Abstract
Background. The epidemiology of congenital malaria was investigated in a hospital-based malaria surveillance study in Papua, Indonesia.
Methods. From April 2005 to January 2010, 4878 delivering women and their newborns underwent prospective clinical review and malaria screening by peripheral blood microscopy.
Findings. Congenital malaria occurred in 8 per 1000 (38/4884) live births, with Plasmodium falciparum accounting for 76.3% (29) and P. vivax for 15.8% (6) of infections. Maternal malaria at delivery (adjusted odds ratio [AOR], 9.5; 95% confidence interval [CI], 4.2–21.5; P < .001), age ≤ 16 years (AOR, 4; 95% CI, 1.4–12.1; P = .011), and prior malaria during pregnancy (AOR, 2.2; 95% CI, 1.1–4.4, P = .022) were independent risk factors for vertical transmission. Of 29 mothers and neonates with contemporaneous peripheral parasitemia, 17% (5) had discordant parasite species, suggesting possible antenatal malaria transmission. Newborns with malaria were at significantly greater risk of low birth weight (AOR, 2.8; 95% CI, 1.2–6.6; P = .002). Following introduction of dihydroartemisinin-piperaquine for uncomplicated malaria in the second and third trimesters of pregnancy, congenital malaria incidence fell from 3.2% to 0.2% (odds ratio, 0.07; 95% CI, .03–.15; P < .001).
Conclusions. Congenital malaria is an important cause of neonatal morbidity in this region co-endemic for P. falciparum and P. vivax malaria. The introduction of artemisinin-combination therapy was associated with a significant risk reduction in the vertical transmission of malaria.
In areas where malaria is endemic, the risk of malaria starts in the early neonatal period, with congenital infections recognized as an important cause of morbidity [1–4]. The asymptomatic nature of infection in the early stages is likely to result in its true incidence being underestimated [5, 6].
In highly endemic areas of Africa, up to a third of infants acquire malaria in utero [7–10]. Vertical transmission is less well described in the Asia Pacific region, descriptions of its epidemiology often relying on case series/reports or maternal or placental malaria studies that are generally of small sample size [11–15]. In the present study we sought to define the risks of vertical malaria transmission along with its treatment and prevention in > 4000 neonates born in the local hospital in Timika, Papua-Indonesia, an area with highly prevalent drug-resistant Plasmodium falciparum and P. vivax [16].
METHODS
Study Site
The study was conducted in southern Papua, Indonesia. This area is largely forested, with both coastal and mountainous areas. Malaria transmission is restricted to the lowland area, where it is associated with 3 mosquito vectors: Anopheles koliensis, A. farauti, and A. punctulatus. Until November 2008, Rumah Sakit Mitra Masyarakat (RSMM) hospital, Timika, was the only hospital in this district servicing an area of 21 522 km2, with a population of ∼200 000 people. The annual incidence of malaria in the region is 876 per 1000 person-years, divided 60:40 between P. falciparum and P. vivax infections [17]. In this region, high levels of antimalarial drug resistance are present in both species, the risk of failure within 28 days reaching 65% after chloroquine monotherapy for P. vivax and 48% after chloroquine plus sulfadoxine-pyrimethamine for P. falciparum [16].
Study Population
The infant mortality rate in southern Papua is estimated as 68 per 1000 live births [18], with malaria being the most common cause of morbidity in the first year of life, followed by diarrhea and lower respiratory tract infections [19]. Malaria accounts for 30% and 14% of infant hospital admissions and outpatient visits, respectively [4]. Due to economic migration, the ethnic origin of the local population is diverse, with highland Papuans, lowland Papuans, and non-Papuans all resident in the region. Hospital policy dictates that all patients presenting with a febrile illness, irrespective of other symptoms, should have blood film examination for malaria.
