Abstract
Background
Partial protective efficacy lasting up to 43 months after vaccination with the RTS,S malaria vaccine has been reported in one cohort (C1) of a Phase IIb trial in Mozambique, but waning efficacy was observed in a smaller contemporaneous cohort (C2). We hypothesized that low dose exposure to asexual stage parasites resulting from partial pre-erythrocytic protection afforded by RTS,S may contribute to long-term vaccine efficacy to clinical disease, which was not observed in C2 due to intense active detection of infection and treatment.
Methodology/Principal Findings
Serum collected 6 months post-vaccination was screened for antibodies to asexual blood stage antigens AMA-1, MSP-142, EBA-175, DBL-α and variant surface antigens of the R29 laboratory strain (VSAR29). Effect of IgG on the prospective hazard of clinical malaria was estimated. No difference was observed in antibody levels between RTS,S and control vaccine when all children aged 1–4 years at enrollment in both C1 and C2 were analyzed together, and no effects were observed between cohort and vaccine group. RTS,S-vaccinated children <2 years of age at enrollment had lower levels of IgG for AMA-1 and MSP-142 (p<0.01, all antigens), while no differences were observed in children ≥2 years. Lower risk of clinical malaria was associated with high IgG to EBA-175 and VSAR29 in C2 only (Hazard Ratio [HR]: 0.76, 95% CI 0.66–0.88; HR: 0.75, 95% CI 0.62–0.92, respectively).
Conclusions
Vaccination with RTS,S modestly reduces anti-AMA-1 and anti-MSP-1 antibodies in very young children. However, for antigens associated with lower risk of clinical malaria, there were no vaccine group or cohort-specific effects, and age did not influence antibody levels between treatment groups for these antigens. The antigens tested do not explain the difference in protective efficacy in C1 and C2. Other less-characterized antigens or VSA may be important to protection.
Trial Registration
ClinicalTrials.gov NCT00197041
Introduction
GlaxoSmithKline Biologicals' adjuvanted RTS,S malaria vaccine candidate has repeatedly demonstrated protective efficacy in clinical trials in Africa [1]. It is composed of the NANP central repeat and C-terminal T-cell multi-epitope of Plasmodium falciparum circumsporozoite protein (CSP), fused with the S-antigen of hepatitis B virus and combined with an AS adjuvant systems [2], either AS02 (QS21, MPL and an oil-in-water emulsion) or AS01 (QS21, MPL and liposomes) [3]. The RTS,S/AS01 formulation is being evaluated in a Phase III efficacy trial.
The generation of high titer anti-CSP antibodies has been extensively documented following RTS,S vaccination of malaria-naïve adult volunteers [4]. Although certain antibody thresholds have been proposed that may be necessary to achieve protection [5], to date, there is no strict anti-CSP IgG correlate of protection derived from studies involving laboratory-based challenge of vaccinated volunteers with the bite of an infectious mosquito. Additionally, an association has been shown between CSP-specific CD4+ T cell responses and protection in a laboratory challenge model [5]. However, information is lacking on immunological correlates of protection in the face of natural exposure to malaria, which the challenge model cannot provide. Similar efforts in African field trials of RTS,S have confirmed the consistent, high titer generation of CSP-specific antibodies, while cell-mediated immune (CMI) responses have not yet been systematically studied [6]. Interestingly, in field studies where efficacy against infection is the primary endpoint, CSP antibodies seemed to correlated with protection [7], [8], whereas no such correlation could be found with protection against clinical manifestation of disease [7], [9], except in a recent trial of RTS,S/AS02 in infants [10] and a recent analysis in children vaccinated with RTS,S/AS01 where anti-CSP antibody titers 6.5 months after vaccination seemed to correlate with protection [11].
Protection has been observed for up to 43 months following vaccination despite declining levels of CSP-specific antibodies [12], [13], and efficacy measurements remained remarkably stable. This long-term protection observed in Mozambican children differs markedly from the waning protection observed in earlier studies of RTS,S/AS02 in Gambian men and U.S. non-immune adults in the U.S. [14], [15]. This unexpected finding suggests that, in addition to anti-CSP antibodies, other factors may contribute to sustained protection, such as anti-CSP CMI, fine specificity and functionality of CSP-specific antibodies, or acquisition of blood stage immunity greater than that which would be acquired naturally [4]. Indeed, a hypothesis that RTS,S vaccination may affect blood stage immunity was proposed at earlier stages of this vaccine's development [16]. The concept of enhancing naturally acquired immunity through interventions that reduce the blood stage parasite burden was proposed to explain long-term efficacy of intermittent preventive treatment in infants [17]. In the case of RTS,S vaccination, it was hypothesized that low dose parasitemia as a result of partial pre-erythrocytic protection may allow for a more effective immune response to asexual blood stage parasites [18], [19]. The alternative to this hypothesis would be that RTS,S vaccination reduces naturally acquired immune responses to blood stage parasites through reduction in high level exposure.
In the Phase IIb trial in children 1–4 years of age performed in Manhiça, Mozambique, the study was divided into two cohorts, each with different follow up methods and schedules [7]. Cohort 1 (C1) was followed for 43 months post-vaccination by passive case detection (PCD) of clinical malaria, and an efficacy of 30.5% against first or only episode of clinical malaria was observed over the entire follow-up period [13]. The estimated entomological inoculation rate in Manhiça District in 2002 was 38 infective bites per person per year [20]. Cohort 2 (C2) was followed for 6 months post-vaccination by active detection of infection (ADI) by fortnightly and monthly household visits and thereafter by PCD; vaccine efficacy was initially 45.0% against first or only episode of infection, but waning efficacy was observed by 6 months post-vaccination. There were no EIR estimates in this area at the time of the trial, but transmission intensity in C2 was notably higher, as deduced by elevated blood stage immunofluorescence antibody test (IFAT) responses at baseline [7]. In addition to transmission intensity, another key difference was prompt clearing of parasitemia during ADI visits in C2, regardless of parasite density or presence of fever.
