Abstract
Background
Crumbs (Crb), a cell polarity gene, has been shown to provide a positional cue for the apical membrane domain and adherens junction during Drosophila photoreceptor morphogenesis. It has recently been found that stable microtubules in developing Drosophila photoreceptors were linked to Crb localization. Coordinated interactions between microtubule and actin cytoskeletons are involved in many polarized cellular processes. Since Spectraplakin is able to bind both microtubule and actin cytoskeletons, the role of Spectraplakin was analyzed in the regulations of apical Crb domain in developing Drosophila photoreceptors.
Methodology/Principal Findings
The localization pattern of Spectraplakin in developing pupal photoreceptors showed a unique intracellular distribution. Spectraplakin localized at rhabdomere terminal web which is at the basal side of the apical Crb or rhabdomere, and in between the adherens junctions. The spectraplakin mutant photoreceptors showed dramatic mislocalizations of Crb, adherens junctions, and the stable microtubules. This role of Spectraplakin in Crb and adherens junction regulation was further supported by spectraplakin's gain-of-function phenotype. Spectraplakin overexpression in photoreceptors caused a cell polarity defect including dramatic mislocalization of Crb, adherens junctions and the stable microtubules in the developing photoreceptors. Furthermore, a strong genetic interaction between spectraplakin and crb was found using a genetic modifier test.
Conclusions/Significance
In summary, we found a unique localization of Spectraplakin in photoreceptors, and identified the role of spectraplakin in the regulation of the apical Crb domain and adherens junctions through genetic mutational analysis. Our data suggest that Spectraplakin, an actin-microtubule cross-linker, is essential in the apical and adherens junction controls during the photoreceptors morphogenesis.
Introduction
Drosophila has been extensively used to study the localization and function of cell polarity proteins [1]. Genetic analysis has identified three groups of cell polarity genes that are required for the organization of apical basal epithelial cell polarity. The formation of cell polarity is dependent on the Crumbs (Crb) complex of Crb, Stardust and Patj, the Par complex of Bazooka (Baz, Par-3), Par-6 and a typical protein kinase C (aPKC), and the Scrib complex of Scrib, Discs-large (Dlg), and Lethal giant larvae (Lgl) [1].
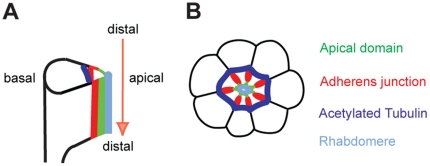
Evolutionary conservation in the structure and function of polarity genes makes the Drosophila eye an excellent model for studying the genetic and molecular basis of retinal cell organization [2], [3]. Photoreceptor cells in Drosophila are formed in eye imaginal discs of third-instar larvae [4]. During pupal development, photoreceptor cells undergo the proximal to distal elongation (Fig 1A) while the apical membrane domain localizes at the center of the photoreceptor clusters surrounded by the adherens junction and the basolateral domains (Fig 1B) [5], [6]. Crb is required for extension of photoreceptors along the distal-proximal axis of the photoreceptor cell [2], [3]. The mammalian homolog of Crb, CRB1, is also localized to the inner segment of photoreceptors, the structure analogous to the rhabdomere stalk, between the outer segment and the adherens junction [3]. Furthermore, mutations in CRB1 causes retinal diseases including retinitis pigmentosa 12 and Leber Congenital Amaurosis in humans [7], [8]. One of the important questions is how the apical Crb/CRB1 is regulated during the photoreceptor morphogenesis. Searching for new genes that interact with Crb will help in understanding the Crb-dependent eye development and degeneration. Here, we found that spectraplakin is one of the genes that affect Crb localization in the eye.
Figure 1. Morphogenesis of Drosophila pupal photoreceptors.
(A) Side view of developing photoreceptors at 45% pd. The photoreceptors elongate from distal to proximal (arrow). (B) Cross-section of 45% pd pupal photoreceptors. Apical domain (green) localizes apical to adherens junction (red) in the center of a photoreceptor cluster. The E-cad localizes at adherens junction (red) which are more basal to the apical domain. The basolateral domains (black) are more basal to the adherens junction (red), and the acetylated-tubulin (blue) localizes at the outside from the adherens junctions (red). The rhabdomere (light blue) localizes at the apical to the apical domain (green).