Data Collection
From April 2004 hospital protocol dictated that all infants born at RSMM were to be screened for malaria within 3 days of birth. After obtaining parental/legal guardian consent, the malaria smear results were documented, and a trained research nurse or research physician performed systematic physical examination of the baby to assess clinical signs, gestational age (using New Ballard Score) [20], and the presence of external congenital malformations. Babies with a birth weight < 2500 g were defined as having a low birth weight (LBW), and those with gestational age of < 37 weeks, as being premature. External congenital malformation was diagnosed based on the International Classification of Diseases 10 [21]. Maternal clinical and laboratory data at delivery were also collected as described elsewhere [22].
If the newborn had malaria, a more detailed questionnaire on clinical and laboratory data was completed. In order to determine late congenital manifestations, all infants younger than 3 months old admitted with malaria to the pediatric wards were identified and invited to be part of the study. In the latter case maternal data at delivery were retrieved from hospital records if the infant was born at RSMM. A research nurse documented history of illness (including fever, restlessness, poor feeding, cough, and breathlessness) and weight of the infants. The infant was examined, and pulse, respiratory rate, and the presence of splenomegaly and hepatomegaly were recorded. Temperature was recorded per axilla, with fever defined as a temperature >37.5°C. Severe anemia was defined as having hemoglobin concentration <5 g/dL [23].
Malaria was diagnosed by microscopy of Giemsa-stained blood films. Slides were read by an expert microscopist and considered negative after review of 200 high-power fields. Parasite counts were determined from the number of parasites per 200 white blood cells (WBC) on Giemsa-stained thick films. Peripheral parasitemia was calculated from the recorded white cell count. A thin smear was also examined to confirm parasite species and used for quantification if parasitemia was > 200 per 200 WBC.
As clinically indicated, venous blood samples (1–5 mL) were drawn from infants admitted with malaria to the pediatric wards for complete blood counts and hemoglobin concentration (using electronic counter—Coulter JT). Venous sampling was not routinely conducted in neonates (age < 28 days) with peripheral malaria parasitemia unless requested by the attending clinician.
All infants with peripheral parasitemia received standard antimalarial therapy and supportive care as per hospital protocol. Before March 2006, young infants and pregnant women with uncomplicated malaria of any species were treated with quinine for 7 days (intravenous quinine was used for severe malaria). After March 2006, local protocols were changed to recommend dihydroartemisinin-piperaquine (DHP) for uncomplicated malaria in infants weighing > 5 kg and in pregnant women in the second and third trimesters of pregnancy. Neonates weighing < 5 kg were treated with oral quinine, and women in the first trimester, with quinine and clindamycin. Neonates and pregnant women with severe malaria were treated with intravenous artesunate [24].
Statistical Analysis
Data from the questionnaire and the laboratory were entered into EpiData 3.02 software (EpiData Association). Data were analyzed using SPSS vs 17.0 for Windows software (SPSS Inc). Normally distributed data were compared by Student t test. Data not conforming to a normal distribution were compared by the Mann–Whitney U test. Categorical data were compared by calculating the χ2 with Yates’s correction or by Fisher’s exact test and the odds ratio (OR) with 95% confidence intervals (CIs). Multiple logistic regression was used to analyze independent risk factors for congenital malaria by entering all significant risk factors in univariate analysis.
Ethical Approval
Ethical approval for this study was obtained from the ethics committees of the National Institute of Health Research and Development, Ministry of Health, Indonesia; and the Human Research Ethics Committee of the Menzies School of Health Research and Department of Health, Darwin, Australia.
RESULTS
Congenital Malaria
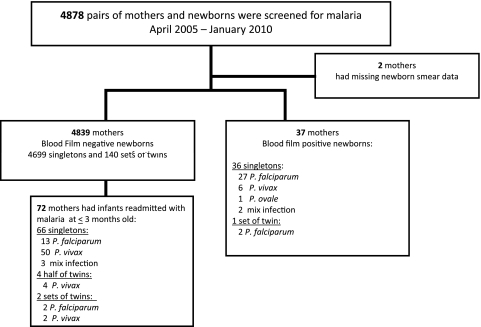
Between April 2004 and January 2010, 4884 (85%) of 5728 babies born at RSMM were screened for malaria (Figure 1). In total, 38 (0.8%) neonates were diagnosed with congenital infection within the first 3 days of life. P. falciparum accounted for 76.3% (29) of infections; P. vivax, for 15.8% (6); P. ovale, for 2.6% (1); and mixed species, for 5.3% (2). There was 1 pair of twins with congenital malaria, both of whom had P. falciparum. The median parasite density of the newborns was 100 μL−1 (range, 50–26 700 μL−1). All newborns were asymptomatic, with the exception of 1 baby with high parasitemia and severe malaria manifest as neonatal sepsis syndrome, as reported previously [25]. The median parasitemia was similar between infants infected with P. falciparum (100 μL−1; range, 50–26 688 μL−1) and P. vivax (125 μL−1; range, 50–300 μL−1; P = 1.0).
