Abstract
Background/Aims
A controversy exists about which statin is preferable for patients with acute myocardial infarction (AMI), and clinical impacts of different statins according to lipophilicity have not been established.
Methods
The 1,124 patients with AMI included in the present study were divided into hydrophilic- and lipophilic-statin groups. In-hospital complications (defined as death, cardiogenic shock, ventricular arrhythmia, infection, bleeding, and renal insufficiency, and other fatal arrhythmias), major adverse cardiac events (MACE), all-cause death, re-myocardial infarction, re-percutaneous coronary intervention (re-PCI), and surgical revascularization were analyzed during a 1-year clinical follow-up.
Results
Baseline characteristics were similar between the two groups, and in-hospital complication rates showed no between-group differences (11.7% vs. 12.8%, p = 0.688). Although MACE at the 1- and 6-month clinical follow-ups occurred more in hydrophilic statin group I (1 month: 10.0% vs. 4.4%, p = 0.001; 6 month: 19.9% vs. 14.2%, p = 0.022), no significant difference in MACE was observed at the 1-year follow-up (21.5% vs. 17.9%, p = 0.172). Both statin groups showed similar efficacy for reducing serum lipid concentrations. A Cox-regression analysis showed that the use of a hydrophilic statin did not predict 1-year MACE, all-cause death, AMI, or re-PCI.
Conclusions
Although short-term cardiovascular outcomes were better in the lipophilic-statin group, 1-year outcomes were similar in patients with AMI who were administered hydrophilic and lipophilic statins. In other words, the type of statin did not influence 1-year outcomes in patients with AMI.
Keywords: Hydroxymethylglutaryl-CoA reductase inhibitors, Myocardial infarction
INTRODUCTION
Statins are hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitors and lipid-lowering agents used for various kinds of dyslipidemia. They also have beneficial effects in ischemic heart disease such as acute coronary syndrome including acute myocardial infarction (AMI) [1-5]. Statins have shown additional pleiotropic effects beyond their lipid-lowering effect; they improve endothelial function, reduce inf lammation and coronary artery thrombus, and decrease left-ventricular mass, blood pressure, left-ventricular fibrosis, cardiac-valve sclerosis, atrial fibrillation, and mortality in patients with diabetes and renal disease [6-11]. Consequently, statins are essential medical therapy for patients with ischemic heart disease and dyslipidemia. However, statins comprise many subtypes based on structural differences, resulting in different pharmacokinetics and efficacy. Although different types of statins have slightly different efficacy, no standard exists for selecting a statin in a clinical setting. Generally, statins are classified into hydrophilic and lipophilic groups based on tissue selectivity. Hydrophilic statins, such as pravastatin and rosuvastatin, have less tissue absorption, except for the liver, and fewer side effects due to lower dependence on the cytochrome p450 enzyme [12]. Several studies have compared lipophilic with hydrophilic statins in a clinical setting [13-15]. In a sub-analysis of the Multicenter Study for Aggressive Lipid-lowering Strategy by HMG-CoA Reductase Inhibitors in Patients with Acute Myocardial Infarction (MUSASHI-AMI) database, hydrophilic statins were shown to be superior to lipophilic statins for preventing new Q-waves and reducing cardiovascular events in normocholesterolemic patients with AMI [13]. Furthermore, inflammation was attenuated by hydrophilic compared with lipophilic statins [14]. However, in a study of patients with coronary artery disease, no significant difference in the incidence of all-cause events was observed with respect to statin lipophilicity [15].
It has not been established which statin is preferable, based on lipophilicity, in patients with AMI. Therefore, we compared the clinical outcomes in patients with AMI who were administered a hydrophilic statin and those administered a lipophilic statin.
