=332) |
HHE only (n=348) |
Trained GP only (n=335) |
No Intervention (n=326) |
|
|---|---|---|---|---|
| At Baseline, % (95% CI) | 35.2 (30.3 to 40.5) | 39.7 (34.7 to 44.9) | 35.5 (30.6 to 40.8) | 40.5 (35.3 to 45.9) |
| At Follow-up, % (95% CI) | 49.7 (44.4 to 55) | 46 (40.8 to 51.2) | 46.3 (41 to 51.6) | 48.8 (43.4 to 54.2) |
| % Change (95% C.I) | 14.5 (11.1 to 18.6) | 6.3 (4.2 to 9.4) | 10.8 (7.9 to 14.5) | 8.3 (5.8 to 11.8) |
The analysis was performed at the patient level so use of more than one antihypertensive medication by a patient was counted as one.
Effectiveness
As reported previously, systolic BP declined in all four groups. The decline in systolic BP (adjusted for age, sex, and baseline BP) was significantly more pronounced in the HHE plus trained GP care group (11 mm Hg, p=0.001) as compared to the other groups (about 6 mm Hg in each). We also detected a significant interaction in the proportion of patients with controlled BP (P < 0.001) between the main effects of GP training and HHE. Thus, a substantially greater proportion of patients (56.9%) achieved controlled BP in the HHE and trained GP group than in the other groups (trained GP-only, 29.0%; HHE-only, 23.0%; no intervention, 27.3%; P for difference among groups <0.003). The mean per-capita expenditure by respondents on all healthcare over 2 years is shown in Table 4. While the direct costs for HHE plus trained GP were highest at $189, the reported productivity loss was greatest for the trained GP group. The expenditure on fruit and vegetables was also highest in the latter, in part responsible for the overall highest costs at $538 for the trained GP group.
Table 4. Cost and Cost Effectiveness by Intervention groups over two years in US $.
| Characteristic | HHE and Trained GP (n = 332) |
HHE Only (n = 348) |
Trained GP Only (n = 335) |
No Intervention (n = 326) |
*p- value |
|---|---|---|---|---|---|
| Patient level costs† | |||||
| On GP Consultations | 45.0 | 32.4 | 29.5 | 36.0 | 0.902 |
| On Medication | 116.0 | 81.3 | 108.6 | 98.7 | 0.862 |
| On Laboratory | 28.2 | 13.6 | 27.7 | 26.4 | 0.303 |
| Total Expenses on Health Care | 189.1 | 127.3 | 165.8 | 161.1 | 0.161 |
| On Fruits & Vegetables | 103.7 | 101.2 | 117.0 | 99.8 | 0.295 |
| Total Patient costs on health‡ | 296.5 | 219.8 | 278.1 | 252.1 | 0.129 |
| Productivity loss @ USD 5.72/- | 195.4 | 166.7 | 258.4 | 205.3 | 0.177 |
| Intervention Cost | 8.0 | 6.7 | 1.3 | - | |
| Mean SBP Effect (95% C.I) | 10.8 (8.9 to 12.8) |
5.6 (3.7 to 7.5) | 5.6 (3.7 to 7.4) | 5.8 (3.9 to 7.7) |
|
| Total Cost§ | 499.9 | 393.2 | 537.8 | 457.4 | |
| ICER∥ | 23 (6 to 99) | Dominated (dominated to 714) |
201 (dominated to 790) |
- | |
| 5% Discounting | |||||
| Mean SBP Effect (95% C.I) | 9.8 | 5.1 | 5.1 | 5.3 | |
| Total Cost § | 464.9 | 365.7 | 500.2 | 425.4 | |
| ICER∥ | 23 (7 to 101) | Dominated (dominated to 730) |
206 (dominated to 807) |
- | |
p-values are calculated based on raw data
Patient level costs computed based on average of inflated baseline and final visit.
Total patient costs on health included GP consultation, medication, laboratory, fruit and vegetable expenses.
