Abstract
Biomechanical factors profoundly influence the processes of tissue growth, development, maintenance, degeneration, and repair. Regenerative strategies to restore damaged or diseased tissues in vivo and create living tissue replacements in vitro have recently begun to harness advances in understanding of how cells and tissues sense and adapt to their mechanical environment. It is clear that biomechanical considerations will be fundamental to the successful development of clinical therapies based on principles of tissue engineering and regenerative medicine for a broad range of musculoskeletal, cardiovascular, craniofacial, skin, urinary, and neural tissues. Biomechanical stimuli may in fact hold the key to producing regenerated tissues with high strength and endurance. However, many challenges remain, particularly for tissues that function within complex and demanding mechanical environments in vivo. This paper reviews the present role and potential impact of experimental and computational biomechanics in engineering functional tissues using several illustrative examples of past successes and future grand challenges.
Introduction: History and Current Status
Although medical implants have ameliorated the consequences of tissue disease and injury for countless patients, the vast majority of implants are currently made from inert materials that do not promote biologic integration or functional tissue regeneration. Tissue autografts and allografts also have inherent limitations, including donor site morbidity, availability, and unacceptable failure rates for many clinical applications. Tissue engineering and regenerative medicine (TE/RM) are the emerging disciplines invoking strategies involving cells and/or bioregulatory factors, often in combination with polymeric materials, to create a tissue in vitro (tissue engineering) or induce tissue growth in vivo (regenerative medicine). TE/RM has the potential to revolutionize the next generation of implants to be more biologically interactive and long lasting. However, many challenges remain, particularly for tissues that function within complex and demanding mechanical environments in vivo. Engineered tissues must be able to function within the context of physiologic loading conditions once implanted. Moreover, it is increasingly apparent that local stresses and strains play a ubiquitous role in modulating cell behavior and thus the tissues they create and maintain. Biomechanical considerations are thus fundamental to the successful development of TE/RM-based clinical therapies.
The concept of a more biologic approach to implants that is represented by TE/RM goes back to before World War II.1 However, research in TE/RM did not really begin until the 1970s. The term “tissue engineering” was coined at a 1987 National Science Foundation meeting, and this was followed by the first tissue engineering workshop at Lake Tahoe in 1988.2 TE/RM research accelerated tremendously in the 1990s and an industry that accompanied research successes during this period was spawned. However, even then, the scientific, financial, and regulatory requirements to bring TE/RM combination products to market remained poorly understood. As a result, early living skin substitute products were initially a commercial failure, despite being a clinical success. Since then, several companies have emerged to produce profitable TE/RM products. Today, the traditional medical device industry is increasingly investing in TE/RM research and development and now recognizes that the convergence of biologics with medical devices will have an enormous impact on patient care.
One of the most underappreciated and difficult challenges in TE/RM has been to engineer or regenerate tissues with sufficient mechanical integrity to restore patient function.3 Musculoskeletal tissues such as articular cartilage, bone, intervertebral disc, ligament, tendon, meniscus, and muscle are all subject to exceptionally high mechanical demands in vivo. Similarly, in the cardiovascular area, biomechanics must be considered in myocardial repair, the design and fabrication of valvular replacements, and the engineering of blood vessel substitutes. As noted in the examples below, biomechanical stimuli may hold the key to producing high strength tissues for implantation. The purpose of this paper is to describe the critical role that biomechanics must play in achieving the goal of advancing from tissue replacement using inert implants to successful treatments based on TE/RM.
The Role of Biomechanics in TE/RM
Biomechanical interactions between cells and biomaterial scaffolds or matrices play a fundamental role in cell attachment, viability, and function. In living skin substitutes used clinically to treat burns and diabetic ulcers, for example, tension generated by fibroblasts in the collagen or fibrin gel that forms the dermal layer is essential to producing constructs with adequate biomechanical integrity. Recent work also indicates that stem-cell differentiation may be modulated by varying the stiffness of the underlying matrix.4,5 As illustrated in the representative examples below, another important role of biomechanics in TE/RM is the use of physical stimuli such as compression, stretching, and tension to create robust tissue constructs for implantation.
