Abstract
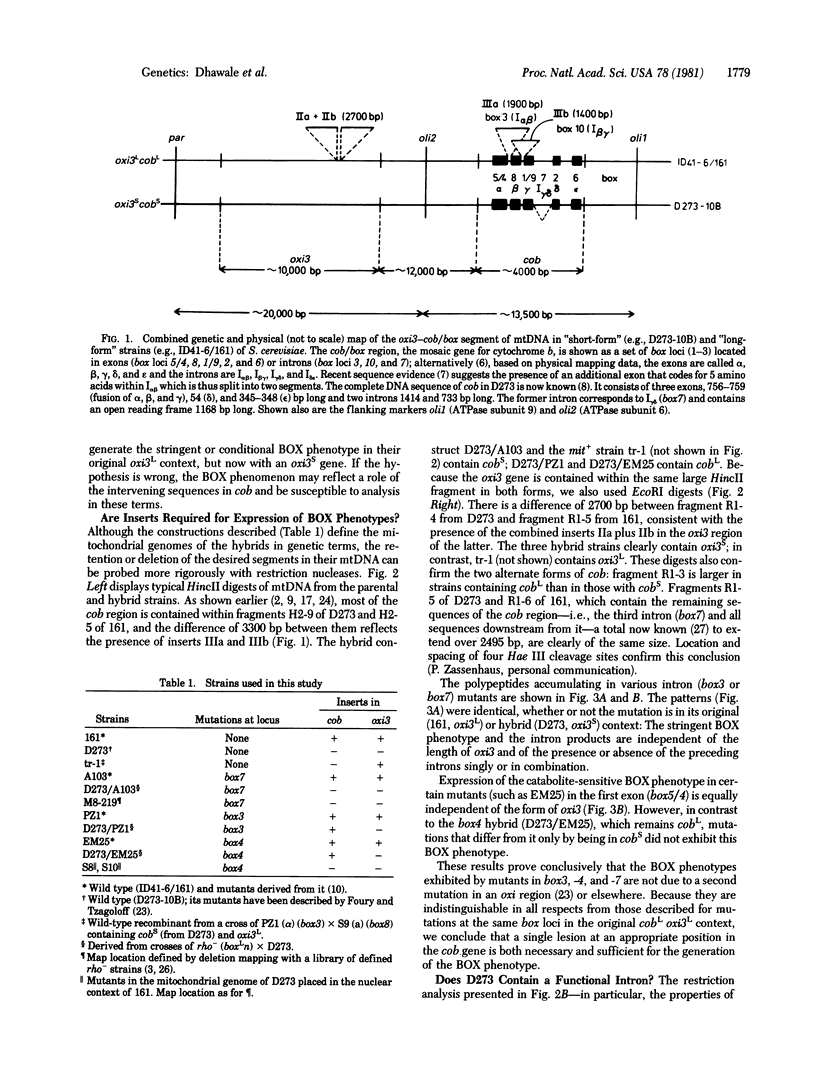
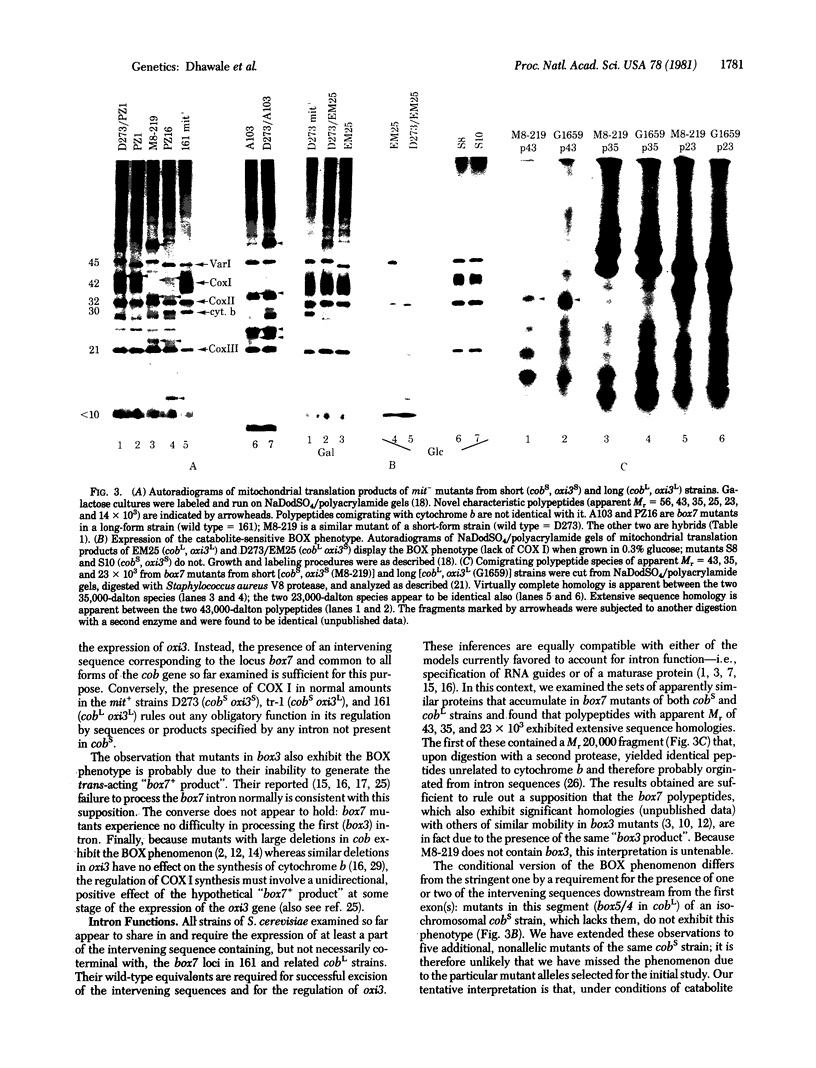
We have studied the mitochondrial DNA and the phenotypes of strains of Saccharomyces cerevisiae with specific intervening sequences in two mosaic genes: cob (the gene for apocytochrome b) and oxi3 (the gene for subunit I of cytochrome oxidase). The results suggest the following. (i) The presence of an intervening sequence downstream encompassing the intron box7 is sufficient for the regulation of oxi3 by cob (BOX phenotype); two sequences (containing intron loci box3 and box10) upstream in cob and two in oxi3 are dispensable. (ii) Strains without the two sequences upstream still contain the downstream sequence and the competence to specify a functional trans-acting element. Mutational lesions in this segment are phenotypically indistinguishable from box7 mutants, including the accumulation of polypeptides with homologous amino acid sequences. (iii) A catabolite-sensitive BOX phenotype, characteristic of mutants in the first exon, requires the simultaneous presence of an adjacent intervening sequence. A model is presented in which a hypothetical product specified by an intron (locus box7) of the cob gene controls the expression of a second mosaic gene (oxi3).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander N. J., Periman P. S., Hanson D. K., Mahler H. R. Mosaic organization of a mitochondrial gene: evidence from double mutants in the cytochrome b region of Saccharomyces cerevisiae. Cell. 1980 May;20(1):199–206. doi: 10.1016/0092-8674(80)90247-0. [DOI] [PubMed] [Google Scholar]
- Alexander N. J., Vincent R. D., Perlman P. S., Miller D. H., Hanson D. K., Mahler H. R. Regulatory interactions between mitochondrial genes. I. Genetic and biochemical characterization of some mutant types affecting apocytochrome b and cytochrome oxidase. J Biol Chem. 1979 Apr 10;254(7):2471–2479. [PubMed] [Google Scholar]
