Abstract
Background. New vaccines against tuberculosis (TB) are urgently needed because the only available vaccine, Mycobacterium bovis bacillus Calmette-Guérin (BCG), fails to protect against pulmonary TB in adults. The recombinant ΔureC hly+ BCG (rBCG) is more efficient than parental BCG (pBCG) against pulmonary TB in preclinical studies and has proven safe and immunogenic in phase I clinical trials.
Methods. In an attempt to identify the mechanisms underlying the superior protection of rBCG, we compared the immune responses elicited after vaccination and subsequent aerosol infection with Mycobacterium tuberculosis (MTB) in mice.
Results. We demonstrate that both rBCG and pBCG induce marked type 1 cytokine responses, whereas only rBCG elicits a profound type 17 cytokine response in addition. We observed earlier recruitment of antigen-specific T lymphocytes to the lung upon MTB infection of rBCG-vaccinated mice. These T cells produced abundant type 1 cytokines after restimulation, resulting in 10-fold reduced bacterial burden 90 days after infection.
Conclusions. Our findings identify a general immunologic pathway for improved vaccination strategies against TB that can also be harnessed by other vaccine candidates.
Every year, up to 2 million people die from tuberculosis (TB) [1]. The only available vaccine against TB is Mycobacterium bovis bacillus Calmette-Guérin (BCG), which was used in humans for the first time in 1921 [2]. To date, 4 billion doses of BCG have been administered, making it the most widely used human vaccine worldwide [3]. Yet, we are still far from having achieved eradication of TB. BCG vaccination prevents tuberculous meningitis and miliary TB in infants [4]. However, protection against other forms of TB, notably pulmonary TB in adolescents and adults, is inconclusive, as emphasized by a meta-analysis that revealed protective efficacies ranging from 0% to 80% [5]. Therefore, new vaccines against TB are urgently needed. At present, new vaccination strategies against TB include recombinant BCG to replace or boost canonical BCG as well as subunit vaccines and nonreplicating viral vector-based vaccines to booster BCG prime [6, 7].
Identification of the immunologic mechanisms that underly protection can facilitate the rational design of novel vaccination strategies for TB prevention. Moreover, biomarkers indicative for protective immunity could serve as surrogate endpoints of clinical outcome in TB vaccine efficacy trials and thus reduce the duration of those trials as well as facilitate testing of larger numbers of vaccine candidates. Observational studies of newly infected, healthy contacts of patients with TB and BCG-vaccinated infants have been initiated to define such biomarkers [8]. Despite extensive research on the immune response to TB, the fundamental elements of protective memory have yet to be elucidated. After BCG vaccination, antigen-specific memory CD4 T cells are difficult to detect because of the paucity of immunodominant antigens. Currently, the most widely used biomarkers are based on elevated frequencies of CD4 T cells producing interferon γ (IFN-γ). Increasing evidence questions the value of IFN-γ as correlate of protection in TB [9, 10]. Undoubtedly IFN-γ does play a crucial role in defense against Mycobacterium tuberculosis (MTB) infection [11], but determination of IFN-γ levels alone can no longer be considered a reliable marker of protective immunity.
We constructed a recombinant ΔureC hly+ BCG (rBCG) strain that expresses membrane-perforating listeriolysin (hly) of Listeria monocytogenes and is devoid of urease C. This rBCG construct induces superior protection against aerogenic challenge with MTB compared with parental BCG (pBCG) [12]. The rBCG construct has successfully proven to be safe and immunogenic in phase I clinical trials. In this study, we compared immune responses after vaccination with rBCG or pBCG in an attempt to identify biomarkers that correlate with protection in a mouse model of TB infection. Our data reveal that rBCG concomitantly induced a balanced combination of type 1 and type 17 cytokine responses, whereas pBCG-induced immunity comprised a type 1 response only. The results of further experiments emphasize the unique role of interleukin (IL) 17 in improved protection against TB and hence point to general mechanisms for consideration in future vaccine design against this major health threat.
MATERIALS AND METHODS
Mice
Female BALB/c mice were kept under specific pathogen-free conditions. Experiments were conducted with the approval of the Landesamt für Gesundheit und Soziales (State Office of Health and Social Affairs, Berlin, Germany).
Bacteria
The MTB strains H37Rv, pBCG, and rBCG that were used have been described elsewhere [12]. Bacteria were grown in Middlebrook 7H9 agar supplemented with glycerol, 0.05% Tween 80, and albumin dextrose catalase. Midlogarithmic cultures were harvested and stored at –80°C. Stocks were titrated prior to use.
Vaccination and MTB Infection
The pBCG or rBCG (106 colony-forming units [CFUs]) were administered subcutaneously. Aerogenic infection was performed using a Glas-Col inhalation exposure system. Bacterial burdens were assessed by mechanical disruption of removed organs in phosphate-buffered saline (PBS) with 0.5% vol/vol Tween 80 and plating serial dilutions onto Middlebrook 7H11 agar plates supplemented with oleic acid-albumin-dextrose-catalase. Colonies were counted after 3 weeks.