Dynamic compressive loading enhances engineered cartilage tissue formation
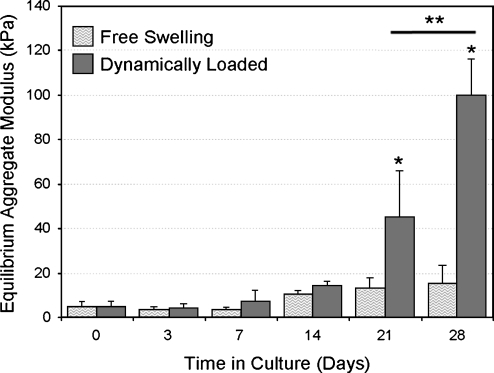
The mechanobiologic regulation of cartilage matrix biosynthesis has been extensively examined, with the understanding that physiologic mechanical forces can regulate tissue development and homeostasis.6 This understanding of cartilage mechanobiology was successfully exploited by the application of cyclic compression to constructs formed by encapsulating primary bovine chondrocytes in agarose hydrogels.7 Dramatic improvements in the compressive stiffness were observed by day 28 in groups that had been exposed to mechanical loading compared with free swelling controls (Fig. 1). A very exciting recent study reported that sequential application of transforming growth factor-β3 and dynamic loading produces engineered cartilage constructs with comparable mechanical properties and composition to native cartilage.8 Similar approaches have been employed to induce and optimize the chondrogenic differentiation of mesenchymal stem cells in three-dimensional (3D) culture9,10 to provide a convenient autologous cell source.
FIG. 1.
Effects of dynamic loading on the mechanical properties of cartilage contstructs.1 Mechanical preconditioning was carried out with three intermittent (separated by 1 h) loading cycles per day of 10% deformation at 1 Hz. Loading was then carried out for 5 days per week for a total of 4 weeks. *, Indicates significant differences between loaded samples and free-swelling controls; **, indicates a significant difference between peak stress of day 21 and day 28 loaded samples.
Cyclic mechanical distension strengthens engineered arteries
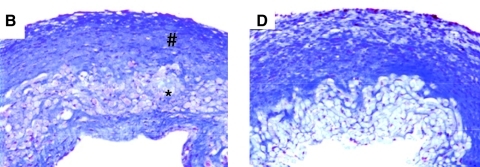
In the field of vascular tissue engineering, a landmark study using cyclic mechanical loading associated with the cardiovascular system, cyclic distension, was reported by Niklason and colleagues.11 In this study, tubular meshes of the synthetic degradable polymer poly(glycolic acid) were seeded with adult bovine aortic smooth muscle cells and placed around distensible silicone tubes for 8 weeks. The silicone tubes were cyclically inflated to 5% radial distension at 165 pulses per minute, and during the 8-week culture period, smooth muscle cells produced significant quantities of collagen as the polymer degraded. The burst pressure of these constructs averaged 2150 mmHg, which was superior to statically incubated controls having burst pressure of 1400 mmHg and compares favorably to the gold standard for coronary bypass grafts, human saphenous vein. The burst pressure differences mirror the histological differences (Fig. 2). Cyclic distention continues to be used as an effective strategy, with a recent study showing tensile strength increasing by a factor of almost threefold when the strain amplitude is increased incrementally rather than being held constant during the incubation period,12 motivating a rationale to define the optimal cyclic distention regimen.
FIG. 2.
Histology of engineered vessels. Cultured for 8 weeks revealed by Masson's Trichrome stain (collagen stains blue). (B) Cyclically stretched. (D) Nonstretched (original magnification × 100). Number sign indicates the dense cellular region; asterisk indicates the residual polymer region. Reproduced with permission from Niklason et al.11 Color images available online at www.liebertonline.com/ten.