Microtubule cytoskeletons play essential roles in determining cell shape, cell polarity, and vesicle trafficking. As a consequence, microtubule reorganization during differentiation is essential for morphogenesis. Despite their importance in cell shape and polarity generation, the organization of microtubules in Drosophila photoreceptors remains relatively unexplored [9], [10], [11]. Therefore, we have recently examined the presence of stabilized microtubules in developing pupal photoreceptors and proposed its potential role in Drosophila photoreceptor development [12], [13]. We identified the presence of stable/acetylated microtubules in developing Drosophila pupal photoreceptors (Fig 1) [12], [13]. It was also found that Spastin, a microtubule-severing ATPase involved in constructing microtubule arrays, helps control the apical localization of Crb [12]. Furthermore, the microtubule-based motors, Kinesin-1 and Kinesin-2, were discovered to involve the Crb localization in developing photoreceptors [14], [15]. Therefore, Spectraplakin, an actin-microtubule cross-linker [16], might have a potential role in the regulation of stable-microtubules and apical Crb during the photoreceptors morphogenesis.
Spectraplakins are a family of actin-microtubule cross-linking proteins that have been highly conserved throughout the animal kingdom [16], [17]. All spectraplakins possess a series of plakin domains that are structurally similar to spectrin repeats. Many also have one or two amino-terminal calponin homology domains, a carboxy-terminal region containing a GAS2-like domain. The calponin homology domains, as two tandem copies, are able to bind tightly to actin filaments, whereas the GAS2 domain interacts with microtubules [18], [19]. Thus, spectraplakins are thought to be molecules with actin- and microtubule-binding sites on either end. This organization suggests that these molecules are able to act as actin-microtubule cross-linkers.
The Spectraplakin family is comprised of mammalian BPAG1/dystonin and ACF7/MACF1, Drosophila Short stop (Shot), and Caenorhabditis elegans Vab-10 [16], [18], [20], [21]. Spectraplakins have important roles in a broad spectrum of cellular contexts, ranging from highly dynamic roles in development or wound healing to structural functions in cell or tissue maintenance [17]. In this study, we characterized the role of Spectraplakin/Shot in photoreceptor development and examined the genetic interaction between the spectraplakin/shot and crb, a cell polarity gene which provides a positional cue for photoreceptor morphogenesis[2], [3].
Results
Genetic interaction between crb and spectraplakin/shot in Drosophila photoreceptors
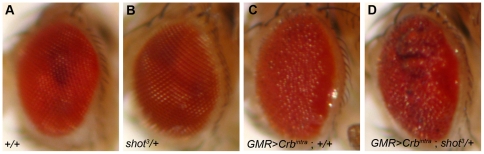
We overexpressed the conserved Crb intracellular domain (Crbintra) [22] using GMR-Gal4 [23], which led to a roughening of the eye's external morphology (Fig 2C) [2]. Using this genetically sensitized condition, we performed a genetic screen to identify additional players that function with Crb to regulate photoreceptor morphogenesis. From a pilot screen, we found that the rough eye phenotype of GMR>Crbintra was dominantly enhanced by reducing the level of shot in the shot/+heterozygous background (Fig 2D), thus suggesting a strong genetic interaction between crb and shot in the Drosophila eye. The enhancement of the rough eye phenotype in the shot/+heterozygous background was very consistent with 100% penetrance (n>100). This genetic interaction data strongly suggests that Shot may provide an additional positional cue for Crb-dependent photoreceptor development.
Figure 2. Genetic interactions between crb and shot in Drosophila eye.
(A–B) Adult eye phenotypes of+/+(A), shot3/+(B), GMR>Crbintra;+/+(C), and GMR>Crbintra; shot3/+(D).
