Abstract
Cells that express MyoD mRNA, the G8 antigen and the bone morphogenetic protein (BMP) inhibitor noggin (Nog) are present in the epiblast before gastrulation. Ablation of “Myo/Nog” cells in the blastocyst results in an expansion of canonical BMP signaling and prevents the expression of noggin and follistatin before and after the onset of gastrulation. Once eliminated in the epiblast, they are neither replaced nor compensated for as development progresses. Older embryos lacking Myo/Nog cells exhibit severe axial malformations. Although Wnts and Sonic hedgehog are expressed in ablated embryos, skeletal muscle progenitors expressing Pax3 are missing in the somites. Pax3+ cells do emerge adjacent to Wnt3a+ cells in vitro; however, few undergo skeletal myogenesis. Ablation of Myo/Nog cells also results in ectopically placed cardiac progenitors and cardiomyocytes in the somites. Reintroduction of Myo/Nog cells into the epiblast of ablated embryos restores normal patterns of BMP signaling, morphogenesis and skeletal myogenesis, and inhibits the expression of cardiac markers in the somites. This study demonstrates that Myo/Nog cells are essential regulators of BMP signaling in the early epiblast and are indispensable for normal morphogenesis and striated muscle lineage specification.
Keywords: MyoD, Noggin, BMP, Blastocyst, Myogenesis
INTRODUCTION
Cells of the epiblast undergo dramatic alterations in position, adhesion and gene expression that lead to the development of the primitive streak, establishment of three germ layers during gastrulation and formation of the nervous system. These events are mediated by complex communication networks involving several families of diffusible proteins, including Wnts, Fibroblast Growth Factors and members of the Transforming Growth Factor family (Gilbert, 2010; Stern, 2006). The response of cells to extracellular factors depends, in part, on where they lie within the concentration gradient and the presence of inhibitors of signal transduction (Ashe and Briscoe, 2006).
In the chick embryo, the first visible sign of primitive streak formation is an increase in cell density in the posterior/medial, stage 2 epiblast (Bellairs, 1986). A hallmark of primitive streak formation is the disappearance of bone morphogenetic protein 4 (BMP4) and its signal transducer, phosphorylated Smad1/5/8 (p-Smad1/5/8), from this region of the epiblast (Faure et al., 2002; Streit et al., 1998; Wilson et al., 2000). The downregulation of BMP signaling in the epiblast was attributed to suppression of BMP4 gene transcription by FGF3 (Wilson et al., 2000) and blocking BMP receptor activation by chordin (Streit et al., 1998). Two other BMP inhibitors, noggin and follistatin, were not detected in the epiblast of the prestreak chick embryo (Chapman et al., 2002; Streit et al., 1998; Wilson et al., 2000); however, both of these BMP inhibitors are expressed in the early Xenopus embryo (Khokha et al., 2005; Wessely et al., 2001). Once gastrulation begins, noggin, follistatin, chordin and other BMP inhibitors are synthesized in an important signaling center located at the anterior end of the primitive streak called Hensen’s node in the chick, the node in mammals and Spemann’s organizer in amphibians (Anderson et al., 2002; Bachiller et al., 2000; Bouwmeester et al., 1996; Capdevila and Johnson, 1998; Chapman et al., 2002; Glinka et al., 1998; Hansen et al., 1997; Hemmati-Brivanlou et al., 1994; Hsu et al., 1998; Khokha et al., 2005; Klingensmith et al., 1999; Lamb et al., 1993; McMahon et al., 1998; Sasai et al., 1994; Smith and Harland, 1992; Streit et al., 1998; Wessely et al., 2001).
The roles of BMP inhibitors in multiple developmental processes have been explored, in part, by adding them to embryonic tissues and by molecular knockout or knockdown of their expression. While addition of noggin or chordin to the embryo induces the formation of ectopic primitive streaks in chick and Xenopus embryos (Frisch and Wright, 1998; Graff et al., 1994; Hawley et al., 1995; Streit et al., 1998; Streit and Stern, 1999b; Suzuki et al., 1997), gastrulation does occur in noggin and chordin single and double homozygous mutant mice (Anderson et al., 2002; Bachiller et al., 2000; Brunet et al., 1998; Klingensmith et al., 1999; McMahon et al., 1998; Stottmann et al., 2006). BMP inhibitors were also shown to induce neural tissue in Xenopus embryos, maintain the expression of neural markers, establish the border between neural and epidermal tissues in the chick embryo, and regulate morphogenesis and patterning of the mouse neural tube (Anderson et al., 2002; Harland and Gerhart, 1997; Hartley et al., 2001; Khokha et al., 2005; Linker and Stern, 2004; Lumsden and Krumlauf, 1996; McMahon et al., 1998; Sasai et al., 1994; Smith and Harland, 1992; Stottmann et al., 2006; Streit et al., 1998; Streit et al., 1997; Streit and Stern, 1999a; Tanabe and Jessell, 1996; Wills, 2010). Chordin null mice exhibit defects in the development of the skeleton, ear, pharynx and cardiovascular system (Bachiller et al., 2000), whereas the absence of follistatin results in musculoskeletal defects and abnormalities in whisker, tooth and skin development (Matzuk et al., 1995). Knockout of noggin in the mouse embryo results in neural tube patterning and fusion defects, enlargement of the notochord, malformations of the heart, lungs, skeleton and esophagus, and a decrease in skeletal muscle in the somites (Anderson et al., 2002; Bachiller et al., 2000; Brunet et al., 1998; Choi et al., 2007; McMahon et al., 1998; Que et al., 2006; Stottmann et al., 2006; Weaver et al., 2003).
A source of noggin in the chick embryo during the period of organogenesis are cells that also express mRNA for the skeletal muscle specific transcription factor MyoD and the cell surface G8 antigen (Gerhart et al., 2006; Gerhart et al., 2009). These cells arise in the early epiblast and are integrated into all three germ layers during gastrulation (Gerhart et al., 2000; Gerhart et al., 2006; Strony et al., 2005). Later they are found in multiple tissues and organs, including the somites, eyes, heart and central nervous system (Gerhart et al., 2001; Gerhart et al., 2006; Gerhart et al., 2009). The importance of MyoD-positive (+) epiblast cells as a noggin delivery system in the eyes and somites was demonstrated by ablating them during early stages of primitive streak formation. Ablated embryos exhibited eye defects, a herniation of organs through the ventral body wall and a severe reduction in skeletal muscle (Gerhart et al., 2006; Gerhart et al., 2009). Myogenic progenitor cells expressing the Pax3 transcription factor were present in the somites of ablated embryos; however, they were unable to differentiate unless they were exposed to exogenous noggin (Gerhart et al., 2006).
In the following study we examined whether MyoD+ cells express noggin while they reside within the epiblast and defined the consequences of ablating them in the blastocyst prior to the formation of the primitive streak. Noggin producing, MyoD+ (Myo/Nog) cells regulate BMP signaling and follistatin expression during the hours leading up to primitive streak formation. The double noggin/follistatin knockdown resulting from the ablation of Myo/Nog cells in the blastocyst has profound consequences on development that includes a disruption in neurulation, an inhibition of the emergence of skeletal muscle progenitor cells and ectopic cardiomyogenic cells in the somites.
MATERIALS AND METHODS
Immunofluorescence Localization and In Situ Hybridization
White Leghorn chick embryos (BE Eggs, York, PA) were staged according to the method of Eyal-Giladi and Kochav (1976) (stages X–XII) or Hamburger and Hamilton (1951) (stage 2 and older embryos). Whole stage X-4 embryos and 10 µm paraffin sections from 3–6 day embryos were double labeled for the G8 antigen and mRNAs by incubating with the G8 IgM MAb and DyLight 488 conjugated goat anti-mouse IgM μ chain (Invitrogen/Molecular Probes, Eugene, OR), followed by incubation in Cy3 labeled 3DNA™ dendrimers (Genisphere, LLC, Hatfield, PA) conjugated with an antisense cDNA sequence for chicken MyoD (5′-TTCTCAAGAGCA AATACTCACCATTTGGTGATTCCGTGTAGTA-3′) (Dechesne et al., 1994) (L34006), chicken noggin (5′-TCTCGTTAAGATCCTTCTCCTTGGGGTCAAA-3′) (Tonegawa and Takahashi, 1998) (NM_204123) or chicken Wnt1 (5′-CAGATCTCGGCCCTTCTCGCTGGAA TCCACAAA-3′) (AY655699.1; Jensen, J.L. et al., Direct Submission) (Gerhart et al., 2004b; Gerhart et al., 2006; Gerhart et al., 2009). Double labeling was also carried out with IgG MAbs to Pax3 (Venters et al., 2004), sarcomeric myosin heavy chain (MF20 MAb) (Bader et al., 1982), neurofilament associated antigen (3A10 MAb) (Furley et al., 1990), the notochord marker NOT1, cardiac troponin I (TI-1 MAb) (Saggin et al., 1989), Shh (5E1 MAb) (Ericson et al., 1996) (all obtained from the Developmental Studies Hybridoma Bank, Iowa City, IA), MyoD1 (NCL-MyoD1 MAb from Vector Laboratories, Burlingame, CA), BMP2 (Sigma-Aldrich, St. Louis, MO) and cardiac troponin T, isoform Ab-1 (MS-295-PO, Thermo Scientific, Fremont, CA), and the following polyclonal antisera: goat anti-mouse noggin (AF719, R&D Systems, Minneapolis, MN), rabbit anti-mouse chordin (ab24562, Abcam, Cambridge, MA), goat anti-human follistatin (sc-23553, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human BMP4 (5674R-100, Biovision, Mountain View, CA), rabbit anti-human p-Smad1/5/8 (9511, Cell Signaling Technology, Danvers, MA), rabbit anti-human Wnt3a (ab28472, Abcam), rabbit anti-human GATA4 (ab25992, Abcam) and rabbit anti-human Smad4 (sc-7154, Santa Cruz Biotechnology). IgG MAbs were tagged with affinity purified, goat anti-mouse IgG conjugated with rhodamine (Jackson ImmunoResearch, West Grove, PA) and fluorescein or rhodamine labeled, rabbit anti-mouse IgG2b or IgG1 (Fitzgerald Industries, Inc., Concord, MA). Polyclonal antibodies were labeled with donkey anti-rabbit or anti-goat IgG conjugated with fluorescein, DyLight 488 or DyLight 549 (Jackson ImmunoResearch, Chemicon and Invitrogen/Molecular Probes). Nuclei were counterstained with Hoechst dye 33258 (1 µg/ml Hanks buffer) (Sigma-Aldrich). Experiments included sections stained with secondary antibodies alone to determine the level of background fluorescence.