Flow-mediated shear in a perfusion bioreactor increases 3D mineralized matrix formation
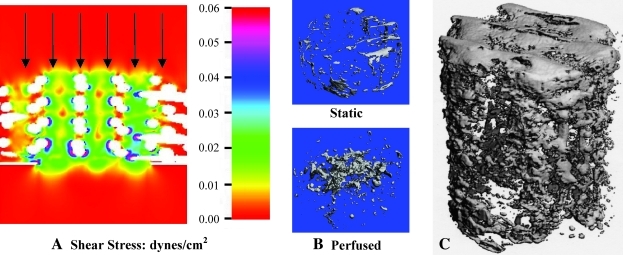
Without a vascular blood supply in vitro, nutrient delivery to cells throughout 3D tissue-engineered constructs grown in static culture must occur by diffusion. Attempts to grow tissues greater than 1 mm in thickness usually result in a thin shell of viable tissue and cells localized at the periphery. Therefore, tissue culture systems that provide dynamic media flow around or within tissue-engineered constructs have been designed to enhance nutrient and waste exchange in vitro. In addition to improving mass transport, dynamic flow bioreactors simultaneously deliver flow-mediated shear stresses to cells seeded within the constructs. In the example shown in Figure 3, fluid flow through 3D polycaprolactone scaffolds seeded with rat marrow-derived progenitor cells was simulated to determine local shear stress distribution (Fig. 3A).13 Experimentally, perfusion not only increased the amount of mineralized matrix by nearly fourfold but also improved the distribution of matrix synthesis compared with the mineralized shells formed in static culture (Fig. 3B). Mineralized constructs created using flow-mediated stimulation and model-based optimization are now approaching clinically relevant scales for treating large bone defects in patients (Fig. 3C).14 Recently, the combination of in vitro–generated extracellular matrix (ECM) and flow-mediated shear stress was shown to synergistically enhance osteoblast differentiation and mineralization within 3D titanium fiber mesh discs.15
FIG. 3.
(A) Three-dimensional Lattice–Boltzmann simulation of local shear stresses resulting from fluid flow through a porous polycaprolactone scaffold (white) seeded with marrow-derived progenitor cells in a perfusion bioreactor. (B) Micro-CT images of mineralized matrix synthesis in perfused constructs compared with constructs cultured under static conditions. (C) 9 mm long mineralized construct produced under dynamic culture using stem cells seeded on a polycaprolactone scaffold. Color images available online at www.liebertonline.com/ten.
Mechanical model predicts development of alignment in an engineered heart valve
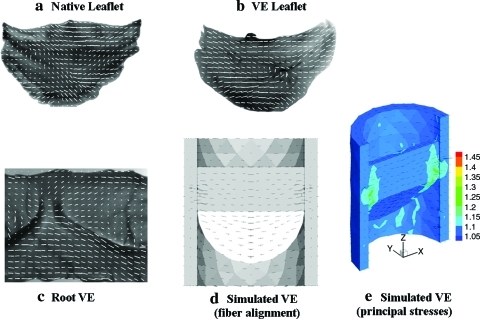
In one approach to heart valve tissue engineering, cell-induced compaction of a biopolymer gel around the surfaces of a casting mold was exploited to achieve the gross fiber alignment pattern of a native valve,16 which is circumferential in the root and lateral across the leaflets as shown in Figure 4. These alignment patterns are known to be critical in normal valve function, allowing leaflet bending for valve closure with minimal stress concentration. A continuum mechanical theory developed to predict this compaction-induced fiber alignment in any geometry17 can be used to simulate the outcome using a heart valve mold geometry. Figure 4 shows that the alignment patterns measured in a valve equivalent formed from human fibroblasts entrapped in fibrin gel agree qualitatively with the simulations.18 These can be used to assess stress concentrations and thereby optimize the design based on underlying biomechanical theory.
FIG. 4.