Cell Isolation, Stimulations, and Flow Cytometry
Cells were purified as described elsewhere [13]. Cells were stimulated with 50 μg/mL purified protein derivative (PPD; SSI) for 20 hours for analysis by multiplex assay or for 6 hours in the presence of 25 μg/mL brefeldin A for intracellular cytokine staining. The following antibodies were used: CD4, IFN-γ, IL-2, Ly6G/C, CD11b, γδ-T-cell receptor, and CD49b (eBioscience). CD8α, tumor necrosis factor α (TNF-α), CD16/CD32, F4/80, and CD11c were purified from hybridoma supernatants and fluorescently labeled. IL-17 was obtained from BD Biosciences. Cells were analyzed using a FACSCanto II or LSRII and FACSDiva software (BD Biosciences). Cytokines were measured using the Bio-Plex Mouse Th1/Th2, IL-17, and IL-6 bead-based immunoassays (Bio-Rad). IL-21 and IL-22 were measured by enzyme-linked immunosorbent assay (R&D systems).
Peritoneal Lavage
The pBCG or rBCG was freshly prepared from midlogarithmic cultures; 106 CFUs were administered intraperitoneally. Recruited cells were obtained from the peritoneal cavity by injection of 5 mL PBS and analyzed by flow cytometry. Cytokine and chemokine levels were determined using the Bio-Plex Mouse Cytokine 23-plex kit (Bio-Rad).
RESULTS
Type 1 and Type 17 Cytokine Responses After rBCG and pBCG Vaccination
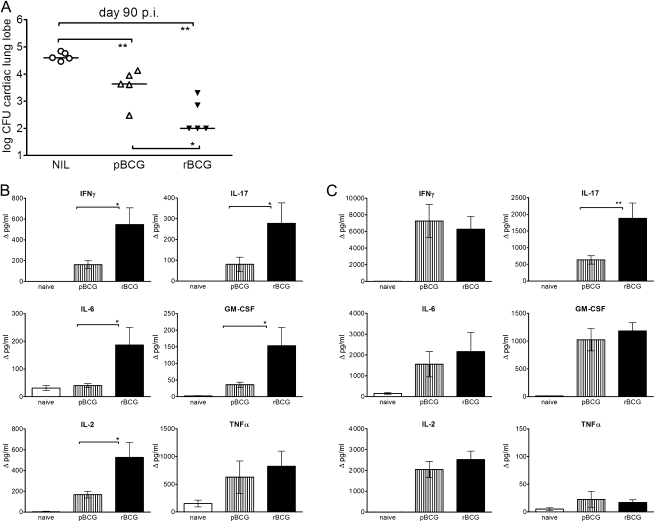
In an attempt to elucidate the immune mechanisms relevant to TB vaccine efficacy, we compared immune responses to rBCG and pBCG in mice. Superior protective efficacy of rBCG had been originally determined after intravenous immunization [12]. Subcutaneous administration of rBCG induced comparable levels of protection and retained the superior efficacy of rBCG over pBCG (Figure 1). We analyzed long-term memory responses 83 days after subcutaneous vaccination with rBCG or pBCG. Cells were restimulated with PPD, and supernatants were analyzed by multiplex assays for cytokines. Immunization with rBCG induced significantly higher cytokine production by cells isolated from the lung compared with pBCG (Figure 1). These cytokines included IFN-γ, IL-2, IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF). In contrast, levels of the type 2 cytokines IL-4, IL-5, and IL-10 were not increased above background levels (data not shown). Intriguingly, approximately 3-fold higher IL-17 concentrations were produced by lung cells from rBCG-vaccinated mice. Note that only a few cells could be isolated from the lungs of uninfected mice. Consequently, overall cytokine concentrations in lungs were lower than those in spleens, where 10-fold higher cell densities could be used for stimulation (Figure 1). In spleens, both vaccines elicited equally strong type 1 responses as reflected by comparable concentrations of IL-2, IL-6, IFN-γ, and GM-CSF. Yet, spleen cells from rBCG-vaccinated mice produced significantly more IL-17 upon restimulation with PPD compared with pBCG-vaccinated animals. Thus, immunization with rBCG, but not pBCG, induced concomitant and strong type 1 and type 17 cytokine responses in lungs and spleens, which were sustained for prolonged periods.
Figure 1.
Superior protection and cytokine induction by recombinant ΔureC hly+ bacillus Calmette-Guérin (rBCG) over parental bacillus Calmette-Guérin (pBCG) vaccination. A, Protection by subcutaneous immunization against infection with Mycobacterium tuberculosis (MTB). Mice were vaccinated 90 days before aerosol infection with 200–400 colony-forming units (CFUs) of MTB, and the bacterial burden was determined 90 days after infection. The cardiac lung lobe was homogenized; the remaining material was used for in vitro restimulation assays. Statistical significance was determined by the Mann-Whitney test with 2-tailed P values. *P < .05; **P < .01. Data are representative of 3 experiments with similar results. B, C, Responses in lungs and spleens, respectively, 83 days after subcutaneous vaccination with rBCG or pBCG. A total of 2.5 × 105 cells (lung) or 2 × 106 cells (spleen) were restimulated with purified protein derivative for 20 hours, and the supernatants were analyzed by multiplex assays. Cytokine concentrations are depicted as means (± SEM) of 4 independent experiments with 3 replicates each. Background cytokine production levels from medium controls were subtracted. Analysis of variance and the Bonferroni multiple comparison test were applied for statistical analysis. *P < .05; **P < .01. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL-2, interleukin 2; IL-6, interleukin 6; IL-17, interleukin 17; NIL, none; TNF-α, tumor necrosis factor α.