METHODS
Study population and statin prescription
In total, 1,124 patients diagnosed with AMI from January 2006 to June 2008 were enrolled. All patients were statin naïve and were prescribed a statin during admission. The kind of statin was not changed during the follow-up period. However, dose was increased to obtain the optimal target range of low-density lipoprotein cholesterol (LDL-C, 70-100 mg/dL) [16,17]. Of these patients, 317 were prescribed a hydrophilic statin, including rosuvastatin (90%; mean dosage, 10.7 mg) or pravastatin (10%, 19.3 mg), and 807 patients received a lipophilic statin, including atorvastatin (53%, 16.1 mg), simvastatin (19.5%, 24.7 mg), or pitavastatin (17%, 2.6 mg), based on the preference of the primary physician or cardiologist. The AMI diagnosis was based on clinical presentation, including increased levels of cardiac biomarkers (creatine kinase-MB, troponin-I, or troponin-T) and 12-lead electrocardiographic findings. Any of the following criteria satisfied an AMI diagnosis: typical rise and gradual fall (troponin) or more rapid rise and fall (creatine kinase-MB) of biochemical markers of myocardial necrosis with at least one of the following: 1) ischemic symptoms; 2) development of pathological Q-waves on electrocardiogram; 3) electrocardiogram changes indicative of myocardial ischemia (ST-segment deviation); or 4) coronary artery intervention [18]. All laboratory data were obtained immediately after admission. However, the lipid panel was checked in a fasting state, and two-dimensional echocardiography was performed before discharge. Of these patients, 1,095 (97.4%) completed the 12-month clinical follow-up.
Coronary angiographic and procedural findings
Coronary arterial lesion type was determined according to the American College of Cardiology/American Heart Association classification. The culprit vessel of patients with non-ST-segment elevation MI (NSTEMI) was determined by the coronary angiographic findings, 12-lead electrocardiogram, two-dimensional echocardiogram, and non-invasive stress test, if possible. In cases of STEMI, a 12-lead electrocardiogram was mainly used to identify the culprit vessel. If indicated, a percutaneous coronary intervention (PCI) was performed. All patients received 100-300 mg aspirin and 300-600 mg clopidogrel as a loading dose before PCI. A 50-70 U/kg dose of unfractionated heparin was used before or during PCI to maintain activated clotting time at 250-300 seconds. After PCI, 100-300 mg aspirin and 75 mg clopidogrel were prescribed daily as a maintenance dose. In cases of hemodynamic instability, an intra-aortic balloon pump (IABP) was inserted to support hypotension.
In-hospital outcomes and clinical endpoints
Baseline clinical characteristics, laboratory findings, and procedural findings were analyzed. In-hospital complications including mortality, periprocedureal cardiogenic shock, ventricular fibrillation or tachycardia, infection, major or minor bleeding, renal insufficiency, and other arrhythmias such as bradycardia or supraventricular tachycardia were evaluated. Clinical follow-up was performed at 1, 6, and 12 months. Endpoints were analyzed as a composite of major adverse cardiac events (MACE: all-cause death, recurrent MI, repeated PCI, and surgical revascularization), cardiac death, non-cardiac death, or MI, and repeated PCI. Additionally, the baseline lipid panel was checked, and a 1-, 6-, and 12-month follow-up lipid panels were checked to observe the efficacy of each statin type. Recurrent MI was defined as recurrent symptoms with new electrocardiographic changes reflecting MI or increased cardiac biomarkers at least twice the normal upper limit [19]. Repeated PCI included target-lesion revascularization, target-vessel revascularization (TVR), and non-TVR [20].
