Abstract
Increased evidence shows that normal stem cells may contribute to cancer development and progression by acting as cancer-initiating cells through their interactions with abnormal environmental elements. We postulate that normal stem cells and cancer stem cells (CSC) possess similar mechanisms of self-renewal and differentiation. CSC can be the key to the elaboration of anti-cancer-based therapy. In this article, we focus on a controversial new theme relating to CSC. Tumorigenesis may have a critical stage characterized as a “therapeutic window”, which can be identified by association of molecular, biochemical and biological events. Identifying such a stage can allow the production of more effective therapies (e.g. manipulated stem cells) to treat several cancers. More importantly, confirming the existence of a similar therapeutic window during the conversion of normal stem cells to malignant CSC may lead to targeted therapy specifically against CSC. This conversion information may be derived from investigating the biological behaviour of both normal stem cells and cancerous stem cells. Currently, there is little knowledge about the cellular and molecular mechanisms that govern the initiation and maintenance of CSC. Studies on co-evolution and interdependence of cancer with normal tissues may lead to a useful treatment paradigm of cancer. The crosstalk between normal stem cells and cancer formation may converge developmental stages of different types of stem cells (e.g. normal stem cells, CSC and embryonic stem cells). The differential studies of the convergence may result in novel therapies for treating cancers.
Keywords: Neural stem cell, Cancer stem cell, Convergence, Therapeutic
INTRODUCTION
The survival rate for patients with solid cancers such as glioblastoma multiforme (GBM) has not improved even though multiple billions of dollars have been invested in cancer research since US president Richard Nixon declared war on cancer in 1971[1]. Cancer cells have been treated as invading aliens, which must be completely destroyed and removed[2]. Emerging evidence, however, argues for the need to view cancer differently. We and others have found that similarities and overlapping mechanisms between induced cell plasticity and cancer formation shed new light on the emerging picture of p53 sitting at the crossroads between two intricate cellular potentials: stem cell vs cancer cell generation[3]. A recent report shows that GBM neovasculature may be driven by cancer stem cells (CSC)[4-6] rather than recruiting mesenchymal endothelial progenitors[7-9]. Here, we propose that normal stem cells and CSC may share the same developmental stages. Understanding this paralleled multi-stage oncogenesis process may imply a differential therapy for treating tumors.
CANCER STEM CELLS
A growing body of evidence demonstrates that brain tumors may arise from a single, self-renewing cell, namely CSC[10]. CSC that have characteristics similar to brain stem cells, play a key role in cancer recurrence and resistance to current therapies[11]. These “bad seeds”- CSC - may have the ability to escape standard therapies, explaining tumor growth and new malignancies[12,13]. CSC have been identified in acute myeloid leukemia, breast cancer[14] and, most recently, brain tumors[15-17]. With a frequency as few as one out of thousands or even millions of tumor cells, CSC must be targeted and eliminated to prevent tumor relapse and to promote a cancer-free life. Cancer cells without stem cell properties may have little or no significance for cancer treatment or patient survival. However, the transplantation of native neural stem cells (“naïve”) increased the survival of the recipient animals presumably by inhibiting tumor outgrowth[18]. Despite exciting initial reports of this anticancer potential, clinical potency of stem cell therapy in animal brain tumor models has proven disappointing. Amassed evidence shows that some normal naïve stem cells may contribute to cancer development and progression either by acting as cancer-initiating cells or through interactions with the environment[19-24]. However, it is believed that not all naïve stem cells have the potential to promote cancer progression, but only some naïve stem cells [e.g. mesenchymal stem cells, vascular progenitor cells (VPC)], possess these abilities to favor tumor formation principally due to their secreted pro-angiogenic and immunomodulatory factors. Only stem cells (e.g. native neural stem cells) reprogrammed or genetically altered to deliver anti-tumoral agents (protein, genes, viral, etc.) can exert a more robust anti-cancer effect[25-28] than naïve neural stem cells as demonstrated by Tyler et al[18]. Nevertheless, it is important and necessary to elucidate the cellular and molecular switch involved during the convergence of normal stem cells to CSCs.