Figure 1.
Study profile.
Newborns with congenital malaria had a lower birth weight (mean, 2764; 95% CI, 2553–2975 g; with 32.4% [12/37] having LBW), compared with newborns without malaria (mean, 3031; 95% CI, 2997–3028 g; 13.3% (642/4838) with LBW; P = .02), the OR for LBW being 3.1 (95% CI, 1.6–6.3; P = .002). The effect of congenital malaria on LBW remained after controlling for other known risk factors in this population (maternal age, parity, maternal malaria, and severe anemia; adjusted odds ratio [AOR], 2.7; 95% CI, 1.1–6.5; P = .03).
Antimalarials were given to all parasitemic newborns, with 32 receiving quinine (oral ± intravenous); 2, intravenous artesunate followed with DHP; and 4, oral DHP alone. All malaria smears were negative at time of discharge from hospital.
Maternal Parasitemia and Vertical Transmission
Data on the blood film examination of the mothers were available in 4878 women, the prevalence of maternal malaria being 19% (928; Table 1). Of the 37 recorded cases of early vertical transmission, 9 (24%) were born from aparasitemic mothers. In the remaining 28 women with malaria at delivery, 5 had neonates with different parasite species, giving rise to discordance in 18% of cases (Table 1).
Table 1.
Vertical Malaria Transmission Profile
| Parasitemia at birtha |
||||||
| Maternal parasitemia | Negative | Plasmodium falciparum | P. vivax | P. ovale | Mixed infection | Overall vertical transmission |
| Negative (n = 3948) | 3939 (99.8) | 8 (0.2) | 1 (0.02) | 0 | 0 | 9 (0.2) |
| P. falciparum (n = 501) | 488 (97.2) | 12 (2.4) | 1 (0.2) | 0 | 0 | 13 (2.5) |
| P. vivax (n = 321) | 314 (97.8) | 3 (0.9) | 4 (1.2) | 0 | 0 | 7 (2.2) |
| P. ovale (n = 3) | 2 (66.7) | 0 (0) | 0 (0) | 1 (33.3) | 0 | 1 (33.3) |
| P. malariae (n = 50) | 49 (98) | 1 (2) | 0 (0) | 0 | 0 | 1 (2) |
| Mixed infection (n = 53) | 47 (88.7) | 4 (7.5)/3 | 0 (0) | 0 | 2 (3.8)/2 | 6 (11) |
All data are no. of cases (%).
Where deliveries included multiple births, vertical transmission was reported if at least 1 of the babies was found to be parasitemic.
Maternal Risk Factors
Maternal risk factors for vertical transmission are shown in Table 2. The risk of vertical transmission was 3% in women with peripheral parasitemia at delivery compared with 0.2% in those aparasitemic (OR, 13.6; 95% CI, 6.4–28.9; P < .001), with the population attributable risk of congenital infection being 70%. The risk of transmission did not vary with magnitude of maternal peripheral parasitemia even after controlling for the species of infection (Table 3). Afebrile mothers with malaria had a risk of transmitting the parasites vertically (2.5%, 16/642) comparable to symptomatic parasitemic mothers (4.2%, 12/284; P = .21). Regardless of parasitemia status at delivery, women with possible history of malaria infection during pregnancy had a 4.1-fold (95% CI, 2.1–7.9) greater risk of vertical transmission to the fetus compared with mothers without any history of malaria infections (P < .001). In the multivariate models, maternal malaria at delivery, history of malaria during pregnancy, and maternal age ≤ 16 years old remained as independent risk factors for vertical transmission (Table 2). Together these factors explained 89% of all congenital infections.