Statistical analysis
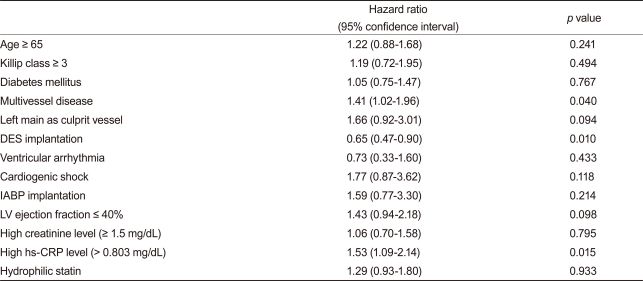
Statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation, and these were compared using the Student t test. Categorical variables were analyzed by the chi-square test. A paired t test was performed to observe significant changes in serum lipid levels. Cox regression analysis was used to compare endpoints between the two groups. Significant variables in the univariate analysis (p < 0.1) for endpoints were included in the Cox-regression analysis. The included variables were age ≥ 65 years, Killip classification ≥ 3/4 on admission, history of diabetes mellitus, multi-vessel disease on coronary angiography, left main stem as a culprit vessel, drug-eluting stent (DES) implantation, ventricular arrhythmia during admission, periprocedural cardiogenic shock, IABP insertion, left ventricular ejection fraction < 40% by two-dimensional echocardiography, high creatinine level (≥1.5 mg/dL), and high-sensitivity C-reactive protein (hs-CRP) level greater than a median value of 0.803 mg/dL. All analyses were two tailed, and all variables were considered significant when the p value was < 0.05.
RESULTS
Clinical characteristics and procedural findings
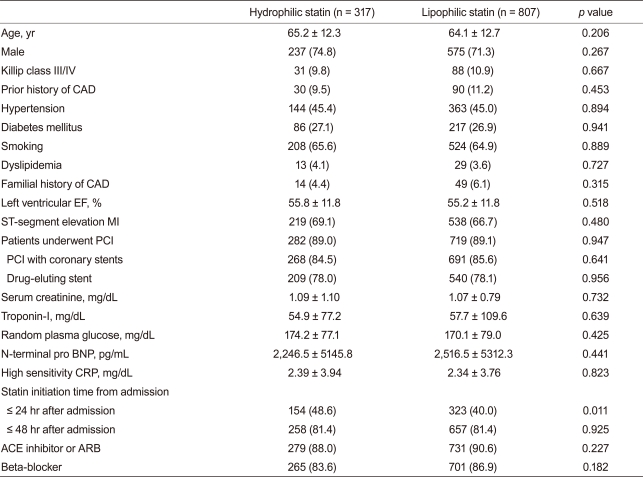
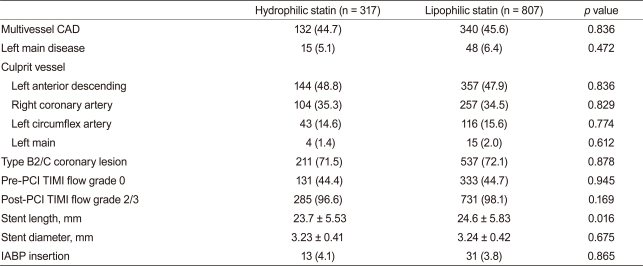
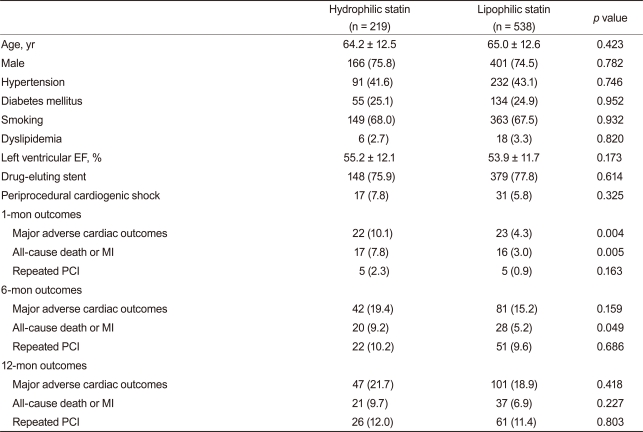
Baseline demographic, laboratory, and clinical findings were similar between the two groups. Additionally, PCI rate (hydrophilic statin, 89.0% vs. lipophilic statin, 89.1%, p = 0.947), PCI with coronary stents (84.5% vs. 85.6%, p = 0.641), and DES implantation rate (78.0% vs. 78.1%, p = 0.956) were not different between the two groups. However, statin initiation time from admission and initiation rate ≤24 hours after admission was higher in the hydrophilic statin group (48.6% vs. 40.0%, p = 0.011) (Table 1). No significant differences in the rate of multi-vessel coronary artery disease, left main stem disease, and the location of the culprit vessel were observed. PCI success rate, presenting as post-PCI thrombolysis in myocardial infarction flow grade ≥ 2/3, was similar between the two groups. However, mean stent length implanted into the culprit vessel was longer in the lipophilic statin group (23.7 ± 5.5 mm vs. 24.6 ± 5.8 mm, p = 0.016) (Table 2).