Here, we examined the antibody immune responses to multiple asexual blood stage antigens at 6 months post-vaccination, the time when waning efficacy is observed in C2 but not in C1. We hypothesized that in C1, antibodies to asexual blood stage antigens in the RTS,S/AS02 group would be higher than those in the control group. Furthermore, we hypothesized that the magnitude of the difference between C2 vaccine groups would be less than that observed in C1. We measured antibodies to leading blood stage vaccine-candidate antigens AMA-1 [21], MSP-142 [22], [23] and EBA-175 [24], as well as variant surface antigens of the R29 P. falciparum culture line (VSAR29) and recombinant DBL-α. The R29 culture line exhibits high levels of rosetting, and DBL-α is encoded within var-1 of R29 [25]. Each of these antigens has been targeted for vaccine development or for further investigation due to their importance in the blood stage parasite lifecycle, such as merozoite invasion, immune evasion and cytoadherence [25]–[27]. Although it is unknown if there is a causal relationship between antibodies to these antigens and protective immunity, some of these antibodies have shown association with protection in seroepidemiological studies [28]–[30].
Methods
Ethics Statement
The study was approved by the Mozambican National Health and Bioethics Committee, the Hospital Clínic of Barcelona Ethics Committee and the PATH Research Ethics Committee, and written informed consent was gathered from parents/guardians.
Study Design
The samples assessed were obtained as part of a Phase IIb proof-of-concept randomized, controlled trial of the RTS,S/AS02 vaccine administered to 1–4 year-old Mozambican children (ClinicalTrials.gov registry number NCT00197041). The trial design has been described in detail in multiple primary research articles [7], [31], [13], [19].
Serum samples were obtained from cross-sectional blood collections at the start of the single-blind phase of follow-up, corresponding to 6 months after third dose [31], [19]. This timepoint was selected, because it provides a period of 6 months of natural exposure following vaccination and coincides with the beginning of the follow-up period where waning efficacy was observed in C2 [19]. Samples were selected to have an equal representation of C1 and C2 and of older and younger children. To do this, samples were stratified by cohort and by two age groups (≥ or <2 years of age) to create 4 subgroups of the full sample set. Samples were randomly selected in each subgroup. Baseline characteristics of the samples selected, including age group, previous episodes and pre-vaccination blood stage antibody titers, are given in Table S1.
Recombinant Proteins
Apical membrane antigen 1 (AMA-1) 3D7 strain [21], PfF2 (fragment II of region II of the 175 kDa erythrocyte binding antigen, EBA-175) [32], and the Duffy binding-like alpha (DBL-α) domain of PfEMP-1 [25] were produced at ICGEB. FMP009 (FVO strain of AMA-1), FMP1 and FMP010 (3D7 and FVO strains of MSP-142, respectively) [22], [23], all were produced at WRAIR.
Suspension array technology
Microsphere coupling
A multiplex suspension array technology (SAT) panel was constructed to quantify IgG responses to P. falciparum antigens using Luminex xMAP™ (Luminex Corp., Austin, Texas) and the Bio-Plex 100 platform (Bio-Rad, Hercules, CA). xMAP™ beads (regions: 4, 10, 15, 34, 40, and 45) were selected for each antigen. Uncoupled polystyrene 5.6 µm COOH-microspheres (Bio-Rad) were coupled to antigen in 200 µL coupling reactions (2.5×106 microspheres). First, microspheres were washed twice with 100 µL of Wash Solution from the Bio-Rad carboxylated microsphere coupling kit. Microspheres were resuspended by sonication and vortexing and activated using Bead Activation buffer. Sulfo-NHS (N-hydroxysulfosuccinimide) and EDC (1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride) (Pierce, Thermo Fisher Scientific Inc., Rockford, IL) were simultaneously added to reaction tubes at 5 mg/mL each in Bead Activation buffer, and reaction tubes were incubated at room temperature with gentle agitation, protected from light for 20 min. Microspheres were washed with 100 µL PBS, sonicated and vortexed. One microgram of corresponding recombinant protein per million microspheres was added to each reaction tube. A prior titration of the antigen concentrations confirmed that as little as 1 µg of each antigen per million microspheres could be used without changing the saturation levels of the microspheres when assayed with hyperimmune plasma (HIP). Reaction tubes were left at 4° C on a shaker overnight, protected from light. Microspheres were then blocked with 250 µL of 1% bovine serum albumin (BSA) in PBS for 30 min on a shaker at room temperature, protected from light, then centrifuged and washed with 500 µL assay buffer (1% BSA/0.05% sodium azide in PBS, filtered). Coupled microspheres were quantified on a Guava PCA desktop cytometer (Guava, Hayward, CA), equal amounts of each analyte combined in multiplex tubes, and stored at 3,000 microspheres/μL at 4° C, protected from light.
Luminex assay and standard curves
The SAT assay developed for these analyses used modified standard curves and a template employed by the Laboratory of Malaria and Vector Research (LMVR, NIH) ELISA reference center [33]. Briefly, 3,000 microspheres per analyte were added to a 96-well round bottom plate per well. A HIP pool from Mozambican volunteers was applied in a 2-fold serial dilution for a starting dilution of 1∶1,500 and incubated for 1 h at room temperature with plate agitation and protection from light. The plate was washed by pelleting microspheres (centrifuge at 800×g for 5 min) and resuspended with wash buffer (0.05% Tween 20/PBS). 100 µL of biotinylated anti-human IgG (Sigma, Tres Cantos, Spain) diluted 1∶2,500 in assay buffer was applied to all wells and incubated at room temperature for 45 min with agitation and protection from light. The plate was washed as before, and 100 µL of streptavidin-conjugated R-phycoerythrin (Invitrogen, Carlsbad, CA) diluted 1∶1,000 in assay buffer was added and incubated at room temperature for 25 min with agitation and protection from light. The plate was immediately read using Bio-Plex Manager version 4.0, and at least 100 microspheres per analyte were acquired per sample. Crude mean fluorescent intensity (MFI) was exported with background fluorescence from blank wells already subtracted. Additionally, Bio-Plex Manager software automatically calculated the regression equation for the curves of each analyte. The 5-parameter logistic regression curve with logarithmic variance weighting was selected due to superior fit with antibody data [34], [35].
