Abstract
Tenofovir (TFV) 1% vaginal gel has been found to decrease sexual transmission of human immunodeficiency virus. To initiate investigations during pregnancy, 16 healthy pregnant women scheduled for cesarean delivery received a single application of TFV gel preoperatively. Maternal serum drug concentrations were determined and fetal cord blood, amniotic fluid, placental tissue, and endometrial tissue specimens were collected. The median maternal peak concentration and cord blood TFV concentrations were 4.3 and 1.9 ng/mL, respectively (∼100- and 40-fold lower than after TFV oral dosing, respectively). No adverse events were related to the use of TFV gel. These findings support ongoing and future investigations of TFV gel in pregnancy.
Clinical Trial Registration: NCT00572273. http://www.clinicaltrials.gov/ct2/show/NCT00540605?term=mtn-002&rank=1.
Heterosexual transmission now accounts for the majority of new infections globally from human immunodeficiency virus (HIV) [1]. Topical microbicides are a promising approach for the prevention of sexual transmission of HIV. CAPRISA 004 found that pericoital use of tenofovir (TFV) 1% vaginal gel lowered the risk of HIV transmission by 39% among heterosexual women [2]. With confirmation of efficacy from ongoing trials, an effective topical microbicide could soon be available for global use.
There are many compelling reasons to investigate topical microbicides, such as TFV gel, in pregnancy [3, 4]. An Institute of Medicine evaluation in 2008 recognized pregnancy as a major methodological challenge to the conduct and interpretation of HIV prevention trials [4]. These challenges include the many deleterious effects that unexpected pregnancies pose on study design, conduct, and analysis. Data from Gray et al [5] also suggest that pregnancy produces a 2-fold increased susceptibility to HIV acquisition. Additionally, incident HIV infection during pregnancy has been demonstrated to increase the odds 15-fold of maternal-to-child transmission (MTCT) [6]. These findings, plus the fact that pregnant women remain sexually active, warrant careful investigation of select candidate microbicides during pregnancy [7].
The objectives of this study were to characterize the maternal and fetal pharmacokinetics of single-dose TFV gel in term gestation, compare systemic absorption in pregnant women with that in nonpregnant women, and provide initial safety data for TFV gel in pregnancy.
METHODS
A phase 1 investigation of maternal single-dose pharmacokinetics and placental transfer of TFV gel among healthy term gravidas was performed by the Microbicide Trials Network (MTN-002) at a single site, Magee-Womens Hospital in Pittsburgh, Pennsylvania, from August 2008 through January 2010. The protocol, sample informed consent forms, and all study materials were reviewed and approved by the Institutional Review Board of the University of Pittsburgh (Pittsburgh, Pennsylvania). All participants underwent a thorough informed consent process. Study gel and applicators were provided by CONRAD (Arlington, Virginia).
HIV-uninfected, healthy pregnant women previously scheduled for cesarean delivery of a singleton infant at term were recruited. Women with hypertension, diabetes, other known maternal disease, or placental or fetal abnormalities were excluded. All participants underwent screening for HIV infection, sexually transmitted infections, and evidence of renal and liver dysfunction prior to enrollment. Before gel administration, physical and pelvic examinations were performed, and maternal blood was collected. A single dose of TFV gel (40 mg TFV) was administered per vagina ∼2 hours before cesarean delivery.
During cesarean delivery and prior to the rupture of amniotic membranes, amniotic fluid was collected in cryovials (∼3–5 mL) for storage at −80°C. Immediately following delivery, samples of cord blood were collected, processed, and frozen. Samples of endometrium (2 cm3 from the endometrial surface) and placenta (2 cm3 from the fetal surface) were also collected and frozen to assess for intracellular drug concentration. TFV diphosphate (TFV-DP) is the active intracellular form of TFV that inhibits HIV-1 reverse transcriptase. Maternal blood specimens were drawn to determine serum TFV concentration at 1, 2, 4, 6, 8, 12, and 24 hours after gel administration. At 24 hours and 2 weeks after delivery, participants were evaluated for adverse events. Clinical and laboratory data were reviewed by a protocol safety review team monthly and by a study monitoring committee throughout the study.