CONVERGENCE OF NORMAL STEM CELL AND CANCER STEM CELL DEVELOPMENT
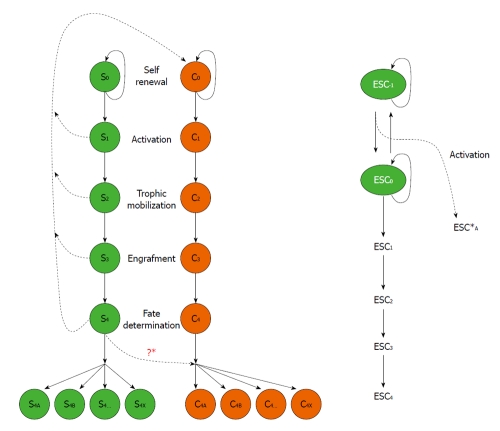
We hypothesize a convergence mechanism for development of different stem cells (normal stem cells, CSC and embryonic stem cells) as illustrated in Figure 1. Normal stem cells, defined as “S”; S0 defines stem cells in a self-renewal stage that actively replicate themselves. S0 are activated by environmental cues to go through different stages: S1 denotes activation, S2 denotes trophic mobilization and migration toward targeted locations, S3 denotes integration and engraftment, S4 denotes terminated differentiation. CSC, defined as “C” in Figure 1, share similar developmental stages: C1, C2, C3, and C4. S0 and C0 stage cells make additional copies of themselves before they go on to make cells of other stages S1, C1; S2, C2; S3, C3, and S4, C4, respectively. However, in the process of stem cell development, it is theoretically possible that genetic mistakes may be made; “S0” may convert to “C0” or CSC, and “S1” to “C1”, etc. The cancer stem cell will then go on to follow the classic steps of differentiation, possibly the same as those of the normal stem cell. The “C1” will be activated to CSC, which are no longer in residency or quiescence. The “C2” cells become migratory and engraft themselves in a targeted tissue to become a “C3” engraftment. The integrated “C3” cells then differentiate to its final “C4” cancer cell stage. The “C4” cells may divide into a heterogeneous population, “C4a” “C4b” “C4c” ¡ “C4x”, derived from not only normal stem cells but also CSC. We know that normal stem cells “S0” replicate and, when activated, go on to “S1”, “S2”, “S3”, and “S4” stages, respectively. The cells in the “S4” stage cells then differentiate into “S4a”, “S4b”, “S4c” ¡ “S4x” which are also heterogeneous in nature.
Figure 1.
Developmental stages of normal stem cell (S) vs cancer stem cell (C). Right panel: Embryonic stem cells (ESC) may undergo similar stages in both normal stem cell and cancer stem cell development. However, at an earlier stage, ESC-1, there is a malignant convergence (See text for details).
Cancer itself can develop in either of two ways. One route is described in which the “S4” cells undergo malignant dedifferentiation. For example, mature glial cells in the brain dedifferentiate to glioma. Thus terminally differentiated cells can ultimately dedifferentiate into “C0” CSC, which remain regulated and produce more CSC. This is the classic origin of tumorigenesis, particularly in adults.
An alternative process that occurs in children involves the normal stem cell “S0” spinning off a “C0”. The “C0” may progress to “C1” “C2” “C3” and “C4”, creating terminally differentiated cancer cells. It is interesting to note that some terminally differentiated stem cells contribute to the establishment of terminally differentiated cancer cells[29]. Accumulated evidence also suggests that factors in the local extracellular milieu contribute to cancer development. For example, glioblastoma by definition must show necrosis, but why is this the case? A glioblastoma has the highest level of neovascularization of any tumor type. It is impossible to make a diagnosis of glioma in the absence of necrosis and neovascularization. Glioblastomas also have to recruit local cells to make blood vessels to support tumor growth. Thus if there is an inhibition of neovascularization, the glioma can only grow to the point of the maximum diffusion of nutrients from pre-existing blood vessels. Beyond that point, the tumor stops growing due to necrosis. Our previous work shows that p53 stops the growth of tumors[30,31]. A surprising recent observation suggests that an integrated differentiated tumor releases trophic factors, recruiting even mesenchymal stem cells (see above) into the area of the tumor. These integrated mesenchymal stem cells support the growth of tumor blood vessels. There is a very important link between “S4” and “C4” cells. In the parallel processes of activation in normal stem cells and CSC, trophic mobilization, engraftment, and commitment, therapeutic intervention may be possible when “S4” cells become“C4”. This dedifferentiation stage makes CSC or malignant conversion, “S0” to “C0”, an alternative treatment target, perhaps most appropriate for children.