Embryos and sections were mounted in Gelmount (Biomeda, Foster City, CA) or Elvanol (Dupont, Wilmington, DE) and analyzed with a Nikon Eclipse E800 epifluorescence microscope (Optical Apparatus) equipped with 4× NA 0.2, 40× oil NA and 60× oil NA 1.4 objectives and the following filters: excitation 530–560, barrier 573–648 for Cy3 and Rhodamine; excitation 465–495, barrier 515–555 for Alexa 488; excitation 330–380, barrier 435–485 for Hoechst dye. Images were captured and produced with the Evolution QE Optronics video camera (Media Cybernetics) and Image Pro Plus image analysis software program (Phase 3 Imaging Systems). Figures were annotated and adjusted for brightness and contrast using Adobe Photoshop 6.0.
Ablating Cells in the Epiblast and Incubation of Embryos
MyoD+ cells were ablated in stage X–XII epiblasts by incubating embryos with the G8 MAb and lysing labeled cells with baby rabbit complement (Cedar Lane, Inc., Hornby, Ontario, Canada) (Gerhart et al., 2006; Gerhart et al., 2008; Gerhart et al., 2009). For these studies, antibody and complement solutions were applied to the vitelline membrane as the embryo resided on the yolk (Gerhart et al., 2008). Similar numbers of cells bound the G8 MAb whether the solutions were applied to isolated embryos or embryos within the vitelline membrane. Control embryos were incubated in Hanks buffer, complement only or the D4 MAb and complement. Hybridoma cells producing the D4 MAb were generated by immunizing BALB/c mice with three inoculations of 106 cells obtained from the posterior 12 pairs of somites and segmental plate mesoderm of stages 12–14 chick embryos, as described previously for the generation of the G8 MAb (Gerhart et al., 2001). Cell lysis and death were visualized by incubating embryos in trypan blue (Gerhart et al., 2006) and staining for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (Roche Diagnostics, Mannheim, Germany). Control and ablated embryos were incubated on the yolk in a weigh boat for 18–48 hours or returned to the shell (Gerhart et al., 2008) and grown for 5–6 days. Curvature of the stage 4 primitive streak was calculated by selecting the point of greatest deviation from linear as the vertex and measuring the angle between lines drawn from the vertex to the rostral and caudal ends of the streak.
Isolation and Microinjection of G8+ and G8− Epiblast Cells
G8+ and G8− epiblast cells were isolated from stage X–XII embryos by removing and dissociating the epiblasts, labeling the cells in suspension with the G8 MAb and rat anti-mouse IgM microbeads, and sorting on a MiniMACS magnetic column (Miltenyi Biotech, Inc) (Gerhart et al., 2007; Gerhart et al., 2004a; Strony et al., 2005). Sorted cells were examined for expression of MyoD and noggin mRNAs, and noggin protein by centrifuging cells onto slides and staining with fluorescent dendrimers to mRNA and the anti-noggin antibody. Nuclei were counterstained with Hoechst dye. Forty five minutes after ablating embryos with the G8 MAb and complement, five G8+ or G8− cells suspended in 9 ηl of a 70% glycerol solution containing fast green dye were microinjected directly into three sites within the posterior/medial epiblast using the Nanoject II microinjector (Drummond Scientific, Broomall, PA).
Preparation of Somite and Cardiac Cell Cultures
Cultures of somite cells were prepared as described previously (George-Weinstein et al., 1994). Untreated or G8 and complement ablated embryos were grown to stage 10–12, removed from the yolk and placed in PBS. Somites 4 to 10–16 (below the level of the heart) were isolated from surrounding tissues of the embryo and dissociated in 0.25% trypsin-0.02% EDTA. Cells were plated at 2 × 104 cells in 15 ul of culture medium containing Dulbecco’s modified Eagle’s medium (DMEM) with 5% fetal bovine serum, 5% horse serum, 1% penicillin/streptomycin (GIBCO/Invitrogen) and 5% chick embryo extract on tissue culture dishes coated with 1% gelatin (Sigma-Aldrich) and human serum fibronectin (GIBCO/Invitrogen). Following cell attachment, dishes were flooded with 1.5 ml serum and hormone free DMEM/F12 medium (GIBCO/Invitrogen). Other dishes were plated with pieces of the neural tube and notochord removed from untreated stage 10–12 embryos at the level of somites 4-12-16, followed by the addition of somite cells. Cells were fixed in 2% formaldehyde on the fifth day in culture. Differences between populations were determined by the Wilcoxon rank sum test. Cardiac cell cultures were prepared by dissecting the hearts from stage 12 embryos, dissociating in trypsin-EDTA and plating and growing cells by the same method used to culture somite cells.
RESULTS
G8+/MyoD+ Cells Express Noggin in the Epiblast
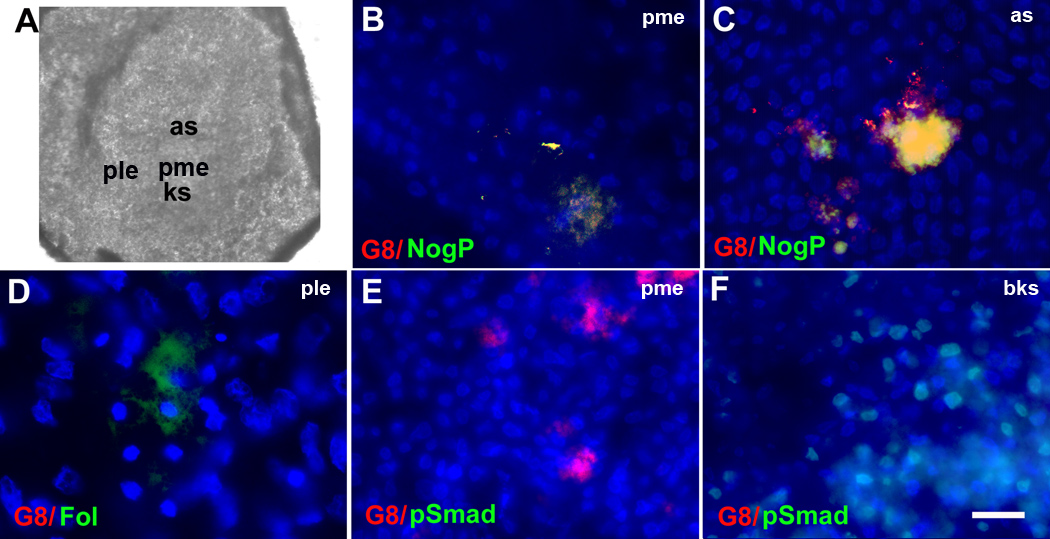
Double label experiments revealed that blastula stage X–XII embryos (Fig. 1A) (Eyal-Giladi and Kochav, 1976) contained 14 ± 5 (mean ± standard deviation, n = 24) G8+/MyoD mRNA+ cells in the posterior/medial epiblast (Fig. 2B). Noggin mRNA and protein were detected in 98% ± 6 (n = 9) and 71% ± 21 (n = 4) of cells labeled with the G8 MAb, respectively (Fig. 2C and D). Only two noggin mRNA+/G8− cells were found in the anterior epiblast of two out of 25 embryos. In the stage 2 embryo (Fig. 1B), G8+ cells were present in the developing primitive streak (Fig. 2G). By stage 2+, G8+ cells continued to occupy the streak as it narrowed and elongated along the midline. The number of G8+ cells increased to 62 ± 13 (n = 15). Noggin was expressed in 98 % ± 7 (n = 15) of G8+ cells (Fig. 2G). Noggin producing, G8+/MyoD+ cells are hereafter called Myo/Nog cells.