Total cost included total patients costs on health, productivity loss and intervention cost
Incremental Cost-Effectiveness Ratio calculated based upon boot strapping
All expenses are expressed in US $, valued of exchanges rate based on July 2007 (1 USD=Pak Rs 61)
Incremental Cost effectiveness ratios
From a societal perspective, the combined intervention of HHE plus trained GP yielded a bootstrapped ICER of $ 23 (95% CI 6 to 99) per mm Hg reduction in systolic BP compared to no intervention (usual care) (Table 4). When discounted at 5% the combined intervention of HHE plus trained GP yielded an ICER of $ 23 (7 to 101) per mm Hg reduction in systolic BP compared to no intervention (usual care). Whether discounted or undiscounted the ICERs associated with the single interventions were less cost-effective (Table 4) as their confidence intervals were wide in magnitude and direction ranging from dominant to positive ICERs. Scatter plots of the bootstrapped ICERs illustrate these results (Figure 1).
Per-capita cost of intervention and cost per CVD DALY averted for Pakistan
Based on hypertension prevalence of about 18% in Pakistani adults aged 15 years and older 2 (and 60% of the population is in this age range) the estimated cost of annual combined intervention cost of HHE and GP training of $3.99 per participant translates into an annual per capita cost of $0.43 for the 170,000,000 population of Pakistan. 26 From a policymaker’s perspective, the HHE plus GP training intervention was estimated to cost $115 per CVD DALY averted. From a societal perspective, the incremental cost of the combined intervention versus usual care was $1226 per CVD DALY averted. Moreover, the additional cost of screening for BP would be about $0.06, thus cumulative per capita cost incurred to scale-up the combined HHE plus GP intervention and BP screening would be US$ 0.49.
Sensitivity analyses
Sensitivity analysis of the outcome parameter by varying change in systolic BP from the trial during computation of 95% limits of ICER confirmed the cost-effectiveness of HHE plus trained GP over the other interventions. Further, exploration of the implications of simultaneous alteration in costs and effects, application of discount rates of 3% and 10% (as recommended by ISPOR)1, additional and reduced costs, as well as application of US cost estimates, all revealed cost-effectiveness of combined HHE plus trained GP over trained GP, HHE or no intervention. (Table 5a). The cost per DALY averted for Pakistan increased from $115 to $230 at the policymaker level, and from $1226 to $2451 at the societal level with modelling declining persistence of cardiovascular benefit from 20% to 10%, respectively. (Table 5b)
Table 5a. Sensitivity Analyses.
| Characteristic | HHE and Trained GP (n = 332) |
HHE Only (n = 348) |
Trained GP Only (n = 335) |
No Intervention (n = 326) |
|---|---|---|---|---|
| Bayesian Probabilistic Sensitivity Analysis | ||||
| Mean SBP Effect (95% CI) mm Hg |
10.8 (8.9 to 12.8) | 5.6 (3.7 to 7.4) | 5.6 (3.7 to 7.5) | 5.8 (3.9 to 7.7) |
| Total Cost* | 499.9 | 393.2 | 537.8 | 457.4 |
| ICER† | 22 (7 to 104) | Dominated (dominated to 416) | 85 (dominated to 642) | - |
| Excluding productivity losses | ||||
| Mean SBP Effect (95% CI) mm Hg |
10.8 (8.9 to 12.8) | 5.6 (3.7 to 7.4) | 5.6 (3.7 to 7.5) | 5.8 (3.9 to 7.7) |
| Total Cost‡ | 304.5 | 226.5 | 279.4 | 252.1 |
| ICER† | 28 (8 to 123) | Dominated (dominated to 248) | 68 (dominated to 267) | - |
| 3% Discounting | ||||
| Mean SBP Effect mm Hg | 10.2 | 5.3 | 5.3 | 5.5 |
| Total Cost* | 477.4 | 375.5 | 513.6 | 436.8 |
| ICER† | 23 (7 to 100) | Dominated (dominated to 726) | 204 (dominated to 802) | - |
| 10% Discounting | ||||
| Mean SBP Effect mm Hg | 9.0 | 4.6 | 4.6 | 4.8 |
