Abstract
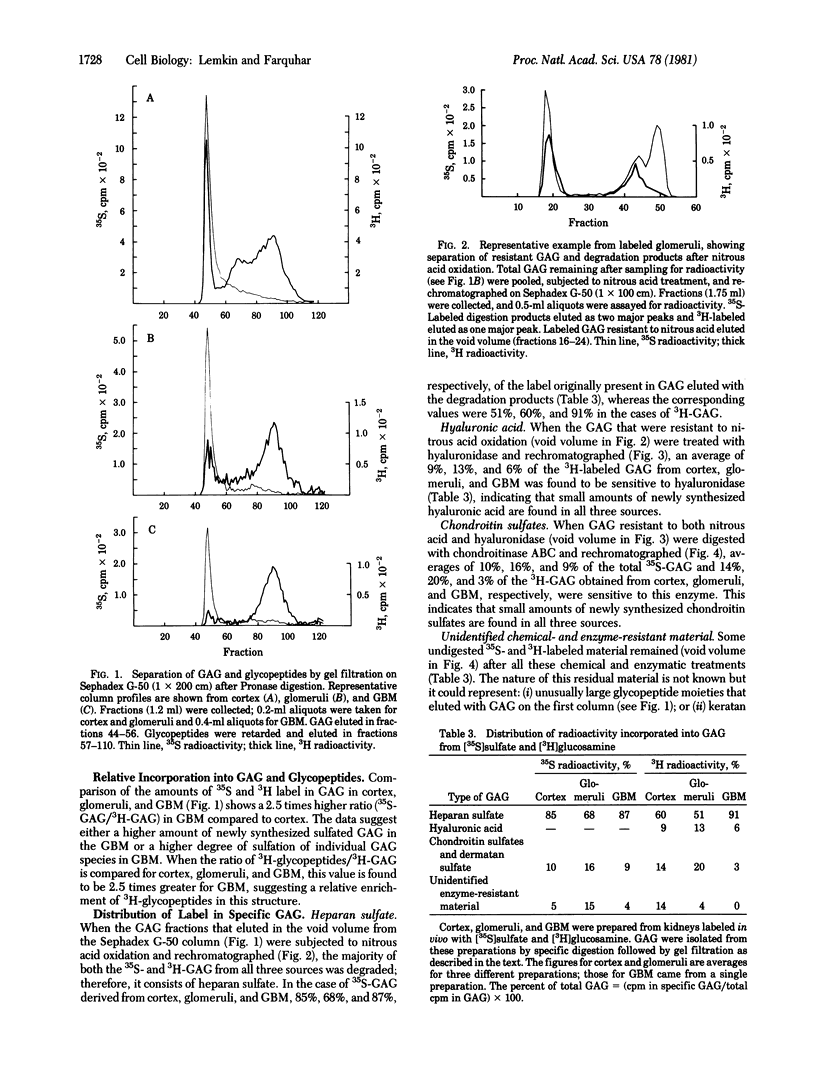
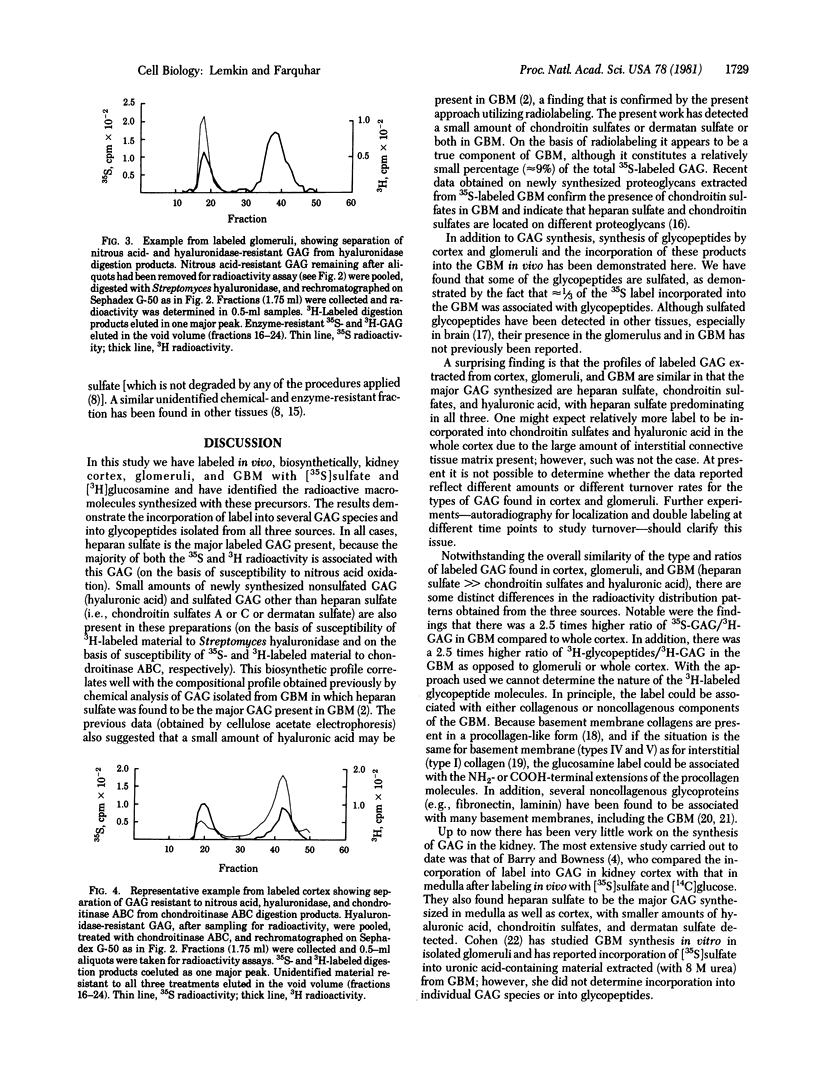
The biosynthesis of glycosaminoglycans (GAG) and glycopeptides was studied in rat kidney cortex, glomeruli, and isolated glomerular basement membranes (GBM). Rats were given four intraperitoneal injections of [35S]sulfate and [3H]glucosamine (over 10 hr) and sacrificed 14 hr after the last injection. Fractions of kidney glomeruli and purified GBM were prepared. The percent of the label incorporated into specific GAG or into glycopeptides was determined by selective degradative techniques in conjunction with gel filtration chromatography using the methods of Hart [Hart, G. W. (1976) J. Biol. Chem. 251, 6513-6521; Hart, G. W. (1978) Dev. Biol. 62, 78-98]. After digestion with Pronase and chromatography on Sephadex G-50, ≈68% of the total 35S radioactivity and 10-15% of the total 3H radioactivity incorporated into cortex, glomeruli, or GBM was found in the GAG fraction, and the remainder (≈32% of 35S radioactivity and 85-90% of the 3H radioactivity) was found in glycopeptide fractions. Treatment of GAG fractions isolated from the three sources (cortex, glomeruli, and GBM) with nitrous acid (which degrades heparan sulfates) indicated that the majority (85%, 65%, and 87%) of the 35S radioactivity as well as the majority (60%, 50%, and 91%) of the 3H radioactivity from all three sources was degraded by this treatment. When nitrous acid-resistant GAG from GBM were subjected to digestion with Streptomyces hyaluronidase (which degrades hyaluronic acid), ≈6% of the 3H-labeled material was sensitive to this treatment. The remaining 35S- and 3H-labeled GAG isolated from GBM were digested with chondroitinase ABC (which degrades chondroitin sulfates A and C and dermatan sulfate). Although the ratios of the types of GAG synthesized by all three sources were similar, in GBM the ratios of 35S- to 3H-labeled GAG and of 3H-labeled glycopeptides to 3H-labeled GAG were higher (2.5 times) than those found for glomeruli. The data demonstrate the synthesis of both sulfated and nonsulfated GAG by rat kidney cortex and glomeruli and their transport to and incorporation into the GBM. Heparan sulfate is the major GAG synthesized by glomeruli, but the glomeruli also synthesize smaller amounts of hyaluronic acid and chondroitin sulfates, which are in part incorporated into GBM. In addition, the renal cortex and the glomeruli synthesize glycopeptides, some of which are sulfated, and incorporate them into GBM.