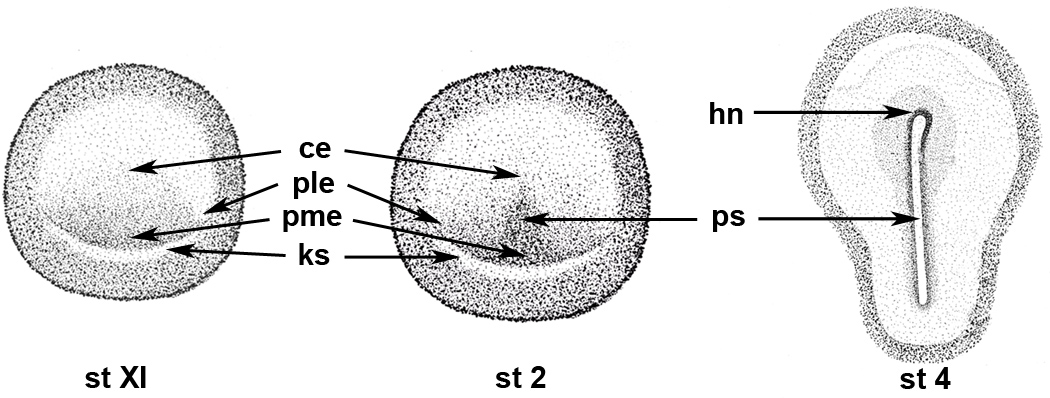
Figure 1. Maps of blastula and gastrula stage chick embryos.
Drawings of stages XI, 2 and 4 embryos are labeled for those regions that are illustrated in the high magnification photomicrographs of Figures 2–4 and 9. pme: posterior/medial epiblast, ks: Koller’s sickle; ple: posterior/lateral epiblast, ce: central epiblast, ps: primitive streak, hn: Hensen’s node
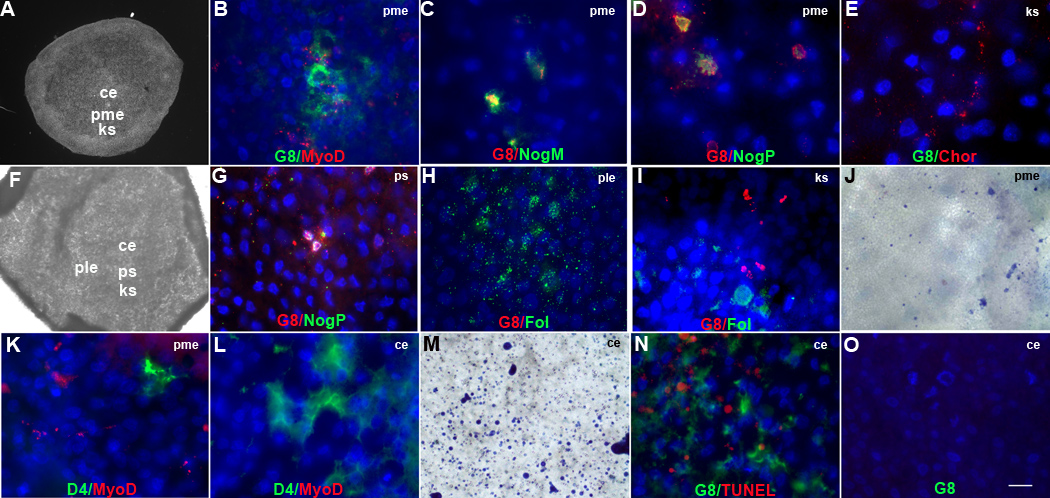
Figure 2. Localization and ablation of G8+ and D4+ cells in the epiblast.
Stage X-2 embryos were double labeled with the G8 or D4 MAb and dendrimers to MyoD or noggin mRNA (NogM), or antibodies to BMP inhibitors. The colors of the fluorescent tags are indicated in each panel. Overlap of red and green appears yellow/white in merged images. Nuclei were stained with Hoechst dye. The photographs of stage XI (A) and stage 2 (F) embryos illustrate the areas shown at high magnification in fluorescence micrographs. G8+ cells co-expressing MyoD (B) and noggin mRNA (C) and noggin protein (NogP) (D) were located in the posterior/medial epiblast (pme). Chordin+ (Chor)/G8− cells were present in a narrow band of cells along Koller’s sickle (ks) in stage XI embryo (E). By stage 2, G8+/noggin+ cells were localized to the developing primitive streak (ps) (G). Staining for follistatin (Fol) was concentrated in the posterior/lateral epiblast (ple) (H) and to a lesser extent along Koller’s sickle where it did not overlap with G8+ cells (I). Trypan blue+ cells were present in the pme following treatment of with G8 and complement (J). D4+ cells lacking MyoD mRNA were present in the pme (K) and central epiblast (ce) (L). Ablation with D4 and complement produced trypan blue+ cells in the ce (M) and pme. G8+ cells accumulated in the ce surrounding TUNEL labeled apoptotic figures (N). Cells stained with the G8 MAb were not present in the ce when both G8+ and D4+ cells were ablated simultaneously in the epiblast (Fig. 1O). Bar, 875 µm in A, F; 9 µm in B–E, G–I, K, L, N, O; 56 µm in J, M.
Myo/Nog cells were examined for the expression of two other BMP inhibitors, chordin and follistatin. Chordin was found in the blastocyst adjacent to Koller’s sickle but was not co-localized with G8 (Fig. 2E). Follistatin was not detected in the embryo until stage 2 when it appeared in 164 ± 11 (n = 5) cells located in the posterior/lateral epiblast and Koller’s sickle (Fig. 2H and I). Staining for follistatin did not overlap with that of G8, noggin or chordin.
Myo/Nog Cells Do Not Reappear in the Epiblast Following Their Ablation in the Blastocyst
The role of Myo/Nog cells in regulating BMP signaling in the early embryo was examined by eliminating them in the blastocyst. Following treatment with G8 and complement (complement), embryos were incubated with trypan blue to visualize lysed cells. The ablation procedure produced 15 ± 3 (n = 7) trypan blue+ cells that were located in the same region of the epiblast as the cells that were fluorescently labeled with the G8 MAb (Fig. 2J).
A control for this experiment was embryos incubated with the D4 MAb and complement. The D4 MAb labeled a cell surface antigen present in a separate population from those that express MyoD (Fig. 2K and L). Some D4+ cells were located in the posterior/medial epiblast (Fig. L) but most resided in the central epiblast (Fig. 2K). Incubation with the D4 MAb and complement lysed 76 ± 12 cells (n = 6) (Fig. 2M). Embryos incubated with the D4 MAb (n = 4), G8 MAb (n = 4) or complement alone (n = 3) contained only 5 ± 3 (n = 11) trypan blue stained cells randomly distributed throughout the epiblast.
The G8 and D4 MAbs were reapplied 18 hours following ablation to monitor whether their target populations re-emerged in the epiblast. No G8+ cells were detected in seven embryos. The remaining two embryos contained two and three G8+ cells with fragmented nuclei, suggesting they were undergoing apoptosis. The decrease in G8+ cells in stage 2 ablated embryos compared to untreated embryos was statistically significant (p < 0.0001). No D4+ cells were observed in six ablated embryos. The specificity of the ablation procedure was further demonstrated by ablating D4+ cells, incubating the embryos overnight and staining with the G8 MAb and TUNEL reagents to mark dead cells. Whereas G8+ cells were present exclusively in the posterior/medial epiblast of unablated embryos, after ablating the D4+ population, cells expressing G8 or MyoD mRNA were also found in the central epiblast where they surrounded TUNEL+ cell remnants (Fig. 2N). G8+ cells did not emerge in the embryo when they were ablated simultaneously with the D4+ cells (Fig. 2O). These experiments demonstrate that incubation with the G8 or D4 MAbs and complement ablates separate populations of cells, few, if any, G8+ and D4+ cells emerge in the embryo within a day after the original populations are eliminated in the blastocyst, and G8+ cells respond to cells undergoing apoptosis.
Ablation of Myo/Nog Cells in the Blastocyst Inhibits Noggin and Follistatin Expression and Expands BMP Signaling
The consequences of Myo/Nog cell ablation on BMP signaling were examined by staining stage 2–4 embryos for BMP inhibitors, BMPs and p-Smad1/5/8, the downstream mediator of canonical BMP signaling (Balemans and Van Hul, 2002). Ablation of Myo/Nog cells in the blastocyst eliminated staining for noggin in three stage 2 embryos (Fig. 3B). A fourth ablated embryo contained two noggin+/G8+ cells. Two noggin+/G8− cells were found in the anterior epiblast of two other ablated embryos. Although follistatin was not detected in Myo/Nog cells (Fig. 3H and I), their ablation in the blastocyst prevented follistatin accumulation in five stage 2 embryos (Fig. 3C). Follistatin was expressed in embryos ablated with the D4 MAb and complement (Fig. 3D). Chordin continued to be synthesized in embryos ablated with the G8 MAb (Fig. 3E), although the intensity of fluorescence was decreased compared to unablated embryos (Fig. 3E).