Table 2.
Maternal Risk Factors for Vertical Malaria Transmission
| Prevalence n/valid cases (%) | Prevalence of cases exposed to factor n/valid cases (%) | Univariate analysis |
PAR | Multivariate analysis |
PAR | |||
| Risk factor | OR (95%CI) | P value | AOR (95%CI) | P value | ||||
| Maternal peripheral parasitemia at birth | 928/4878 (19) | 28/37 (75) | 13.6 (6.4–28.9) | <.001 | 70% | 9.5 (4.2–21.5) | <.001 | 68% |
| No. | 3950/4878 (81) | 9/37 (24) | … | … | … | 1 | 1 | … |
| P. falciparum malaria | 501/4878 (10) | 13/37 (35) | 11.6 (4.9–27.4) | <.001 | 32% | 7.6 (2.9–19.4) | <.001 | 30% |
| P. vivax malaria | 321/4878 (6.6) | 7/37 (18.9) | 9.7 (3.6–26.4) | <.001 | 17% | 6.9 (2.4–19.3) | <.001 | 16% |
| Maternal fever | 365/4877 (7.5) | 13/37 (35) | 6.9 (3.5–13.7) | <.001 | 30% | 1.6 (.8–3.4) | .207 | 13% |
| Maternal history of febrile illness | 925/4881 (19) | 18/37 (48.6) | 4 (2.1–7.9) | <.001 | 36% | 2.2 (1.1–4.4) | .022 | 26% |
| Young mother (< 16 years old) | 118/4879 (2.4) | 4/37 (10.8) | 5 (1.7–14.4) | .012 | 8.6% | 4.1 (1.4–12.1) | .011 | 8.2% |
| Primipara | 1527/4878 (31) | 12/37 (32.4) | 1.05 (.5–2.1) | .860 | … | … | … | … |
| Severe anemia | 333/4740 (6.8) | 3/36 (8.3) | 1.2 (.4–3.9) | .74 | … | … | … | … |
| Preterm delivery | 748/4865 (15.3) | 8/37 (21.6) | 1.5 (.7–3.3) | .260 | … | … | … | |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio; PAR, population attributable risk, calculated as [(proportion of cases exposed to risk factor) × (OR – 1)]/OR.
Table 3.
Maternal Parasitemia at Delivery and Vertical Malaria Transmission
| Maternal parasitemia (geometric mean/μL) | Vertical malaria transmission |
P value | |
| Present | Absent | ||
| P. falciparum | 403 (81–1998) | 1480 (1211–1998) | .098 |
| P. vivax | 2186a | 665 (200–1998) | … |
One case only.
Malaria Treatment in Pregnancy
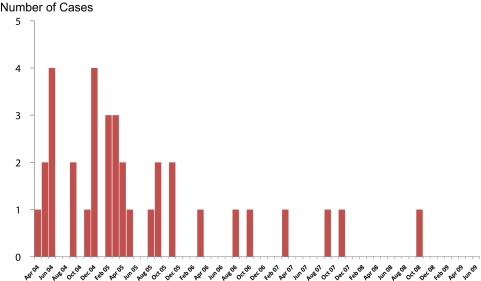
In total 905 pregnant women described a prior febrile episode during their pregnancy and were treated with antimalarial medication, with vertical transmission of infection observed in 18 (2%) births. DHP was introduced as first-line treatment of uncomplicated malaria in the second and third trimesters of pregnancy since March 2006. Babies born of mothers with a history of DHP treatment during pregnancy had a significantly lower risk of malaria (0.2%, 1/471) compared with those with prior exposure to chloroquine/quinine treatment (4.9%, 17/348; OR, 0.04; 95% CI, .005–.3; P < .001). The number of congenital malaria cases declined significantly following the change in malaria treatment policy, from 3.2% (29/907) before policy change to 0.2% (9/3912) after (OR, 0.07; 95% CI, .03–.15; P < .001). There has been no reported congenital malaria case since October 2008 (Figure 2). After controlling for other confounding factors, malaria treatment policy change remained as a protective factor for congenital malaria (AOR, 0.18; 95% CI, .032–.89; P = .036).