Tenofovir Assays.
TFV and TFV-DP concentrations were determined by previously described LC-MS/MS methods validated for serum, amniotic fluid, and vaginal tissue; assay validation report and standard operating procedures were approved by the National Institutes of Health cross-network Clinical Pharmacology Quality Assurance program [8, 9]. Both assays used 13C5-TFV as internal standard. Samples were extracted by protein precipitation, separated on Zorbax Eclipse XDB-C18 column, and detected using positive electrospray ionization. The lower limit of detection for the TFV assay was 0.3 ng/mL with a precision of <11% and accuracy in the range of ±14%. TFV-DP in tissue lysates (70% methanol) was extracted and isolated using a single solid-phase extraction following which TFV-DP was hydrolyzed to TFV and injected into the reverse phase ultra performance liquid chromatography–tandem mass spectrometry system using electrospray ionization with detection via negative ion multiple reaction monitoring as described elsewhere [8]. TFV-DP lower limit of quantitation was 50 fmol TFV-DP/sample, precision <15%, and accuracy in the range of ±15%. The TFV-DP and TFV concentrations were normalized by tissue weight.
Binomial exact 95% confidence intervals (CIs) for the proportion of women with detectable TFV concentration at a given anatomic site were computed. Maternal serum pharmacokinetic estimates of TFV included peak concentration (Cmax) and time to peak concentration (Tmax). The ratio of TFV concentrations in each matrix relative to maternal serum was calculated using a simple linear interpolation (based on the serum sample preceding and following a nonblood sample) to estimate the maternal serum concentration at the time the nonserum sample was taken. Ratios of tissue concentration relative to serum assume similar specific gravity in serum and tissue to establish a rough equivalence of units (pg/mL = ng/mg).
RESULTS
Two-hundred twelve women were approached on the basis of potential eligibility for participation, and 21 women were interested in participating, eligible for the study, and subsequently enrolled. Sixteen women received TFV gel, 4 women delivered prior to gel placement, and 1 participant subsequently declined further participation. Of the 16 women who received gel, 8 were white, 7 were black, and 1 was Native American. The median age (± standard deviation) was 27.2 ± 4.0 years.
TFV vaginal gel was very well tolerated by the participants, with no vulvovaginal complaints related to the study product. A total of 146 adverse events were recorded, including 112 among mothers and 34 among newborns. Ninety-four (84%) of the maternal adverse events were mild or moderate, and 18 (16%) were severe. None of the adverse events were assessed to be related to study product. Nearly all maternal adverse events were related to the pregnancy, surgery, and/or postoperative recovery. Of those adverse events that occurred among infants, 33 (97%) were mild, 1 was moderate, and none were related to the study product.
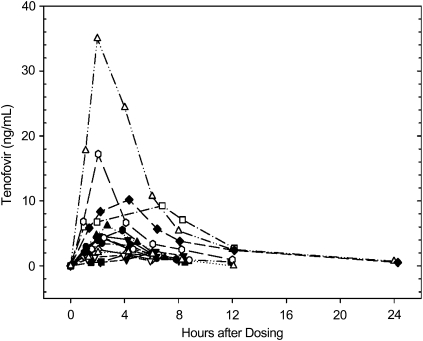
All 16 women (95% CI, 79%–100%) had detectable concentrations of TFV in their serum during the 24-hour period after dosing (Figure 1). The median Cmax for the mothers was 4.3 ng/mL (interquartile range [IQR], 2.2–7.0 ng/mL) (Table 1). The median Tmax was 2.6 hours (IQR, 2.1–4.3 hours) after gel administration. Of note, only 5 (31%) of 16 (95% CI, 11%–59%) women had detectable TFV 12 hours after dosing, and only 2 (12.5%; 95% CI, 2%–38%) had detectable concentrations at 24 hours.