Localization of Spectraplakin/Shot in Drosophila pupal photoreceptors
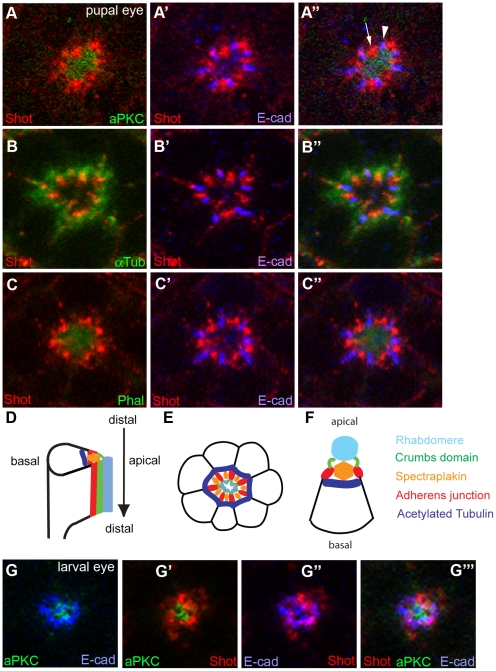
After finding genetic interaction between crb and shot, it was then necessary to determine the localization of Shot in developing wild type photoreceptors. We utilized the anti-Shot monoclonal antibody, mAb Rod1 (Developmental Studies Hybridoma Bank, DSHB of Univ of Iowa) [24] to examine the localization of Shot in the mid-stage developing pupal eyes (45% pupal development stage, pd), which was examined by immunostaining and confocal microscopy. Previously, Shot was reported to localize at adherens junctions in embryonic and follicular epithelia cells [20], or with microtubules in oocytes [25]. We investigated where Shot localizes compared to other subcellular markers of aPKC (apical membrane domain) and E-cadherin (adherens junction) [26], [27], [28], and cytoskeleton markers of acetylated tubulin (stable microtubule) [12], [29] and phalloidin (F-actin, rhabdomere) [4] in mid-stage pupal photoreceptors. In Drosophila pupal photoreceptors, Shot is highly enriched in between the adherens junctions (E-cad), at the basal side of the Crb, and at the apical side of the microtubules (Fig 3A and 3B). This data strongly indicates that Shot is concentrated at the rhabdomere terminal web (RTW) [30], [31], [32], [33] in the developing photoreceptors. The RTW is considered to serve as a transition zone for the constant delivery of proteins needed for the maintenance and function of the rhabdomere [30], [31], [32], [33]. The “RTW” might be the place where the stable microtubules and fibrous actins (F-actins) meet (Fig 3D–3F), based on the localization of Shot, an actin-microtubule cross-linker. Therefore, Shot might have a potential role in the stable microtubules and rhabdomere, and thereby in the localizations of Crb and adherens junctions in photoreceptor cells (Fig 3D–3F). It is possible that the differential localization of Shot from the apical and adherens junction could be the cell-type and developmental-stage specific. The localization of Shot at the RTW in the pupal stage could be mainly due to the actin-microtubule junctions. Therefore, it is possible, the Shot localizes at a different location in the absence of the fibrous-actin of rhabdomere which is formed during the mid-stage pupal eyes [5], [6]. However, the differential localization pattern of Shot from aPKC and E-cad was also observed in the eye discs of third-instar larvae (Fig 3G). Therefore, the localization of Shot at the RTW in pupal eyes might be independent of the rhabdomere.
Figure 3. Localization of Shot in Drosophila photoreceptors.
(A–F) Localization of Shot in pupal photoreceptors (45% pd). (A and B) Shot (A and B, red) localizes in between the E-cad (A' and B', blue), at the basal side of the aPKC (apical marker, A, green), and at the apical side of the microtubules (B, green). (C) Shot localizes at the basal side of phalloidin (Phal, rhabdomere marker, C and C''). (D–F) Schematic representation of mid-pupal photoreceptor and localization of Shot was labeled (orange). Shot (orange) localizes in between adherens junction (red), at the basal side of the apical Crumbs domain (green), at the apical side of the stable microtubule (blue), and at the basal side of the rhabdomere (light blue). (G) Localization of Shot in eye discs of third-instar larvae. Shot localizes at the basal side of the apical domain (aPKC, G'), and in between adherens junction (E-cad, G'').
Loss-of-function analysis of spectraplakin/shot in Drosophila pupal photoreceptors
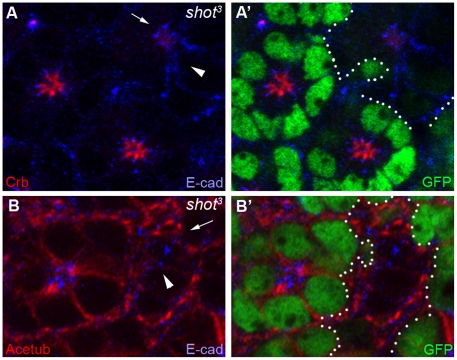
To examine whether Shot is required for photoreceptor morphogenesis in mid-stage pupal eye development, we generated mosaic eyes of a null mutation of shot, shot3 [34], [35], using a genetic mosaic technique of FLP/FRT [36]. The allele shot3 is genetically an amorphic/null allele that lacks Shot protein expression [20] and has been completely rescued by the UAS-ShotA transgene [19]. In the absence of Shot in shot3 mutants, the apical Crb domain (Fig 4A, arrow) was dramatically reduced, and adherens junction (Fig 4A, arrowhead, E-cad, blue) was mislocalized from the apical center toward the basolateral areas (Fig 4). Furthermore, stable microtubules [12] were also disrupted (Fig 4B). These mutational analyses of the null allele of shot mutation strongly indicated that the Shot is indispensable in the photoreceptor morphogenesis, and is required for the correct positioning and/or targeting of apical domain, adherens junctions and stable microtubules during the photoreceptor development.