| Total Cost* | 434.9 | 342.1 | 467.9 | 398.0 |
| ICER† | 24 (7 to 104) | Dominated (dominated to 749) | 211 (dominated to 828) | - |
| Assuming increased use of thiazides by patients receiving care by trained GP groups§ |
||||
| Mean SBP Effect mm Hg | 10.8 | 5.6 | 5.6 | 5.8 |
| Total Cost§ | 459.3 | 393.2 | 499.8 | 457.4 |
| ICER† | 10 (3 to 46) | Dominated (dominated to 714) | 155 (dominated to 611) | - |
| Additional patient cost inputs | ||||
| On Meat consumption | 8.8 | 9.2 | 11.0 | 9.1 |
| On smoking | 0.6 | 1.0 | 1.0 | 1.0 |
| Mean SBP Effect mm Hg | 10.8 | 5.6 | 5.6 | 5.8 |
| Total Cost∥ | 313.9 | 236.7 | 291.4 | 262.2 |
| ICER† | 27 (7 to 116) | Dominated (dominated to 227) | 92 (dominated to 361) | - |
| Additional patient cost and productivity losses | ||||
| Productivity loss for care giver @ USD 5.72/- |
52.9 | 44.5 | 69.3 | 60.2 |
| Mean SBP Effect mm Hg | 10.8 | 5.6 | 5.6 | 5.8 |
| Total Cost# | 562.1 | 447.9 | 619.1 | 527.8 |
| ICER† | 17 (5 to 75) | Dominated (dominated to 883) | 248 (dominated to 973) | - |
| Excluding cost of fruit and vegetables | ||||
| Mean SBP Effect mm Hg | 10.8 | 5.6 | 5.6 | 5.8 |
| Total Cost** | 392.5 | 300.7 | 425.5 | 366.5 |
| ICER† | 13 (4 to 55) | Dominated (dominated to 755) | 143 (dominated to 558) | - |
| US costs based on purchasing power parity | ||||
| Mean SBP Effect mm Hg | 10.8 | 5.6 | 5.6 | 5.8 |
| Total Cost†† | 1346.2 | 1058.9 | 1448.4 | 1231.9 |
| ICER† | 62 (17 to 266) | Dominated (dominated to 1923) | 542 (dominated to 2127) | - |
| US costs based on individually modified estimates | ||||
| Mean SBP Effect mm Hg | 10.8 | 5.6 | 5.6 | 5.8 |
| Total Cost‡‡ | 11744.3 | 9140.0 | 12139.2 | 10943.2 |
| ICER† | 416 (113 to 1860) | Dominated (dominated to 19722) | 3011 (dominated to 11809) | - |
Total cost includes total patient costs on health, productivity loss and intervention cost as in the main model
Incremental Cost-Effectiveness Ratio calculated based upon boot strapping
The costs include total patient costs on health and intervention costs as in the main model.
Cost of medications reduced by a conservative estimate of 50% in 70% of patients on antihypertensive medications in HHE plus GP and trained GP only groups assuming they were on generic thiazide costing less than 50% of their current medication.
Additional patient cost inputs include cost of smoking cigarettes and expense of meat at baseline and two years of follow-up. All current smokers were assumed to smoke 1 pack per day priced at average market rate of most commonly sold brand in 2004 for baseline and in 2007 for follow-up. The actual expenditure on meat was recorded during the study.
Additional patient cost and productivity losses. In addition to the patient cost of cigarettes and meat, the productivity loss of the caregiver’s time was also accounted for.
Total cost includes consultation, medication, diagnostics, productivity loss and intervention cost.
1 international US = 22.65 Pakistan rupees in terms of purchasing power parity. All costs in rupees were multiplied by 22.7.
Total cost computed with the following multiplication factors 85 for GP consultation visits (assuming $70 per visit in the US (http://www.nber.org/papers/w14568.pdf) and applied to average of $0.73 per visit in the communities in Pakistan; 10 for academic faculty, 15 for medication costs assuming replacement with generics; 10 for diagnostic tests; 171 for productivity loss (based on average wage $885/week for 2007 in the US as per US Department of Labor Bureau of labor statistics Quarterly Census of Employment and Wages, Annual Averages 2007); 10 for community health workers’ salaries (assuming minimum hourly wage $ 7.75) and 2 for transportation (gas price $2 per/L in the US).