Enhanced Early Response of MTB-Specific T Cells Upon Infection With Virulent MTB in rBCG-Vaccinated Mice
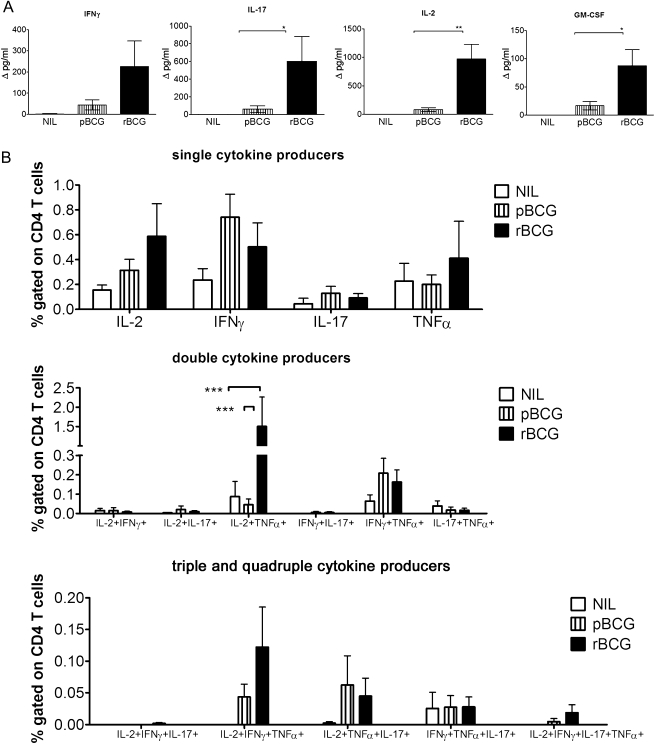
T-helper (Th) 17 cells have been linked to improved immune surveillance [14]. We compared antigen-specific T-cell responses in vaccinated animals upon aerosol infection with virulent MTB, 7 days after infection. Marked production of IFN-γ, IL-17, IL-2, and GM-CSF by lung cells from rBCG-vaccinated mice was detected 20 hours after restimulation with PPD (Figure 2). In contrast, these cytokines were barely secreted by cells from pBCG-vaccinated mice. In nonvaccinated animals infected with MTB, cytokine production levels were below the detection limit, in agreement with previous reports that MTB-specific T cells do not appear before 3 weeks after MTB infection [14]. Type 2 cytokines IL-4, IL-5, and IL-10 were not detected, and levels of TNF-α, IL-12p70, and IL-6 were only barely above background levels at this early time point after MTB challenge (data not shown).
Figure 2.
Vaccination with recombinant ΔureC hly+ bacillus Calmette-Guérin (rBCG) confers enhanced early responses of antigen-specific T cells in the lung upon aerosol infection with Mycobacterium tuberculosis (MTB). A, Cytokine secretion by lung cells 7 days after aerosol infection with 200–400 colony-forming units of MTB. A total of 2 × 105 cells were stimulated with purified protein derivative (PPD) for 20 hours, and the supernatants were analyzed by multiplex assay. Cytokine concentrations are depicted as means (± SEM) of 2 independent experiments with 3 replicates each. Background cytokine production levels from medium controls were subtracted. B, Analysis of cells restimulated with PPD for 6 hours in the presence of brefeldin A by multicolor flow cytometry. Frequencies of responding CD4 T cells are depicted as means (± SEM) of 3 independent experiments with 3 replicates each. Analysis of variance and the Bonferroni multiple comparison test were applied for statistical analysis. *P < .05; **P < .01; ***P < .001. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL-2, interleukin 2; IL-17, interleukin 17; NIL, none; pBCG, parental BCG; TNF-α, tumor necrosis factor α.
We determined cytokine production levels by lung T cells by flow cytometry 7 days after MTB infection (Figure 2). Cells were stimulated with PPD for 6 hours, followed by intracellular cytokine staining for IL-2, IL-17, IFN-γ, and TNF-α. In nonvaccinated control mice, a small proportion of CD4 T cells produced IL-2, TNF-α, or IFN-γ. In vaccinated mice, CD4 T cells secreted IL-2, IFN-γ, TNF-α, and IL-17. Frequencies of single-cytokine-producing cells were highest, albeit not significant, in the rBCG group. In vaccinated animals, we detected multifunctional T cells, which are implicated in protective immunity [15]. Multiple-cytokine–producing T cells were predominantly IL-2+TNF-α+ double producers, and levels were significantly increased upon rBCG vaccination. Triple-producer cells were almost exclusively IL-2+IFN-γ+TNF-α+, and levels were slightly increased in rBCG-vaccinated mice compared with those in pBCG-vaccinated mice. In addition, IL-2+TNF-α+IFN-γ+IL-17+ quadruple-positive cells exclusively appeared in the rBCG group, albeit at very low frequencies.