Table 1.
Baseline demographic, laboratory, and clinical findings
Values are presented as mean ± SD or number (%).
CAD, coronary artery disease; EF, ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; BNP, B-type natriuretic peptide; CRP, C-reactive protein; ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blocker.
Table 2.
Angiographic findings and procedural characteristics
Values are presented as number (%).
CAD, coronary artery disease; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction; IABP, intra-aortic ballooning pump.
In-hospital outcomes and endpoints during clinical follow-up
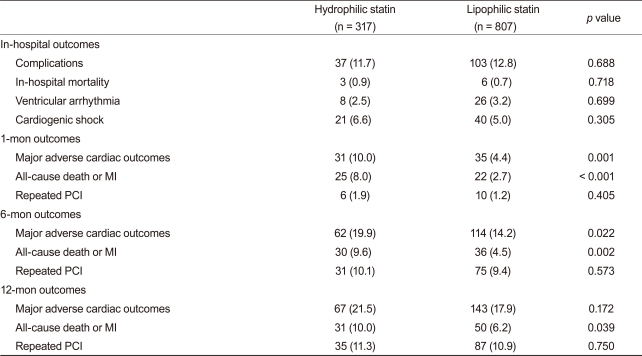
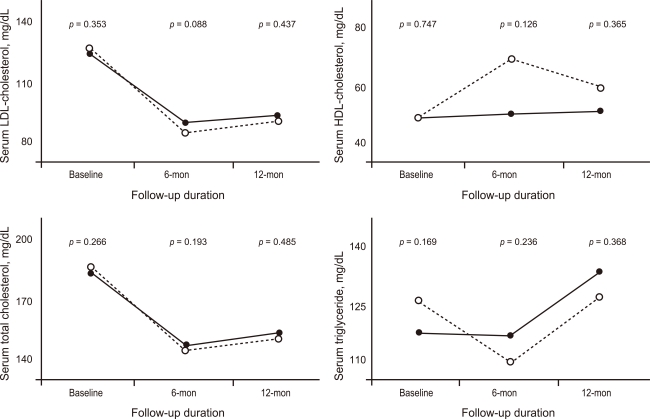
The rates of in-hospital complications were similar between the hydrophilic group and lipophilic group (11.7% vs. 12.8%, p = 0.688). During the 12-month clinical follow-up, 210 endpoints were identified (18.9% of all patients). Although 1- and 6-month MACE rates were higher in the hydrophilic statin group, the 12-month MACE rate was not different between the hydrophilic group and lipophilic group (21.5% vs. 17.9%, p = 0.172). No difference was observed for repeated PCI between the two groups. However, death and MI rate were higher in the hydrophilic statin group at the 12-month follow-up based on a crude analysis (10.0% vs. 6.2%, p = 0.039) (Table 3). The levels of LDL-C, high-density lipoprotein-cholesterol (HDL-C), triglyceride (TG), and total cholesterol (TC) were similar between the two groups at the 1-, 6-, and 12-month follow-ups (Fig. 1). None of the statins changed the levels of serum HDL-C or TG. However, they reduced the levels of LDL-C and TC at the 12-month follow-up (LDL-C, p < 0.001 and TC, p < 0.001, respectively).
Table 3.
In-hospital and clinical outcomes
Values are presented as number (%).
MI, myocardial infarction; PCI, percutaneous coronary intervention.
Figure 1.