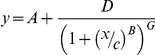 |
where A is the lower asymptote, B is the slope at the inflection point, C is the concentration at the inflection point, D is the upper asymptote, and G is a factor of asymmetry.
The back-calculated dilution corresponding to 15,000 MFI, roughly the middle point of the linear region of a fully saturated sigmoidal curve with the Bio-Plex 100 system, was assigned the arbitrary value of 15,000 Antibody Units (AU), and the "undiluted units" of the HIP pool for each analyte was calculated by multiplying 15,000 units by the corresponding dilution factor. This value was registered with the HIP pool to create a reference standard for these antigens. This reference standard was repeated on each assay day in a 14-point 2-fold dilution series, starting at a dilution of 1∶1,500 with the corresponding unit values (the large range of the dilution series encompasses different saturation points for each antigen). Test plasmas were incubated with the multiplex microspheres at a final dilution of 1∶500 in duplicate, and the assay was performed as described for the standard curves. Based on the standard curves for each analyte, AU concentration for each antigen was calculated by the Bio-Plex Manager software. Samples where microsphere aggregation exceeded 50% were excluded from analysis.
Anti-VSA antibody assays
VSA antibody assays were performed as described previously [36]. The R29 line of P. falciparum was selected for anti-VSA assays because, compared to other field and laboratory isolates, it was the most immunogenic in preliminary studies in this population (Dobaño et al. unpublished). Briefly, synchronized cultures of the R29 line were cryopreserved and thawed for each assay day. Sterile 96-well round bottom plates were blocked with 200 µL/well of 1% BSA/PBS and left overnight at 4° C. Erythrocyte pellets were measured and resuspended in 1% BSA/PBS to 1% hematocrit, and 95 µL of erythrocyte suspension was added to each well. A 1∶20 dilution, or 5 µL of serum, was added to wells without replicates, and 4 wells were left as controls: a blank, two compensation controls and a HIP positive control. Plates were incubated for 30 min at room temperature with mild agitation and washed three times by centrifuging the plate at 1,200×g for 2 min, carefully flicking off the supernatant, and resuspending the pellets with 200 µL of 1% BSA/PBS. Pellets were resuspended in 100 µL of polyclonal rabbit anti-human IgG (Dako Cytomation, Glostrup, Denmark) diluted in 1∶200 in 1% BSA/PBS and incubated for 30 min at room temperature with gentle agitation. Plates were washed and pellets resuspended in 100 µL of Alexa Fluor 488 donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA) diluted 1∶1,000 and ethidium bromide (Ecogen, Barcelona, Spain) diluted 1∶1,000 from a 1% stock in 1% BSA/PBS and incubated for 30 min at room temperature with gentle agitation and protection from light. Plates were washed as before and two additional times with PBS. Pellets were resuspended in 200 µL of PBS, transferred to cytometer acquisition tubes containing 200 µL of PBS, acquired on a 4-color FACS Calibur and analyzed using CellQuest Pro v5.2.1 (BD, Franklin Lakes, NJ). A gate for infected events was established using infected erythrocytes and ethidium bromide stain. A minimum of 1,000 infected events and up to 5,000 were acquired. Geometric mean MFI was reported for both infected and uninfected events, and an overall MFI value for specific VSAR29 antibodies was calculated by subtracting the MFI of uninfected events from MFI of infected events.
Procedures for normalization of data were as follows. A 6-step 2-fold serial dilution of HIP was added in duplicate to each assay plate, starting with a 1∶20 dilution to generate a reference curve for inter-assay variation and dynamic assay normalization. A standardized mean fluorescence intensity (MFI) score was assigned to each sample, as previously described [37], based on the hyperimmune plasma (HIP) titration. Samples with specific MFI above the highest titer (1∶20) were assigned a score of 6, between that and the next titer (1∶40) a score of 5, and so forth, and all samples with specific MFI below the lowest titer (1∶640) were given a score of 0. Additionally, the HIP titrations for all plates were averaged, and 6 "normalization constants" were generated, one for each dilution of the HIP, which were the ratio of MFI on the plate to the mean of all plates. All samples were normalized by multiplying the crude MFI by the normalization constant corresponding to the standardized MFI score for that sample (e.g. a sample with MFI score of 6 would be normalized with the normalization constant for the 1∶20 HIP dilution for that plate). This method allowed for more accurate adjustments across the MFI spectrum than by normalizing all values by a single dilution of a positive control.
Statistical Analysis
All antibody data were exported in Microsoft Excel, organized and imported into STATA version 11 (STATA Corp., Texas). Luminex values were divided by 1,000 for analysis. Data was log-transformed using the natural logarithm. Differences between groups were analyzed by the reverse cumulative distribution function [38] and the Wilcoxon rank-sum test. Correlations between antibody responses to different antigens were assessed by Pearson's correlation coefficient (ρ), and p-values were adjusted by the Bonferroni correction. High correlation was considered for values of ρ greater than 0.7, and moderate correlation was considered for ρ greater than 0.5. Continuous data were analyzed for variables interacting with antibody levels using an Ordinary Least Square (OLS) linear regression model adjusting for age group, cohort, blood stage antibodies at baseline (based on IFAT performed for the main trial) [7], previous malaria episodes, present infection and batch of experiments. Interactions with the vaccine group and the adjusting variables were tested with an F-test. Data were categorized into 5 groups: below range, lower/middle/higher tertiles and above range. Categorical data were analyzed using an Ordered Politomous Logisitc Regression (OPLR) model adjusting for the same variables as above. Continuous and categorical data were compared for time-to-first clinical episode in the single-blind follow-up time period from 6–18 months post-vaccination [31] using univariate and step-wise multivariate Cox proportional hazard models on all children, stratified by cohort and adjusting for vaccine group, age group, IFAT at baseline, and previous clinical malaria episodes. Maternal antibodies were not considered as a confounding factor, because the youngest children in the study were nearly 2 years of age at the time of sampling. Results were considered statistically significant for a p-value <0.05.