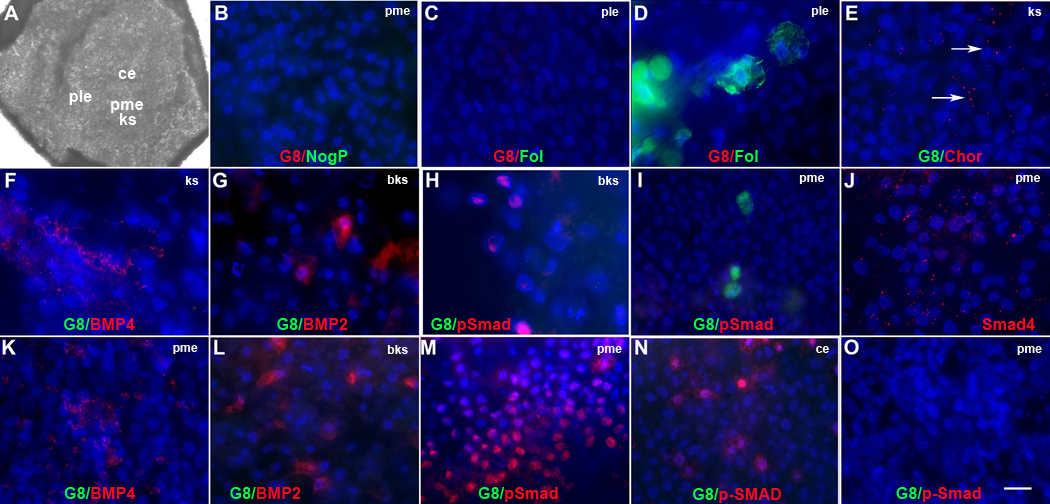
Figure 3. Effects of Myo/Nog cell ablation on the expression of regulators of BMP signaling in the stage 2 embryo.
Untreated embryos and G8 or D4/complement treated stage X–XII embryos were incubated to stage 2 and double labeled with antibodies. The colors of the fluorescent tags are indicated in each panel. The photograph of the stage 2 embryo (A) illustrates the regions shown in the fluorescence micrographs. Overlap of red and green appears yellow/white in merged images. After ablating G8+ cells in the stage X–XII epiblast, neither G8 (B, C, E–I, K–N), noggin (NogP) (B) nor follistatin (Fol) (C) was detected in the stage 2 embryo. Ablation with the D4 MAb did not affect follistatin expression (D). Staining for chordin (Chor) was reduced along Koller’s sickle (ks) in G8 ablated embryos (E). In untreated stage 2 embryos, BMP4+ cells surrounded Koller’s sickle (F) and BMP2 was present below Koller’s sickle (bks) (G). P-Smad1/5/8 was detected only below Koller’s sickle (H) and did not overlap with G8+ cells in the posterior/medial epiblast (pme) (I). Cytoplasmic staining for Smad4 was present in the pme (J). In G8 ablated embryos, staining for BMP4 was increased in the pme (K), BMP2 remained below Koller’s sickle (L) and p-Smad1/5/8 was expanded into the pme (M) and central epiblast (ce) (N). P-Smad1/5/8 was not present above Koller’s sickle in embryos ablated with the D4 MAb (O). Bar, 1,250 µm in A; 9 µm in B–T.
In untreated stage 2–2+ embryos, BMP4 was present in clusters of cells in the anterior/lateral epiblast and the posterior portion of the developing primitive streak (Fig. 3F). BMP2 was synthesized in the area opaca below Koller’s sickle (Fig. 2G). While nuclear p-Smad1/5/8 was observed in the region of the area opaca containing BMPs 2 and 4 (Fig. 3H), p-Smad1/5/8 was not detected within the epiblast (Fig. 3I). Cytoplasmic, but not nuclear staining for Smad4, p–Smad1/5/8’s binding partner, was found in the posterior/medial epiblast (Fig. 3J).
Following ablation of Myo/Nog cells, staining for BMP4 was broadened in the posterior/medial epiblast (Fig. 3K). The staining pattern of BMP2 in the area opaca of G8 ablated embryos was similar to that of control embryos (Fig. 3L and G). While unablated and D4 ablated embryos lacked detectable levels of p-Smad1/5/8 above Koller’s sickle (Fig. 3I and O), G8 ablated embryos displayed p-Smad1/5/8 staining throughout the posterior and central regions of the epiblast (Fig. 3M and N). The increase in staining for p-Smad1/5/8 within the epiblast indicates that embryos lacking Myo/Nog cells exhibit abnormal BMP signaling by the initiation of primitive streak formation.
One to two days following ablation, 63% of ablated embryos had formed a primitive streak; however, the morphology of the streak differed from that of control embryos. Whereas the primitive streaks of untreated embryos (Fig. 1C and 4A) or embryos treated with complement alone or the D4 MAb and complement (Fig. 4B and C) were linear along the midline or appeared slightly curved (168° ± 6, n = 9), the streaks of G8 ablated embryos had more pronounced curves (144° in Fig. 4D) or were shortened or displaced laterally (Fig. 4E). Hensen’s node was either difficult to detect or appeared thicker than the nodes of control embryos.
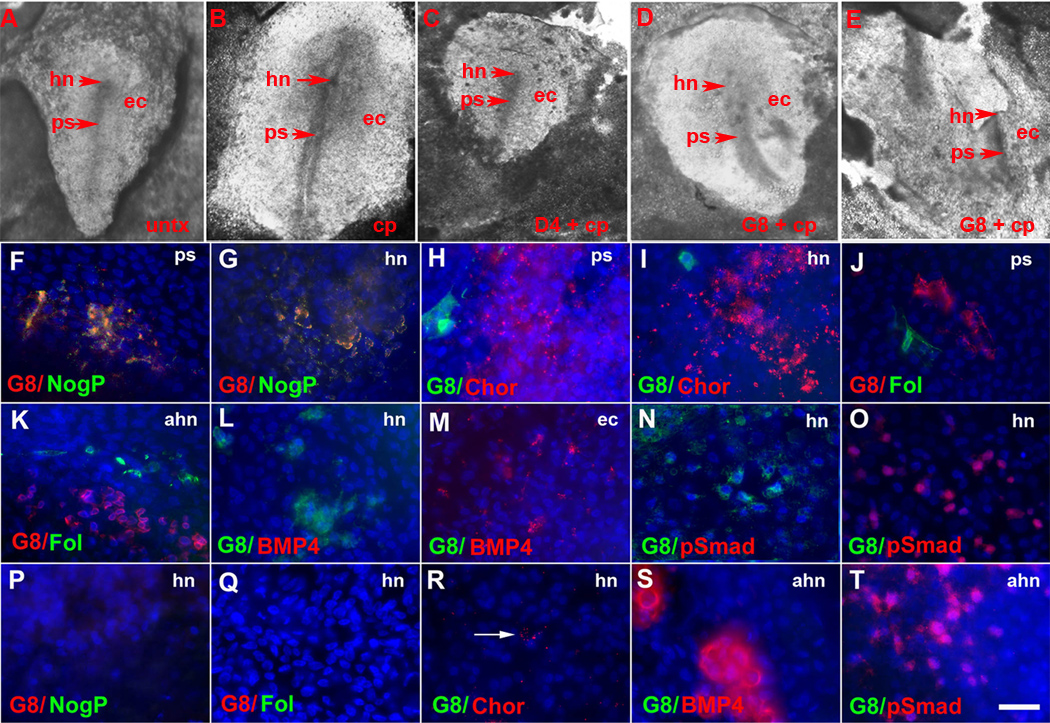
Figure 4. Effects of Myo/Nog cell ablation on the expression of regulators of BMP signaling in gastrulating embryos.
Untreated embryos (untx) and embryos incubated with complement (cp) only or D4 and complement at stage X–XII displayed a normal primitive streak (ps) by stage 3–4 (A–C). G8 ablated embryos had primitive streaks of abnormal morphology and/or positioning (D, E). Stage 3–4 embryos were double labeled with antibodies. The photographs of the embryos in A and D demonstrate the regions shown in the photomicrographs of F–O and P–T, respectively. The colors of the fluorescent tags are indicated in each panel. Overlap of red and green appears yellow in merged images. In untreated embryos, G8+/noggin+ (NogP) cells were present in the primitive streak (ps) (F) and Hensen’s node (hn) (G). Chordin (Chor) was found in the primitive streak (H) and Hensen’s node (I). Staining for follistatin (Fol) was observed in the anterior primitive streak (J) and above Hensen’s node (ahn) (K). BMP4 (L) and p-Smad1/5/8 (N) were absent in Hensen’s node but present in the lateral ectoderm (ec) (M and O). Staining for chordin, follistatin, BMP4 and p-Smad1/5/8 did not overlap with G8 (H–O). Neither G8, noggin nor follistatin was detected in Hensen’s node in embryos ablated with G8 and complement (P and Q). Staining for chordin was weaker in Hensen’s node of G8 ablated embryos (R) compared to untreated embryos (I). BMP4 (S) and p-Smad1/5/8 (T) were present above Hensen’s node in G8 ablated embryos. Bar, 135 µm in A–E and 9 µm in F–T.