Fiber alignment in (a) native leaflet, (b) 3 week valve-equivalent leaflet, and (c) 3 week valve-equivalent root as determined by polarized light imaging. The orientations of the white lines correspond to the local average fiber direction, and their lengths are proportional to the local average retardation, a measure of the fiber alignment strength. Simulation of (d) gel compaction in the mold, and (e) principal stresses based on the anisotropic biphasic theory of tissue-equivalent mechanics.17 Black lines indicate principal fiber alignment directions in the root (triangulated gray area) and leaflet (solid white and gray interior areas) in (d) and directions of principal stresses in (e). Reproduced with permission from Robinson et al.18 Color images available online at www.liebertonline.com/ten.
Sustained growth of axons via continuous mechanical tension
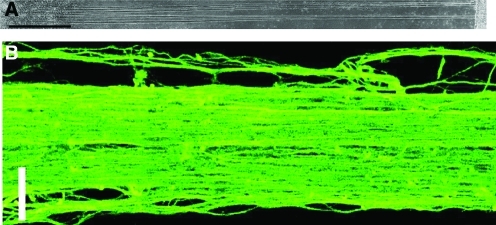
Repair of nerve injuries in the central nervous system could potentially be treated by transplantation of neurons projecting axons that span the nerve gap. Using a microstepper motor system to incrementally separate two membranes initially in close (50 μm) proximity (to allow for spontaneously growing axons from preadherent neurons on one membrane to anchor to the other membrane), it was reported that rapid growth rates of fasciculated axons could be sustained19 as seen in Figure 5. By optimizing the in vitro stretch growth process, axon growth rates of up to 1 cm/day have recently been achieved.20,21 This axon growth method has been used to create 10 cm long transplantable nervous tissue constructs that have been shown to survive 4 weeks in a preclinical spinal cord injury model.22
FIG. 5.
Representative images of axon tracts after 7 days of stretch induced growth. (A) Phase micrograph showing tracts between neurons adherent on membranes at both ends (scale bar = 1 mm). (B) Confocal micrograph of immunostained microtubule protein of coalescing axons in a single tract (scale bar = 25 μm). Reproduced with permission from Smith et al.19 Color images available online at www.liebertonline.com/ten.
Molecular basis for regulating tissue growth and function
Although much has been learned regarding the role of physical stress and the mechanical environment on tissue growth from an experimental approach, studies are also underway to understand the underlying molecular processes that mediate these effects. Among those being actively investigated are (i) stretch activated ion channels, (ii) changes in protein activity associated with force-induced conformational change, and (iii) direct action on gene expression due to forces transmitted to the nucleus. Each of these requires an understanding of how forces are transmitted from the tissues to the resident cells, down to the level of the individual force-sensing proteins. Certain locations in the cell tend to be sites of force concentration—cell–matrix or cell–cell adhesions—and, not surprisingly, these also appear to be where some of the force-sensitive molecules are found.
In the specific context of TE/RM, the role of the ECM on, for example, cell differentiation processes needs to be investigated at the molecular scale. In this instance, aside from externally applied force, forces generated by the internal acto-myosin contractile apparatus create forces on these same adhesion plaques, activating specific signaling pathways that regulate cell function. Interestingly, the cells apparently use this as a mechanism for sensing the stiffness of their environment and elicit an appropriate response. Although our understanding of these processes has progressed enormously just during the past several years, this remains a vigorous area of research.