Figure 2.
Number of congenital malaria cases per month. Treatment policy for pregnant women in second and third trimesters changed to dihydroartemisinin-piperaquine in March 2006.
Possible Late Manifestation of Congenital Infection
Of 4878 hospital deliveries, 74 infants from 72 mothers (66 singletons, 4 half twins, and 2 sets of twin) were readmitted to the hospital in the first 3 months of life with symptomatic malaria. All of these infants had been screened at birth with negative blood films. On readmission, P. falciparum accounted for 20% (15), P. vivax for 76% (56), and mixed species for 4% (3) of the infections. One infant (92 days old) with mixed P. falciparum and P. vivax malaria had severe anemia and died within 24 hours of hospitalization. All other children were discharged from hospital after a median of 3 days’ (interquartile range, 1–2) inpatient stay.
The major maternal factors associated with admission of an infant to hospital with malaria in the first 3 months of life were maternal malaria at delivery (OR, 3.3; 95% CI, 2.1–5.3; P < .001) and a history of malaria during pregnancy (OR, 1.8; 95% CI, 1.1–3; P = .024). LBW infants and twins had a 2.3-fold (95% CI, 1.3–3.8; P = .005) and 3.5-fold (95% CI, 1.6–7.9; P = .01) higher risk of malaria in early life compared with those with normal birth weight and singletons, respectively.
DISCUSSION
This large surveillance study defines the epidemiology of congenital malaria and its outcome after treatment in Papua, Indonesia, an area co-endemic for both vivax and falciparum malaria. Congenital malaria was observed in 0.8% of live births, with the risk of vertical transmission rising almost 10-fold in women with peripheral parasitemia at delivery. This incidence was significantly lower than that reported from areas of high malaria transmission in Africa, which, with some exceptions [26], has generally ranged from 5% to 33% [8–10] and even higher (19%–50%) in women with peripheral parasitemia [7, 9, 10]. Contrary to previous hypotheses, these findings suggest that vertical transmission may be determined by other factors than just the level of malaria exposure and associated maternal immunity [27].
In almost 20% of cases the parasite species in the baby was discordant with the peripheral parasitemia of the mother. Vertical transmission of malaria occurring antenatally is well described in both African and Southeast Asian settings [8, 9, 11, 14, 25, 28, 29], and this may explain some of the materno-neonatal discordance in parasite species. In addition, the vertical transmission of infection may reflect the species of placental infection, in which case the peripheral parasitemia may be an incidental finding. However, because we did not routinely examine placental tissue, we were unable to investigate this further.
Most reports of congenital malaria originate from sub-Saharan Africa, where the majority of infections are due to P. falciparum. The epidemiology of congenital infection in Asia and South America is less well described. In these regions, where P. falciparum and P. vivax are co-endemic, vertical transmission can occur with either species [11, 14]. In the present study we routinely screened all babies within 3 days of birth and were thus able to reliably exclude infections acquired early in the postnatal period. P. vivax congenital infection (alone or mixed) occurred in 1.6 per 1000 live births and accounted for 22% of all congenital infections. Although the median P. falciparum parasitemia observed in newborns in Papua was higher (100 μL−1; range, 50–26 688 μL−1) than that reported in Africa (48 μL−1; range, 8–200 μL−1) [9], infections in Papua were almost all asymptomatic, compared with a third of infections being symptomatic in an African study undertaking active screening. These findings could not be explained by early detection since in both studies active screening was conducted at birth.
Fetal exposure to malarial parasites in utero has been proposed to modify the immune response of newborns, increasing their susceptibility to symptomatic malaria infections at birth and later in life [30–32]. In Africa, where peripheral parasitemia and placental malaria are highly prevalent, this increased susceptibility is associated with a greater risk of neonatal parasitemia and clinical malaria [27, 33]. In such settings neonatal screening is often reserved for symptomatic infants. However, in lower-transmission areas the consequence of leaving asymptomatic congenital malaria untreated may be significantly associated with greater malaria morbidity in early life [4]. As such, early detection by active screening of all babies is warranted where resources permit.