All expenses are expressed in US $, valued of exchanges rate based on July 2007 (1 USD=Pak Rs 61)
Table 5b. Sensitivity Analysis: Effect of decline in persistence of effect on CVD DALYs.
| Benefit in CVD DALYs saved annually |
Cost per CVD DALYs averted annually based on Intervention Cost for policy maker |
*Cost per CVD DALYs annually saved based on incremental cost at societal level |
*Cost per CVD DALYs averted annually based on incremental cost derived from probabilistic sensitivity analysis at societal level |
|---|---|---|---|
| 20% | 115 | 1226 | 1208 |
| 16% | 144 | 1532 | 1510 |
| 12% | 192 | 2043 | 2014 |
| 10% | 230 | 2451 | 2416 |
based on incremental total health care costs between combined HHE plus GP intervention versus usual care
The approach of comparing the least to the next more costly intervention (ie HHE compared with the combined intervention of HHE plus trained GP) yielded an ICER of $77.8 (19.9 to 275.8) per mm Hg reduction in BP, which translates into $ 3600 per CVD DALY saved.
From a societal perspective for the US, based on the prevalence of hypertension of 24% as observed in the third National Health and Nutrition Examination Survey (NHANES III) in adults aged 18 years and older 27 (and about 80% population in this age range), the estimated cost per DALY was I$5,770 for the international purchasing power parity cost replacement, and $40,466 per DALY averted for itemized replacement of costs.
Discussion
We found that the combined intervention of HHE by lay health workers and management by trained GPs was more cost effective for lowering BP compared to trained GP, HHE or no intervention in the communities in Karachi. The combined HHE plus GP group was the most cost-effective compared to other interventions, was associated with the bootstrapped ICER of $ 23 (6 to 99) per mm Hg reduction in systolic BP compared to usual care, and remained so in 97.7% of 1000 bootstrapped replications. According to the US Panel on Cost-effectiveness in Health and Medicine there are no absolute standards for accepting an intervention as “cost-effective” or “not cost-effective” on the basis of cost effectiveness ratios. 28 However, the incremental cost effectiveness ratio (ICER), offers a comparative estimate relative to the next best alternative, and is therefore more informative for decision making.
Our analyses indicate that from the policymakers’ perspective the combined HHE and trained GP intervention was the most costly (annual per participant $3.99) of the three interventions. (Table 2) However, the combined programme was also the most effective. On the other hand the HHE only intervention was costly (annual per participant $3.34) and less effective compared to no intervention; whereas, the trained GP only intervention had lower costs (annual per participant $0.65) but lower effects. On the other hand from a societal perspective, the incremental costs relative to usual care were lowest in HHE, albeit there was no difference in BP levels compared to usual care (Figure 1). The trained GP group had high costs and no difference in benefit. However, the combined HHE and trained GP intervention had higher costs but also produced a greater benefit than usual care; thus remained the most cost-effective intervention.