Future Impact of Biomechanics in TE/RM
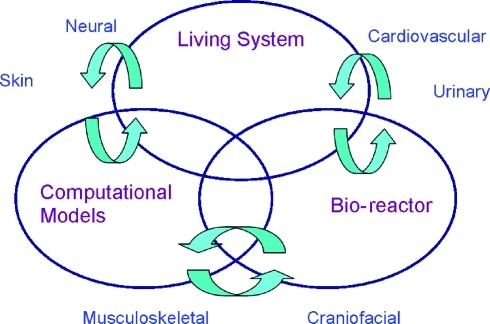
In this section, three primary areas where biomechanics will play a key role in the advancement of TE/RM in the next 10–15 years are described: (i) bioreactors that use optimal mechanical conditioning strategies to grow tissues for implantation and as models for in vitro studies; (ii) living systems' models that allow for the in vivo measurement of mechanical stresses and strains of native tissues to define the environment that implanted artificial tissues are subject to and must function in; and (iii) computational models that can be used to predict the growth and function of tissues, accounting for their complex nature and behavior. These areas are inextricably related and relevant to the engineering of a broad range of tissues as shown in the diagram of Figure 6.
FIG. 6.
Schematic illustrating the interactions required among in vivo living system, in vitro bioreactor, and computational models necessary to understand the influence of biomechanics on tissue repair and regeneration. Representative applications that will benefit from research in these strategic areas are included around the Venn diagram. Color images available online at www.liebertonline.com/ten.
Dynamic bioreactor systems
Critical to the success of tissue growth in vitro is an understanding of how a cell integrates extracellular stimuli into responses of interest (e.g., proliferation, differentiation, and secretion of ECM being responses that are essential to the tissue growth), and controlling how these stimuli are presented to cells in a growing tissue within a bioreactor. There is a large and growing literature showing that cells respond to a variety of forces in addition to a myriad of biomolecular stimuli.23 These forces include pressure and fluid shear acting on cells and cellular deformations associated with compressive and tensile forces transmitted through the cellular substratum.
Although correlations between these forces and cell responses are abundant, elucidation of the mechanotransduction pathways is far from complete. The conversion of external forces to signaling pathways involves intracellular forces and associated molecular deformations (e.g., gap junctions on the cell membrane, the cytoskeleton, and DNA). The integration of these pathways with those associated with, for example, growth factor signaling is even less understood, but ultimately must be defined to provide a basis for bioreactor design. Since the application of mechanical stimuli invariably occurs in the context of biomolecular stimuli, biomechanicians will continue to contribute in these areas.
A wide array of bioreactor designs has been proposed to provide for nutrient delivery (including growth factors) and/or mechanical stimulation. Many of these designs are based on engineering design principles, such as nutrient delivery rates based on convection and diffusion of nutrients, and deformation of the tissue construct due to applied stretching, compression, distention, etc. The continued role of biomechanicians in designing mechanical stimulation systems is obvious, although much greater sophistication in the design will occur, as elaborated below. It should be noted that since tissues are inherently hydrated and compliant, biomechanicians will play a central role in the analysis of the convective nutrient delivery rates as well as the mechanical stimulation; for example, the popular approach of applying cyclic deformation to growing tissue constructs involves a very complicated and potentially significant interstitial flow of culture medium (flow of fluid relative to the deforming ECM network) and associated convective delivery of nutrients.
A central challenge in advancing the field of TE/RM will be to design and, more importantly, operate bioreactors that “close the loop,” that is, connect the applied stretching, compression, and distention, to the forces experienced by the cell and the nutrient delivery to the cell, utilizing knowledge of the mechano/chemotransduction pathways. The combination of noninvasive imaging of key variables at the cellular scale (e.g., strains in the pericellular ECM and cell nucleus) in combination with multiscale models that make this connection will enable bioreactor operation that optimizes the stimuli for tissue growth in contrast to the current paradigm of operating with a constant (or arbitrarily altered) set of bioreactor conditions.