Results
Of the 2022 children aged 1-4 years enrolled, sera from 6 months post-vaccination in 580 randomized individuals who completed according-to-protocol (ATP) criteria throughout the single-blind phase (6–18 months post-vaccination) were assayed by Luminex and flow cytometry. Samples were evenly distributed between C1 and C2 and between the < 2 years and ≥2 years age groups.
Antibody responses in RTS,S and control children
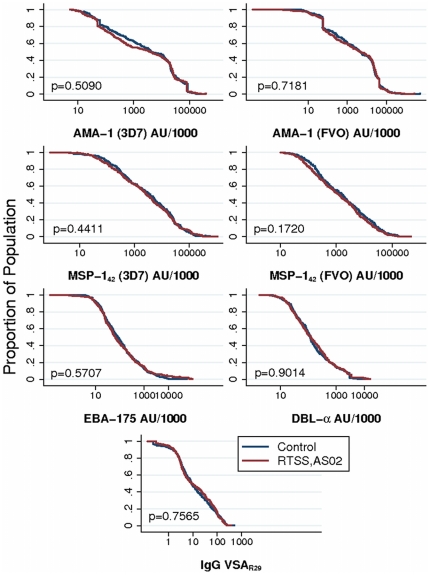
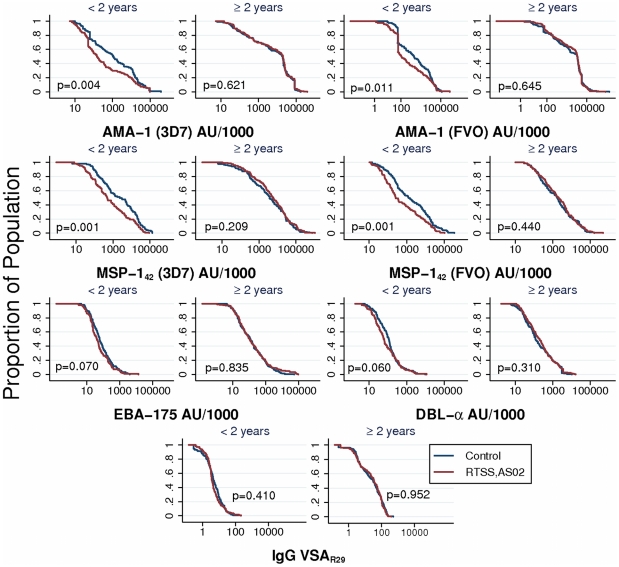
There were no significant differences in the distribution of IgG responses to any of the antigens tested between RTS,S/AS02 vaccine candidate and control groups in the crude (Figure 1 & Table S2), or adjusted (Table 1) analyses, and this was the case for both cohorts separately (Table S3). However, the effect of RTS,S/AS02 vaccination on blood stage antibodies was different by age group (Figure 2), with lower IgG levels for the 3D7 and FVO strains of AMA-1 (p = 0.0104 & p = 0.0147, respectively) and MSP-142 (p = 0.0009 & p = 0.0142, respectively) in the RTS,S group compared to control, among younger (< 2 years) children but not in the older (≥ 2 years) children (Table 2 & Table S4). No difference was seen in either age group for antibodies against EBA-175, DBL-α, or VSAR29.
Figure 1. Reverse cumulative distribution of crude IgG responses by treatment group.
The graphs represent the pooled antibody responses of children 1–4 years (cohorts 1 & 2 together) who received RTS,S/AS02 vaccine or control vaccine.
Table 1. Multivariate linear regression model showing the effect of RTS,S vaccination on levels of IgG to blood stage antigens, compared to control vaccination, adjusted by cohort, age, IFAT titer at baseline, batch of experiments, previous episodes and present infection.
| Antigen | Prop. Diffa | 95% CIb | p | R2 c |
| AMA-1 (3D7) | 0.82 | 0.61–1.10 | 0.190 | 0.63 |
| AMA-1 (FVO) | 0.90 | 0.66–1.24 | 0.525 | 0.62 |
| MSP-142 (3D7) | 0.97 | 0.72–1.32 | 0.867 | 0.48 |
| MSP-142 (FVO) | 0.87 | 0.64–1.18 | 0.365 | 0.34 |
| EBA-175 | 1.08 | 0.83–1.41 | 0.547 | 0.38 |
| DBL-α | 1.13 | 0.91–1.40 | 0.258 | 0.43 |
| VSAR29 | 1.07 | 0.87–1.33 | 0.502 | 0.49 |
Proportional difference refers to the proportional effect per log-increase in antibody level.
Confidence Interval.
R2 value of the OLS regression model was <0.65 in all cases, indicating that only a portion of the variability of IgG data is explained in the model.
Figure 2. Reverse cumulative distribution of antibodies in RTS,S/AS02 vaccine vs. control vaccine groups, stratified by age group.
The graphs represent the pooled antibody responses (cohorts 1 & 2) who received RTS,S/AS02 vaccine or control vaccine.