Control stage 3–4 embryos contained Myo/Nog cells within and around Hensen’s node, in the primitive streak (Fig. 4F and G) and at the base of the streak. Following ablation of Myo/Nog cells in the blastocyst, no G8+ cells were found in 12 stage 4 embryos (Fig. 4P–T). Three out of four ablated embryos lacked staining for noggin (Fig. 4P). The fourth embryo contained six Noggin+/G8− cells in the anterior ectoderm. Follistatin, which was normally expressed in Hensen’s node, in a discrete arc of cells above the node (Fig. 4J and K) and at the base of the streak, was not detected in two out of four embryos (Fig. 4Q). Two other embryos contained only a few follistatin+ cells in the lateral epiblast. Staining for chordin in Hensen’s node and the primitive streak was weaker in ablated than control embryos (Fig. 5I and R). BMP4 was expressed in groups of cells in the lateral ectoderm adjacent to the anterior portion of the primitive streak (Fig. 4M). An additional cluster of BMP4+ cells was observed above the node in ablated embryos (Fig. 4S). Whereas p-Smad1/5/8 was not detected in the area surrounding Hensen’s node (Fig. 4N) or primitive streak in control embryos, ablated embryos contained strong p-Smad1/5/8 staining above the node (Fig. 4T) and along the margins of the streak. These analyses demonstrate that although the majority of embryos do form a primitive streak after ablating Myo/Nog cells in the blastocyst, the morphology of the streak is abnormal and BMP signaling is deregulated in the epiblast.
Figure 5. Effects of Myo/Nog cell ablation on morphogenesis.
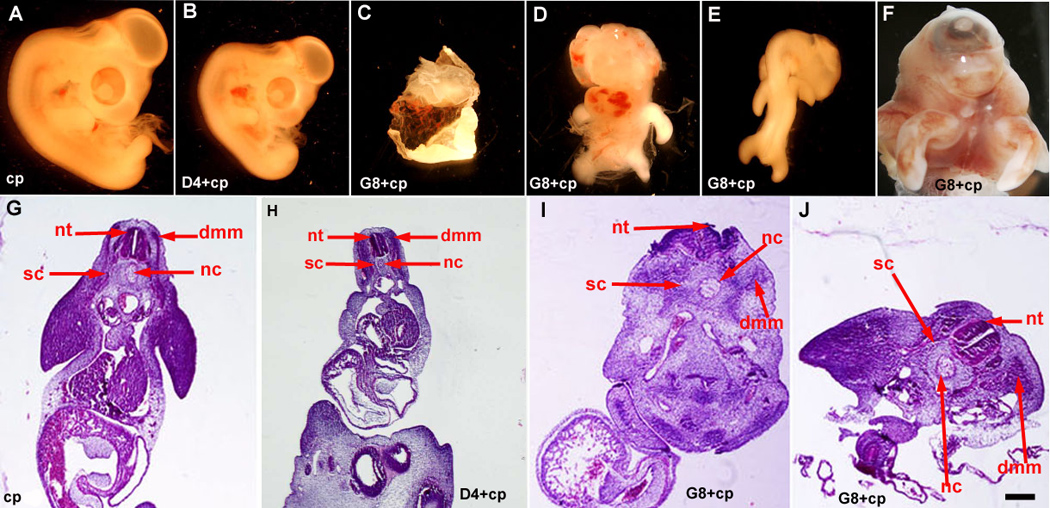
Stage X–XII embryos were incubated with complement (cp) only and G8 or D4 MAb and complement, and grown to stage 25–26. Whole embryos and tissue sections stained with H&E were examined for malformations. Treatment with complement alone did not adversely affect morphogenesis (A and G). Embryos treated with D4 and complement were smaller than control embryos (B and H). Ablation of G8+ cells produced cyst-like structures (C), herniated organs, exenchephaly, anopthalmia, facial defects, abnormally projecting limbs (D), curvature of the body axis (E) and cyclopia (F). G8 ablated embryos also exhibited an open neural tube (nt) and misshapen dermomyotome/myotome (dmm), sclerotome (sc) and notochord (nc) at the level of the heart (I). The neural tube was closed at the level of the mesonephros (J). Bar, 2,500 µm in A–F; 135 µm in G, I, J; 41 µm in H.
Ablation of Myo/Nog Cells in the Blastocyst Disrupts Morphogenesis
The effects of eliminating G8+ and D4+ cells in the blastocyst on morphogenesis was determined by examining the structure of day 4–6 embryos. Ablation of D4+ epiblast cells resulted in a reduction in the overall size of the embryo, but otherwise the embryos appeared to have a normal morphology (n = 5) (Fig. 5B). Other control embryos treated with Hanks buffer or MAb or complement only (n = 28) had no visible morphological defects (Fig. 5A).
Ablation of Myo/Nog cells in 28 stage X–XII embryos produced defects of varying severity. Of the embryos treated with the G8 MAb and complement, 33% formed cyst-like structures or masses of morphologically unidentifiable, vascularized tissue (Fig. 5C). Five embryos had missing or shortened trunks. All embryos that developed a trunk exhibited ventral organ herniations, 11% had a twisted body axis, 50% had limbs that projected dorsally or laterally instead of ventrally (Fig. 5D–F), and two embryos were missing a head. Malformations of the head, facial prominences and eyes were present in 89% of ablated embryos. Examples of these defects were anopthalmia, micropthalmia, cyclopia, facial clefting, absence of facial structures (Fig. 5D–F) and an anterior proboscis. Externally visible brain defects, including a lack of a forebrain vesicle, exencephaly (Fig. 5D), open anterior neuropore and retroflexure of the forebrain into hindbrain, were observed in 57% of the embryos. At the level of the heart and above, the neural tube was open (Fig. 5I) and appeared horizontal in some sections due to undulations along its length. Axial defects improved below the heart. The neural tube was fused at the level of the mesenephros (Fig. 5J). The dermomyotome/myotome, sclerotome and notochord were present in ablated embryos (Fig. 5I and J). In some sections, the shape of the dermomyotome/myotome appeared distorted, a possible effect of undulations of the neural tube Fig. 5J). Ninety three percent of stage X–XII ablated embryos exhibited a more severe phenotype than embryos in which G8+ cells were lysed at stage 2 (Gerhart et al., 2006).
Ablation of Myo/Nog Cells Affects Skeletal Myogenesis and Neurogenesis
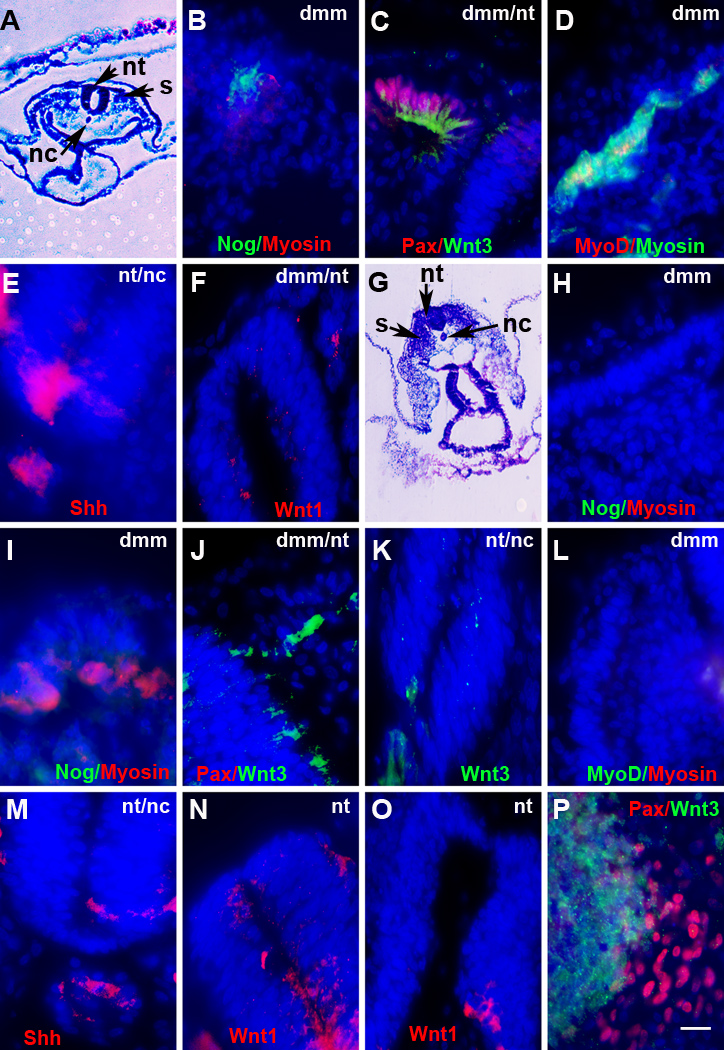
No G8+ cells were observed in sections through two 5-day embryos following ablation of Myo/Nog cells in the blastocyst (Fig. 6D). A third embryo contained three G8+ cells in one of the left somites and in the notochord. Neither noggin nor follistatin was found in sections from different levels of two embryos (Fig. 6D and F). The third embryo contained two noggin+/G8− cells in the neural tube. The somites lacked detectable levels of Pax3 and MyoD (Fig. 6H and J). Small clusters of Pax3+/G8− cells were present outside of the somites.. BMP inhibitors and skeletal muscle markers were expressed in the somites of control embryos treated with the D4 MAb and complement (Fig. 6C, E, G, I and K). Sarcomeric myosin was abundant in the myotome of D4 ablated embryos but was not detected in most somite cells in ablated embryos (Fig. 6L). Staining for neurofilament associated antigen was weaker in the spinal cord and NOT1 was reduced in the notochord of G8 ablated embryos (Fig. 6N and P) compared to embryos ablated with the D4 MAb and complement (Fig. 6M and O). Similar results were obtained when day 2–2.5 embryos were examined for the expression of markers of skeletal myogenesis. At the level of the heart, somites from untreated embryos (Fig. 7B–D), but not ablated embryos (Fig. 7H, I, L), contained noggin, Pax3 and MyoD. A few sarcomeric myosin+ cells were present in the somites of control and ablated embryos (Fig. 7D and I).