Living system models
Several factors have contributed to the slow trickle of new clinical products derived from tissue regenerative technologies. One factor has been an over-reliance in the field on qualitative assessment methods such as tissue histology as indicators of success. It is now recognized that quantitative measures are required to fully evaluate the ability of tissue-engineered constructs or other regenerative strategies to restore tissue function. Moreover, in vivo models are ultimately essential to test TE/RM technologies in a living system environment that includes revascularization, remodeling, and immune and inflammatory responses, which will clearly influence clinical success.3
Another factor has been the lack of appreciation of the exceptionally complex and challenging in vivo mechanical environment within which many of the tissues of interest in regenerative medicine function. This has led to the need to better establish normal 3D in vivo forces and displacements for activities of daily living in animal models of human disease and injury. Further, it is essential to determine how in vivo mechanical signals affect cell phenotype and matrix synthesis, revascularization, and tissue integration following the implantation of tissue-engineered constructs. Mechanical loading due to normal functional activities can be destructive to the regenerative process either by causing outright failure or shunting repair down a deleterious (e.g., fibrotic) pathway. Conversely, mechanical signals can also have a stimulatory effect on tissue regeneration and in some cases (e.g., bone) are believed to be necessary to achieve full restoration of function. One major challenge facing the field is to integrate mechanobiology principles into regenerative medicine strategies to exploit positive adaptive responses to mechanical signals in vivo. This might be done, for example, by design of tissue-engineered constructs that initially bear full in vivo loading but then resorb at a rate consistent with the formation of new tissue and thereby gradually transfer mechanical signals to the regenerating tissue.
Strategies for tissue regeneration must thus be evaluated using in vivo models to assess their ability to functionally integrate with a living host, remodel, and restore tissue function. Critical features of living system models are that they are repeatable, controllable, robust, discriminatory, and mimic a specific biological phenomena and/or the human clinical condition of interest. The most valuable in vivo models are those that can be integrated with noninvasive imaging methods that provide longitudinal monitoring of tissue regeneration at the molecular, cellular, and tissue levels and allow quantitative measurement of functional restoration via biomechanical testing. Such models are necessary to adequately explore the large potential design space of tissue-engineered constructs and to objectively discriminate the relative effectiveness of different TE/RM strategies.
Computational models
Computational modeling is the component of the integrated triad in tissue engineering and regenerative medicine that provides the theoretical-based computational tools that can be used to interpret and predict behavior for, and between, in vivo and in vitro systems.
Computational modeling in the context of this paper can be defined as the set of computational methods and algorithms that are used to solve a well-posed mathematical description of a physiological problem at the wide range of length or time scales relevant to tissue growth and homeostasis. The computational models ultimately serve as tools for analysis. In most cases, the mathematics is motivated by experimental observations of behavior that suggest, for example, that bone behaves like a nonlinear solid or cartilage like a multiphase material. Hypotheses about such mechanistic behaviors form the basis for mathematical equations that may be continuous, discrete, stochastic, etc., and are usually so complex that computer-based methods are needed for the solution. In addition, simulation plays a critical role in the interpretation of the response to stress or sensation of the mechanical environment. A combination of advances in computational power, improved molecular dynamics algorithms, and a rapid growth in the shear number of angstrom-level structures that are known has opened the door for significant simulations of protein conformational change. To be useful, however, a link must be made to stresses at the macroscale, and this requires computational methods that can span orders of magnitude in both length and time scales.
The foremost challenge and opportunity in this field, then, is advancing the multiscale and multicomponent, predictive computational modeling tools to analyze complex tissue and organ systems with:
accurate input variables such as complex microstructural characteristics and material properties
salient biological features that govern the multiple time scales and multiple processes (mechanical, chemical, and electrical)
rigorously validated experimental outcomes
patient-specific clinical applications.
In what follows, we elaborate briefly on the key points of this challenge:
Multiscale and multicomponents: computational models will be needed at time and length scales that span from those appropriate to cellular mechanotransduction to those appropriate to tissue mechanics, involving numerous molecular components. It will also be necessary to understand how to efficiently transfer information contained in the aforementioned variables across those scales.
Accurate input: computational models are only as accurate as the input data provided. These data include spatial and temporal distributions in geometric properties, microstructural characteristics, and material properties.
