Abstract
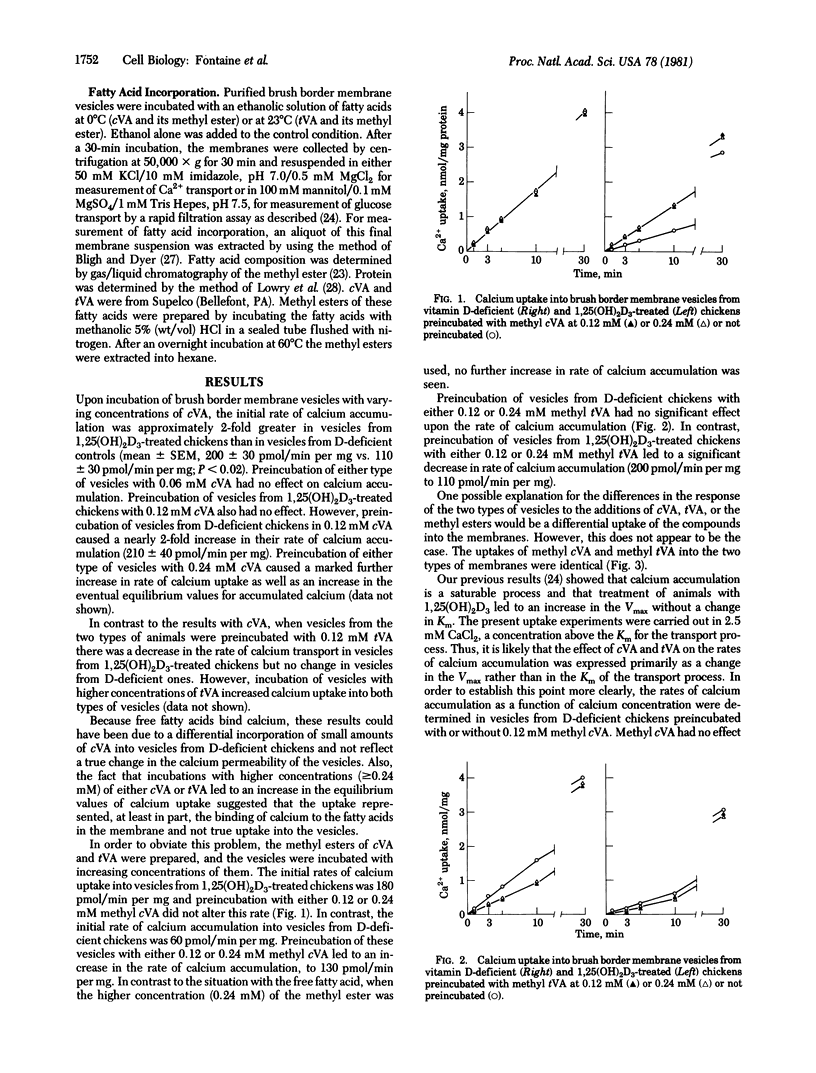
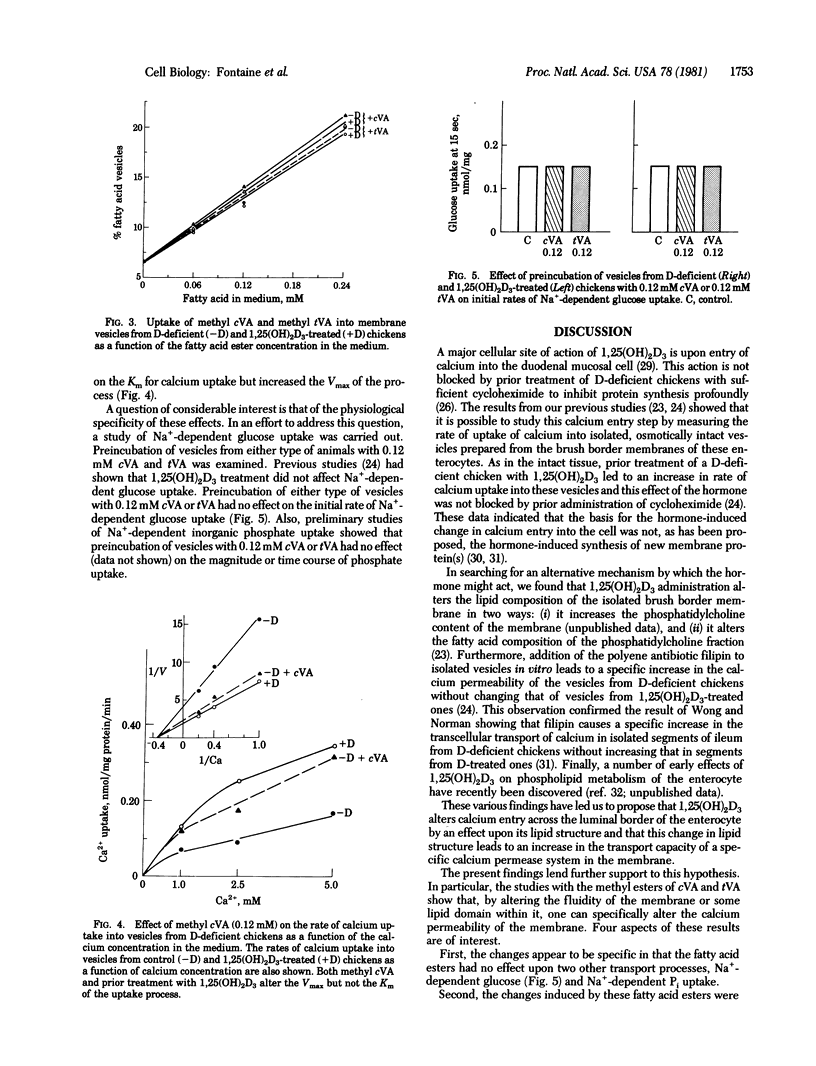
Isolated vesicles prepared from the brush border membranes of chicken duodenal mucosal cells (enterocytes) take up calcium by a passive but saturable process. The rate of uptake (Vmax) is increased 2.5- to 3-fold, with no change in Km, in vesicles prepared from 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]-treated chickens compared to vesicles from vitamin D-deficient controls. Preincubation of vesicles with either cis- or trans-vaccinic acid (cVA or tVA, respectively) or their methyl esters in vitro also alters the rates of calcium transport. Methyl cVA causes an increase in rate of calcium uptake into vesicles from vitamin D-deficient chickens but not in those from 1,25(OH)2D3-treated chickens. This increase is 80-90% of that seen after 1,25(OH)2D3 treatment. Higher concentrations of methyl cVA produce no further increases. Conversely, methyl tVA causes a decrease in rate of calcium uptake in vesicles from 1,25(OH)2D3-treated chickens but no change in vesicles from vitamin D-deficient controls. This decrease reduces the rate of calcium uptake to nearly the same value as seen in vesicles from vitamin D-deficient controls. Higher concentration of methyl tVA produce no further suppression of uptake rate. The changes seen were in the Vmax and not in the Km of the transport process. The fatty acids did not alter the process of Na+-dependent glucose uptake in the same membrane. These data demonstrate that a small alteration in the lipid structure of this membrane can specifically shift the activity of the calcium transport process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloj S. M., Lee G., Grollman E. F., Beguinot F., Consiglio E., Kohn L. D. Role of phospholipids in the structure and function of the thyrotropin receptor. J Biol Chem. 1979 Sep 25;254(18):9040–9049. [PubMed] [Google Scholar]
- Amatruda J. M., Finch E. D. Modulation of hexose uptake and insulin action by cell membrane fluidity. The effects of temperature on membrane fluidity, insulin action, and insulin binding. J Biol Chem. 1979 Apr 25;254(8):2619–2625. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bikle D. D., Zolock D. T., Morrissey R. L., Herman R. H. Independence of 1,25-dihydroxyvitamin D3-mediated calcium transport from de novo RNA and protein synthesis. J Biol Chem. 1978 Jan 25;253(2):484–488. [PubMed] [Google Scholar]
- Chapman D. Phase transitions and fluidity characteristics of lipids and cell membranes. Q Rev Biophys. 1975 May;8(2):185–235. doi: 10.1017/s0033583500001797. [DOI] [PubMed] [Google Scholar]