Figure 6. Effects of Myo/Nog cell ablation on the expression of markers for skeletal muscle, neurons and notochord cells.
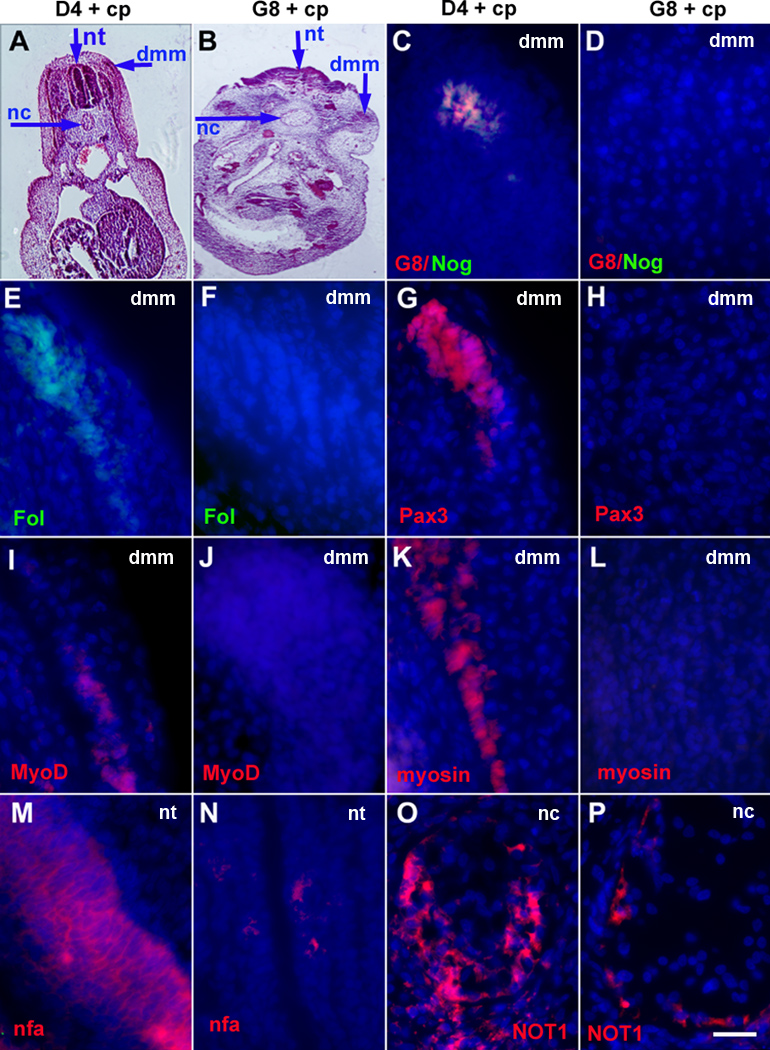
Stage X–XII embryos were treated with complement (cp) to ablate G8+ or D4+ cells, grown to stage 25–26, sectioned and labeled with antibodies. The colors of the fluorescent tags are indicated in each panel. The areas illustrated in the low magnification images of H&E stained sections are shown in the fluorescence micrographs. Overlap of red and green appears yellow in merged images. D4 ablated embryos contained G8+/noggin+ (Nog) cells (C) and cells with follistatin (Fol) (E), Pax3 (G), MyoD (I) and sarcomeric myosin (K) in the dermomyotome/myotome (dmm). Embryos ablated with the G8 MAb lacked G8 (D), noggin (D), follistatin (F), Pax3 (H), MyoD (J) and sarcomeric myosin (L) in the somites. Staining for neurofilament associated antigen (nfa) in the neural tube (N) and NOT1 in the notochord (P) were reduced in G8 ablated embryos compared to D4 ablated embryos (M and O). Bar, 27 µm in A; 56 µm in B; 9 µm in C–P.
Figure 7. Effects of Myo/Nog cell ablation on the expression of skeletal muscle markers, Shh and Wnts.
Untreated (A–F) and G8 and complement treated (G–P) stage X–XII embryos were incubated to stage 12–26. Sections at the level of the heart were labeled with antibodies and dendrimers. The colors of the fluorescent tags are indicated in each panel. The areas illustrated in A and G are shown in the fluorescence photographs. Overlap of red and green appears yellow in merged images. Noggin, Pax3 (Pax), and MyoD were expressed in the stage 12–17 dermomyotome/myotome (dmm) of the somites (s) of untreated (B–D) but not G8 ablated embryos (H, I, J, L). Sarcomeric myosin+ cells were present in the dmm of control embryos (D) and in some (I), but not all sections (H, L) from ablated embryos. Untreated and ablated embryos contained Shh in the notochord (nc) and floor plate of the neural tube (nt) (E, M) and Wnt3a in the nt and dmm (C, J, K). Wnt1 mRNA was localized to the dorsal nt of untreated embryos (F) and dorsal and ventral nt in ablated embryos (N, O). Pax3+ cells were adjacent to Wnt3a+ cells in cultures of somite cells from stage 12–13, G8/complement treated embryos (P). Bar, 56 µm in A and G; 9 µm in B–F, H–P.
An inhibition of the emergence of Pax3+ skeletal muscle progenitors in the somites of ablated embryos could result from a lack of appropriate signals from axial structures. Stage 12–16 embryos were analyzed for the expression of Sonic hedgehog (Shh), Wnt3a and Wnt1, factors that promote the development of skeletal muscle progenitor cells in the somites (Borycki et al., 1998; Christ, 2003; Fan et al., 1997; Munsterberg et al., 1995; Stern et al., 1995; Tajbakhsh et al., 1998; Wagner et al., 2000). Shh was expressed in the notochord and floor plate in both untreated and ablated embryos (Fig. 7E and M). At the level of the heart, Wnt3a was localized to the dorsal neural tube, dorsal ectoderm and a few cells in the somites of untreated and two out of three ablated embryos (Fig. 7C and J). Wnt3a was also found in the ventral neural tube and notochord of the third ablated embryo (Fig. 7K). Wnt1 mRNA was observed in the dorsal neural tube of control and ablated embryos (Fig. 7F and N). Two ablated embryos also had Wnt1+ cells in the ventral neural tube (Fig. 7N and O). The variations in staining indicate that ablation of Myo/Nog cells affects the expression patterns of Wnts1 and 3a.
Subpopulations of Somite Cells from Embryos Lacking Myo/Nog Cells Synthesize Pax3 in vitro and Cardiac Markers in vitro and In Vivo
The potential of somite cells from ablated embryos to express muscle genes was tested in vitro under conditions that were optimized to promote skeletal myogenesis (George-Weinstein et al., 1994). The majority of somite cells from untreated stage 12–13 embryos differentiated into skeletal muscle in culture; however, only a small percentage of somite cells from ablated embryos synthesized MyoD, sarcomeric myosin or the skeletal muscle specific 12101 antigen (Table 1). Approximately one third of the cells from ablated embryos contained nuclear Pax3 staining. Cells expressing Pax3 were adjacent to Wnt3a+ cells (Fig. 7P).
Table 1. Behavior of somite cells from embryos lacking Myo/Nog cells in vitro.
Stage X–XII embryos treated with the G8 MAb and complement were grown for two days. Right and left somites were removed from ablated (Abl) and untreated (Untx) embryos. Some somite cells from ablated embryos were cultured with a piece of the neural tube (NT) and notochord (NC) dissected from untreated embryos. Cells were stained with antibodies to G8, muscle proteins and noggin on the fifth day in culture. A minimum of 400 cells were scored per culture. Values are the mean ± standard deviation of the percent of the total number of cells that were stained with antibody. The number of cultures scored is indicated in parentheses. With the exception of Wnt3a, statistically significant differences were found between untreated and ablated cultures with or without the neural tube and notochord (MyoD, myosin, 12101, GATA4 and CTpI: p < 0.0001; Pax3, G8, and noggin: p ≤ 0.07, 0.02, 0.01, respectively).