Salient biological features that govern processes: at the core of this challenging area is determining the quantitative mechanisms, expressed in mathematical form, that link the scales noted above and reflect the multiple, coupled processes, such as mechanical, chemical, and electrical, typically in play.
Validated outcomes: the models will first have to be shown capable of reproducing a range of experimentally measured outcomes, and then of providing accurate predictions of outcomes for a range of relevant physiological and biological input variables.
Patient-specific clinical applications: once the predictive capabilities of a computational model are established, it must be applied on a patient-specific basis. Critical issues here will include the ability to gather patient-specific input data and the ability to carry out the computations in a timely fashion.
As an example, a computational model for growth of a tissue in a bioreactor or after implantation of a scaffold might include the forces and nutrient transport to and within the material at one scale, their effects on cell behavior, such as migration, proliferation, and ECM deposition, and scaffold alteration at another scale, their effects on cell metabolism and gene expression at yet another scale, and the prescription of tissue mechanical and structural properties in terms of the cells and ECM to close the loop. A validated model would then provide an engineering tool for the optimal design and operation of the bioreactor and scaffold.
Grand Challenges
With current technologies, we are limited in our ability to engineer only tissues that possess modest structural and functional complexities. Thus, several challenges remain for TE/RM to widely succeed. These include challenges in mimicking the complex structure and function of the native ECM by controlling cell interactions with polymeric scaffolds so as to promote deposition of ECM that mimics native ECM, and in creating the complex architecture associated with native tissues and organs. Continued successes will greatly depend on our ability to fabricate stimuli-sensitive scaffolds that present an appropriate template for microstructural complexity and can be transformed by cells with appropriate stimulation (by the cells or via external intervention) into functional tissues. Apart from the cellular interaction with the scaffold via the proteins/peptides it presents, the biomechanical properties of the scaffold are crucial for the ensuing tissue growth since they mediate the strains and stresses imposed upon the cells in bioreactors designed to apply mechanical conditioning and in the patient after implantation. They may also play a role in developing the vascularization that is vital for oxygenation and nutrient exchange in many tissues, particularly metabolic organs. Listed below are some of the grand challenges associated with TE/RM for which biomechanics must be a primary consideration.
Limbs
Studies thus far have demonstrated the feasibility of developing various component tissues required for assembling a functional limb using a cell-scaffold approach. However, integrating individual components into a functional organ system pose unique challenges. Apart from integrating the vascular and nervous systems, one of the most important challenges will be developing and integrating tissues such as cartilage, tendon, and ligament, all of which have unique biomechanical functions. Another grand challenge will be in mimicking the structure and biomechanical properties of tissue interfaces, particularly articular joints.
Spinal cord
Surprisingly, not much research into the mechanical response of the spinal cord to loading, under either normal or abnormal conditions, has been performed to date. Mechanical cues play an important role in directed axonal growth as highlighted earlier. Development of suitable in vitro and in vivo models to evaluate these responses will be crucial in developing optimized constructs and implantation techniques for spinal cord repair.
Heart
There is a chronic shortage of donor hearts for transplantation. There are many efforts underway toward tissue-engineered blood vessels, heart valves, and cardiac tissue. All three components play a critical biomechanical role in the cardiac cycle leading to blood ejection, and recent tissue engineering strategies for all three components involve the controlled application of stresses and strains. An important challenge with high-impact consequences will be the ability to integrate these components into a functional heart replacement with sufficient pumping capacity, while also meeting the major challenge of microvascularization for the oxygenation demands of cardiac tissue.
In conclusion, biomechanical factors have a significant, yet incompletely understood influence on tissue growth, development, maintenance, degeneration, and repair. Recent advances in our understanding of the role of biomechanics in normal physiology and pathophysiology have begun to be harnessed to develop regenerative strategies to restore damaged or diseased tissues in vivo and create living tissue replacements in vitro. Many challenges remain, especially in relation to complex tissues, but the potential impact on health care is enormous.
Disclosure Statement
No competing financial interests exist.