Changes in the lipid panel at follow-up (solid line, lipophilic-statin group; dotted line, hydrophilic-statin group. p value represents the statistical difference between the lipid values of the hydrophilic- and lipophilic-statin groups). HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Multivariate analysis for MACE
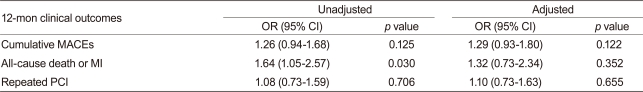
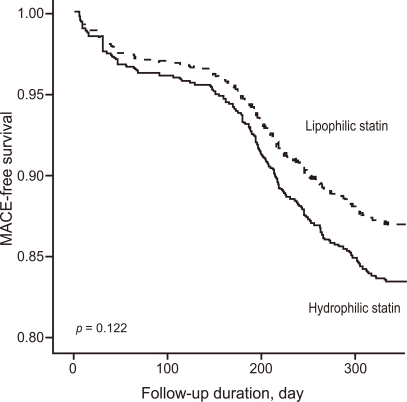
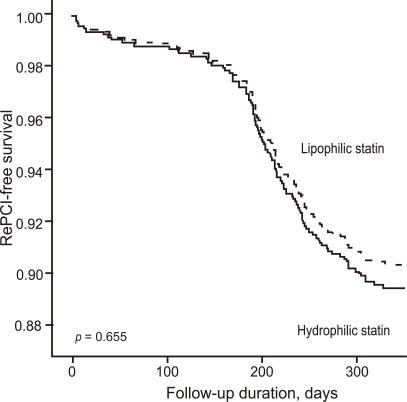
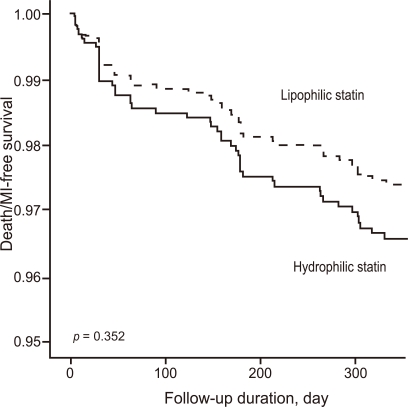
After adjusting for confounding factors, the hydrophilic statins did not predict cumulative MACE (hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.93-1.80; p = 0.122), all-cause death or MI (HR, 1.32; 95% CI, 0.73 to 2.34; p = 0.352), and repeated PCI (HR, 1.10; 95% CI, 0.73 to 1.63; p = 0.655) (Table 4 and Figs. 2-4). Results of the 1-year multivariate analysis for MACE are presented in Table 5.
Table 4.
Adjusted 12-mon clinical outcomes for hydrophilic statins compared with lipophilic statins
OR, odds ratio; CI, confidence interval; MACE, major adverse cardiac events; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Figure 2.
Major adverse cardiac event (MACE)-free survival rates during follow-up.
Figure 4.
Repeated percutaneous coronary intervention (RePCI)-free survival rates during follow-up.
Table 5.
Multivariate analysis for 1 year MACE
MACE, major adverse cardiac events; DES, drug-eluting stent; IABP, intra-aortic balloon pump; LV, left ventricle; hs-CRP, high sensitivity C-reactive protein.
DISCUSSION
No differences were observed in the 12-month clinical outcomes in patients with AMI based on statin lipophilicity. Few clinical and basic studies have compared hydrophilic and lipophilic statins. Two studies could not demonstrate which type of statin was better in an AMI setting [13,15]. In a study comparing the two types of statins, cognitive impairment associated with statins use in 24,595 patients was significantly different in the two types of statins. Moreover, the lipophilic, but not the hydrophilic, statin induced cell death in gynecological cancers expressing high levels of HMG-CoA reductase, and enhanced phagocytosis in human peripheral blood [21-23]. Similarly, clinical and basic studies have not standardized which statin type is preferable. Therefore, our study was designed to compare the clinical outcomes between the two types of statin in patients with AMI.