Evidence supporting this scheme has emerged recently. One of the first developmental stages-specific factors is repressor element 1-silencing transcription/neuron-restrictive silencer factor (REST/NRSF). REST/NRSF is required to maintain the adult neural stem cell (NSC) pool and orchestrate stage-specific differentiation[32]. REST/NRSF recruits CoREST and mSin3A corepressors to stem cell chromatin for the regulation of pro-neuronal target genes to prevent precocious neuronal differentiation in cultured adult NSCs. Selective transplantation of ESC-derived VPCs in appropriate differentiation stages, contributes to adult neovascularization[33]. Another example, PW1 is involved in staging the self-renewing stem cells in a wide array of adult tissues[34]. Conditional Pten deletion in quiescent, and nestin-expressing radial glia-like precursors (RGL) initially promotes their activation and symmetric self-renewal but ultimately leads to terminal astrocytic differentiation and RGL depletion in the adult hippocampus[35]. However, little is known about the convergence of stem cells with tumorigenesis stages.
CLINICAL RELEVANCE OF STEM CELL CONVERGENCE
What can we do to stop normal cells from becoming a tumor? How do we take tumor potential away from embryonic stem cells? It is crucial to first address the malignant convergence from “ESC0” to “ESC-1,” the first step of tumorigenesis (i.e. focus on the first step of the Genesis) (Figure 1, right panel). In our organotypic slice culture model, we can identify “stage-specific” cell populations as “ESC0” to “ESC-1” cells vs “S4” to “C4”[36]. Furthermore, we can perform a gene array subtraction for genetic profiling of these subpopulations to determine the molecular switching mechanism for the “malignant conversion”. For example, in considering the cross-over of “S4” to “C4”, most patients at this stage are given the high doses of chemotherapy, which may promote the convergence.
During the CSC differentiation process, there is a time when they are sensitive to chemotherapy which can be defined as a “therapeutic window”[37-39]. Intracranial placement of tumor xenografts under transparent glass cranial windows in nude rats models allows direct serial inspection of human brain tumor growth that can be used to study stage-specific tumor responses to therapeutics[40]. Chemotherapy results in unwanted killing of normal stem cells, which are necessary to help support the growth of the tumor. Following osmotic disruption of the blood-brain barrier (BBB) in humans, the time course to closure of the BBB, or the so-called therapeutic window, has important clinical implications for the design of therapeutic protocols[41]. Three-dimensional magnetic resonance spectroscopic imaging provides a unique biochemical “window” to study cellular metabolism non-invasively[42]. This has already demonstrated the potential for improved diagnosis, staging, and treatment planning in brain and prostate cancer. Certain agents like the VEGFR2 blockade create a “normalization window” - a period during which combined radiation therapy gives the best outcome[43]. This window is characterized by an increase in tumour oxygenation, which is known to enhance radiation response. The determination of this therapeutic window can allow maximization of the efficacy of the immunotherapy[44]. A non-invasive imaging system can be used to pin-point this therapeutic window[45].
Normal stem cells, which travel to tumors to support their growth, are subject to as much killing as the “trojan horse” of chemotherapy or radiation. However, therapeutic success relies on finding an effective strategy to select a stem cell subpopulation at a suitable stage when the cells are competitive and capable of targeting brain tumors. We have proposed the concept of a “therapeutic window” for stem cells, which may be defined more specifically a “biochemical therapeutic window”, or even a “molecular therapeutic window” determined from genetic description. This selective process may produce more effective stem cells to treat cancers[46].