Figure 1.
Maternal serum tenofovir concentration in nanograms per milliliter (y-axis, linear scale) versus hours after dosing (x-axis) curves are shown for all 16 women who participated in the study. Each woman is indicated with a different symbol (legend not shown). Only values above the limit of quantitation (0.3 ng/mL), except for predose values, are shown.
Table 1.
Tenofovir Concentrations in Anatomic Locations Sampled
| Median value (interquartile range) |
|||
| Sample location | Percentage (proportion) of samples with detectable concentrationa | Tenofovirb | Other/Maternal Serum Ratioc |
| Maternal Cmax serum | 100 (16/16) | 4.3 (2.2–7.0) ng/mL | … |
| Maternal delivery serum | 100 (16/16) | 3.1 (1.6–5.0) ng/mL | 1.0 |
| Fetal cord blood | 94 (15/16) | 1.9 (1.0–3.0) ng/mL | 0.53 (0.47–0.61) |
| Amniotic fluid | 79 (11/14) | 0.5 (<0.3–2.2) ng/mL | 0.21 (0.07–0.39) |
| Placental tissue | 81 (13/16) | 2.6 (1.6–4.4) pg/mg | 0.79 (0.64–1.36)d |
| Endometrial tissue | 53 (8/15) | 1.8 (<0.3–3.6) pg/mg | 1.12 (0.62–1.89)d |
| Tenofovir diphosphatee | TFV-DP/TFV molar ratioe | ||
| Placental tissue | 13 (2/16) | 0.27 fmol/mg; 0.63 fmol/mg | 0.0014; 0.0033 |
| Endometrial tissue | 7 (1/15) | 0.41 fmol/mg | 0.0325 |
Abbreviation: Cmax, peak concentration.
Percentage (no. of samples quantifiable/no. of samples available) above the lower limit of assay quantitation.
Tenofovir concentrations are at the time of delivery for all matrices, except maternal Cmax serum.
Based on a single extravascular sample concentration (obtained from cord, amniotic fluid, placental, or endometrial tissue) divided by concurrent (linearly interpolated) maternal serum concentration; calculated only if both samples are above the limit of quantitation.
For ratios involving serum and tissue homogenate, we assume similar specific gravity for each matrix; therefore, 1 ng/mL = 1 pg/mg.
Because only 2 placental tissue samples and 1 uterine tissue sample had detectable tenofovir diphosphate, all data are shown for tenofovir diphosphate concentration and tenofovir diphosphate/tenofovir molar ratios.
TFV was detectable in 15 (94%) of 16 (95% CI, 70%–100%) cord blood samples and in 11 of 14 (79%; 95% CI, 49%–95%) amniotic fluid samples available for analysis. The median cord blood TFV concentration was 1.9 ng/mL (IQR, 1.0–3.0 ng/mL). This extrapolated to a median cord blood:maternal blood ratio of 0.5 (IQR, 0.5–0.6). Thus, fetal exposure was approximated at half the maternal serum concentration. The median amniotic fluid concentration noted in the 11 women was 0.5 ng/mL (IQR, <0.3–2.2 ng/mL). This extrapolated to an amniotic fluid:maternal serum ratio of 0.2 (IQR, 0.1–0.4) and an amniotic fluid:fetal serum ratio of 0.5 (IQR, 0.2–0.7).