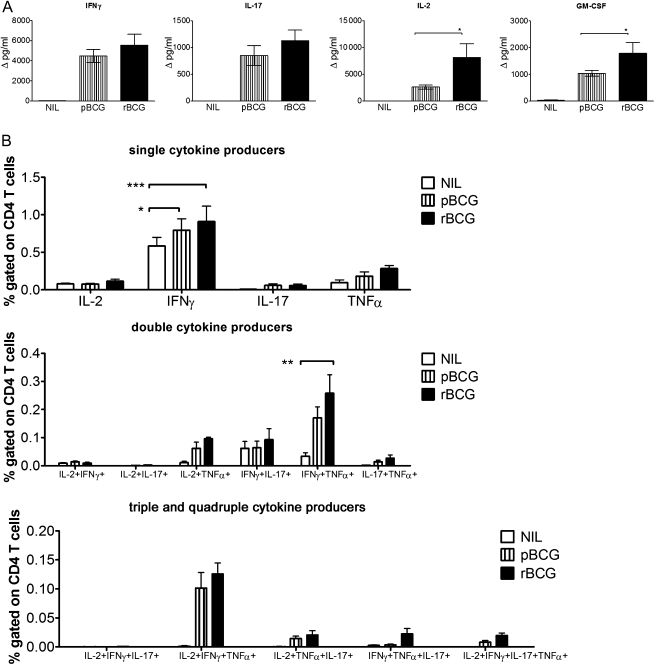
Splenic T cells produced similar cytokine patterns to those of pulmonary T cells (Figure 3). IL-2 and GM-CSF production was significantly higher in rBCG-vaccinated animals than in pBCG-vaccinated animals and below the detection level in nonvaccinated controls, even though a small percentage of CD4 T cells produced IFN-γ in nonvaccinated controls. In sum, vaccination with either pBCG or rBCG induced CD4 T cells secreting IFN-γ and TNF-α with higher frequencies of single and multiple producers in rBCG-vaccinated mice compared with pBCG-vaccinated mice. This is consistent with the finding that IL-17 accelerates recruitment of IFN-γ–producing cells to the site of infection [14]. Different levels of cytokine production and frequencies of producer cells were not due to different bacterial burdens, as confirmed by comparable numbers of CFUs in lungs and spleens (data not shown). Total numbers of T-regulatory cells increased upon infection to a comparable degree in vaccinated groups (data not shown). At 7 days after infection, CD4 T cells were the main cytokine producers; cytokine-producing CD8 T cells were detected with lower frequencies in lungs and spleens albeit with similar patterns (data not shown).
Figure 3.
Vaccination with recombinant ΔureC hly+ bacillus Calmette-Guérin (rBCG) increases purified protein derivative (PPD)–specific responses in the spleen upon aerosol infection with Mycobacterium tuberculosis (MTB). A, Cytokine secretion by spleen cells 7 days after aerosol infection with 200–400 colony-forming units of MTB. A total of 2 × 106 cells were restimulated with PPD for 20 hours, and the supernatants were analyzed by multiplex assays. Cytokine concentrations are depicted as means (± SEM) of 4 independent experiments with 3 replicates each. Background cytokine production levels from medium controls were subtracted. B, Analysis of cells restimulated with PPD for 6 hours in the presence of brefeldin A by multicolor flow cytometry. Frequencies of responding CD4 T cells are depicted as means (± SEM) of 3 independent experiments with 3 replicates each. Analysis of variance and the Bonferroni multiple comparison test were applied for statistical analysis. *P < .05; **P < .01; ***P < .001. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL-2, interleukin 2; IL-17, interleukin 17; NIL, none; pBCG, parental BCG; TNF-α, tumor necrosis factor α.
Vaccination With rBCG Confers Potent Immune Responses During Persistent Infection
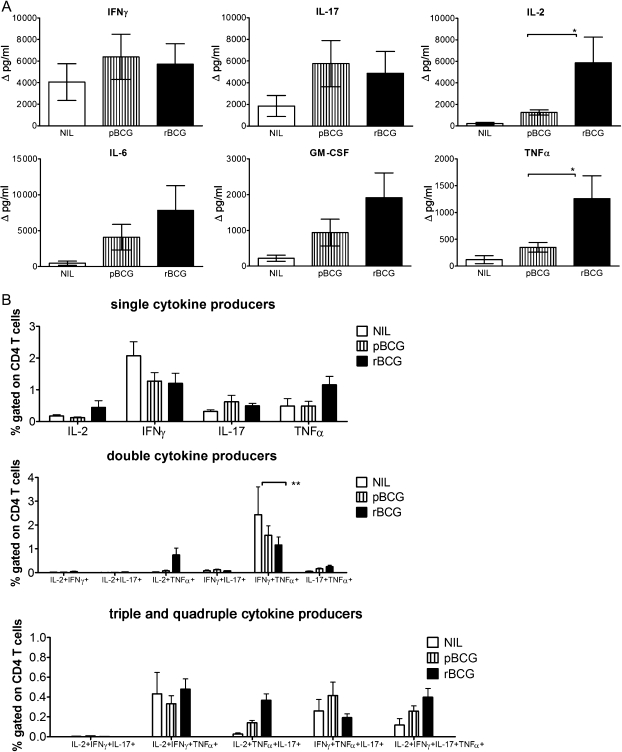
We analyzed MTB-specific immune responses 90 days after infection, when lung bacterial burdens were approximately 10-fold lower in rBCG-immunized animals than in the pBCG group and 100-fold lower than in nonvaccinated controls. Cells from lungs of vaccinated mice and untreated controls were restimulated with PPD for 20 hours, and cytokine concentrations were measured by multiplex assays (Figure 4). Cytokines detected upon restimulation were predominantly of type 1. However, we could not detect differences in IFN-γ or IL-17 levels between the 2 vaccinated groups during persistent infection. In contrast, amounts of IL-2, IL-6, GM-CSF, and TNF-α were higher in rBCG-vaccinated mice. In all groups, IL-4 and IL-5 levels were below the detection limit (data not shown). In addition, analysis of lung cells by multicolor flow cytometry revealed predominantly cytokine-producing CD4 T cells during persistent infection (Figure 4). CD4 T cells secreting only IFN-γ were detected in all groups with similar frequencies. Upon vaccination, CD4 T cells producing IL-2, IFN-γ, TNF-α, or IL-17 in different combinations could also be detected. Intriguingly, frequencies of responding CD4 T cells did not differ significantly between rBCG- and pBCG-vaccinated mice despite higher concentrations of IL-2 and TNF-α in supernatants. We assume that both vaccines increased frequencies of antigen-specific CD4 T cells in the lung during persistent MTB infection, with rBCG-induced T cells becoming more potent cytokine producers. CD8 T cells almost exclusively secreted IFN-γ with comparable frequencies in all groups, and multifunctional CD8 T-cell counts were barely above the background (data not shown).