PERSPECTIVES AND FUTURE DIRECTIONS
To begin to unravel the biological behaviour of both normal tem cells and CSC, we have proposed conceptual models in order to help facilitate the design of new studies. Based upon our current studies, we postulate that a critical stage, defined as a “therapeutic window”, can be thoroughly characterized by defining associated molecular[47,48], biochemical[49] and biological events[50]. Within this experimental framework, data obtained may support or contradict the hypothetical models, thereby shaping stage-defined biological models. Information obtained from these stage-specific stem cell studies will allow us to further explore the detailed mechanisms underlying the prospective roles of stage-specific molecules in stem cell development. Advances in our understanding of stem cell behaviour may extend application of stem cell transplantation, with stage-specific matching of normal stem cells and brain tumor stem cells. Advances in diagnosis and treatment of childhood cancers are expected to emerge from these coordinated stem cell studies, hopefully culminating in better cancer survival prognosis with a reduction in the risks of acute and late-stage adverse consequences of treatment.
ACKNOWLEDGMENTS
We thank Brent A Dethlefs; Philip H Schwartz, PhD; Henry J Klassen, MD-PhD; Saul Puszkin, PhD; Michael P Lisanti, MD, PhD; Richard G Pestell, MD, PhD; Joan S Brugge, PhD; and Robert A Koch, PhD for their support and enthusiasm.
Footnotes
Supported by CHOC Children’s Foundation and CHOC Neuroscience Institute (to Li SC); Austin Ford Tribute Fund; and the W. M. Keck Foundation (to Li SC); Grant R21CA134391 from the National Institutes of Health, and Grant AW 0852720 from the National Science Foundation (to Zhong JF)
Peer reviewers: Niels Olsen Saraiva Camara, MD, Associate Professor, Laboratory of Transplantation Immunobiology, Department of Immunology, Institute of Biomedical Sciences IV, University of São Paulo, Rua Prof. Dr. Lineu Prestes 1730, Cidade Universitária, 05508-900, São Paulo, Brazil; Gregor Barr Adams, Assistant Professor, Cell and Neurobiology, Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC, Keck School of Medicine, University of Southern California, 1450 Biggy Street (NRT 4507), Los Angeles, CA 90033, United States
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
References
- 1.Drake N. Forty years on from Nixon's war, cancer research 'evolves'. Nat Med. 2011;17:757. doi: 10.1038/nm0711-757. [DOI] [PubMed] [Google Scholar]
- 2.Daga A, Bottino C, Castriconi R, Gangemi R, Ferrini S. New Perspectives in Glioma Immunotherap. Curr Pharm Des. 2011:Epub ahead of print. doi: 10.2174/138161211797249206. [DOI] [PubMed] [Google Scholar]
- 3.Li SC, Jin Y, Loudon WG, Song Y, Ma Z, Weiner LP, Zhong JF. Increase developmental plasticity of human keratinocytes with gene suppression. Proc Natl Acad Sci U S A. 2011;108:12793–12798. doi: 10.1073/pnas.1100509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Ping YF, Bian XW. Cancer stem cells switch on tumor neovascularization. Curr Mol Med. 2011;11:69–75. doi: 10.2174/156652411794474383. [DOI] [PubMed] [Google Scholar]
- 7.Greenfield JP, Jin DK, Young LM, Christos PJ, Abrey L, Rafii S, Gutin PH. Surrogate markers predict angiogenic potential and survival in patients with glioblastoma multiforme. Neurosurgery. 2009;64:819–26; discussion 826-7. doi: 10.1227/01.NEU.0000343742.06625.DB. [DOI] [PubMed] [Google Scholar]
- 8.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergers G. Bone marrow-derived cells in GBM neovascularization. In: Van Meir EG., editor. CNS cancer, cancer drug discovery and development. New York: Humana Press; 2009. pp. 749–773. [Google Scholar]