- Bos J. L., Osinga K. A., Van der Horst G., Hecht N. B., Tabak H. F., Van Ommen G. J., Borst P. Splice point sequence and transcripts of the intervening sequence in the mitochondrial 21S ribosomal RNA gene of yeast. Cell. 1980 May;20(1):207–214. doi: 10.1016/0092-8674(80)90248-2. [DOI] [PubMed] [Google Scholar]
- Church G. M., Slonimski P. P., Gilbert W. Pleiotropic mutations within two yeast mitochondrial cytochrome genes block mRNA processing. Cell. 1979 Dec;18(4):1209–1215. doi: 10.1016/0092-8674(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Claisse M. L., Slonimski P. P., Johnson J., Mahler H. R. Mutations within an intron and its flanking sites: patterns of novel polypeptides generated by mutants in one segment of the cob-box region of yeast mitochondrial DNA. Mol Gen Genet. 1980 Feb;177(3):375–387. doi: 10.1007/BF00271476. [DOI] [PubMed] [Google Scholar]
- Cordell B., Bell G., Tischer E., DeNoto F. M., Ullrich A., Pictet R., Rutter W. J., Goodman H. M. Isolation and characterization of a cloned rat insulin gene. Cell. 1979 Oct;18(2):533–543. doi: 10.1016/0092-8674(79)90070-9. [DOI] [PubMed] [Google Scholar]
- Crick F. Split genes and RNA splicing. Science. 1979 Apr 20;204(4390):264–271. doi: 10.1126/science.373120. [DOI] [PubMed] [Google Scholar]
- Dujon B. Mutants in a mosaic gene reveal functions for introns. Nature. 1979 Dec 20;282(5741):777–778. doi: 10.1038/282777a0. [DOI] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Foury F., Tzagloff A. Assembly of the mitochondrial membrane system. XIX. Genetic characterization of mit- mutants with deficiencies in cytochrome oxidase and coenzyme qh2-cytochrome c reductase. Mol Gen Genet. 1976 Nov 24;149(1):43–50. doi: 10.1007/BF00275959. [DOI] [PubMed] [Google Scholar]
- Haid A., Schweyen R. J., Bechmann H., Kaudewitz F., Solioz M., Schatz G. The mitochondrial COB region in yeast codes for apocytochrome b and is mosaic. Eur J Biochem. 1979 Mar;94(2):451–464. doi: 10.1111/j.1432-1033.1979.tb12913.x. [DOI] [PubMed] [Google Scholar]