Figure 4.
Immune responses in the lung during persistent Mycobacterium tuberculosis (MTB) infection in mice vaccinated with recombinant ΔureC hly+ bacillus Calmette-Guérin (rBCG). A, Cytokine secretion by lung cells 90 days after aerosol infection with 200–400 colony-forming units of MTB. Cells were restimulated with purified protein derivative (PPD) for 20 hours, and the supernatants were analyzed by multiplex assays. Cytokine concentrations are depicted as means (± SEM) of 2 independent experiments with 3 replicates each. Background cytokine production levels from medium controls were subtracted. B, Analysis of cells restimulated with PPD for 6 hours in the presence of brefeldin A by multicolor flow cytometry. Frequencies of responding CD4 T cells are depicted as means (± SEM) of 2 independent experiments with 3 replicates each. Analysis of variance and the Bonferroni multiple comparison test were applied for statistical analysis. *P < .05; **P < .01. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL-2, interleukin 2; IL-6, interleukin 6; IL-17, interleukin 17; NIL, none; pBCG, parental BCG; TNF-α, tumor necrosis factor α.
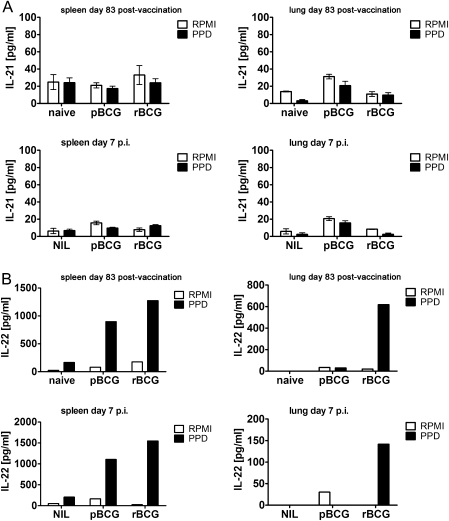
Vaccination Causes IL-22 but Not IL-21 Production
Th17 cells can produce additional effector cytokines such as IL-21 [16] and IL-22 [17]. IL-22–producing cells have been identified in patients with TB, but these cells seem to be distinct from IL-17–producing cells [18]. We did not detect IL-21 after PPD stimulation of cells from vaccinated and subsequently MTB-infected mice (Figure 5). IL-22 was produced at elevated concentrations by PPD-stimulated splenocytes (Figure 5) in immunized mice, but concentrations did not further increase early after infection with MTB. IL-22 production by lung cells was only observed in the rBCG-vaccinated group and decreased after aerosol MTB infection.
Figure 5.
Vaccination with recombinant ΔureC hly+ bacillus Calmette-Guérin (rBCG) induces production of interleukin (IL) 22 but not IL-21. A, B, IL-21 and IL-22 secretion, respectively, by cells from spleens (2 × 106 cells) or lungs (2 × 105 cells) of mice 83 days after vaccination and subsequent aerosol infection with 200–400 colony-forming units of Mycobacterium tuberculosis. IL-21 concentrations were measured from 3 samples per group; means (± SEM) are depicted. For IL-22, samples from 1 group were pooled. Data are representative of 2 (day 83 after vaccination) and 5 (day 7 after infection) similar experiments. Cells were restimulated with purified protein derivative (PPD) for 20 hours, and the supernatants were analyzed by enzyme-linked immunosorbent assay. Abbreviations: NIL, none; pBCG, parental BCG; RPMI, Roswell Park Memorial Institute medium.
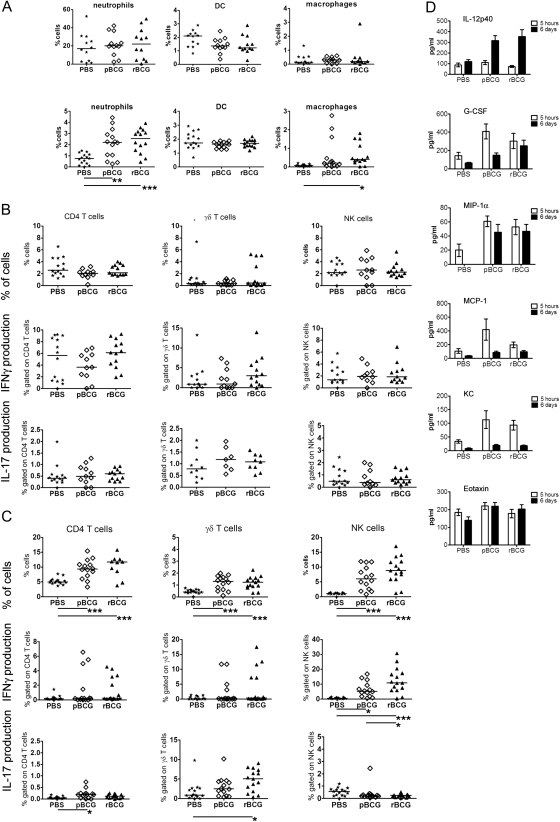
rBCG Causes Increased Recruitment of γδ T Cells and Natural Killer Cells Without Significantly Altering Antigen-Presenting Cell Populations
We wanted to better understand the mechanisms underlying IL-17 induction after immunization with rBCG. To this end, rBCG and pBCG were administered intraperitoneally, and immigrant cells were isolated from the peritoneal cavity 5 hours or 6 days after administration and analyzed by flow cytometry. Neutrophils (defined as Gr1high, CD11bhigh, MHCII−, and CD11c−) rapidly entered the peritoneal cavity and remained at elevated counts on day 6 after infection in the pBCG and rBCG groups to the same extent. Frequencies of peritoneal macrophages (defined as CD11bhigh, F4/80high, Gr1−, MHCIIhigh, and CD11c−) and dendritic cells (defined as CD11c+, CD11blo, and Gr1−) remained unchanged. In sum, no significant differences in antigen-presenting cell (APC) recruitment were detectable between the rBCG and pBCG groups (Figure 6).