- 10.Dirks PB. Brain tumour stem cells: the undercurrents of human brain cancer and their relationship to neural stem cells. Philos Trans R Soc Lond B Biol Sci. 2008;363:139–152. doi: 10.1098/rstb.2006.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 14.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 15.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 17.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 18.Tyler MA, Ulasov IV, Sonabend AM, Nandi S, Han Y, Marler S, Roth J, Lesniak MS. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16:262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 20.Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, Stoicov C, Kurt-Jones E, Grossman SR, Lyle S, et al. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res. 2007;67:10889–10898. doi: 10.1158/0008-5472.CAN-07-2665. [DOI] [PubMed] [Google Scholar]
- 22.Pisati F, Belicchi M, Acerbi F, Marchesi C, Giussani C, Gavina M, Javerzat S, Hagedorn M, Carrabba G, Lucini V, et al. Effect of human skin-derived stem cells on vessel architecture, tumor growth, and tumor invasion in brain tumor animal models. Cancer Res. 2007;67:3054–3063. doi: 10.1158/0008-5472.CAN-06-1384. [DOI] [PubMed] [Google Scholar]
- 23.Bagley RG, Weber W, Rouleau C, Teicher BA. Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy. Cancer Res. 2005;65:9741–9750. doi: 10.1158/0008-5472.CAN-04-4337. [DOI] [PubMed] [Google Scholar]
- 24.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 25.Yip S, Aboody KS, Burns M, Imitola J, Boockvar JA, Allport J, Park KI, Teng YD, Lachyankar M, McIntosh T, et al. Neural stem cell biology may be well suited for improving brain tumor therapies. Cancer J. 2003;9:189–204. doi: 10.1097/00130404-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Gao Y, Tokuyama T, Yamamoto J, Yokota N, Yamamoto S, Terakawa S, Kitagawa M, Namba H. Genetically engineered neural stem cells migrate and suppress glioma cell growth at distant intracranial sites. Cancer Lett. 2007;251:220–227. doi: 10.1016/j.canlet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Kim Y, Jo MY, Kim HS, Jin Y, Kim SU, Jin J, Joo KM, Nam DH. Combined treatment of tumor-tropic human neural stem cells containing the CD suicide gene effectively targets brain tumors provoking a mild immune response. Oncol Rep. 2011;25:63–68. [PubMed] [Google Scholar]
- 28.Mercapide J, Rappa G, Anzanello F, King J, Fodstad O, Lorico A. Primary gene-engineered neural stem/progenitor cells demonstrate tumor-selective migration and antitumor effects in glioma. Int J Cancer. 2010;126:1206–1215. doi: 10.1002/ijc.24809. [DOI] [PubMed] [Google Scholar]
- 29.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broaddus WC, Liu Y, Steele LL, Gillies GT, Lin PS, Loudon WG, Valerie K, Schmidt-Ullrich RK, Fillmore HL. Enhanced radiosensitivity of malignant glioma cells after adenoviral p53 transduction. J Neurosurg. 1999;91:997–1004. doi: 10.3171/jns.1999.91.6.0997. [DOI] [PubMed] [Google Scholar]
- 31.Loudon WG, Abraham SR, Owen-Schaub LB, Hemingway LL, Hemstreet GP, DeBault LE. Identification and selection of human lymphokine activated killer cell effectors and novel recycling intermediates by unique light-scattering properties. Cancer Res. 1988;48:2184–2192. [PubMed] [Google Scholar]
- 32.Gao Z, Ure K, Ding P, Nashaat M, Yuan L, Ma J, Hammer RE, Hsieh J. The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells. J Neurosci. 2011;31:9772–9786. doi: 10.1523/JNEUROSCI.1604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurugi-Kobayashi T, Itoh H, Yamashita J, Yamahara K, Hirai H, Kobayashi T, Ogawa M, Nishikawa S, Nishikawa S, Nakao K. Effective contribution of transplanted vascular progenitor cells derived from embryonic stem cells to adult neovascularization in proper differentiation stage. Blood. 2003;101:2675–2678. doi: 10.1182/blood-2002-06-1877. [DOI] [PubMed] [Google Scholar]