Eight (53%) of 15 (95% CI, 27%–79%) and 13 (81%) of 16 (95% CI, 54%–96%) participants had detectable TFV in endometrial and placental tissues, respectively. The actual median concentrations for TFV in these tissues were very low, at 1.8 pg/mg (IQR, below quantitative limits to 3.6) in endometrium and 2.6 pg/mg (IQR, 1.6–4.4) in placenta. The median concentration for TFV-DP in both tissues was below the limit of quantification. The concentration of TFV-DP was above detection limits in only 1 (7%) of the subjects’ endometrial biopsy specimens (0.41 fmol/mg [0.18 ng/mL]) and 2 (13%) of the subjects’ placental biopsy specimens (0.27 fmol/mg [0.12 ng/mL] and 0.63 fmol/mg [0.28 ng/mL]). On a molar basis, the tissue TFV-DP concentration was 3.3% of the TFV concentrations in the 1 endometrial tissue biopsy and 0.14%–0.33% in the 2 placental tissue biopsies where TFV-DP was detectable.
DISCUSSION
This investigation demonstrates that single-dose TFV 1% vaginal gel use in term pregnancy produces measurable but very low concentrations of TFV in the maternal serum. Specifically, the median Cmax noted herein is ∼100-fold lower than the 443 ng/mL Cmax noted after a maternal dose of 600 mg oral TFV used for the prevention of MTCT [10]. Furthermore, the observed Cmax is 70-fold lower than steady-state blood TFV concentrations (303–375 ng/mL) seen in HIV-infected patients on a 300-mg tenofovir disoproxil fumarate daily treatment regimen [11]. TFV does cross the placenta after maternal vaginal dosing, as expected [10, 12]. However, the overall fetal exposure is also very low at roughly 40-fold less than after maternal oral dosing for prevention of MTCT [10]. These low cord blood levels are augmented by the finding that neither TFV nor TFV-DP concentrated in the uterine or placental tissues. In addition, the gel was well tolerated by the 16 women and their infants, with no concerning safety signals. These findings justify further investigation with increased dosing frequency in pregnancy.
TFV gel was chosen as the first topical HIV prevention agent to study in pregnancy because this formulation is the furthest along of all antiretroviral-based topical microbicides and has an excellent track record of safety [5, 10]. Tenofovir is classified as a pregnancy class B agent by the US Food and Drug Administration [13]. Moreover, cumulative data from the Antiretroviral Pregnancy Registry on TFV provide a high level of reassurance among >1500 pregnant women exposed to oral TFV, with over half of these exposures occurring in the first trimester [14].
Perhaps the most compelling reason to perform controlled investigations of TFV gel in pregnancy is the potential upcoming availability of this agent for HIV prevention [5]. If efficacy is substantiated, many sexually active women will be using these products soon, and inadvertent pregnancy exposure will occur. Additionally, the increased risk of MTCT from incident HIV during pregnancy suggests that it is a critical time to prevent new HIV infection [6]. Data assessing the safety of TFV gel in pregnancy are thus necessary and timely.
The current study is a first step in gathering a portfolio of information to address the safety of TFV gel use in pregnancy. The pharmacokinetics values generated for MTN-002 are very similar to those values noted in HIV Prevention Trials Network (HPTN) 050, which was the first study to investigate TFV gel among nonpregnant, sexually active women; after a single dose, the median Cmax was found to be <3.0 ng/mL (IQR, <3.0 to 4.3), and the median Tmax was found to be 4 hours (IQR, 2–6) (data on file in HPTN 050 database) [15]. These similarities suggest that pregnancy does not alter the absorption of this vaginally administered product. A higher percentage of participants in the current study (100%) had detectable serum TFV concentrations, compared with the percentage in HPTN 050 (33%), which is likely attributable to the 10-fold increased sensitivity of the current assay (level of quantification, 0.3 ng/mL), compared with 3 ng/mL, which was the level of quantification used for analysis in HPTN 050 [15].