Figure 4. Shot is essential for photoreceptor morphogenesis in the mid-stage developing pupal eyes.
(A and B) Mid-stage pupal eyes (45% pd) with shot3 null mutant clones marked by the absence of the GFP and dotted lines (green, A' and B'). Crb (red, A, arrow) was almost absent, and E-cad (blue, A, arrowhead) was mislocalized from the apical center toward the surrounded distal areas (arrow, A). Stable microtubules (Acetub, red) were also mislocalized in the shot3 mutants (arrow, B).
Gain-of-function analysis of spectraplakin/shot in Drosophila pupal photoreceptors
The loss-of-function analysis of the shot mutation (Fig 4) strongly suggests that shot is essential to maintain the apical Crb domain, adherens junctions, and stable microtubules during photoreceptor development. Therefore we hypothesize that Shot might have an active role for the positioning of Crb/E-cad/Acetub in photoreceptors. To test this hypothesis, a gain-of-function analysis of shot was conducted using an eye-specific Gal4 driver, GMR-Gal4 [23], in order to increase shot expression in the developing photoreceptors.
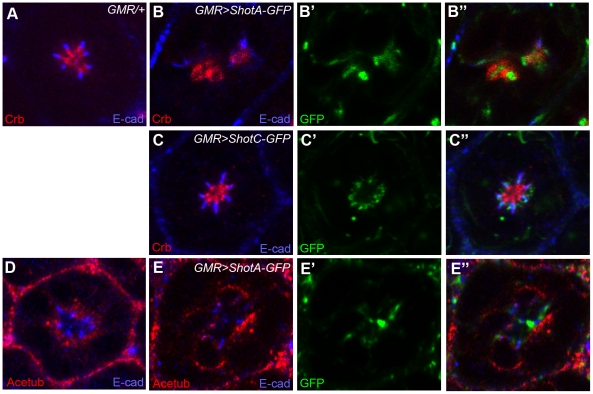
Several isoforms of Shot are ShotA, ShotB, and ShotC based on the alternative RNA splicing from the unique shot gene in Drosophila [16]. The Shot isoforms vary at their amino-terminus while having identical central and carboxy-terminal domains. The ShotC isoform differs from ShotA by lacking one calponin homology domain which is necessary for actin-binding ability. Therefore, ShotA has an actin-binding activity, but ShotC does not [18], [19]. The previously established UAS-ShotA-GFP and UAS-ShotC-GFP were used to test the effects of Shot overexpression in photoreceptor development. Overexpression of ShotA using GMR-Gal4 in mid-stage pupal photoreceptors resulted in the dramatic mislocalized distribution of ShotA-GFP. In this situation, concurrent mislocalizations of Crb and E-cad (Fig 5B) occurred in the situation of ShotA-GFP overexpression. The Shot, Crb and E-cad were mingled together in this mislocalized situation (Fig 5B''). These results suggest that the ShotA-overexpression induce the complete cell polarity defects including the mislocalizations of the Crb and E-cad.
Figure 5. Overexpression of Shot causes the mislocalization of apical domain, adherens junction, and stable microtubules.
Pupal eyes (45% pd) with Shot overexpression driven by GMR-GAL4 at 22°C were examined by Crb (apical domain marker, red, A–C), E-cad (adherens junction marker, A–E) and acetylated-tubulin (Acetub, stable microtubules marker, red, D–E). (A, D) control, GMR-GAL4/+, (B, E) GMR-GAL4/UAS-ShotA-GFP, (C) GMR-GAL4/UAS-ShotC-GFP.
In the condition of ShotA-GFP overexpression, the most dramatic mislocalization happened in the localization of stable microtubules (Fig 5E). The stable microtubules were completely displaced from the apical areas to the basolateral areas in the ShotA-GFP overexpression situation (Fig 5E). Therefore, the primary target of Shot might be the stable microtubules. Then, the defected microtubules might further affect the Crb and E-cad, but other possibilities of direct targeting of Shot toward Crb and/or E-cad cannot be excluded.
When the ShotC-GFP, lacking the actin-binding activity, was expressed, the ShotC-GFP precisely localized at the “RTW” in the photoreceptors, and did not cause the mislocalization defects in Crb and E-cad (Fig 5C), although the expression level of ShotA-GFP and ShotC-GFP were similar based on the level of GFP tag (Fig 5B' and 5C'). These data suggest that the ShotA-induced mislocalizations of Crb/E-cad/Acetub require the active calponin homology domain for actin-binding activity. However, the targeting of Shot to the RTW might not require the calponin homology domain having the actin-binding activity, because the ShotC isoform localizes at the RTW without the actin-binding activity.