Figure 6.
Recombinant ΔureC hly+ bacillus Calmette-Guérin (rBCG) causes increased recruitment of γδ T cells and natural killer (NK) cells without significantly altering antigen-presenting cell (APC) populations. A, Analysis of cell populations in peritoneal lavage fluid by flow cytometry. Cells were recruited to the peritoneal cavity upon intraperitoneal administration of 106 colony-forming units of rBCG or parental BCG (pBCG). APCs were harvested from the peritoneum 5 hours (upper panel) and 6 days (lower panel) after intraperitoneal administration of rBCG or pBCG. B, Intracellular cytokine staining (ICS) of T-cell populations 5 hours after injection. Cells were stimulated with αCD3/αCD28 antibodies for 18 hours in the presence of brefeldin A. C, ICS of T-cell populations 6 days after administration. Cells were restimulated with purified protein derivative for 18 hours in the presence of brefeldin A. Data are presented as a summary of 3 independent experiments with 5 mice per group. Horizontal lines indicate the median values. Analysis of variance and the Bonferroni multiple comparison test were applied for statistical analysis. *P < .05; **P < .01. D, Cytokines and chemokines detected in peritoneal lavage fluid analyzed by multiplex assay, depicted as mean concentrations (± SEM); summary of 3 independent experiments with 5 mice per group. Abbreviations: DC, dendritic cells; G-CSF, granulocyte colony-stimulating factor; IFN-γ, interferon γ; IL-12p40, interleukin 12p40; IL-17, interleukin 17; KC, keratinocyte chemoattractant; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; PBS, phosphate-buffered saline.
Peritoneal cells harvested 5 hours after vaccination were subjected to polyclonal stimulation (Figure 6) with αCD3/αCD28 antibodies. Frequencies of CD4 T cells and natural killer (NK) cells were comparable between all groups, as were levels of IFN-γ and IL-17 production. Six days after administration, these cells increased numerically and reached the highest frequencies in the rBCG group (Figure 6). PPD was used for restimulation of cells on day 6. CD4 T cells did not produce appreciable amounts of IFN-γ or IL-17, whereas a substantial proportion of NK cells produced IFN-γ after rBCG administration. The γδ T cells have been identified as a major source of early IL-17 in TB [19]. Increased, albeit not significant, proportions of γδ T cells producing IFN-γ were identified 5 hours after administration of rBCG. This cell population further increased numerically, and markedly higher frequencies of γδ T cells producing IL-17 were detected in the rBCG group 6 days after vaccination. CD8 T cells were not detected in the peritoneal cavity in any of the groups. In addition, we analyzed cytokines and chemokines in the peritoneal cavity by multiplex assay of peritoneal lavage fluid (Figure 6). Levels of monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, granulocyte colony-stimulating factor (G-CSF), and keratinocyte chemoattractant (KC) were rapidly increased upon injection; levels of eotaxin remained unchanged and IL-12p40 was detected in higher concentrations after 6 days. However, we could not detect any significant differences in these measures between the pBCG and rBCG groups. Levels of IL-1β, IL-6, IL-9, IL-13, MIP-1β, and regulated upon activation, normal T-cell expressed and secreted could also be measured in comparable concentrations, whereas IL-12p70, IFN-γ, IL-1α, IL-2, IL-3, IL-4, IL-5, IL-10, IL-17, GM-CSF, and TNF-α concentrations were below the detection limit (data not shown). Thus, rBCG and pBCG induced recruitment of APCs as well as chemokine and cytokine production at the site of administration to a similar extent. Intriguingly, proportions of γδ T cells secreting IL-17 and NK cells producing IFN-γ were most abundant after rBCG administration.
DISCUSSION
The identification of biomarkers is crucial for rational design of novel TB vaccines. These markers could also form the basis for definition of surrogate markers to predict endpoints of clinical outcome in TB vaccine efficacy trials and thus provide guidelines for improvement of current vaccine candidates. The importance of key cytokines that activate macrophage antimycobacterial capacities including IFN-γ [20] and TNF-α [21], and the necessity for IL-2 in the expansion of memory cells [22], are well established and thus commonly used to monitor TB vaccine trials.