African infants with congenital malaria are at greater risk of LBW than aparasitemic infants [7, 10], and a similar observation was apparent in our Papuan study. Premature delivery associated with maternal malaria could not explain this increased risk, suggesting that other factors such as placental infection and insufficiency may play a crucial role in reducing fetal growth [34].
Our study highlights the importance of ensuring the effective treatment of maternal malaria. DHP has excellent efficacy against both multidrug-resistant P. falciparum and P. vivax. In March 2006 local guidelines for the treatment of maternal malaria in the second and third trimesters of pregnancy were changed from chloroquine plus sulfadoxine-pyrimethamine or quinine to DHP. Over the ensuing 3 years this policy has been associated with a large reduction in the incidence of congenital malaria. Indeed we have not witnessed a case of congenital malaria since October 2008, despite screening almost 1416 babies for malaria. Chloroquine and sulfadoxine-pyrimethamine have been the main treatment options for maternal malaria in most parts of Africa [9, 35, 36], but these policies are threatened by high rates of aminoquinoline and antifolate drug resistance. This may in part explain the high rates of congenital malaria reported from a number of sites in the continent [7, 9, 10] and further emphasizes the need for exploring more effective and reliable treatment strategies in pregnancy. In Kenya, congenital malaria has fallen in recent years in parallel with the fall in general community malaria burden, suggesting that malaria control interventions targeting the whole community may also contribute to reductions in congenital malaria [26].
There are a number of limitations to our study. We were reliant on microscopy of peripheral blood film for diagnosing malaria, and this is likely to have underestimated the real burden of congenital malaria by missing low-parasitemia infections and misdiagnosis of the species of infection [37]. The use of polymerase chain reaction diagnostics in previous reports may have detected a higher proportion of infections [29]. In addition, the lack of cord blood parasitemia and placental malaria data in our study will have also underestimated the true burden of congenital malaria [9, 29]. The risks to the baby documented in this article are therefore conservative estimates.
Currently, there are no consensus international guidelines for the case management of congenital malaria. In Africa asymptomatic congenital malaria is often left untreated if the parasites are cleared within 3 days [10]. Chloroquine and sulfadoxine-pyrimethamine have been used previously for treating congenital falciparum malaria [7, 38], but with increasing antimalarial drug resistance, these treatments can no longer be advocated [16, 39].
At our study site all infants with positive blood films were treated with antimalarial drugs irrespective of symptomatology; however, treatment options in this region are limited [4]. Due to the high prevalence of multidrug resistance in both P. falciparum and P. vivax [16], local guidelines recommend that infants <5 kg in weight with malaria should be treated with either a 7-day course of oral quinine or, if unable to tolerate oral medication, intravenous artesunate followed with DHP. In the current report all infants responded well to treatment, although they were not systematically followed up after discharge from hospital. The reality is that compliance with unsupervised 7-day courses of oral quinine is rare, and as a result, late recurrence rates are high [16]. Therefore, evidence for the safety and efficacy of reliable short-course treatment regimens is urgently required for malaria in neonates and young infants.
In conclusion, our study provides insights into the epidemiology of congenital malaria in endemic areas outside Africa. The introduction of a highly effective artemisinin combination therapy for malaria treatment in the second and third trimesters of pregnancy was associated with a marked decrease in the risk of congenital malaria. Routine screening of newborns for asymptomatic parasitemia followed by prompt, effective antimalarial treatment could reduce malaria burden in early life even further.
Notes
Acknowledgments.
We are grateful to the Mimika District Government; Lembaga Pengembangan Masyarakat Amungme Kamoro; the staff of the National Institute of Health Research and Development–Menzies School of Health Research, Timika, research facility; Dr Maurits J. Okoseray and Pak Erens Meokbun; the heads of the District Health Office; and Dr Rose McGready for her support and advice in carrying out the study and analysis.
Financial support.
