Abstract
The most common pancreatic cancer is adenocarcinoma. Primary adenosquamous cell carcinoma of the pancreas is very rare and aggressive. A 46-year-old man presented with a 3-month history of dyspepsia and a 7-kg weight loss. The physical examination showed tenderness of the right upper quadrant of the abdomen. There was no jaundice. Amylase and lipase were elevated. CA 19-9 was elevated to 566.7 U/mL. Gastroduodenoscopy showed a hard ulceroinfiltrative mass with a yellowish exudate that bled readily on touch in the second portion of the duodenum. Abdominal computed tomography showed a 7.1 × 6.3-cm heterogeneously enhancing mass in the pancreatic head. The pancreatic mass had invaded the duodenum wall, gastric antrum, and gastroduodenal artery sheath. Fine-needle aspiration biopsy of the pancreatic mass revealed adenosquamous cell carcinoma, anaplastic type. We concluded that an adenosquamous cell carcinoma of pancreas had invaded the duodenal mucosa causing ulceration.
Keywords: Pancreas; Carcinoma, Adenosquamous
INTRODUCTION
Pancreatic cancer is the second most common gastrointestinal malignancy in the United States [1] and the fifth most common malignancy in Korea [2]. More than 95% of malignant neoplasms of the pancreas arise from exocrine glands. Ductal adenocarcinoma accounts for 80-85% of pancreatic tumors [3,4]. By contrast, adenosquamous cell carcinoma of the pancreas is very rare and aggressive [4] and accounts for 1-4% of all exocrine malignancies of the pancreas based on autopsy and surgical specimens [5]. A rare case of pancreatic adenosquamous cell carcinoma with duodenal invasion is reported, and the literature is reviewed.
CASE REPORT
A 46-year-old man presented with a 3-month history of dyspepsia and a 7-kg weight loss. He had no history of any disease. He did not smoke and drank alcoholic beverages in moderation. On admission, the patient's vital signs were stable. His family history was negative for malignancy. Direct tenderness was noted over the right upper quadrant area of the abdomen, but rebound tenderness and Murphy's sign were absent. The remainder of the examination was unremarkable. The complete blood count showed a white cell count of 7,800/mm3 (70.9% neutrophils), hemoglobin 10.3 g/dL, hematocrit 29.2%, and platelet count of 283,000/mm3. The blood chemistry showed a fasting glucose level of 107 mg/dL, urea nitrogen 19 mg/dL, creatinine 0.8 mg/dL, total protein 6.1 g/dL, albumin 4.0 g/dL, aspartate transaminase 13 IU/L, alanine aminotransferase 12 IU/L, alkaline phosphatase 238 IU/L, total bilirubin 0.6 mg/dL, total cholesterol 170 mg/dL, and triglyceride 64 mg/dL. The serum amylase was elevated slightly to 170 U/L, and the serum lipase to 338 U/L. The carcinoembryonic antigen (CEA) was 3.3 ng/mL, α-fetoprotein was 1.5 ng/mL, and the CA 19-9 was elevated to 566.7 ng/mL. Gastroduodenoscopy showed a hard ulceroinfiltrative mass with a yellowish exudate that bled readily on touch at the second portion of the duodenum (Fig. 1A). Initially, we thought that he had duodenal cancer. However, a biopsy of the duodenum showed adenosquamous cell carcinoma (Fig. 1B). Abdominal computed tomography showed a 7.1 × 6.3-cm heterogeneously enhancing mass in the pancreatic head. The main pancreatic duct was dilated due to compression by the mass. The tumor mass invaded the duodenum, gastric antrum, and gastroduodenal artery sheath. Multiple enlarged lymph nodes were seen around the left gastric vessel, greater omentum, and small bowel mesentery (Fig. 2A). Fine-needle aspiration biopsy of the pancreatic mass revealed adenosquamous cell carcinoma, anaplastic type (Fig. 2B). We diagnosed primary adenosquamous cell carcinoma of the pancreas that had invaded the duodenum, resulting in an ulceroinfiltrative lesion. The patient refused palliative management and was discharged.
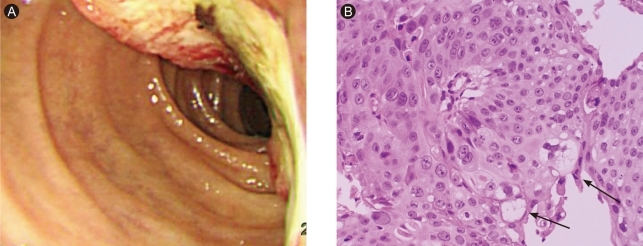
Figure 1.
(A) Gastroduodenoscopy showed hard ulceroinfiltrative mass with yellowish exudates and easy touch bleeding at second portion of duodenum. (B) The tumor cells showed mostly squamous differentiation with focal adenocarcinoma, showing cytoplasmic mucins (arrows) (H&E, × 200).