In an attempt to better understand immune mechanisms underlying the superior protection of rBCG over pBCG and to harness our knowledge for identification of biomarkers of vaccine efficacy, we compared long-term memory immune responses elicited by rBCG and its parental strain, pBCG. Responses differed in both quantitative and qualitative terms. We detected an increased abundance of type 1 cytokines as well as IL-17 in the lung following vaccination with rBCG. Analysis of vaccine-induced immune responses in the lungs is obviously not feasible in the context of clinical trials. Therefore, we also analyzed systemic long-term memory responses. Intriguingly, comparable concentrations of type 1 promoting cytokines were detected in both pBCG and rBCG groups. In contrast, IL-17 production by splenocytes was significantly elevated upon rBCG vaccination. Thus, we conclude that the systemic release of IL-17, rather than of IFN-γ or IL-2, qualified as a potential marker of superior protection induced by rBCG.
Th17 cells contribute to antimicrobial defense by attracting and activating neutrophils [23], which are among the first cells recruited in response to IL-17. It has been shown that IL-17 is dispensable during primary MTB infection [24, 25] but gains importance in memory responses [14]. In addition, recent reports on the expression of CCR6 on human Th17 cells [26, 27] point to a positive feedback loop, because CCR6 is the receptor for CCL20 produced by neutrophils [28] and CCL20-CCR6 has been implicated in immunopathogenesis of TB [29]. Thus, type 17 cytokines could promote enhanced recruitment of antigen-specific memory T cells to the sites of bacterial residence. Analysis of vaccine-induced immune responses 7 days after infection with MTB revealed that vaccination with rBCG indeed led to an enhanced early response of effector cells at the sites of bacterial replication. We observed increased frequencies of antigen-specific CD4 T cells and elevated production of IL-2, IL-17, IFN-γ, and GM-CSF by lung and spleen cells. Multifunctional CD4 T cells coproducing IL-2, IFN-γ, and TNF-α were first implicated in successful vaccination strategies against Leishmania major [15] and later also against MTB [30]. Recently, these polyfunctional CD4 T cells were also detected in TB vaccine trials [31, 32]. We detected polyfunctional CD4 T cells upon rBCG and pBCG vaccination and subsequent infection; however, the composition of cytokines (IL-2, IL-17, IFN-γ, and TNF-α) in double and triple producers varied considerably between experiments as well as between individual animals. If multifunctional cells were a true correlate of protection, then their overall frequencies, which were higher in the rBCG group, rather than their composition, seem relevant.
Th17 cells are considered to be instrumental in inflammatory and autoimmune diseases such as collagen-induced arthritis [33], experimental autoimmune encephalomyelitis (EAE) [33, 34], and allergic airway hypersensitivity [33, 35]. This pathogenic role is usually associated with development of a profound IL-21–mediated inflammatory response. We never detected IL-21 after vaccination and subsequent MTB infection above background levels (data not shown). A recent report described a pathological role for IL-17 after repeated BCG vaccination and MTB exposure [36]. In contrast, the single administration of rBCG described here elicited a balanced type 1/type 17 cytokine response, and increased IL-17 abundance was only detected prior to and early after MTB infection. During persistent infection, IFN-γ and IL-17 production was comparable between the pBCG and rBCG groups. In addition, we never observed signs of autoimmunity or excessive inflammation upon vaccination with rBCG for up to 200 days after MTB infection.
In an attempt to compare data from mouse experiments with data from human studies, we analyzed cytokine profiles of frozen peripheral blood mononuclear cells from a phase I clinical trial with rBCG and pBCG. In a limited number of samples (3 samples from pBCG-vaccinated donors and 7 samples from rBCG-vaccinated donors), we detected IL-17 production after rBCG vaccination but not after pBCG vaccination (data not shown). Th17 cells have been detected in peripheral blood samples from MTB-infected humans [18]. Recently, IL-17–producing CD4 T cells have been reported in samples from adolescents vaccinated with a modified vaccinia virus Ankara expressing Ag85A [32, 37]. Thus, it is tempting to consider IL-17 as a correlate of protection in TB vaccine trials.
Why did rBCG but not pBCG induce IL-17 production? Immunization with rBCG and pBCG caused recruitment of APC as well as chemokine and cytokine producers to a similar extent. Intriguingly, proportions of γδ T cells secreting IL-17 and NK cells producing IFN-γ were highly abundant after rBCG vaccination. This is consistent with reports that IL-17 is rapidly produced by γδ T cells [19, 38] as well as NK T cells [39]. NK cells, counts of which were also increased upon vaccination with rBCG, are an important early source of IFN-γ. These initial events could pave the way for the development of a sustained, long-lived memory T-cell response.
We have shown elsewhere that components released from rBCG reside in the cytosol of infected macrophages [12]. Thus, rBCG-derived pathogen-associated molecular patterns could activate intracellular signaling cascades. Nucleotide-binding oligomerization domain (NOD)-2, for example, is a cytosolic pattern recognition receptor and its engagement has been linked to the development of type 17 memory T-cell responses [40]. It is further known that apoptosis induced during bacterial infection promotes generation of Th17 cells [41]. We have obtained evidence that rBCG induces increased apoptosis compared with pBCG [12], which could contribute to increased development of IL-17–producing T cells. Activation of inflammasomes could also contribute to type 17 responses via production of IL-1β [42]. NOD-like receptor family, pyrin domain-containing 3, for example, has been found to sense the presence of listeriolysin through changes in adenosine triphosphate concentrations [43]. Thus, induction of IL-17 secretion could require a complex interplay of intracellular stimuli and increased apoptosis that are induced upon rBCG but not pBCG vaccination.