- Coleman R. Membrane-bound enzymes and membrane ultrastructure. Biochim Biophys Acta. 1973 Apr 3;300(1):1–30. doi: 10.1016/0304-4157(73)90010-5. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Sakura J. D., Small D. M., Shipley G. G. Protein-lipid interactions: recombinants of the proteolipid apoprotein of myelin with dimyristoyllecithin. Biochemistry. 1977 May 31;16(11):2313–2319. doi: 10.1021/bi00630a001. [DOI] [PubMed] [Google Scholar]
- Goodman D. B., Allen J. E., Rasmussen H. Studies on the mechanism of action of aldosterone: hormone-induced changes in lipid metabolism. Biochemistry. 1971 Oct 12;10(21):3825–3831. doi: 10.1021/bi00797a004. [DOI] [PubMed] [Google Scholar]
- Goodman D. B., Wong M., Rasmussen H. Aldosterone-Induced Membrane Phospholipid Fatty Acid Metabolism in the Toad Urinary Bladder. Biochemistry. 1975 Jul;14(13):2803–2809. doi: 10.1021/bi00684a003. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., McCain T. A. Basic and clinical concepts related to vitamin D metabolism and action (first of two parts). N Engl J Med. 1977 Nov 3;297(18):974–983. doi: 10.1056/NEJM197711032971804. [DOI] [PubMed] [Google Scholar]
- Hirata F., Viveros O. H., Diliberto E. J., Jr, Axelrod J. Identification and properties of two methyltransferases in conversion of phosphatidylethanolamine to phosphatidylcholine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1718–1721. doi: 10.1073/pnas.75.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M. K., White H. B., 3rd Long-range order in biomembranes. Adv Lipid Res. 1977;15:1–60. doi: 10.1016/b978-0-12-024915-2.50007-4. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980 Feb 25;255(4):1286–1295. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine Y. K. Physical studies of membrane structure. Prog Biophys Mol Biol. 1972;24:1–74. doi: 10.1016/0079-6107(72)90003-x. [DOI] [PubMed] [Google Scholar]
- Lien E. L., Goodman D. B., Rasmussen H. Effects of an acetyl-coenzyme A carboxylase inhibitor and a sodium-sparing diuretic on aldosterone-stimulated sodium transport, lipid synthesis, and phospholipid fatty acid composition in the toad urinary bladder. Biochemistry. 1975 Jun 17;14(12):2749–2754. doi: 10.1021/bi00683a030. [DOI] [PubMed] [Google Scholar]
- Limas C. J. Effect of phospholipid methylation on beta-adrenergic receptors in the normal and hypertrophied rat myocardium. Circ Res. 1980 Oct;47(4):536–541. doi: 10.1161/01.res.47.4.536. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D., Barrantes F. J. Immobilized lipid in acetylcholine receptor-rich membranes from Torpedo marmorata. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4329–4333. doi: 10.1073/pnas.75.9.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D-3 on phosphate uptake into chick intestinal brush border membrane vesicles. Biochim Biophys Acta. 1980 Jun 20;599(1):13–23. doi: 10.1016/0005-2736(80)90052-8. [DOI] [PubMed] [Google Scholar]