Although these pharmacokinetic data are reassuring, larger-scale investigations with repeat dosing inclusive of varying times in pregnancy are currently underway to better delineate the safety and pharmacokinetics of this candidate HIV prevention agent. The first such study, MTN-008, is currently enrolling 90 term and near-term (≥34 weeks gestation) pregnant women who use TFV gel for 7 consecutive days. This approach will allow for investigation of the potential for TFV concentration in the fetal blood after repeated dosing. Pending reassuring safety data, subsequent international investigations will include larger numbers at sequentially earlier gestational ages who will be exposed to longer durations of gel use (28 consecutive days). These data, in addition to information from an ongoing HIV prevention agent exposure pregnancy registry among trial participants with inadvertent first trimester exposure to HIV prevention agents (MTN-016), will provide data on TFV gel use in all stages of pregnancy. This information will likely be available contemporaneously with pending efficacy data on the use of TFV gel in nonpregnant women and aim to enable informed global use.
The high level of safety noted in this single-dose study is also reassuring. Nearly all of the maternal adverse events were related to the pregnancy or surgical procedure. Likewise, all infant adverse events were benign and unrelated to the study product. Ongoing and future investigations with extended dosing will better delineate the safety profile of TFV gel in this important patient population.
Notes
Financial support.
This work was supported by the Division of AIDS, US National Institute of Allergy and Infectious Diseases, the US Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Mental Health, US National Institutes of Health (grant 1-U01-AI068633-0); and CONRAD (Arlington, Virginia).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. 2009 AIDS epidemic update. http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. Accessed 1 November 2010. [Google Scholar]
- 2.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;5996:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raymond EG, Taylor D, Cates W, Jr, et al. Pregnancy in effectiveness trials of HIV prevention agents. Sex Transms Dis. 2007;34:1035–9. doi: 10.1097/OLQ.0b013e3180e90586. [DOI] [PubMed] [Google Scholar]
- 4.Lagakos S, Gabel A. Methodological challenges in biomedical HIV prevention trials. Washington, DC: The National Academies Press; 2008. [Google Scholar]
- 5.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–8. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 6.Birkhead GS, Pulver WP, Warren BL, et al. Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York. Obstet Gynecol. 2010;115:1247–55. doi: 10.1097/AOG.0b013e3181e00955. [DOI] [PubMed] [Google Scholar]
- 7.Solberg DA, Butler J, Wagner NN. Sexual behavior in pregnancy. N Engl J Med. 1973;288:1098–103. doi: 10.1056/NEJM197305242882105. [DOI] [PubMed] [Google Scholar]
- 8.King T, Bushman L, Anderson PL, Delahunty T, Ray M, Fletcher CV. Quantitation of zidovudine triphosphate concentrations from human peripheral blood mononuclear cells by anion exchange solid phase extraction and liquid chromatography-tandem mass spectroscopy; an indirect quantitation methodology. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:248–57. doi: 10.1016/j.jchromb.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Keller MJ, Madan RP, Torres NM, et al. Development of biomarkers of microbicide efficacy and safety in a phase I study of tenofovir vaginal gel. Plos One . In press. [Google Scholar]
- 10.Mirochnick M, Kafulafula G, Kreitchmann R, et al. Program and abstracts from the 16th Conference on Retroviruses and Opportunistic Infections. (Montreal, Canada) Alexandria, VA: CROI: 2009. Pharmacokinetics (PK) of tenofovir disoproxil fumarate (TDF) after administration to HIV-1 infected pregnant women and their newborns [abstract #T-137] [Google Scholar]
- 11.Barditch-Crovo P, Deeks SG, Collier A, et al. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45:2733–9. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarantal AF, Marthas ML, Shaw JP, et al. Administration of 9-[2-(R)-(phophonomethoxy)propyl]adenine (PMPA) to gravid and infant rhesus macaques (Macacca mulatta): safety and efficacy studies. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:323–33. doi: 10.1097/00042560-199904010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Mayer KH, Maslankowski LA, Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–51. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 14.United States Food and Drug Administration. FDA pregnancy categories. http://depts.washington.edu/druginfo/Formulary/Pregnancy.pdf. Accessed 15 October 2010. [Google Scholar]
- 15.The Antiretroviral Pregnancy Registry. Semiannual report through 7-31-2010. http://www.apregistry.com/index.htm. Accessed 1 April 2011. [Google Scholar]