- 34.Besson V, Smeriglio P, Wegener A, Relaix F, Nait Oumesmar B, Sassoon DA, Marazzi G. PW1 gene/paternally expressed gene 3 (PW1/Peg3) identifies multiple adult stem and progenitor cell populations. Proc Natl Acad Sci U S A. 2011;108:11470–11475. doi: 10.1073/pnas.1103873108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li SC, Loudon WG. A novel and generalizable organotypic slice platform to evaluate stem cell potential for targeting pediatric brain tumors. Cancer Cell Int. 2008;8:9. doi: 10.1186/1475-2867-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laramore GE. Neutron radiotherapy for high grade gliomas: the search for the elusive therapeutic window. Int J Radiat Oncol Biol Phys. 1990;19:493–45; discussion. doi: 10.1016/0360-3016(90)90564-z. [DOI] [PubMed] [Google Scholar]
- 38.Strother D, Ashley D, Kellie SJ, Patel A, Jones-Wallace D, Thompson S, Heideman R, Benaim E, Krance R, Bowman L, et al. Feasibility of four consecutive high-dose chemotherapy cycles with stem-cell rescue for patients with newly diagnosed medulloblastoma or supratentorial primitive neuroectodermal tumor after craniospinal radiotherapy: results of a collaborative study. J Clin Oncol. 2001;19:2696–2704. doi: 10.1200/JCO.2001.19.10.2696. [DOI] [PubMed] [Google Scholar]
- 39.Aguirre-Ghiso JA. The problem of cancer dormancy: understanding the basic mechanisms and identifying therapeutic opportunities. Cell Cycle. 2006;5:1740–1743. doi: 10.4161/cc.5.16.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foltz RM, McLendon RE, Friedman HS, Dodge RK, Bigner DD, Dewhirst MW. A pial window model for the intracranial study of human glioma microvascular function. Neurosurgery. 1995;36:976–84; discussion 984-5. doi: 10.1227/00006123-199505000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Siegal T, Rubinstein R, Bokstein F, Schwartz A, Lossos A, Shalom E, Chisin R, Gomori JM. In vivo assessment of the window of barrier opening after osmotic blood-brain barrier disruption in humans. J Neurosurg. 2000;92:599–605. doi: 10.3171/jns.2000.92.4.0599. [DOI] [PubMed] [Google Scholar]
- 42.Kurhanewicz J, Vigneron DB, Nelson SJ. Three-dimensional magnetic resonance spectroscopic imaging of brain and prostate cancer. Neoplasia. 2000;2:166–189. doi: 10.1038/sj.neo.7900081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Li SC, Zhong JF. Twisting immune responses for allogeneic stem cell therapy. World J Stem Cells. 2009;1:30–35. doi: 10.4252/wjsc.v1.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li SC, Tachiki LM, Luo J, Dethlefs BA, Chen Z, Loudon WG. A biological global positioning system: considerations for tracking stem cell behaviors in the whole body. Stem Cell Rev. 2010;6:317–333. doi: 10.1007/s12015-010-9130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li SC, Wang L, Jiang H, Acevedo J, Chang AC, Loudon WG. Stem cell engineering for treatment of heart diseases: potentials and challenges. Cell Biol Int. 2009;33:255–267. doi: 10.1016/j.cellbi.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Auda-Boucher G, Bernard B, Fontaine-Pérus J, Rouaud T, Mericksay M, Gardahaut MF. Staging of the commitment of murine cardiac cell progenitors. Dev Biol. 2000;225:214–225. doi: 10.1006/dbio.2000.9817. [DOI] [PubMed] [Google Scholar]
- 48.Candeliere GA, Rao Y, Floh A, Sandler SD, Aubin JE. cDNA fingerprinting of osteoprogenitor cells to isolate differentiation stage-specific genes. Nucleic Acids Res. 1999;27:1079–1083. doi: 10.1093/nar/27.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elghetany MT, Patel J. Assessment of CD24 expression on bone marrow neutrophilic granulocytes: CD24 is a marker for the myelocytic stage of development. Am J Hematol. 2002;71:348–349. doi: 10.1002/ajh.10176. [DOI] [PubMed] [Google Scholar]
- 50.Li SC, Han YP, Dethlefs BA, Loudon WG. Therapeutic window, a critical developmental stage for stem cell therapies. Curr Stem Cell Res Ther. 2010;5:297–293. [PMC free article] [PubMed] [Google Scholar]