In summary, we demonstrate that vaccination with rBCG leads to generation of type 17 cytokine production in addition to profound type 1 cytokine production. The IL-17 produced could enhance recruitment of antigen-specific T cells to the lung. Ultimately, this cascade of events results in earlier containment of MTB and hence to superior protection by rBCG compared with pBCG. Because IL-17 seems to be instrumental for enhanced recruitment of antigen-specific T cells to the sites of MTB replication, future TB vaccines should be tailored to concomitantly induce balanced type 1 and type 17 cytokine responses.
Notes
Acknowledgments.
We thank M. L. Grossman for help in preparing the manuscript and C. Köberle for the FACS data analyzer software.
Financial support.
This work was supported by the European Commission's Framework Programme 6 TBVAC (LSHP-CT-2003-503367 to S. H. E. K.); and the European Commission's Framework Programme 7 NEWTBVAC (Health-F3-2009-241745 to S. H. E. K.).
Potential conflicts of interest.
S. H. E. K. and L. G. are coinventors of the DureC hly1 rBCG vaccine. S. H. E. K. is member of the Scientific Advisory Board of Vakzine Projekt Management (VPM). VPM is the exclusive licensee for ΔureC hly + rBCG. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Tuberculosis fact sheet no. 104 March. http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed 21 August 2010. [Google Scholar]
- 2.Calmette A. Sur la vaccination préventive des enfants nouveau-nés contre tuberculose par le BCG. Ann Inst Pasteur. 1929;41:201–32. [Google Scholar]
- 3.Reece ST, Kaufmann SH. Rational design of vaccines against tuberculosis directed by basic immunology. Int J Med Microbiol. 2008;298:143–50. doi: 10.1016/j.ijmm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 6.Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006;4:469–76. doi: 10.1038/nrmicro1419. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann SH, Baumann S, Nasser EA. Exploiting immunology and molecular genetics for rational vaccine design against tuberculosis. Int J Tuberc Lung Dis. 2006;10:1068–79. [PubMed] [Google Scholar]
- 8.Fletcher HA. Correlates of immune protection from tuberculosis. Curr Mol Med. 2007;7:319–25. doi: 10.2174/156652407780598520. [DOI] [PubMed] [Google Scholar]
- 9.Goldsack L, Kirman JR. Half-truths and selective memory: interferon gamma, CD4+ T cells and protective memory against tuberculosis. Tuberculosis. 2007;87:465–73. doi: 10.1016/j.tube.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Tchilian EZ, Desel C, Forbes EK, et al. Immunogenicity and protective efficacy of prime-boost regimens with recombinant ΔureC hly+ Mycobacterium bovis BCG and modified vaccinia virus ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infect Immun. 2009;77:622–31. doi: 10.1128/IAI.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearl JE, Saunders B, Ehlers S, Orme IM, Cooper AM. Inflammation and lymphocyte activation during mycobacterial infection in the interferon-gamma-deficient mouse. Cell Immunol. 2001;211:43–50. doi: 10.1006/cimm.2001.1819. [DOI] [PubMed] [Google Scholar]
- 12.Grode L, Seiler P, Baumann S, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115:2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kursar M, Koch M, Mittrucker HW, et al. Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol. 2007;178:2661–5. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 14.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 15.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional Th1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 16.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory Th17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang SC, Tan XY, Luxenberg DP, et al. IL-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scriba TJ, Kalsdorf B, Abrahams DA, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–70. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 20.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 22.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–3. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khader SA, Pearl JE, Sakamoto K, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–95. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 25.Steinman L. A brief history of Th17, the first major revision in the Th1/Th2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 26.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–21. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Rohowsky-Kochan C. Regulation of IL-17 in human CCR6+ effector memory T cells. J Immunol. 2008;180:7948–57. doi: 10.4049/jimmunol.180.12.7948. [DOI] [PubMed] [Google Scholar]
- 28.Scapini P, Laudanna C, Pinardi C, et al. Neutrophils produce biologically active macrophage inflammatory protein-3alpha (MIP-3alpha)/CCL20 and MIP-3beta/CCL19. Eur J Immunol. 2001;31:1981–8. doi: 10.1002/1521-4141(200107)31:7<1981::aid-immu1981>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Lee JS, Lee JY, Son JW, et al. Expression and regulation of the CC-chemokine ligand 20 during human tuberculosis. Scand J Immunol. 2008;67:77–85. doi: 10.1111/j.1365-3083.2007.02040.x. [DOI] [PubMed] [Google Scholar]
- 30.Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–64. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abel B, Tameris M, Mansoor N, et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med. 2010;181:1407–17. doi: 10.1164/rccm.200910-1484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scriba TJ, Tameris M, Mansoor N, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol. 2010;40:279–90. doi: 10.1002/eji.200939754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 34.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellings PW, Kasran A, Liu Z, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 36.Cruz A, Fraga AG, Fountain JJ, et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207:1609–16. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Cassan SC, Pathan AA, Sander CR, et al. Investigating the induction of vaccine-induced Th17 and regulatory T cells in healthy, Mycobacterium bovis BCG-immunized adults vaccinated with a new tuberculosis vaccine, MVA85A. Clin Vaccine Immunol. 2010;17:1066–73. doi: 10.1128/CVI.00047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. Gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–7. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rachitskaya AV, Hansen AM, Horai R, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–71. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–9. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs Th17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 42.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–74. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]