Current prevention strategies promoted by WHO focus on combination drug treatment (low dose antihypertensives, statin and aspirin) to manage patients’ overall cardiovascular risk profile which is considered cost-effective for most low and middle income countries. 14 While such an approach is attractive, the use of multiple drugs is more expensive and combined formulations are not yet available. Moreover, the use of risk charts and the clinical assessment required is more complicated than making a BP measurement. A systematic review of use of risk charts did not show any strong evidence of improvements in care or risk factor outcomes 29
We utilized the existing healthcare infrastructure in Pakistan to deliver the intervention, hence ensuring logistical feasibility. The community general practitioners trained during the study were mostly private contractors who continued to provide care on their usual fee-for service model. Consistent with the pattern of out-of-pocket coverage for medications in Indo-Asia, antihypertensive drugs were also primarily bought by the patients. The door to door service of HHE replicated the existing publically funded Lady Health Workers Programme of Pakistan (LHWP) in terms of credentials, frequency of visits, and duty hours of the home educators. The LHWP has been implemented for about two decades, and provides immunization, basic maternal and preventive child care services. 30 The synergistic benefit of HHE and GP training on BP lowering in our study was probably due to mutual reinforcement of health care messages. 6 Thus, we show that modest investment in strengthening the existing private and public health sector for BP control can lead to substantial improvement in patient outcomes from a societal perspective. Moreover, the demonstration of success in a largely private physician dominated healthcare infrastructure suggests that the observed benefit is likely to enhance further in the long term by reduction in market failure in the prevention of chronic disease. 31
Our results indicate that the majority (>60%) of direct healthcare costs in hypertensive subjects were spent on medications. The proportional cost of medications became even more substantial after two years in the two groups receiving care from trained GPs. (Table 3b) The subjects in these groups were also more likely to be receiving antihypertensive agents perhaps reflecting the effectiveness of the update in management guidelines that underscored the value of appropriate use of antihypertensive agents. (Table 3b) These findings are supported by a nested study in our population linking GP training with better adherence to antihypertensive medications which was also linked with improved BP control. 32 Additional efforts to overcome barriers to thiazide diuretics are likely to further enhance economic efficiency of the intervention, as indicated by our results where overall costs reduced by more than 50% with an assumption that these agents were used as first-line anti-hypertensive agents by patients assigned to trained GPs (Table 5b)
The combination of low cost and effectiveness in achieving target BP with the HHE plus trained GP programme compares favourably with the results for a similar programme deemed cost-effective in Georgia, USA, where BP control rates of 68% were achieved with per-capita expenditure of $7.8. 33 While outcomes data are not reported, a train the trainers intervention for BP control costing the same has also been successfully implemented across Kyrgyzstan, a lower middle income country. The estimated annual per capita combined intervention cost of $0.43 (or $0.49 inclusive of BP screening) is about 10% of the current national budgetary allocation to health ($700 million) in Pakistan, and only 14% of the current $2.97 per capita spending on maternal and child health programs. 26 Moreover, even this cost will be somewhat offset when the program is implemented on the existing LHWP, as considerable resource sharing is expected. The per capita GDP of Pakistan is about $881 (World Bank estimate for 2007)); and the cost per CVD DALY saved from a societal perspective of the combined HHE plus trained GP was $1226 per DALY saved.
According to the World Health Organization, an intervention that saves one DALY for less than three times the gross domestic product (GDP) per capita is considered cost-effective, while one that saves a DALY for less than one GDP is considered very cost-effective. 34These recommendations are consistent with the <$ 1000 per CVD DALYS saved being highly cost effective for low income countries by The Disease Control Priorities Project (DCPP). 35 Moreover, all the sensitivity analyses indicate that the estimates associated with the combined intervention fall within the range qualifying it to be cost-effective. Furthermore, the $115 per DALY saved with the combined intervention from the policymaker’s perspective is comparable to the existing maternal and neonatal care programs in South Asia costing $127-394 per DALY averted .8 We believe our findings have significant public health implications for a substantial proportion of the world’s population in Indo-China, Africa, as well as other countries where the prevalence of hypertension is high, the mixed public-private healthcare system is comparable for up-scaling strategies using non-physician workforce and training existing healthcare providers to achieve a similar effect, and a significant share of the health care costs are borne out-of pocket. 23, 36,37Thus, the combined intervention of GP training plus HHE intervention could potentially be an effective and affordable intervention for many low income communities worldwide. 28,38