Table 2. F-test for interactions between RTS,S vaccine group and key variables on antibody levels.
| Prop. Diff.a (95% CI) <2 years | Prop. Diff.a (95% CI) >2 years | p-value interaction RTS,S x Age group | |
| AMA-1 (3D7) | 0.51 (0.32–0.81) | 1.11 (0.76–1.63) | 0.0104 |
| AMA-1 (FVO) | 0.55 (0.34–0.91) | 1.23 (0.82–1.84) | 0.0147 |
| MSP-142 (3D7) | 0.52 (0.33–0.82) | 1.46 (0.98–2.17) | 0.0009 |
| MSP-142 (FVO) | 0.54 (0.34–0.87) | 1.17 (0.79–1.74) | 0.0142 |
Results are proportional difference in antibody levels associated with being in the RTS,S/AS02 vaccine group.
Proportional difference refers to the proportional effect per log-increase in antibody level to blood stage antigens; CI: Confidence Interval.
As previously shown, antibodies were generally higher in the older age groups, in the region of higher transmission intensity (C2), in children with previous malaria episodes or infection at the time of sampling, and in those with higher pre-vaccination IFAT titers (Table S5).
Correlation of antibody responses to different antigens
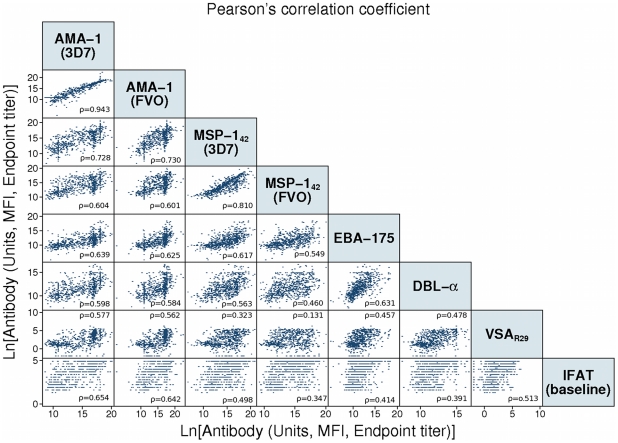
Antibody responses to the different antigens were not systematically correlated (Figure 3). Most antibody combinations were moderately (15/28) to highly (4/28) correlated. Correlation was high between AMA-1 3D7 and FVO (ρ: 0.94, p<0.001) or MSP-142 3D7 and FVO strains (ρ: 0.81, p<0.001), indicating high levels of cross-strain recognition. Prior to performing assays, HIP was titrated against AMA-1 and MSP-142 3D7- and FVO-coated microspheres in singleplex and multiplex to confirm a minimal level of cross-reactivity in the positive control plasma (data not shown).
Figure 3. Correlation of antibody responses to merozoite and VSA antigens within the individual.
A matrix of log-transformed antibody units or mean fluorescence intensities (VSAR29) against the selected blood stage antigens shows correlation of antibody responses between antigens. Pearson's correlation coefficient (ρ) is included in each antibody combination panel (p-value<0.001 for all correlations).
Antibody responses in relation to protection from clinical malaria
We calculated the effect of antibodies at 6 months post-vaccination on the hazard of clinical malaria, analyzed as continuous and categorized data. There were no divergences in the continuous or categorical models, and only the continuous models are reported here. In C1, there was no evidence of any association between antibodies and risk of clinical malaria. The hazard to the first or only episode of clinical malaria was significantly higher in children with previous malaria episodes (hazard ratio [HR]: 2.95, p<0.001), and lower in the older age group of children (HR: 0.54, p = 0.015). In C2, there was a lower risk of clinical malaria associated with higher IgG to EBA-175 and VSAR29 (Table 3 & Table S6), as well as a lower risk in the older age group (HR: 0.57, p = 0.041). The HR was, again, significantly higher in children with previous malaria episodes (HR: 1.91, p = 0.004).
Table 3. Cox proportional hazards model showing effect of IgG levels on risk of having a clinical malaria episode from 6–18 months post-vaccination.
| HRa | p | 95% CIb | |
| Cohort 1 | |||
| AMA-1 (3D7) | 0.97 | 0.409 | 0.90–1.05 |
| AMA-1 (FVO) | 0.99 | 0.731 | 0.92–1.06 |
| MSP-142 (3D7) | 0.95 | 0.160 | 0.87–1.02 |
| MSP-142 (FVO) | 0.94 | 0.107 | 0.87–1.01 |
| EBA-175 | 1.01 | 0.910 | 0.92–1.10 |
| DBL-α | 0.97 | 0.649 | 0.87–1.09 |
| VSAR29 | 1.12 | 0.055 | 1.00–1.26 |
| Cohort 2 | |||
| AMA-1 (3D7) | 0.97 | 0.577 | 0.88–1.07 |
| AMA-1 (FVO) | 0.97 | 0.589 | 0.88–1.07 |
| MSP-142 (3D7) | 0.92 | 0.033 | 0.85–0.99 |
| MSP-142 (FVO) | 0.92 | 0.056 | 0.85–1.00 |
| EBA-175b | 0.81 | 0.000 | 0.74–0.90 |
| DBL-α | 0.92 | 0.099 | 0.83–1.02 |
| VSAR29 b | 0.80 | 0.001 | 0.70–0.92 |
Only the univariate model is shown.
Hazard Ratio is the proportional effect on the hazard per doubling of antibody levels.
Confidence Interval. cSignificant in step-wise multivariate model (adjusted by vaccination group, age, previous clinical malaria cases and baseline immunofluorescence antibody test titers); VSA = Variant surface antigen.
Discussion
We tested a hypothesis that vaccination of children with the partially protective, pre-erythrocytic stage RTS, S adjuvanted vaccine candidate would elicit a broadly stronger asexual blood stage immune response that may be associated with long-term protection from clinical disease. Furthermore, we proposed that this observation would explain the waning efficacy observed in C2 and in newborns [8], [12]. From the onset, we were confronted with the question of which antigens to select among the nearly 2,000 antigens of the asexual blood stage (www.plasmodb.org). Our selection was limited by the current knowledge and availability of blood stage antigens, and we used targets expressed on the surface of merozoites or infected erythrocytes, thus accessible to the immune system and probably under immune pressure [39], and which have known functions in erythrocyte invasion, immune evasion and cytoadherence.