In conclusion, the role of Shot in Crb, E-cad and stable microtubules during photoreceptor development was identified, based on the loss-of-function (Fig 4) and gain-of-function (Fig 5) phenotypes of shot mutations.
crb is required in Shot localization during Drosophila photoreceptor elongation
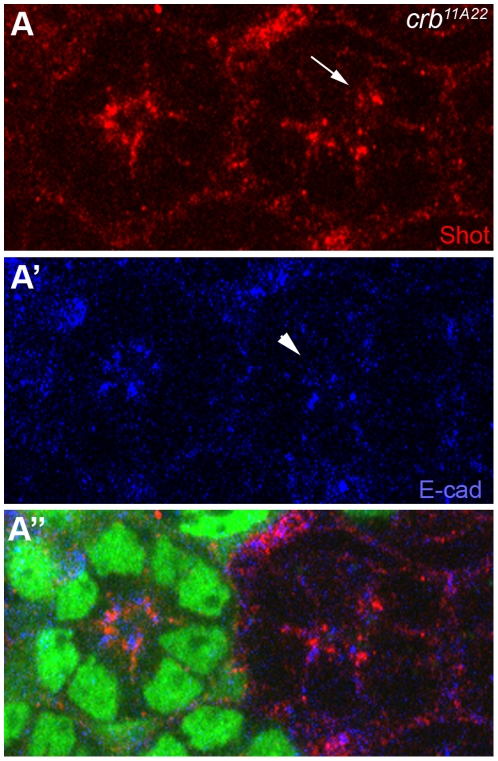
The genetic interaction between shot and crb, shows that the shot/+ heterozygote dominantly enhanced crb gain-of-function phenotype (Fig 2). This genetic interaction data suggests that Shot may provide an additional positional cue for Crb-dependent photoreceptor development. Furthermore, shot mutation analysis shows Shot's role in the Crb's localization in photoreceptors (Fig 4 and 5). Therefore , it is necessary to analyze Crb's role in the Shot's localization in photoreceptors. We generated mosaic eyes of a null mutation of crb, crb11A22 [37], using a genetic mosaic technique [36]. The allele crb11A22 is genetically an amorphic/null allele and lacks Crb protein expression [2], [26], [37]. In the crb null mutants, the E-cad of adherens junction marker was mislocalized from apical to basal (Fig 6A', arrowhead) as previously reported [2], [3]. The Shot localization was also mislocalized in the crb mutants (Fig 6A, arrow), as much as the E-cad (Fig 6A', arrowhead). This data indicates that Crb is essential for Shot localization at the “RTW” in the photoreceptors.
Figure 6. Crb is essential for Shot localization in the mid-stage developing pupal eyes.
(A) Pupal eyes (45% pupal stage) with crb11A22 null mutant clones marked by the absence of the GFP (green, A''). (A) Shot (red, A, arrow) and adherens junction (E-cad, blue, A', arrowhead) were mislocalized from apical to basal (A and A', arrow and arrowhead).
Discussion
We investigated where Shot localizes compared to apical membrane domain, adherens junction, stable microtubule, and rhabdomere (Fig 3), in mid-stage pupal photoreceptors. The localization results of Shot in pupal eyes strongly indicate that Shot localizes in between the adherens junctions, at the basal side of the apical domain, and at the apical side of the stable microtubules (Fig 3). The RTW, where the Shot localizes, may be the interface where the stable microtubules and F-actins of rhabdomere meet together. Since Shot has an actin-microtubule cross-linking activity, Shot might cross-link the two cytoskeletons of actin and microtubules at the RTW.
This genetic interaction data of shot and crb (Fig 2) strongly suggests that Shot may provide an additional cue for Crb in photoreceptor development because the rough-eye phenotype caused by Crb overexpression was further enhanced by reduced shot gene dosage (shot/+). The relationship between crb and shot might be one of the following possibilities; (i) shot acts at the upstream of crb, (ii) shot acts at the downstream of crb, or (iii) shot and crb control the parallel pathway in photoreceptor development. From comparative genetic analysis (Fig 4 and 6), Crb and Shot require each other reciprocally to localize at their target sites of rhabdomere stalk (apical domain) and RTW.