This work was supported by the Wellcome Trust–National Health and Medical Research Council (Wellcome Trust ICRG GR071614MA-, NHMRC ICRG ID 283321). J. R. P. is supported by an AusAID Australian Leadership Award; N. M. A., by an NHMRC Practitioner Fellowship; and R. N. P., by a Wellcome Trust Senior Research Fellowship in Clinical Science (091625). The Timika Translational Research Facility is supported by AusAID.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Afolabi BM, Salako LA, Mafe AG, et al. Malaria in the first 6 months of life in urban African infants with anemia. Am J Trop Med Hyg. 2001;65:822–7. doi: 10.4269/ajtmh.2001.65.822. [DOI] [PubMed] [Google Scholar]
- 2.Slutsker L, Khoromana CO, Hightower AW, et al. Malaria infection in infancy in rural Malawi. Am J Trop Med Hyg. 1996;55(Suppl 1):71–6. doi: 10.4269/ajtmh.1996.55.71. [DOI] [PubMed] [Google Scholar]
- 3.Ibhanesebhor SE. Clinical characteristics of neonatal malaria. J Trop Pediatr. 1995;41:330–3. doi: 10.1093/tropej/41.6.330. [DOI] [PubMed] [Google Scholar]
- 4.Poespoprodjo JR, Fobia W, Kenangalem E, et al. Vivax malaria: a major cause of morbidity in early infancy. Clin Infect Dis. 2009;48:1704–12. doi: 10.1086/599041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menendez C, Mayor A. Congenital malaria: the least known consequence of malaria in pregnancy. Semin Fetal Neonatal Med. 2007;12:207–13. doi: 10.1016/j.siny.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Brabin BJ. Congenital malaria—a recurrent problem. Ann Trop Paediatr. 2007;27:95–8. doi: 10.1179/146532807X192453. [DOI] [PubMed] [Google Scholar]
- 7.Larkin GL, Thuma PE. Congenital malaria in a hyperendemic area. Am J Trop Med Hyg. 1991;45:587–92. doi: 10.4269/ajtmh.1991.45.587. [DOI] [PubMed] [Google Scholar]
- 8.Fischer PR. Congenital malaria: an African survey. Clin Pediatr (Phila) 1997;36:411–3. doi: 10.1177/000992289703600706. [DOI] [PubMed] [Google Scholar]
- 9.Falade C, Mokuolu O, Okafor H, et al. Epidemiology of congenital malaria in Nigeria: a multi-centre study. Trop Med Int Health. 2007;12:1279–87. doi: 10.1111/j.1365-3156.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- 10.Okafor UH, Oguonu T, Onah HE. Risk factors associated with congenital malaria in Enugu, south eastern Nigeria. J Obstet Gynaecol. 2006;26:612–6. doi: 10.1080/09638280600902893. [DOI] [PubMed] [Google Scholar]
- 11.Pengsaa K. Congenital malaria in Thailand. Ann Trop Paediatr. 2007;27:133–9. doi: 10.1179/146532807X192507. [DOI] [PubMed] [Google Scholar]
- 12.Valecha N, Bhatia S, Mehta S, Biswas S, Dash AP. Congenital malaria with atypical presentation: a case report from low transmission area in India. Malar J. 2007;6:43. doi: 10.1186/1475-2875-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuurkamp GJ, Paika RL, Spicer PE, Kereu RK. Congenital malaria due to Plasmodium vivax: a case study in Papua New Guinea. P N G Med J. 1986;29:309–12. [PubMed] [Google Scholar]
- 14.McGready R, Davison BB, Stepniewska K, et al. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg. 2004;70:398–407. [PubMed] [Google Scholar]
- 15.Lehner PJ, Andrews CJ. Congenital malaria in Papua New Guinea. Trans R Soc Trop Med Hyg. 1988;82:822–6. doi: 10.1016/0035-9203(88)90006-5. [DOI] [PubMed] [Google Scholar]
- 16.Ratcliff A, Siswantoro H, Kenangalem E, et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans R Soc Trop Med Hyg. 2007;101:351–9. doi: 10.1016/j.trstmh.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karyana M, Burdarm L, Yeung S, et al. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar J. 2008;7:148. doi: 10.1186/1475-2875-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidayat M. Rapid survey on maternal mortality in Papua Province. Papua, Indonesia: Provincial Health Office; 2001. [Google Scholar]
- 19.Mimika . Mimika District Health Office report. Timika, Indonesia: Mimika District Health Office; 2006. District Health Office. [Google Scholar]