Our analysis has potential limitations. Most studies of cost effectiveness use the metric of disability or quality adjusted life years based on deaths and disabilities from myocardial infarctions and strokes. However, the follow-up duration in COBRA was short as it was not powered to assess differences in these hard outcomes. Instead, the primary measure of effectiveness was determined by reduction in BP, an acceptable outcome measure for cost effectiveness studies 39,40, which is well documented to translate into reduction in cardiovascular morbidity and mortality. 22, 41 Second, we did not take into account any cost incurred during hospitalizations of subjects in the four groups. However, the number of hospitalizations was too few to make a significant difference to the results (less than 1% of subjects). Nevertheless, a larger study for a longer duration is likely to show an even greater benefit of the combined HHE plus trained GP intervention on potential relief from the financial burden of catastrophic illness due to acute cardiovascular events, which often perpetuates the cycle of poverty in low income countries. 42 Third, our study was conducted in a developing country setting, hence generalizabilty to high income settings with greater opportunities for physician education and public awareness remains unknown. 43Further, the cost differentials, especially those related to healthcare, would be limited. However, sensitivity analysis assuming similar benefit and replacing costs incurred in Pakistan with those estimated for the US setting for comparable services using international purchasing power parity (I$5,770 per CVD DALY averted) as well as after itemized replacements with rather excessive healthcare costs in the US (40,644 per CVD DALY averted), both were within one per capita GDP of the US (World Bank estimate ($46,627 for 2007, hence revealed consistent findings. Thus, the combined intervention of HHE by lay health workers plus trained GP is likely to be cost effective in industrialized countries with high human resource costs as well. Finally, about 22% of subjects were lost to follow-up. However, they were balanced among the randomized groups. 6 Moreover, we analyzed both costs and effects on intention-to-treat principle. The strengths of our study are the use of empirical cost and effectiveness data and the consistency of results over a range of sensitivity analyses including those not accounting for productivity losses, as well as including additional cost inputs which further enhanced the benefit of the combined HHE plus trained GP intervention by lowering ICER to 17 (5 to 75) (Table 5a), and after assuming a substantial reduction in effect, albeit evidence from meta-analysis of clinical trials suggests persistence of effect. 44Further, our study was built on the existing health system which supports the feasibility, acceptability and high likelihood of wide implementation and sustainability if the intervention is scaled up. Thus we believe our findings are robust and of high validity.
In conclusion, the present analyses show that the combined intervention of HHE plus trained GP in management of hypertension is potentially affordable and significantly more cost effective than usual care or either strategy alone in the communities of Karachi, Pakistan. These results provide valuable guidance to policymakers for up-scaling the combined cost-effective intervention now as well as conducting further in-depth research on improving CVD outcomes in Pakistan and other resource-challenged countries with a rising burden of high blood pressure demanding immediate public health attention. 45
High blood pressure contributes the greatest attributable risk to mortality worldwide. However, evidence on economically efficient strategies to lower blood pressure (BP) from low- and middle-income countries (LIMCs) remains scarce.The Control of Blood Pressure and Risk Attenuation (COBRA) trial randomized 1341 hypertensive subjects in 12 randomly selected communities in Karachi, Pakistan, to three intervention programs compared to usual care: 1) combined home health education (HHE) by lay health workers plus management by trained general practitioner (GP); 2) HHE only; 3) trained GP only.The HHE intervention was modeled on the existing Lady Health Workers Programme in Pakistan and provided education on healthy diet, physical activity, and tobacco cessationin the home setting at 3 monthly intervals. GP training was an annual one-day session on optimal hypertension management.
Total costs and effect were assessed at baseline and 2 years to estimate incremental cost effectiveness ratios (ICER) based on (a) intervention cost; b) cost of physician consultation, medications and diagnostics, changes in lifestyle, and productivity loss and(c) change in systolic BP. We found that combined HHE plus GP intervention led to most BP reduction for the cost (incremental cost effectiveness ratio of $ 23 per mm Hg reduction in systolic BP compared to usual care), and remained so in 97.7% of 1000 bootstrapped replications.The estimated cost of the combined intervention was $115 per disability adjusted life year(DALY) averted from the policymaker’s and $1226 per DALY averted from the societal perspective, and $0.43 per capita population per year would be needed to scale up the intervention in Pakistan. Thus, the combined intervention of GP training plus HHE intervention could potentially be an effective and affordable strategy for many LMICs worldwide urgently in need of efforts to prevent and control hypertension.
Acknowledgements
We would like to thank all members of the Hypertension Research Group. . We would also like to acknowledge Mark J Sculpher, Professor of Economics at University of York, UK, for reviewing the paper and providing critical comments.
Funding Source: The study was financially supported by a research award (070854/Z/03/Z) from the Wellcome Trust, UK. The design, conduct, analysis, interpretation and presentation of the data were the responsibility of the authors, with no involvement from the funder.