Our genetic analysis of the shot (Fig 4) mutation strongly indicates that Shot modulates the apical Crb membrane domain during rhabdomere elongation. The apical membrane modulation activity of spectraplakin was further confirmed by spectraplakin overexpression which caused a dramatic misplacement of the apical membrane domain (Fig 5). It is postulated that the spectraplakin might affect the actin (rhabdomere) and/or microtubules based on its dual binding capacity for actin/microtubule, and its activity as an actin/microtubule cross-linker [16]. Therefore, its role in photoreceptor morphogenesis might require its dual actin/microtubule binding, which will be supported by the absence of Crb-mislocalization activity of ShotC, which lacks the actin-binding domain. The Crb-mislocalization activity of ShotA might be based on its dual actin/microtubule binding ability.
One important point of the potential cross-talk between the crb and shot is their different spatial localizations in photoreceptors. How does one protein affect the other that is at a different localization in the cell? There might be at least several following possibilities; (i) a potential interaction when they co-localize during the trafficking before their final targeting, (ii) a potential interaction in previous developmental time, or (iii) a potential interaction at the interface where the two subcellular compartments meet. The “RTW” is the place where the microtubules and F-actins (rhabdomere) meet each other. Therefore, the Shot might have a potential role in the regulation of stable microtubules and rhabdomere, and thereby the localizations of Crb and adherens junctions might be affected in photoreceptor cells.
Shot has a microtubule organizing activity. Therefore, the expected result in the loss of shot is the defects of stable microtubules which were observed in the loss-of-function study of shot mutation (Fig 4B). Furthermore, the stable microtubules were mostly defected in the gain-of-function of ShotA-GFP overexpression (Fig 5E). Therefore, the primary target of Shot seems to be the stable microtubules. Then, the defected microtubules may further affect the Crb and E-cad through the microtubule-based trafficking [14], [15] and/or other microtubule-based cell polarity [38]. But the other possibilities of direct targeting of Shot toward Crb/E-cad or actin-based cell polarity cannot be excluded. ShotA-GFP overexpression results in ectopic localization of Acetub around the cells (Fig 5E) which could be caused by the direct binding of ShotA-GFP to the Acetub. However, another possibility of the indirect mislocalization of Acetub caused by the mislocalized “RTW” by the ShotA-GFP cannot be excluded.
Another potential possibility of Spectraplakin's function in the regulation of apical membrane domain might be through microtubule plus-end-tracking proteins (+TIPS). The +TIPS belong to the class of microtubule-associated proteins, and link microtubule ends with apical actin cytoskeleton [39], [40]. In Drosophila muscle-tendon junctions, Shot regulates microtubule cytoskeleton by forming a complex with the EB1 and APC of +TIPS [41], [42]. Therefore, there is a potential possibility of Shot and +TIPS interaction in apical domain control during photoreceptor morphogenesis.
We have shown that Spectraplakin/Shot is required for correct localization of Crb, adherens junctions, and stable microtubules in the photoreceptors and disruption of Shot function affects photoreceptor morphogenesis. Our data strongly suggests that Spectraplakin/Shot plays important functions in the modulation of cell membrane domains including the apical Crb domains of photoreceptors during pupal eye development. Evolutionary conservation in the structure and function of eye morphogenesis genes makes the Drosophila eye an excellent model for studying the genetic and molecular basis of retinal cell organization. Future work will help to uncover other genes that might affect the Crb positioning during the extensive morphological growth phase of the Drosophila pupal eye. Given the high degree of evolutionary conservation of Crb and Spectraplakin genes from Drosophila to higher mammals including humans, similar cooperative mechanism between Crb and Spectraplkain could have a role in the development and degeneration of human photoreceptor.
Materials and Methods
Genetics
All Drosophila strains were grown and maintained at room temperature. Mitotic recombination was induced by using the FLP/FRT method for clonal analysis [36]. shot3 is a null allele lacking detectable Shot protein [34], and has been completely rescued by the UAS-ShotA transgene [19]. shot3 mutant clones were produced by eye-specific expression of ey-Flp [43] or GMR-flp [44] in y w ey-Flp or GMR-flp/+; FRT42D shot3 / FRT42D Ubi-GFP, or y w ey-Flp/+; FRTG13 shot3 / FRTG13 Ubi-GFP. crb11A22 is a null allele of crb [36]. crb11A22 mutant clones were produced in y w ey-Flp/+; FRT82D crb11A22 / FRT82D Ubi-GFP. Overexpression of Shot was induced by crossing UAS-ShotA-GFP or UAS-ShotC-GFP [19] with GMR-GAL4 [23] at room temperature (22°C). shot3, UAS-ShotA-GFP, UAS-ShotC-GFP, and GMR-Gal4 were obtained from the Bloomington Stock Center at Indiana University.