In our study, more patients were prescribed a hydrophilic than a lipophilic statin within 24 hours after admission. Although controversy remains, early statin therapy has been reported to improve cardiovascular outcome in patients with AMI [4,24]. However, the univariate analysis in our study did not show that early statin therapy influenced clinical outcome. Additionally, the prevalence of dyslipidemia on admission was relatively low in our registry. This might be associated with enrollment of statin naïve patients. In our study, both statin groups had reduced serum LDL-C and TC at the 12-month follow-up. The TC-lowering effect was considered secondary to the LDL-C-lowering effect. Although statins do not influence the levels of serum HDL-C and TG, they reduce LDL-C [12]. The 12-month lipid profiles appeared worse than those at 6 months. However, longer follow-up periods and larger-scale studies are needed to confirm this issue. Short-term MACE occurred more frequently in the hydrophilic-statin group. Variables not included in our registry such as cardiopulmonary resuscitation, symptom-to-door time, staged PCI, and door-to-PCI time may have influenced these results. After correcting for confounding factors, the Cox regression analysis of different rates of death or MI at the 12-month follow-up revealed no difference between the two groups.
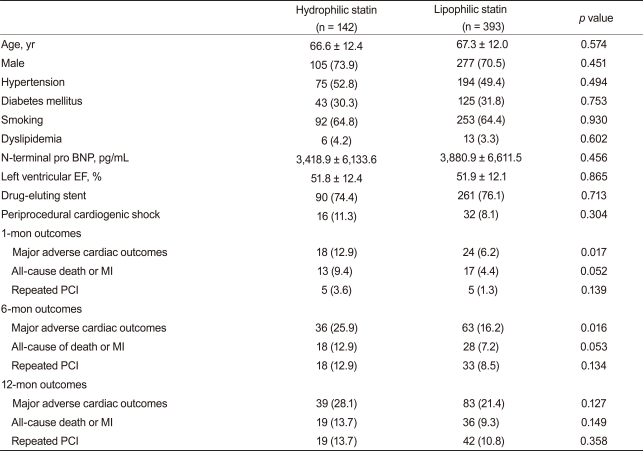
We conducted a subgroup analysis by the presence of ST-segment elevation. No differences in baseline characteristics and cardiovascular risks were observed in 757 patients with STEMI in our registry. Clinical endpoints showed a similar tendency compared with the total AMI group analysis. The 12-month composite MACE, death or MI, and repeated PCI rates were also similar between the two groups (Table 6). hs-CRP is a marker for inflammation and is associated with adverse cardiovascular outcomes [25]. In a subgroup analysis of 535 patients with high hs-CRP (greater than the median value of 0.803 mg/dL), no differences in 12-month clinical endpoints were observed (Table 7).
Table 6.
Clinical characteristics and outcomes in the STEMI subgroup
Values are presented as mean ± SD or number (%).
STEMI, ST-segment elevation myocardial infarction; EF, ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Table 7.
Clinical characteristics and outcomes in patients with high hs-CRP
Values are presented as mean ± SD or number (%).
hs-CRP, high sensitivity C-reactive protein; BNP, B-type natriuretic peptide; EF, ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Our study had several limitations. First, selection bias may have been present because of the retrospective nature of the study. Second, although we included as many variables as possible, other variables may have affected our results. For example, undergoing cardiopulmonary resuscitation, symptom-to-intervention time, and high statin loading dose may have affected the clinical results. Third, statin selection was not standardized; it was the decision of the physician or cardiologist. Fourth, side effects of statin therapy such as myopathy or hepatotoxicity were not compared between the two groups. Fifth, we could not collect detailed information about all-cause death or MI. Because the differences in the 1- and 6-month clinical outcomes between the two groups were mainly caused by differences in all-cause death and MI, we should have had detailed information about death and MI. Finally, reducing periprocedural MI is a current issue for statin therapy, and statin use is associated with a reduced rate of periprocedural MI [26]. However, the subjects in our study had AMI but not angina. Consequently, it was difficult to compare the periprocedural MI rate between the two groups; thus, a large-scaled randomized controlled trial will be needed.