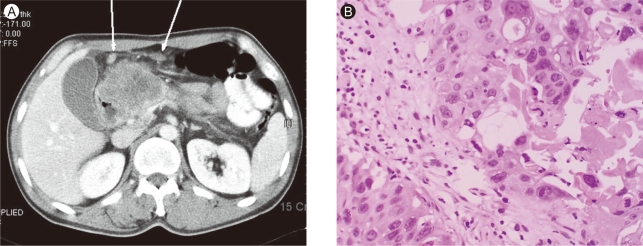
Figure 2.
(A) In abdominal pelvis CT, it showed 7.1 × 6.3 cm-sized heterogeneous enhancing mass in pancreatic head portion. Multiple lymph node enlargements were seen around left gastric vessel, greater omentum, and small bowel mesentery (arrows). (B) Fine needle aspiration biopsy of the pancreatic mass revealed mostly squamous differentiation with focal adenocarcinoma with anaplastic type (H&E, × 200).
DISCUSSION
Pancreatic cancer includes ductal adenosquamous carcinoma, endocrine neoplasms, cystic tumors, solid pseudopapillary tumors, acinar cell carcinoma, adenocarcinoma, squamous cell carcinoma, primary lymphoma of the pancreas, and metastatic lesions to the pancreas [6]. Pancreatic cancer originates mainly from the exocrine duct cells, with more than 80% showing exocrine adenomatous morphology [4]. Pancreatic adenosquamous cell carcinoma is very rare. Based on autopsy studies and surgical specimens, it accounts for 1-4% of all exocrine malignancies of the pancreas [5]. A review of data taken from various cancer registries on 6,668 cases of exocrine pancreatic cancer treated between 1950 and 1985 revealed 36 cases (0.005%) of squamous carcinoma and 68 cases (0.01%) of adenosquamous carcinoma [7].
Male and old age (over 60) are predominant [5,8]. Common symptoms are abdominal pain, weight loss, and painless jaundice [8,9]. The mass most commonly involves the pancreatic head [8,9]. There are no specific radiologic findings that distinguish this tumor from common ductal cell adenocarcinoma of the pancreas [5]. Nabae et al. [10] stated that the presence of central necrosis in a huge infiltrative pancreatic tumor was suggestive of adenosquamous carcinoma of the pancreas.
The histogenesis of adenosquamous cell carcinoma of the pancreas remains uncertain, and several different hypotheses have been offered: 1) a preexisting adenocarcinoma undergoing squamous cell transformation; 2) heterotopic squamous epithelium undergoing a malignant change; and 3) a stem cell capable of differentiating into either a squamous or glandular cell undergoing a malignant change [5,11,12]. Morohoshi et al. [4] proposed a similar hypothesis.
A pathological diagnosis can be made using percutaneous needle aspiration or at surgery. Rahemtullah et al. [8] reported that the cytological features derived from a fine-needle aspiration biopsy are diagnostic of adenosquamous cell carcinoma of the pancreas. A few patients undergo surgery because most of them are reported to have stage IV disease at the time of presentation [8]. Our patient's diagnosis was adenosquamous cell carcinoma of the pancreas based on a percutaneous needle aspiration biopsy.
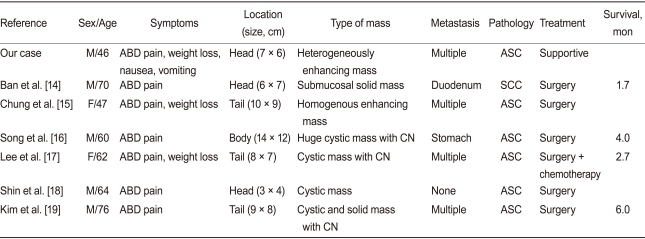
Major surgery does not have a substantial benefit for patients with adenosquamous cell carcinoma of the pancreas, especially those with severe comorbidities [13]. The prognosis of patients with adenosquamous carcinoma of the pancreas is less favorable than that of patients with common ductal cell carcinoma of the pancreas. Smit et al. [13] reported that the survival averaged 5.7 months for 72 patients with adenosquamous carcinoma of the pancreas, and only five patients survived for more than 1 year. Kardon et al. [9] reported that the overall survival was 12.5 months for patients treated with resection and adjuvant chemotherapy and 3.0 months for the patients who received no or palliative chemotherapy. Most patients had a poor prognosis despite aggressive surgery with or without adjuvant therapy [13]. In Korea, seven cases (including our case) have been reported (one case of squamous cell carcinoma, six cases of adenosquamous cell carcinoma) [14-19]. These cases showed a male predominance (M:F = 5:2) and a mean patient age of 61.7 ± 11.1 years. The common symptoms were abdominal pain in seven (100%) and weight loss in four (57.1%). No case had jaundice. Lipase was elevated in three (42.9%), CA19-9 in two (28.6%), and CEA in one (14.3%). On CT, the masses were located in the pancreas head in three (42.9%), body in one (14.3%), and tail in three (42.9%). Central necrosis was observed in three (42.9%), and metastasis in six (85.7%). The median survival was 3.3 months, and the mean survival was 3.6 ± 1.9 months. The prognosis in all cases was very poor, although one case was treated with adjuvant chemotherapy. Table 1 summarizes the clinical findings for the Korean cases.