Immunohistochemistry
Fluorescent immunostaining and confocal analysis of pupal eyes were carried out as reported [12], [13], [14], [26], [27], [28], [29]. Dissected pupal eyes were fixed for 20 min at room temperature in 4% paraformaldehyde. A 15 minute acetone treatment was performed after fixation for rat anti-Crb staining [45]. The fixed pupal eyes were permeabilized by incubation for 10 min at room temperature with 0.5% NP-40 in 50 mM Tris-Cl (pH 6.8), 150 mM NaCl, and 5 mg/ml BSA. The tissues were then incubated overnight at 4°C to primary antibodies in 0.5% NP-40 in 50 mM Tris-Cl (pH 6.8), 150 mM NaCl, and 1 mg/ml BSA. The following primary antibodies were used: mouse anti-acetylated tubulin (Sigma), 1∶1000; rabbit anti-α-tubulin (Abcam), 1∶200, rat anti-E-cadherin (Dcad2, DSHB) [46], 1∶20; mouse anti-Shot (Rod1, DSHB) [24], 1∶20; rat anti-Crb [45], 1∶400; sheep anti-GFP (Biogenesis), 1∶100; rabbit anti-aPKCζ (Santa Cruz), 1∶500. Immune complexes were detected by incubation for 4 hrs at 4°C with donkey Cy3-, Cy5-, or FITC-conjugated secondary antibodies (Jackson ImmunoResearch). F-actin was stained by FITC-phalloidin (Sigma) or TRITC-phalloidin (Sigma) at a concentration of 1 µM. The stained tissues were mounted in Vectashield (Vector Laboratories). Fluorescent images were acquired on an Olympus FV 1000 confocal microscope (Olympus) equipped with a 60x oil-immersion objective lens (Plan-Aprochromat, NA 1.42, WD 0.15 mm). The image analysis and quantification were analyzed using ImageJ (NIH). The size, contrast and brightness of the resulting images were adjusted with Photoshop CS (Adobe Systems).
Acknowledgments
The authors thank Manzoor Bhat (Univ of North Carolina), Kwang-Wook Choi (KAIST, Korea), Hugo J. Bellen (Baylor College of Medicine), Chi-Hon Lee (NIH, Developmental Studies Hybridoma Bank, Univ of Iowa), and Bloomington Stock Center (Indiana Univ) for fly stocks and reagents.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by Ophthalmology Research Grant of Knights Templar Eye Foundation (http://www.knightstemplar.org/ktef/), and University Research Committee Grant of Baylor University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 2.Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- 3.Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, et al. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- 4.Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- 5.Longley RL, Jr, Ready DF. Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev Biol. 1995;171:415–433. doi: 10.1006/dbio.1995.1292. [DOI] [PubMed] [Google Scholar]
- 6.Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- 7.den Hollander AI, Heckenlively JR, van den Born LI, de Kok YJ, van der Velde-Visser SD, et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet. 2001;69:198–203. doi: 10.1086/321263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.den Hollander AI, ten Brink JB, de Kok YJ, van Soest S, van den Born LI, et al. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat Genet. 1999;23:217–221. doi: 10.1038/13848. [DOI] [PubMed] [Google Scholar]
- 9.Fan SS, Ready DF. Glued participates in distinct microtubule-based activities in Drosophila eye development. Development. 1997;124:1497–1507. doi: 10.1242/dev.124.8.1497. [DOI] [PubMed] [Google Scholar]
- 10.Fischer JA, Acosta S, Kenny A, Cater C, Robinson C, et al. Drosophila klarsicht has distinct subcellular localization domains for nuclear envelope and microtubule localization in the eye. Genetics. 2004;168:1385–1393. doi: 10.1534/genetics.104.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosley-Bishop KL, Li Q, Patterson L, Fischer JA. Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr Biol. 1999;9:1211–1220. doi: 10.1016/s0960-9822(99)80501-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, League GP, Nam SC. Role of spastin in apical domain control along the rhabdomere elongation in Drosophila photoreceptor. PLoS One. 2010;5:e9480. doi: 10.1371/journal.pone.0009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Rogers AK, League GP, Nam SC. Genetic interaction of centrosomin and bazooka in apical domain regulation in Drosophila photoreceptor. PLoS One. 2011;6:e16127. doi: 10.1371/journal.pone.0016127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.League GP, Nam SC. Role of kinesin heavy chain in Crumbs localization along the rhabdomere elongation in Drosophila photoreceptor. PLoS One. 2011;6:e21218. doi: 10.1371/journal.pone.0021218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukhopadhyay B, Nam SC, Choi KW. Kinesin II is required for cell survival and adherens junction positioning in Drosophila photoreceptors. Genesis. 2010;48:522–530. doi: 10.1002/dvg.20642. [DOI] [PubMed] [Google Scholar]