| Untx | Abl | Abl + NT/NC | |
|---|---|---|---|
| Pax3 | 22 ± 10 (12) | 37 ± 17 (8) | 39 ± 21 (5) |
| MyoD | 22 ± 12 (14) | 2 ± 4 (13) | 6 ± 5 (5) |
| Myosin | 65 ± 15 (13) | 18 ± 17 (13) | 4 ± 8 (5) |
| 12101 | 42 ± 18 (11) | 1 ± 2 (9) | 4 ± 6 (5) |
| G8 | 55 ± 17 (4) | 2 ± 3 (4) | 0.2 ± 0.1 (5) |
| Wnt3a | 22 ± 21 (12) | 31 ± 17 (8) | 19 ± 3 (5) |
| Noggin | 31 ± 24 (5) | 0.5 ± 0.4 (4) | 0.4 ± 0.6 (4) |
| GATA4 | 1 ± 1 (14) | 50 ± 17 (16) | 50 ± 10 (4) |
| CTpI | 0.3 ± 0.4 (14) | 18 ± 11 (15) | 13 ± 7 (4) |
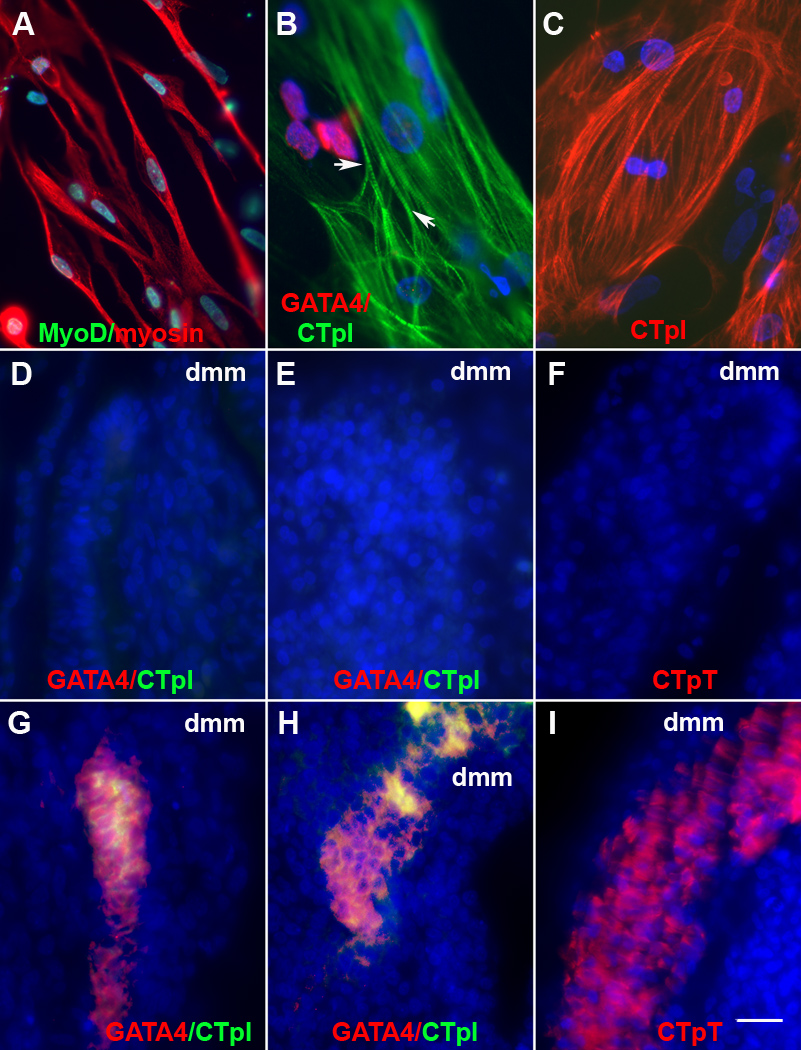
While some somite cells from ablated embryos synthesized sarcomeric myosin in vitro and in vivo, the MF20 MAb used to monitor terminal differentiation stains both skeletal and cardiac muscle (Bader et al., 1982). The muscle lineages were further distinguished by staining for GATA4 synthesized in cardiac progenitor cells (Heikinheimo et al., 1994) and cardiac troponin I (CTpI) specifically expressed in cardiomyocytes (Dhoot et al., 1978; Toyota and Shimada, 1981). Only a few somite cells from normal embryos were labeled with either antibody in vitro (Table 1). The percentages of GATA4+ and CTpI+ cells were increased dramatically in cultures of somite cells from ablated embryos (Table 1, Fig. 8B). In some cells, CTpI appeared in a striated pattern along myofibrils (Fig. 8B). Most CTpI+ cells were broad, contained one or two nuclei and resembled cardiomyocytes in cultures prepared from the heart, whereas the skeletal myofibers were long and thin, and some were multinucleated (Fig. 8A–C). The expression of cardiac and skeletal muscle markers was not significantly affected by the addition of pieces of the neural tube and notochord obtained from untreated embryos (Table 1).
Figure 8. Expression of cardiac markers in somite cells from embryos lacking Myo/Nog cells.
Somite cultures were prepared from untreated and G8 and complement treated stage 12–13 embryos. Somite cells from untreated embryos formed sarcomeric myosin+, elongated skeletal myotubes (A). Cultures from ablated embryos contained GATA4+ and cardiac troponin I+ (CTpI) cells (B). Some cardiomyocytes contained striations (arrows in B). CTpI+ cells in somite cultures resembled cultured cardiomyocytes from the stage 12 heart (C). In vivo, the somites of G8 ablated (G, H) but not untreated (D, E) stage 12–16 (D, G) and stage 25–26 embryos (E and H) contained GATA4+ and CTpI+ cells. Cardiac troponin T (CTpT) was expressed in stage 25–26 ablated (I) but not untreated embryos (F). Bar, 9 µm.
Cardiac progenitors and cardiomyocytes were also present in the somites of ablated, but not control embryos, in vivo (Fig. 8D, E, G and H). GATA4+ and CTpI+ cells were found primarily in the myotome (Fig. 8G and H). At the level of the heart, the myotomes of stage 12–13 ablated embryos contained 0–26 GATA4+ cells and 0–9 CTpI+ cells per section. In 5–6 day embryos, sections contained 0–12 GATA4+ cells and 0–32 CTpI+ cells in the myotome. Cardiac progenitors and cardiomyocytes were also found in the sclerotome (stage 12–13: 0–11 GATA4+ cells and 0–16 CTpI+ cells per section; day 5–6: 0–10 GATA4+ cells and 0–5 CTp+ cells and 0–16 CTpI+ cells per section) (Fig. 8). Staining for cardiac troponin T displayed a similar pattern to that of CTpI (Fig. 8H and I). These in vitro and in vivo experiments demonstrate that cardiomyogenic cells are ectopically located in the somites following ablation of Myo/Nog cells in the blastocyst.
Reintroduction of Myo/Nog Cells into Ablated Embryos Represses BMP signaling in the Epiblast and Improves Morphogenesis and Differentiation in Older Embryos
Stage X–XII embryos treated with the G8 MAb and complement were implanted with G8+ cells isolated from the epiblasts of other blastocysts to test whether they could rescue the Myo/Nog ablation phenotype. Directly following magnetic cell sorting, 98% ± 4 (n = 4), 91% ± 4 (n = 4) and 58 % ± 9 (n = 7) of the cells in the G8+ population were stained for MyoD and noggin mRNAs and noggin protein, respectively. In the G8−negative (G8−) subpopulation, only 5% ± 5 (n = 4), 1% ± 2 (n = 4) and 0% (n = 8) were positive for MyoD mRNA and noggin mRNA and protein, respectively.
G8+ or G8− cells were microinjected into the posterior/medial epiblast 45 minutes after ablating Myo/Nog cells. By stage 2, 55 ± 7 (n = 3) G8+ cells were positioned in the posterior/medial epiblast above Koller’s sickle and in the anterior portion of the developing streak of ablated embryos (Fig. 9B, C and E). All G8+ cells expressed noggin (Fig. 9B and C). Implantation of G8+ cells into ablated embryos restored the normal pattern of follistatin expression (Fig. 9D) (n = 3), although the intensity of staining appeared weaker than that of control embryos (Fig. 2H and I). Importantly, returning G8+ cells to ablated embryos eliminated p-Smad1/5/8 staining within the epiblast (Fig. 9E) but did not affect p-Smad1/5/8/ staining below Koller’s sickle (Fig. 9F) (n = 3), demonstrating that the implanted G8+ cells repressed canonical BMP signaling in the epiblast.
Figure 9. Effects of reintroducing Myo/Nog cells into ablated embryos on regulators of BMP signaling in the stage 2 epiblast.
G8+ cells isolated from stage X–XII embryos were injected into the epiblast after the indigenous population of G8+ cells was ablated with complement. Embryos were grown to stage 2 and double labeled with antibodies. The photograph of the stage 2 embryo (A) illustrates the regions shown in the fluorescence micrographs. The colors of the fluorescent tags are indicated in each panel. Overlap of red and green appears yellow in merged images. Implanted G8+ cells synthesized noggin in the posterior/medial epiblast (pme) (B) and in the anterior portion of the developing primitive streak (as) (C), restored expression of follistatin in the posterior/lateral epiblast (ple) (D) and inhibited p-Smad1/5/8 accumulation in the epiblast in the pme (E). P-Smad1/5/8 was present below Koller’s sickle (bks) (F). Bar, 900 µm in A; 9 µm in B–F.