No differences were observed in the distribution of antibodies to AMA-1 (3D7 & FVO), MSP-142 (3D7 & FVO), EBA-175, DBL-α and VSAR29 when comparing all children in the RTS,S/AS02 and control vaccine groups, suggesting that RTS,S/AS02 neither enhances nor impairs antibodies naturally acquired against blood stage antigens. This finding contrasts with a recent report on blood stage antibodies in a Phase IIb trial of RTS,S in Kenya and Tanzania, which found a significant reduction in blood stage antibodies in RTS,S-immunized children [40]. However, when stratifying by age, AMA-1 and MSP-142 antibodies were lower in the RTS,S/AS02 group in children <2 years of age at enrollment. In this case, the results from Bejon et al. agree with ours, given that the ages of all children in that trial were below 2 years of age [9]. The reduction in episodes of malaria and, thus, exposure to parasites afforded by RTS, S/AS02, thereby reduces levels of IgG to some blood stage antigens, particularly antigens shown to be markers of exposure [41]–[43]. The absence of a detectable reduction in the older age group may be explained by the relatively high antibody levels already acquired in these children; reduced exposure in the RTS, S group may have a less dramatic effect on these antibody levels.
The hypothesis that waning efficacy in C2 is a result of interrupted exposure to low dose parasitemia hinges on a differential profile of blood stage antibodies from those of C1, which is not supported by these data. One explanation is that these antigens may merely serve as strong markers of exposure, which is expected to be lower overall in the RTS,S group. IgG to all antigens tested were higher in C2 and may indicate an already advanced level of blood stage immunity, where RTS,S gives less impact on blood stage antibodies. Lower antibodies in younger children may also be linked to exposure. Whereas blood stage antibodies to AMA-1 and MSP-142 in older children may have already reached a plateau, partial pre-erythrocytic protection afforded by RTS,S may reduce the levels of antibodies to antigens that are strongly associated with exposure in the younger children. We have no evidence that these lower levels of antibodies in younger RTS,S-vaccinated children result in an impairment of blood stage immunity over the 43 months of follow-up. Further studies should confirm this result. The difference in transmission intensity between cohorts remains a potential factor in vaccine efficacy that cannot be controlled in these data, but which may explain the waning efficacy observed in C2 through potential mechanisms such as increased likelihood of inoculation, greater exposure to parasite genetic diversity and a higher level of challenge to strain-specific or variant-specific naturally acquired immunity.
Interestingly, children with higher IgG to EBA-175 and VSAR29 at 6 months post-vaccination had a lower hazard of clinical malaria up to 18 months, but this result was only observed in C2. We found that vaccine group did not modify antibody levels to these antigens differently in C1 and C2, thus showing no interaction between vaccine group and cohort and suggesting that protection in C1 may be mediated by other immune factors. It is possible that the children living in Ilha Josina (C2) experience a faster build-up of immunity than those living in Manhiça (C1) due to the differences in transmission intensity, perhaps indicating a more “mature” immune response in C2. This is supported by the clinical patterns of disease in these populations [44], where the age pattern of disease is shifted to earlier age in Ilha Josina, compared to Manhiça (Aide et al., Guinovart et al., in preparation). It follows that affinity maturation of antibodies in C2 may also occur earlier than in C1 and account for differences in the protective effect of the antibodies. Thus, further characterization of the antibodies evaluated here, particularly fine specificity [45], subclasses of IgG [29], affinity and/or avidity [46] and in vitro functional capacity, could show further differences, both between cohorts and in prospective risk of disease.
Could other asexual blood stage antigens be more relevant to immunity? Evidence from a study in Mali before and after the transmission season suggests that a number of previously uncharacterized antigens may be better candidates for discrimination of protection from clinical disease [47]. A broader repertoire of antibody responses is shown to be associated with reduced risk of clinical malaria [48]. Anti-VSA antibodies and, particularly, how rapidly they are acquired in succession, may be important factors in parasite control [49]. The moderate correlation between antibodies against different antigens suggests that the selection may not be representative of the blood stage antibody immune response as a whole, and that different antibody levels could be expected within individuals for other antigens. It remains to be seen if these antibodies are acquired differently in RTS,S-vaccinated versus control-vaccinated individuals.
In conclusion, antibodies to the immunodominant blood stage antigens evaluated here were neither higher nor lower in RTS,S-immunized children compared to the control vaccine group, as a whole. In younger children, there seemed to be a reduction in antibodies against some antigens in the RTS,S group, although there is no evidence in this study that these lower antibody levels translate to an impairment of naturally acquired immunity. Based on our findings, antibodies to the common blood stage antigens tested do not explain the difference in long-term efficacy between C1 and C2. The data are not sufficient to reject the hypothesis that the RTS,S vaccine facilitates immunity upon exposure to blood stage parasites, as it is not known if the antigens selected are representative of, or relevant to, the overall protective antibody response of asexual blood stage antigens, and CMI responses were not addressed. Further characterization of naturally acquired blood stage antibodies must be done for the duration of the follow-up period to highlight protective antibody responses and effects of RTS,S vaccination in these two cohorts.
Supporting Information
Baseline characteristics of sample selection.
(DOCX)
Univariate analysis of vaccine group and antibody levels are presented in Tables S2A-S2G. Data are presented as geometric mean antibody units or geometric mean of mean fluorescence intensity with standard deviations (SD).
(DOCX)
Univariate analysis of vaccine group and antibody levels by study cohort.
(DOCX)
F-test for interaction between RTS,S vaccine group and adjusting variables of multivariate linear regression model. Data are displayed as p-values, where p<0.05 is considered a significant interaction.