Keywords: [35S]sulfate, [3H]glucosamine, heparan sulfate, hyaluronic acid, chondroitin sulfate
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry D. N., Bowness J. M. Identification and turnover of glycosaminoglycans in rat kidneys. Can J Biochem. 1975 Jun;53(6):713–720. doi: 10.1139/o75-098. [DOI] [PubMed] [Google Scholar]
- Cifonelli J. A., King J. Structural studies on heparins with unusually high N-acetylglucosamine contents. Biochim Biophys Acta. 1973 Sep 14;320(2):331–340. doi: 10.1016/0304-4165(73)90313-9. [DOI] [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of 2-acetamido-2-deoxy-D-glucose residues in mammalian heparins. Carbohydr Res. 1972 Feb;21(2):173–186. doi: 10.1016/s0008-6215(00)82144-8. [DOI] [PubMed] [Google Scholar]
- Clark C. C., Kefalides N. A. Carbohydrate moieties of procollagen: incorporation of isotopically labeled mannose and glucosamine into propeptides of procollagen secreted by matrix-free chick embryo tendon cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):34–38. doi: 10.1073/pnas.73.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. P. Glycosaminoglycans are integral constituents of renal glomerular basement membrane. Biochem Biophys Res Commun. 1980 Jan 29;92(2):343–348. doi: 10.1016/0006-291x(80)90339-3. [DOI] [PubMed] [Google Scholar]
- Cohn R. H., Banerjee S. D., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Nature of glycosaminoglycan and organization of extracellular materials. J Cell Biol. 1977 May;73(2):464–478. doi: 10.1083/jcb.73.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad G. W., Hart G. W. Heparan sulfate biosynthesis by embryonic tissues and primary fibroblast populations. Dev Biol. 1975 Jun;44(2):253–269. doi: 10.1016/0012-1606(75)90396-6. [DOI] [PubMed] [Google Scholar]
- Courtoy P. J., Kanwar Y. S., Hynes R. O., Farquhar M. G. Fibronectin localization in the rat glomerulus. J Cell Biol. 1980 Dec;87(3 Pt 1):691–696. doi: 10.1083/jcb.87.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. R., Bernfield M. R. The basal lamina of the postnatal mammary epithelium contains glycosaminoglycans in a precise ultrastructural organization. Dev Biol. 1980 Jan;74(1):118–135. doi: 10.1016/0012-1606(80)90056-1. [DOI] [PubMed] [Google Scholar]
- Hart G. W. Biosynthesis of glycosaminoglycans by the separated tissues of the embryonic chick cornea. Dev Biol. 1978 Jan;62(1):78–98. doi: 10.1016/0012-1606(78)90094-5. [DOI] [PubMed] [Google Scholar]
- Hart G. W. Biosynthesis of glycosaminolgycans during corneal development. J Biol Chem. 1976 Nov 10;251(21):6513–6521. [PubMed] [Google Scholar]
- Hay E. D., Meier S. Glycosaminoglycan synthesis by embryonic inductors: neural tube, notochord, and lens. J Cell Biol. 1974 Sep;62(3):889–898. doi: 10.1083/jcb.62.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAKOWER C. A., GREENSPON S. A. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun;51(6):629–639. [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Isolation of glycosaminoglycans (heparan sulfate) from glomerular basement membranes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4493–4497. doi: 10.1073/pnas.76.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. K., Margolis R. U. Sulfated glycopeptides from rat brain glycoproteins. Biochemistry. 1970 Oct 27;9(22):4389–4396. doi: 10.1021/bi00824a020. [DOI] [PubMed] [Google Scholar]
- Meezan E., Hjelle J. T., Brendel K., Carlson E. C. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975 Dec 1;17(11):1721–1732. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Synthesis of sulfated glycosaminoglycans by embryonic corneal epithelium. Dev Biol. 1973 Dec;35(2):318–331. doi: 10.1016/0012-1606(73)90027-4. [DOI] [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Toole B. P. Epithelial collagens and glycosaminoglycans in the embryonic cornea. Macromolecular order and morphogenesis in the basement membrane. J Cell Biol. 1974 Sep;62(3):815–830. doi: 10.1083/jcb.62.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]