- Max E. E., Goodman D. B., Rasmussen H. Purification and characterization of chick intestine brush border membrane. Effects of 1alpha(OH) vitamin D3 treatment. Biochim Biophys Acta. 1978 Aug 4;511(2):224–239. doi: 10.1016/0005-2736(78)90316-4. [DOI] [PubMed] [Google Scholar]
- Melancon M. J., Jr, DeLuca H. F. Vitamin D stimulation of calcium-dependent adenosine triphosphatase in chick intestinal brush borders. Biochemistry. 1970 Apr 14;9(8):1658–1664. doi: 10.1021/bi00810a002. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Czech M. P. Sensitivity of the adipocyte D-glucose transport system to membrane fluidity in reconstituted vesicles. J Biol Chem. 1979 Sep 25;254(18):8744–8747. [PubMed] [Google Scholar]
- O'Doherty P. J. 1,25-Dihydroxyvitamin D3 increases the activity of the intestinal phosphatidylcholine deacylation-reacylation cycle. Lipids. 1979 Jan;14(1):75–77. doi: 10.1007/BF02533571. [DOI] [PubMed] [Google Scholar]
- Orly J., Schramm M. Fatty acids as modulators of membrane functions: catecholamine-activated adenylate cyclase of the turkey erythrocyte. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3433–3437. doi: 10.1073/pnas.72.9.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Cowden M., Kimelberg H. Role of cholesterol in membranes. Effects on phospholipid-protein interactions, membrane permeability and enzymatic activity. Biochim Biophys Acta. 1973 Nov 30;330(1):8–26. doi: 10.1016/0005-2736(73)90280-0. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Thompson P. A., Czech M. P. Coordinate modulation of D-glucose transport activity and bilayer fluidity in plasma membranes derived from control and insulin-treated adipocytes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):915–918. doi: 10.1073/pnas.77.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Fontaine O., Max E. E., Goodman D. B. The effect of 1alpha-hydroxyvitamin D3 administration on calcium transport in chick intestine brush border membrane vesicles. J Biol Chem. 1979 Apr 25;254(8):2993–2999. [PubMed] [Google Scholar]
- Schaeffer B. E., Zadunaisky J. A. Stimulation of chloride transport by fatty acids in corneal epithelium and relation to changes in membrane fluidity. Biochim Biophys Acta. 1979 Sep 4;556(1):131–143. doi: 10.1016/0005-2736(79)90425-5. [DOI] [PubMed] [Google Scholar]
- Sinensky M., Minneman K. P., Molinoff P. B. Increased membrane acyl chain ordering activates adenylate cyclase. J Biol Chem. 1979 Sep 25;254(18):9135–9141. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Wong R. G., Norman A. W. Studies on the mechanism of action of calciferol. VIII. The effects of dietary vitamin D and the polyene antibiotic, filipin, in vitro, on the intestinal cellular uptake of calcium. J Biol Chem. 1975 Apr 10;250(7):2411–2419. [PubMed] [Google Scholar]