(DOCX)
Multivariate analysis of vaccine group and antibody levels. The linear regression model was adjusted by cohort, age, IFAT titer at baseline, batch of experiments, previous episodes and present infection.
(DOCX)
Cox proportional hazards model showing effect of IgG levels on risk of having a clinical malaria episode from 6 – 18 months post-vaccination using a multivariate step-wise model adjusting for treatment, cohort, age group, and previous clinical malaria episodes.
(DOCX)
Acknowledgments
We wish to thank the children and parents who participated in this trial, without whom this study would not be possible. We thank Diana Barrios, Alfons Jimenez, Pau Cistero and Laura Puyol for laboratory support and assistance. We thank Joe Cohen, Amanda Leach, Marc Lievens, Johan Vekemans and Erik Jongert of GSK Biologicals for study design critique and review. Jean François Stallaert of GSK Biologicals provided assistance in sample procurement.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Program for Appropriate Technology in Health-Malaria Vaccine Initiative, the Fundación Ramon Areces, the Agència de Gestió d'Ajuts Universitaris i de Recerca [2010FI_B 00168 to JC] and the Spanish Ministry of Science and Innovation [RYC-2008-02631 to CD]. The Centro de Investigaçao em Saúde da Manhiça receives core support from the Spanish Agency for International Cooperation and Development. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Human Vaccines. 2010;6:90–6. doi: 10.4161/hv.6.1.9677. [DOI] [PubMed] [Google Scholar]
- 2.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. The New England Journal of Medicine. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 3.Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Review of Vaccines. 2007;6:723–39. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 4.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malaria Journal. 2009;8:312. doi: 10.1186/1475-2875-8-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. The Journal of Infectious Diseases. 2009;200:337–46. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 6.Casares S, Brumeanu T-D, Richie TL. The RTS,S malaria vaccine. Vaccine. 2010;28:4880–94. doi: 10.1016/j.vaccine.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. The Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 8.Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, et al. Safety of the RTS, S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. The Lancet. 2007;370:1543–1551. doi: 10.1016/S0140-6736(07)61542-6. [DOI] [PubMed] [Google Scholar]
- 9.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. The New England Journal of Medicine. 2008;359:2521–32. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aide P, Aponte JJ, Renom M, Nhampossa T, Sacarlal J, et al. Safety, immunogenicity and duration of protection of the RTS,S/AS02(D) malaria vaccine: one year follow-up of a randomized controlled phase I/IIb trial. PLoS One. 2010;5:e13838. doi: 10.1371/journal.pone.0013838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: a randomised controlled trial. The Lancet Infectious Diseases. 2011;11:102–9. doi: 10.1016/S1473-3099(10)70262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aide P, Dobaño C, Sacarlal J, Aponte JJ, Mandomando I, et al. Vaccine. [Epub ahead of print]; 2011. Four year immunogenicity of the RTS,S/AS02(A) malaria vaccine in Mozambican children during a phase IIb trial. [DOI] [PubMed] [Google Scholar]
- 13.Sacarlal J, Aide P, Aponte JJ, Renom M, Leach A, et al. Long-term safety and efficacy of the RTS,S/AS02A malaria vaccine in Mozambican children. The Journal of Infectious Diseases. 2009;200:329–36. doi: 10.1086/600119. [DOI] [PubMed] [Google Scholar]
- 14.Bojang KA, Milligan PJM, Pinder M, Vigneron L, Alloueche A, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. The Lancet. 2001;358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 15.Stoute JA, Kester KE, Krzych U, Wellde BT, Hall T, et al. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. The Journal of Infectious Diseases. 1998;178:1139–44. doi: 10.1086/515657. [DOI] [PubMed] [Google Scholar]
- 16.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, et al. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. The Journal of Infectious Diseases. 2001;183:640–7. doi: 10.1086/318534. [DOI] [PubMed] [Google Scholar]
- 17.Schellenberg D, Menendez C, Aponte JJ, Kahigwa E, Tanner M, et al. Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. The Lancet. 2005;365:1481–1483. doi: 10.1016/S0140-6736(05)66418-5. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland CJ, Drakeley CJ, Schellenberg D. How is childhood development of immunity to Plasmodium falciparum enhanced by certain antimalarial interventions? Malaria Journal. 2007;6:161. doi: 10.1186/1475-2875-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinovart C, Aponte JJ, Sacarlal J, Aide P, Leach A, et al. Insights into long-lasting protection induced by RTS,S/AS02A malaria vaccine: further results from a phase IIb trial in Mozambican children. PLoS One. 2009;4:e5165. doi: 10.1371/journal.pone.0005165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacarlal J, Aponte JJ, Aide P, Mandomando I, Bassat Q, et al. Safety of the RTS,S/AS02A malaria vaccine in Mozambican children during a Phase IIb trial. Vaccine. 2008;26:174–84. doi: 10.1016/j.vaccine.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Kocken CHM, Withers-Martinez C, Dubbeld MA, Wel A van der, Hackett F, et al. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infection and Immunity. 2002;70:4471–6. doi: 10.1128/IAI.70.8.4471-4476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angov E. Development and pre-clinical analysis of a Plasmodium falciparum Merozoite Surface Protein-142 malaria vaccine. Molecular and Biochemical Parasitology. 2003;128:195–204. doi: 10.1016/s0166-6851(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 23.Angov E, Hillier CJ, Kincaid RL, Lyon JA. Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLoS One. 2008;3:e2189. doi: 10.1371/journal.pone.0002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. The Journal of Clinical Investigation. 2010;120:4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayor A, Rovira-Vallbona E, Srivastava A, Sharma SK, Pati SS, et al. Functional and immunological characterization of a Duffy binding-like alpha domain from Plasmodium falciparum erythrocyte membrane protein 1 that mediates rosetting. Infection and Immunity. 2009;77:3857–63. doi: 10.1128/IAI.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–4. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 27.Triglia T, Healer J, Caruana SR, Hodder AN, Anders RF, et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Molecular Microbiology. 2000;38:706–18. doi: 10.1046/j.1365-2958.2000.02175.x. [DOI] [PubMed] [Google Scholar]