- 20.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. International classification of diseases and related health problems. Geneva, Switzerland: World Health Organization; 2007. http://apps.who.int/classifications/apps/icd/icd10online/. Accessed 28 August 2011. [Google Scholar]
- 22.Poespoprodjo JR, Fobia W, Kenangalem E, et al. Adverse pregnancy outcomes in an area where multidrug-resistant Plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis. 2008;46:1374–81. doi: 10.1086/586743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Catalogue of health indicators: a selection of important health indicators recommended by WHO programmes. http://www.who.int/hac/techguidance/tools/en/Selected%20Health%20Indicators%20.pdf. Accessed 28 August 2011. [Google Scholar]
- 24.World Health Organization. Guidelines for the treatment of malaria. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 25.Poespoprodjo JR, Hasanuddin A, Fobia W, et al. Severe congenital malaria acquired in utero. Am J Trop Med Hyg. 2010;82:563–5. doi: 10.4269/ajtmh.2010.09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mwaniki MK, Talbert AW, Mturi FN, et al. Congenital and neonatal malaria in a rural Kenyan district hospital: an eight-year analysis. Malar J. 2010;9:313. doi: 10.1186/1475-2875-9-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGregor IA. Epidemiology, malaria and pregnancy. Am J Trop Med Hyg. 1984;33:517–25. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra I, Mungai P, Muchiri E, Kwiek JJ, Meshnick SR, King CL. Umbilical cord–blood infections with Plasmodium falciparum malaria are acquired antenatally in Kenya. J Infect Dis. 2006;194:176–83. doi: 10.1086/505150. [DOI] [PubMed] [Google Scholar]
- 29.Tobian AA, Mehlotra RK, Malhotra I, et al. Frequent umbilical cord–blood and maternal-blood infections with Plasmodium falciparum, P. malariae, and P. ovale in Kenya. J Infect Dis. 2000;182:558–63. doi: 10.1086/315729. [DOI] [PubMed] [Google Scholar]
- 30.Broen K, Brustoski K, Engelmann I, Luty AJ. Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Mol Biochem Parasitol. 2007;151:1–8. doi: 10.1016/j.molbiopara.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Dent A, Malhotra I, Mungai P, et al. Prenatal malaria immune experience affects acquisition of Plasmodium falciparum merozoite surface protein-1 invasion inhibitory antibodies during infancy. J Immunol. 2006;177:7139–45. doi: 10.4049/jimmunol.177.10.7139. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra I, Dent A, Mungai P, et al. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 2009;6:e1000116. doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41:1370–4. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adebami OJ, Owa JA, Oyedeji GA, Oyelami OA, Omoniyi-Esan GO. Associations between placental and cord blood malaria infection and fetal malnutrition in an area of malaria holoendemicity. Am J Trop Med Hyg. 2007;77:209–13. [PubMed] [Google Scholar]
- 35.Steketee RW, Wirima JJ, Slutsker L, et al. Malaria parasite infection during pregnancy and at delivery in mother, placenta, and newborn: efficacy of chloroquine and mefloquine in rural Malawi. Am J Trop Med Hyg. 1996;55(Suppl 1):24–32. doi: 10.4269/ajtmh.1996.55.24. [DOI] [PubMed] [Google Scholar]
- 36.Nosten F, McGready R, Mutabingwa T. Case management of malaria in pregnancy. Lancet Infect Dis. 2007;7:118–25. doi: 10.1016/S1473-3099(07)70023-3. [DOI] [PubMed] [Google Scholar]
- 37.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–40. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Hindi RD, Azimi PH. Congenital malaria due to Plasmodium falciparum. Pediatrics. 1980;66:977–9. [PubMed] [Google Scholar]
- 39.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–18. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]