In conclusion, although, short-term cardiovascular outcomes were better in the lipophilic-statin group, long-term outcomes were similar in the hydrophilic- and lipophilic-statin groups in patients with AMI. In other words, statin type did not influence long-term outcomes in patients with AMI.
Figure 3.
Death/myocardial infarction (MI)-free survival rates during follow-up.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea funded by the Korean Government (MEST), Republic of Korea (2010-0020261), and by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (A084869).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 4.Lenderink T, Boersma E, Gitt AK, et al. Patients using statin treatment within 24 h after admission for ST-elevation acute coronary syndromes had lower mortality than non-users: a report from the first Euro Heart Survey on acute coronary syndromes. Eur Heart J. 2006;27:1799–1804. doi: 10.1093/eurheartj/ehl125. [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 7.Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 8.Dangas G, Badimon JJ, Smith DA, et al. Pravastatin therapy in hyperlipidemia: effects on thrombus formation and the systemic hemostatic profile. J Am Coll Cardiol. 1999;33:1294–1304. doi: 10.1016/s0735-1097(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 9.Zile MR. Treating diastolic heart failure with statins: "phat" chance for pleiotropic benefits. Circulation. 2005;112:300–303. doi: 10.1161/CIRCULATIONAHA.105.551887. [DOI] [PubMed] [Google Scholar]
- 10.Yoon HE, Song JC, Hyoung BJ, et al. The efficacy and safety of ezetimibe and low-dose simvastatin as a primary treatment for dyslipidemia in renal transplant recipients. Korean J Intern Med. 2009;24:233–237. doi: 10.3904/kjim.2009.24.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JS, Kim YJ, Choi JY, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010;25:27–35. doi: 10.3904/kjim.2010.25.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26(4 Suppl 3):III32–III38. doi: 10.1002/clc.4960261507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamoto T, Kojima S, Ogawa H, et al. Usef ulness of hydrophilic vs lipophilic statins after acute myocardial infarction: subanalysis of MUSASHI-AMI. Circ J. 2007;71:1348–1353. doi: 10.1253/circj.71.1348. [DOI] [PubMed] [Google Scholar]
- 14.Kai T, Arima S, Taniyama Y, Nakabou M, Kanamasa K. Comparison of the effect of lipophilic and hydrophilic statins on serum adiponectin levels in patients with mild hypertension and dyslipidemia: Kinki Adiponectin Interventional (KAI) Study. Clin Exp Hypertens. 2008;30:530–540. doi: 10.1080/10641960802251925. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M, Yamazaki T, Hayashi D, et al. Pleiotropic effects of statins on cardiovascular events in the Japanese Coronary Artery Disease study. Int J Cardiol. 2008;129:294–296. doi: 10.1016/j.ijcard.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Pat ients With Unstable Angina/Non-ST-Elevat ion Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Myocardial infarction redefined: a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–1513. doi: 10.1053/euhj.2000.2305. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 20.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 21.Glasser SP, Wadley V, Judd S, et al. The association of statin use and statin type and cognitive performance: analysis of the reasons for geographic and racial differences in stroke (REGARDS) study. Clin Cardiol. 2010;33:280–288. doi: 10.1002/clc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato S, Smalley S, Sadarangani A, et al. Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase. J Cell Mol Med. 2010;14:1180–1193. doi: 10.1111/j.1582-4934.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salman H, Bergman M, Djaldetti M, Bessler H. Hydrophobic but not hydrophilic statins enhance phagocytosis and decrease apoptosis of human peripheral blood cells in vitro. Biomed Pharmacother. 2008;62:41–45. doi: 10.1016/j.biopha.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Li YH, Wu HL, Yang YH, Tsai HS, Chao TH. Effect of early versus late in-hospital initiation of statin therapy on the clinical outcomes of patients with acute coronary syndrome. Int Heart J. 2007;48:677–688. doi: 10.1536/ihj.48.677. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 26.Pasceri V, Patti G, Nusca A, et al. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110:674–678. doi: 10.1161/01.CIR.0000137828.06205.87. [DOI] [PubMed] [Google Scholar]