- 16.Roper K, Gregory SL, Brown NH. The ‘spectraplakins’: cytoskeletal giants with characteristics of both spectrin and plakin families. J Cell Sci. 2002;115:4215–4225. doi: 10.1242/jcs.00157. [DOI] [PubMed] [Google Scholar]
- 17.Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 18.Leung CL, Sun D, Zheng M, Knowles DR, Liem RK. Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J Cell Biol. 1999;147:1275–1286. doi: 10.1083/jcb.147.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Kolodziej PA. Short Stop provides an essential link between F-actin and microtubules during axon extension. Development. 2002;129:1195–1204. doi: 10.1242/dev.129.5.1195. [DOI] [PubMed] [Google Scholar]
- 20.Roper K, Brown NH. Maintaining epithelial integrity: a function for gigantic spectraplakin isoforms in adherens junctions. J Cell Biol. 2003;162:1305–1315. doi: 10.1083/jcb.200307089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferson JJ, Leung CL, Liem RK. Plakins: goliaths that link cell junctions and the cytoskeleton. Nat Rev Mol Cell Biol. 2004;5:542–553. doi: 10.1038/nrm1425. [DOI] [PubMed] [Google Scholar]
- 22.Klebes A, Knust E. A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr Biol. 2000;10:76–85. doi: 10.1016/s0960-9822(99)00277-8. [DOI] [PubMed] [Google Scholar]
- 23.Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Lee S, Zadeh AD, Kolodziej PA. Distinct sites in E-cadherin regulate different steps in Drosophila tracheal tube fusion. Development. 2003;130:5989–5999. doi: 10.1242/dev.00806. [DOI] [PubMed] [Google Scholar]
- 25.Roper K, Brown NH. A spectraplakin is enriched on the fusome and organizes microtubules during oocyte specification in Drosophila. Curr Biol. 2004;14:99–110. [PubMed] [Google Scholar]
- 26.Nam SC, Choi KW. Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development. 2003;130:4363–4372. doi: 10.1242/dev.00648. [DOI] [PubMed] [Google Scholar]
- 27.Nam SC, Choi KW. Domain-specific early and late function of Dpatj in Drosophila photoreceptor cells. Dev Dyn. 2006;235:1501–1507. doi: 10.1002/dvdy.20726. [DOI] [PubMed] [Google Scholar]
- 28.Nam SC, Mukhopadhyay B, Choi KW. Antagonistic functions of Par-1 kinase and protein phosphatase 2A are required for localization of Bazooka and photoreceptor morphogenesis in Drosophila. Dev Biol. 2007;306:624–635. doi: 10.1016/j.ydbio.2007.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen TW, Chen G, Funkhouser LJ, Nam SC. Membrane domain modulation by Spectrins in Drosophila photoreceptor morphogenesis. Genesis. 2009;47:744–750. doi: 10.1002/dvg.20555. [DOI] [PubMed] [Google Scholar]
- 30.Karagiosis SA, Ready DF. Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development. 2004;131:725–732. doi: 10.1242/dev.00976. [DOI] [PubMed] [Google Scholar]
- 31.Chang HY, Ready DF. Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science. 2000;290:1978–1980. doi: 10.1126/science.290.5498.1978. [DOI] [PubMed] [Google Scholar]
- 32.Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoh AK, O'Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Harris KL, Whitington PM, Kolodziej PA. short stop is allelic to kakapo, and encodes rod-like cytoskeletal-associated proteins required for axon extension. J Neurosci. 2000;20:1096–1108. doi: 10.1523/JNEUROSCI.20-03-01096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prokop A, Uhler J, Roote J, Bate M. The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons. J Cell Biol. 1998;143:1283–1294. doi: 10.1083/jcb.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 37.Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- 38.Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes Dev. 2007;21:483–496. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- 39.Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 40.Lansbergen G, Akhmanova A. Microtubule plus end: a hub of cellular activities. Traffic. 2006;7:499–507. doi: 10.1111/j.1600-0854.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 41.Subramanian A, Prokop A, Yamamoto M, Sugimura K, Uemura T, et al. Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Curr Biol. 2003;13:1086–1095. doi: 10.1016/s0960-9822(03)00416-0. [DOI] [PubMed] [Google Scholar]
- 42.Slep KC, Rogers SL, Elliott SL, Ohkura H, Kolodziej PA, et al. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J Cell Biol. 2005;168:587–598. doi: 10.1083/jcb.200410114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 44.Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 45.Bhat MA, Izaddoost S, Lu Y, Cho KO, Choi KW, et al. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 46.Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994;165:716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]