Footnotes
Conflict of Interest Disclosures: “None”
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration Information: Registration number NCT00327574, http://clinicaltrials.gov/ct2/show/NCT00327574
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Jafar TH, Levey AS, Jafary FH, White F, Gul A, Rahbar MH, Khan AQ, Hattersley A, Schmid CH, Chaturvedi N. Ethnic subgroup differences in hypertension in Pakistan. J Hypertens. 2003;21:905–912. doi: 10.1097/00004872-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Ali SZ. Health for all in Pakistan: achievements, strategies and challenges. East Mediterr Health J. 2000;6:832–837. [PubMed] [Google Scholar]
- 4.Jafar TH, Jessani S, Jafary FH, Ishaq M, Orakzai R, Orakzai S, Levey AS, Chaturvedi N. General practitioners’ approach to hypertension in urban Pakistan: disturbing trends in practice. Circulation. 2005;111:1278–1283. doi: 10.1161/01.CIR.0000157698.78949.D7. [DOI] [PubMed] [Google Scholar]
- 5. http://www.iom.edu/Reports/2010/Promoting-Cardiovascular-Health-in-the-Developing-World-A-Critical-Challenge-to-Achieve-Global-Health.aspx.
- 6.Jafar TH, Hatcher J, Poulter N, Islam M, Hashmi S, Qadri Z, Bux R, Khan A, Jafary FH, Hameed A, Badruddin SH, Chaturvedi N. Community-based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Ann Intern Med. 2009;151:593–601. doi: 10.7326/0003-4819-151-9-200911030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JT, Neumann PJ, Weinstein MC. Does preventive care save money? Health economics and the presidential candidates. N Engl J Med. 2008;358:661–663. doi: 10.1056/NEJMp0708558. [DOI] [PubMed] [Google Scholar]
- 8.Jamison D. Disease Control Priorities in Developing Countries. 2nd edition 3-36. Oxford University Press; New York: 2006. Investing in health. [Google Scholar]
- 9.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH, DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 11.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, Mc GTS. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004;18:139–185. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- 12.Gold MR, Siegel JE, Russell, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. Oxford University; New York: 1996. [Google Scholar]
- 13.Household Integrated Economic Survey (HIES) 2007-08 . Government of Pakistan Statistics Division. Federal Bureau of Statistics; Jun, 2009. [Google Scholar]
- 14.Lim SS, Gaziano TA, Gakidou E, Reddy KS, Farzadfar F, Lozano R, Rodgers A. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370:2054–2062. doi: 10.1016/S0140-6736(07)61699-7. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, Cook J, Glick H, Liljas B, Petitti D, Reed S. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005;8:521–533. doi: 10.1111/j.1524-4733.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 16.Hay JW, Smeeding J, Carroll NV, Drummond M, Garrison LP, Mansley EC, Mullins CD, Mycka JM, Seal B, Shi L. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR Drug Cost Task Force report--Part I. Value Health. 13:3–7. doi: 10.1111/j.1524-4733.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 17.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ. 1997;6:327–340. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Severens JL, Brunenberg DE, Fenwick EA, O’Brien B, Joore MA. Cost-effectiveness acceptability curves and a reluctance to lose. Pharmacoeconomics. 2005;23:1207–1214. doi: 10.2165/00019053-200523120-00005. [DOI] [PubMed] [Google Scholar]
- 19.Fox-Rushby JA, Hanson K. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy Plan. 2001;16:326–331. doi: 10.1093/heapol/16.3.326. [DOI] [PubMed] [Google Scholar]
- 20.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 23.Mendis S, Fukino K, Cameron A, Laing R, Filipe A, Jr., Khatib O, Leowski J, Ewen M. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ. 2007;85:279–288. doi: 10.2471/BLT.06.033647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 25.Anderson GF. Controlling U.S. health spending: opportunities for academic health centers. Acad Med. 2006;81:807–811. doi: 10.1097/00001888-200609000-00008. [DOI] [PubMed] [Google Scholar]