Table 1.
Summary of the cases reported in Korea
ABD, abdominal; ASC, adenosquamous cell carcinoma; SCC, squamous cell carcinoma; CN, central necrosis.
In Korea, adenosquamous cell carcinoma of pancreas is very rare and aggressive. It has a poor prognosis and short survival with any treatment. Our case had a pancreatic mass with duodenal invasion. Multiple enlarged lymph nodes were seen around the left gastric vessel, greater omentum, and small bowel mesentery. We recommended adjuvant treatment, but the patient refused.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Korea National Statistical Office. Annual report on the cause of death statistics. Daejeon: Korea National Statistical Office; 2007. [Google Scholar]
- 3.Cylwik B, Nowak HF, Głowińska L. Malignant neoplasms of the pancreas: a study based on autopsy data from 1953 to 1982 in Bialystok, Poland. II. A survey of 195 cases. Neoplasma. 1984;31:605–613. [PubMed] [Google Scholar]
- 4.Morohoshi T, Held G, Kloppel G. Exocrine pancreatic tumours and their histological classification: a study based on 167 autopsy and 97 surgical cases. Histopathology. 1983;7:645–661. doi: 10.1111/j.1365-2559.1983.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 5.Madura JA, Jarman BT, Doherty MG, Yum MN, Howard TJ. Adenosquamous carcinoma of the pancreas. Arch Surg. 1999;134:599–603. doi: 10.1001/archsurg.134.6.599. [DOI] [PubMed] [Google Scholar]
- 6.Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol. 2006;12:3180–3185. doi: 10.3748/wjg.v12.i20.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyer KL, Marshall JB, Metzler MH, Poulter JS, Seger RM, Diaz-Arias AA. Squamous cell carcinoma of the pancreas: report of an unusual case and review of the literature. Dig Dis Sci. 1992;37:312–318. doi: 10.1007/BF01308190. [DOI] [PubMed] [Google Scholar]
- 8.Rahemtullah A, Misdraji J, Pitman MB. Adenosquamous carcinoma of the pancreas: cytologic features in 14 cases. Cancer. 2003;99:372–378. doi: 10.1002/cncr.11855. [DOI] [PubMed] [Google Scholar]
- 9.Kardon DE, Thompson LD, Przygodzki RM, Heffess CS. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol. 2001;14:443–451. doi: 10.1038/modpathol.3880332. [DOI] [PubMed] [Google Scholar]
- 10.Nabae T, Yamaguchi K, Takahata S, et al. Adenosquamous carcinoma of the pancreas: report of two cases. Am J Gastroenterol. 1998;93:1167–1170. doi: 10.1111/j.1572-0241.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- 11.Motojima K, Tomioka T, Kohara N, Tsunoda T, Kanematsu T. Immunohistochemical characteristics of adenosquamous carcinoma of the pancreas. J Surg Oncol. 1992;49:58–62. doi: 10.1002/jso.2930490114. [DOI] [PubMed] [Google Scholar]
- 12.Makiyama K, Takuma K, Zea-Iriarte WL, et al. Adenosquamous carcinoma of the pancreas. J Gastroenterol. 1995;30:798–802. doi: 10.1007/BF02349652. [DOI] [PubMed] [Google Scholar]
- 13.Smit W, Mathy JP, Donaldson E. Pancreatic cytology and adenosquamous carcinoma of the pancreas. Pathology. 1993;25:420–422. doi: 10.3109/00313029309090873. [DOI] [PubMed] [Google Scholar]
- 14.Ban JY, Lee JH, Park SH, Kim JH, Ahn HS. Squamous cell carcinoma of the pancreas with invasion of duodenum and pylorus. J Korean Surg Soc. 2006;71:387–391. [Google Scholar]
- 15.Chung JC, Choi SH, Jang KT, et al. Adenosquamous carcinoma of the pancreas. J Korean Surg Soc. 2006;71:69–72. [Google Scholar]
- 16.Song H, Choi IS, Choi WJ, Yoon DS, Mok WK, Min HS. Adenosquamous carcinoma of the pancreas. Korean J Hepatobiliary Pancreat Surg. 2003;7:164–167. [Google Scholar]
- 17.Lee TH, Chung JP, Yoon DS, et al. A case of adenosquamous carcinoma of the pancreas. Korean J Gastroenterol. 2003;41:154–158. [Google Scholar]
- 18.Shin ES, Myung SJ, Kim MH, et al. A case of adenoequamous carcinoma of the pancreas with unusual pancreatographic findings. Korean J Gastrointest Endosc. 1998;18:129–135. [Google Scholar]
- 19.Kim KU, Cho MY, Cho NC, Yoon KS, Kim DS, Rhoe BS. Adenosquamous carcinoma of the pancreas associated with cyst formation: a care report. Korean J Gastroenterol. 1994;26:885–891. [Google Scholar]