- 28.Okenu DM, Riley EM, Bickle QD, Agomo PU, Barbosa A, et al. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infection and Immunity. 2000;68:5559–66. doi: 10.1128/iai.68.10.5559-5566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infection and Immunity. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards JS, Stanisic DI, Fowkes FJI, Tavul L, Dabod E, et al. Association between Naturally Acquired Antibodies to Erythrocyte-Binding Antigens of Plasmodium falciparum and Protection from Malaria and High-Density Parasitemia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51:e50–60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 31.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. The Lancet. 2005;366:2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 32.Pandey KC, Singh S, Pattnaik P, Pillai CR, Pillai U, et al. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Molecular and Biochemical Parasitology. 2002;123:23–33. doi: 10.1016/s0166-6851(02)00122-6. [DOI] [PubMed] [Google Scholar]
- 33.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, et al. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little JA. Comparison of Curve Fitting Models for Ligand Binding Assays. Chromatographia. 2004;59:S177-S181-S181. [Google Scholar]
- 35.Gottschalk PG, Dunn JR. The five-parameter logistic: a characterization and comparison with the four-parameter logistic. Analytical Biochemistry. 2005;343:54–65. doi: 10.1016/j.ab.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 36.Serra-Casas E, Menéndez C, Bardají A, Quintó L, Dobaño C, et al. The effect of intermittent preventive treatment during pregnancy on malarial antibodies depends on HIV status and is not associated with poor delivery outcomes. The Journal of Infectious Diseases. 2010;201:123–31. doi: 10.1086/648595. [DOI] [PubMed] [Google Scholar]
- 37.Vestergaard LS, Lusingu JP, Nielsen MA, Mmbando BP, Dodoo D, et al. Differences in human antibody reactivity to Plasmodium falciparum variant surface antigens are dependent on age and malaria transmission intensity in northeastern Tanzania. Infection and Immunity. 2008;76:2706–14. doi: 10.1128/IAI.01401-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed GF, Meade BD, Steinhoff MC. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics. 1995;96:600–3. [PubMed] [Google Scholar]
- 39.Conway DJ, Cavanagh DR, Tanabe K, Roper C, Mikes ZS, et al. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nature Medicine. 2000;6:689–92. doi: 10.1038/76272. [DOI] [PubMed] [Google Scholar]
- 40.Bejon P, Cook J, Bergmann-Leitner E, Olotu A, Lusingu J, et al. Effect of the Pre-erythrocytic Candidate Malaria Vaccine RTS,S/AS01E on Blood Stage Immunity in Young Children. The Journal of Infectious Diseases. 2011;204:9–18. doi: 10.1093/infdis/jir222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SLR, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5108–13. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quelhas D, Puyol L, Quintó L, Serra-Casas E, Nhampossa T, et al. Impact of intermittent preventive treatment with sulfadoxine-pyrimethamine on antibody responses to erythrocytic-stage Plasmodium falciparum antigens in infants in Mozambique. Clinical and vaccine Immunology : CVI. 2008;15:1282–91. doi: 10.1128/CVI.00044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenhouse B, Ho B, Hubbard A, Njama-Meya D, Narum DL, et al. Antibodies to Plasmodium falciparum Antigens Predict a Higher Risk of Malaria But Protection From Symptoms Once Parasitemic. The Journal of Infectious Diseases. 2011;204:19–26. doi: 10.1093/infdis/jir223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aponte JJ, Menendez C, Schellenberg D, Kahigwa E, Mshinda H, et al. Age interactions in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Medicine. 2007;4:e242. doi: 10.1371/journal.pmed.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okech BA, Corran PH, Todd J, Joynson-Hicks A, Uthaipibull C, et al. Fine Specificity of Serum Antibodies to Plasmodium falciparum Merozoite Surface Protein, PfMSP-119, Predicts Protection from Malaria Infection and High-Density Parasitemia. Infection and Immunity. 2004;72:1557–1567. doi: 10.1128/IAI.72.3.1557-1567.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferreira MU, Kimura EAS, Souza JM de, Katzin AM. The Isotype Composition and Avidity of Naturally Acquired Anti-Plasmodium falciparum Antibodies: Differential Patterns in Clinically Immune Africans and Amazonian Patients. Am J Trop Med Hyg. 1996;55:315–323. doi: 10.4269/ajtmh.1996.55.315. [DOI] [PubMed] [Google Scholar]
- 47.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6958–63. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osier FHA, Fegan G, Polley SD, Murungi L, Verra F, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infection and Immunity. 2008;76:2240–8. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cham GKK, Turner L, Kurtis JD, Mutabingwa T, Fried M, et al. Hierarchical, domain type-specific acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 in Tanzanian children. Infection and Immunity. 2010;78:4653–9. doi: 10.1128/IAI.00593-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of sample selection.
(DOCX)
Univariate analysis of vaccine group and antibody levels are presented in Tables S2A-S2G. Data are presented as geometric mean antibody units or geometric mean of mean fluorescence intensity with standard deviations (SD).
(DOCX)
Univariate analysis of vaccine group and antibody levels by study cohort.
(DOCX)
F-test for interaction between RTS,S vaccine group and adjusting variables of multivariate linear regression model. Data are displayed as p-values, where p<0.05 is considered a significant interaction.
(DOCX)
Multivariate analysis of vaccine group and antibody levels. The linear regression model was adjusted by cohort, age, IFAT titer at baseline, batch of experiments, previous episodes and present infection.
(DOCX)
Cox proportional hazards model showing effect of IgG levels on risk of having a clinical malaria episode from 6 – 18 months post-vaccination using a multivariate step-wise model adjusting for treatment, cohort, age group, and previous clinical malaria episodes.
(DOCX)