References
- 1.Carrel A. Lindberg C. The Culture of Organs. The Canadian Medical Association Journal. New York: Paul B. Hoeber, Inc., Medical Book Department of Harper & Brothers; 1938. [Google Scholar]
- 2.Skalak R. Fox C. NSF Workshop: UCLA Symposia on Molecular and Cellular Biology. Molecular and Cellular Biology, New Series. New York: Alan R. Liss, Inc.; 1988. [Google Scholar]
- 3.Butler D.L. Goldstein S.A. Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 4.Engler A.J. Rehfeldt F. Sen S. Discher D.E. Microtissue elasticity: measurements by atomic force microscopy and its influence on cell differentiation. Methods Cell Biol. 2007;83:521. doi: 10.1016/S0091-679X(07)83022-6. [DOI] [PubMed] [Google Scholar]
- 5.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Guilak F. Sah R.L. Setton L.A. Physical regulation of cartilage metabolism. In: Mow V.C., editor; Hayes W.C., editor. Basic Orthopaedic Biomechanics. second. Philadelphia, PA: Lippincott-Raven; 1997. pp. 179–207. [Google Scholar]
- 7.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 8.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S. Ateshian G.A. Hung C.T. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C.Y. Hagar K.L. Frost L.E. Sun Y. Cheung H.S. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22:313. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- 10.Mauck R.L. Byers B.A. Yuan X. Tuan R.S. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 11.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 12.Syedain Z.H. Tranquillo R.T. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci USA. 2008;105:6537. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter B. Zauel R. Stockman H. Guldberg R. Fyhrie D. 3-D computational modeling of media flow through scaffolds in a perfusion bioreactor. J Biomech. 2005;38:543. doi: 10.1016/j.jbiomech.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Porter B.D. Lin A.S. Peister A. Hutmacher D. Guldberg R.E. Noninvasive image analysis of 3D construct mineralization in a perfusion bioreactor. Biomaterials. 2007;28:2525. doi: 10.1016/j.biomaterials.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Datta N. Pham Q.P. Sharma U. Sikavitsas V.I. Jansen J.A. Mikos A.G. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci USA. 2006;103:2488. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neidert M.R. Tranquillo R.T. Tissue-engineered valves with commissural alignment. Tissue Eng. 2006;12:891. doi: 10.1089/ten.2006.12.891. [DOI] [PubMed] [Google Scholar]
- 17.Barocas V.H. Tranquillo R.T. An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J Biomech Eng. 1997;119:137. doi: 10.1115/1.2796072. [DOI] [PubMed] [Google Scholar]
- 18.Robinson P.S. Johnson S.L. Evans M.C. Barocas V.H. Tranquillo R.T. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng. 2008;14:83. doi: 10.1089/ten.a.2007.0148. [DOI] [PubMed] [Google Scholar]
- 19.Smith D.H. Wolf J.A. Meaney D.F. A new strategy to produce sustained growth of central nervous system axons: continuous mechanical tension. Tissue Eng. 2001;7:131. doi: 10.1089/107632701300062714. [DOI] [PubMed] [Google Scholar]
- 20.Pfister B.J. Iwata A. Meaney D.F. Smith D.H. Extreme stretch growth of integrated axons. J Neurosci. 2004;24:7978. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfister B.J. Iwata A. Taylor A.G. Wolf J.A. Meaney D.F. Smith D.H. Development of transplantable nervous tissue constructs comprised of stretch-grown axons. J Neurosci Methods. 2006;153:95. doi: 10.1016/j.jneumeth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Iwata A. Browne K.D. Pfister B.J. Gruner J.A. Smith D.H. Long-term survival and outgrowth of mechanically engineered nervous tissue constructs implanted into spinal cord lesions. Tissue Eng. 2006;12:101. doi: 10.1089/ten.2006.12.101. [DOI] [PubMed] [Google Scholar]
- 23.Janmey P.A. McCulloch C.A. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]