- Halbreich A., Pajot P., Foucher M., Grandchamp C., Slonimski P. A pathway of cytochrome b mRNA processing in yeast mitochondria: specific splicing steps and an intron-derived circular DNA. Cell. 1980 Feb;19(2):321–329. doi: 10.1016/0092-8674(80)90506-1. [DOI] [PubMed] [Google Scholar]
- Hanson D. K., Miller D. H., Mahler H. R., Alexander N. J., Perlman P. S. Regulatory interaction between mitochondrial genes. II. Detailed characterization of novel mutants mapping within one cluster in the cob2 region. J Biol Chem. 1979 Apr 10;254(7):2480–2490. [PubMed] [Google Scholar]
- Kreike J., Bechmann H., Van Hemert F. J., Schweyen R. J., Boer P. H., Kaudewitz F., Groot G. S. The identification of apocytochrome b as a mitochondrial gene product and immunological evidence for altered apocytochrome b in yeast strains having mutations in the COB region of mitochondrial DNA. Eur J Biochem. 1979 Nov;101(2):607–617. doi: 10.1111/j.1432-1033.1979.tb19755.x. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Morimoto R., Rabinowitz M. Physical mapping of the yeast mitochondrial genome: derivation of the fine structure and gene map of strain D273-10B and comparison with a strain (MH41-7B) differing in genome size. Mol Gen Genet. 1979 Feb 16;170(1):25–48. [PubMed] [Google Scholar]
- Nishioka Y., Leder A., Leder P. Unusual alpha-globin-like gene that has cleanly lost both globin intervening sequences. Proc Natl Acad Sci U S A. 1980 May;77(5):2806–2809. doi: 10.1073/pnas.77.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Solioz M., Schatz G. Mutations in putative intervening sequences of the mitochondrial cytochrome b gene of yeast produce abnormal cytochrome b polypeptides. J Biol Chem. 1979 Oct 10;254(19):9331–9334. [PubMed] [Google Scholar]
- Van Ommen G. J., Boer P. H., Groot G. S., De Haan M., Roosendaal E., Grivell L. A., Haid A., Schweyen R. J. Mutations affecting RNA splicing and the interaction of gene expression of the yeast mitochondrial loci cob and oxi-3. Cell. 1980 May;20(1):173–183. doi: 10.1016/0092-8674(80)90245-7. [DOI] [PubMed] [Google Scholar]