- 26.World Development Indicators . The World Bank. Washington DC. USA: 2009. [Google Scholar]
- 27.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. Jama. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 28.Garg CC, Karan AK. Reducing out-of-pocket expenditures to reduce poverty: a disaggregated analysis at rural-urban and state level in India. Health Policy Plan. 2009;24:116–128. doi: 10.1093/heapol/czn046. [DOI] [PubMed] [Google Scholar]
- 29.Brindle P, Beswick A, Fahey T, Ebrahim S. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart. 2006;92:1752–1759. doi: 10.1136/hrt.2006.087932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haines A, Sanders D, Lehmann U, Rowe AK, Lawn JE, Jan S, Walker DG, Bhutta Z. Achieving child survival goals: potential contribution of community health workers. Lancet. 2007;369:2121–2131. doi: 10.1016/S0140-6736(07)60325-0. [DOI] [PubMed] [Google Scholar]
- 31.Watts JJ, Segal L. Market failure, policy failure and other distortions in chronic disease markets. BMC Health Serv Res. 2009;9:102. doi: 10.1186/1472-6963-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qureshi NN, Hatcher J, Chaturvedi N, Jafar TH. Effect of general practitioner education on adherence to antihypertensive drugs: cluster randomised controlled trial. BMJ. 2007;335:1030. doi: 10.1136/bmj.39360.617986.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson GF, Chu E. Expanding priorities--confronting chronic disease in countries with low income. N Engl J Med. 2007;356:209–211. doi: 10.1056/NEJMp068182. [DOI] [PubMed] [Google Scholar]
- 34.Baltussen RMPM, Adam T, Tan-Torres Edejer T, Hutubessy RCW, Acharya A, Evans DB, CJL M. Making choices in health: WHO guide to costeffectiveness analysis. World Health Organization; Geneva: 2003. [Google Scholar]
- 35.Disease Control Priorities in Developing Countries. www.dcp2.org. [PubMed]
- 36.Yang G, Kong L, Zhao W, Wan X, Zhai Y, Chen LC, Koplan JP. Emergence of chronic non-communicable diseases in China. Lancet. 2008;372:1697–1705. doi: 10.1016/S0140-6736(08)61366-5. [DOI] [PubMed] [Google Scholar]
- 37.Agyemang C, Bruijnzeels MA, Owusu-Dabo E. Factors associated with hypertension awareness, treatment, and control in Ghana, West Africa. J Hum Hypertens. 2006;20:67–71. doi: 10.1038/sj.jhh.1001923. [DOI] [PubMed] [Google Scholar]
- 38.Lange JM, Schellekens OP, Lindner M, van der Gaag J. Public-private partnerships and new models of healthcare access. Curr Opin HIV AIDS. 2008;3:509–513. doi: 10.1097/COH.0b013e3283031c67. [DOI] [PubMed] [Google Scholar]
- 39.Logan AG, Milne BJ, Achber C, Campbell WP, Haynes RB. Cost-effectiveness of a worksite hypertension treatment program. Hypertension. 1981;3:211–218. doi: 10.1161/01.hyp.3.2.211. [DOI] [PubMed] [Google Scholar]
- 40.Shiner T, Simons L, Parkinson H, Khanbhai A, Karthikeyan VJ, Nandhara G, Beevers DG. The financial cost of optimising blood pressure control. J Hum Hypertens. 2005;19:83–84. doi: 10.1038/sj.jhh.1001778. [DOI] [PubMed] [Google Scholar]
- 41.Hypertension Detection and Follow-up Program Cooperative Group Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Jama. 1979;242:2562–2571. [PubMed] [Google Scholar]
- 42.Clarke PM, Glasziou P, Patel A, Chalmers J, Woodward M, Harrap SB, Salomon JA. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. 7:e1000236. doi: 10.1371/journal.pmed.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health programmes. 3rd ed. Oxford University Press; Oxford: 2005. [Google Scholar]
- 44.Kostis WJ, Thijs L, Richart T, Kostis JB, Staessen JA. Persistence of mortality reduction after the end of randomized therapy in clinical trials of blood pressure-lowering medications. Hypertension. 56:1060–1068. doi: 10.1161/HYPERTENSIONAHA.110.160291. [DOI] [PubMed] [Google Scholar]
- 45.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]