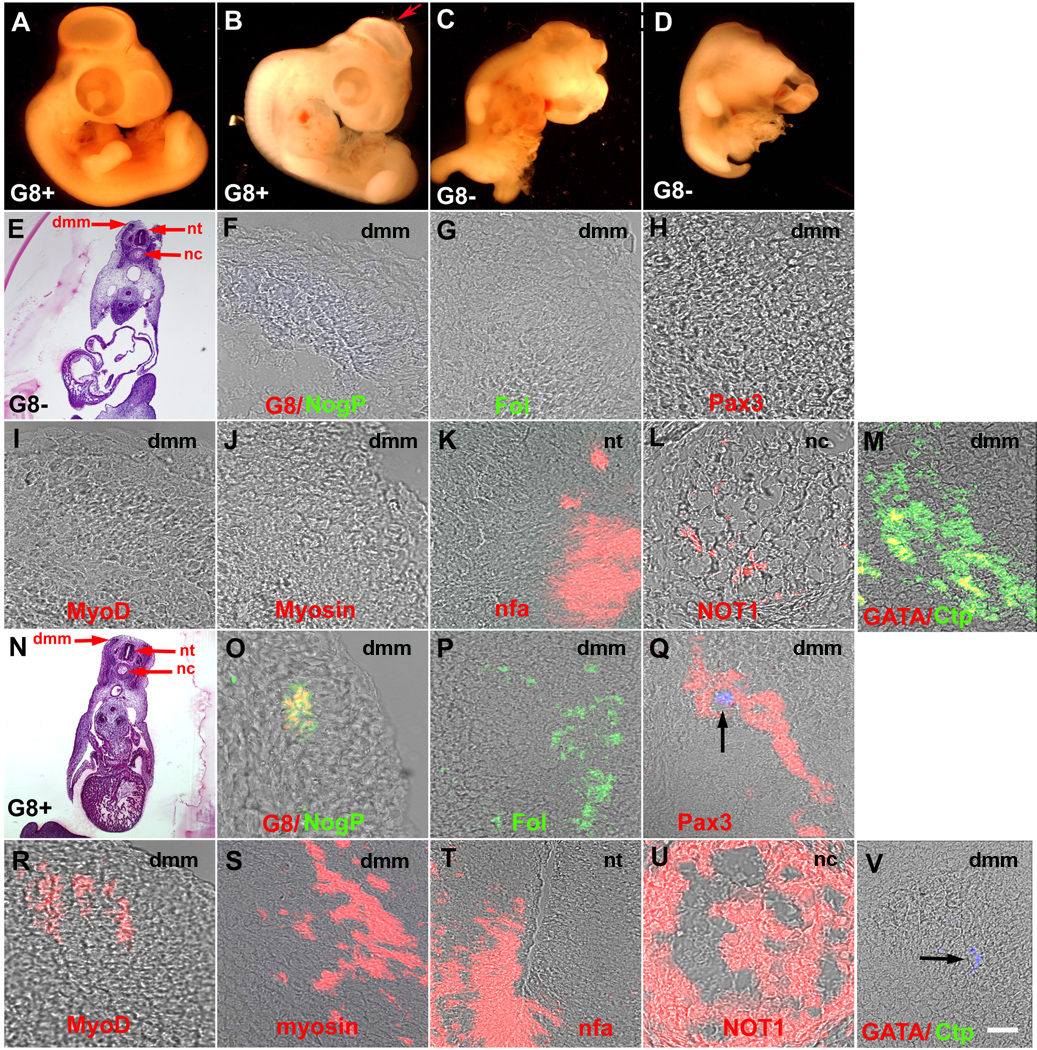
Ablated embryos implanted with G8+ cells were grown for six days and examined for malformations. Five out of nine of these embryos had no externally visible defects (Fig. 10A). Three embryos exhibited a partial correction of the defects in which an intact body wall and normal limbs were accompanied by an open anterior neuropore (Fig. 10B), missing eye or twisted body axis. Only one embryo had the full spectrum of defects observed in ablated embryos. Ablated embryos injected with G8− epiblast cells (n = 8) continued to exhibit head and body wall defects (Fig. 10C and D). Injection of Hank’s buffer diluted in the glycerol/fast green solution did not produce visible malformations in four ablated embryos.
Figure 10. Effects of reintroducing Myo/Nog cells into ablated embryos on morphogenesis and expression of BMP inhibitors and cell type specific markers in 5-day embryos.
G8+ and G8− cells isolated from stage X–XII embryos were injected into the epiblast after the indigenous population of G8+ cells was ablated with complement. Embryos were grown to stage 24–26, analyzed for gross abnormalities, sectioned and labeled with antibodies. Implantation of G8+ cells (A and B), but not G8− cells (C and D), completely or partially restored morphogenesis. The embryo in B exhibited an open anterior neuropore (arrow). Structures identified in the H&E stained sections of ablated embryos implanted with G8+ (E) or G8− cells (N) are shown in the merged images of DIC and fluorescence. Implantation of G8+ cells (F–M) but not G8− cells (O–V) restored expression of noggin (Nog), follistatin (Fol), Pax3, MyoD and sarcomeric myosin in the dermomyotome/myotome (dmm), increased expression of neurofilament associated antigen (nfa) in the neural tube (nt) and NOT1 in the notochord (nc), and eliminated GATA4 and cardiac troponin I (CtpI) in the somites. Hoechst stained, injected G8+ cells are visible in the somite (arrows in H and V). Bar, 2,500 µm in A–D; 135 µm in E, N; 9 µm in F–M, O–V.
G8− cells that had been stained with Hoechst dye prior to implantation were found in axial and paraxial structures; however, they did not restore the expression of BMP inhibitors or markers of skeletal muscle, neurons and notochord cells, and GATA4 and CTpI remained in the somites (Fig. 10F–M). Hoechst+/G8+ cells were integrated into tissues where they are normally found in the embryo (Gerhart et al., 2006), including the somites (Fig. 10Q and V). Reintroduction of G8+ cells into ablated embryos promoted the expression of noggin, follistatin, Pax3, MyoD and sarcomeric myosin in the somites, increased the synthesis of neurofilament associated antigen in the neural tube and NOT1 in the notochord, and eliminated staining for GATA4 and CTpI in the somites (Fig. 10O–V). These experiments illustrate that returning Myo/Nog cells to the epiblast fully or partially rescues the phenotype of embryos ablated with the G8 MAb and complement.
DISCUSSION
Our quest for elucidating the molecular and cellular events involved in the development of the skeletal muscle lineage led to the discovery of Myo/Nog cells, a unique population that plays a critical role in regulating cell behavior in a variety of tissues (Gerhart et al., 2006; Gerhart et al., 2004; Gerhart et al., 2009). While Myo/Nog cells are capable of forming skeletal myofibers when cultured under permissive conditions (Gerhart et al., 2004a; Strony et al., 2005), in vivo, they release noggin to regulate the differentiation of skeletal muscle progenitor cells in the somites and eye morphogenesis (Gerhart et al., 2006; Gerhart et al., 2009). In this study we revealed that MyoD mRNA+/G8+ cells are an indispensible source of noggin in the epiblast itself and their elimination in the blastocyst has profound consequences on embryonic development.
Ablation of Myo/Nog cells in the blastocyst disrupts the development of axial and paraxial structures. Although these effects can be explained by aberrant BMP signaling during gastrulation and neurulation, comparisons of embryos in which Myo/Nog cells were ablated in stage 1 versus stage 2 embryos illustrate a more complicated scenario for the roles of these cells during early development. The defects that arise when Myo/Nog cells are ablated in the blastocyst are more severe than when they are targeted six hours later during early stages of primitive streak formation (Gerhart et al., 2006; Gerhart et al., 2009). The likely cause of the more severe phenotype with earlier ablation is the expansion of BMP signaling that occurs prior to the initiation of gastrulation. Deregulation of BMP signaling in the blastula and gastrula is likely to affect other signaling pathways and contribute to abnormal morphogenesis and altered differentiation during neurogenesis and somitogenesis.
The first structural sign of disrupted development is a dysmorphic or absent primitive streak. These effects of ablating Myo/Nog cells in the early embryo are consistent with the results of Streit et al. (1998) who demonstrated that misexpression of BMP4 inhibits primitive streak formation. It is not clear whether Myo/Nog cells actively or passively accumulate in the developing and elongating streak. However, their capacity to migrate in response to apoptosis within the epiblast raises the possibility that Myo/Nog cells play a role in the convergence and extension movements along the midline of the embryo apart from their production of noggin.
In many respects, embryos lacking Myo/Nog cells resemble noggin null mouse embryos that exhibit neural tube defects, enlargement of the notochord and decreased skeletal muscle in the somites (Anderson et al., 2002; Bachiller et al., 2000; Brunet et al., 1998; Choi et al., 2007; McMahon et al., 1998; Que et al., 2006; Stottmann et al., 2006; Weaver et al., 2003). One difference between these embryos is that follistatin is present in the somites of noggin null embryos (McMahon et al., 1998) but not in chick embryos lacking Myo/Nog cells. Chordin and other BMP inhibitors may compensate for the lack of noggin more effectively in the mouse than the chick embryo to restrain BMP signaling and promote follistatin expression. Variation in chordin expression in chick embryos lacking Myo/Nog cells may be responsible for the range in severity of the Myo/Nog ablation phenotype that resembles either the double noggin/chordin knockout mouse with defects in forebrain, eye and facial development (Anderson et al., 2002; Bachiller et al., 2000) or the triple noggin/chordin/follistatin knockdown in Xenopus embryos that lack neural and dorsal mesoderm tissue (Khokha et al., 2005).
Another difference between embryos lacking Myo/Nog cells and noggin null embryos is that Pax3+ skeletal muscle progenitor cells are present in the murine somites. Both ablated chick and noggin−/− embryos express Shh and Wnt1 in the notochord and neural tube (present study and McMahon et al., 1998). Culturing somite cells from chick embryos lacking Myo/Nog cells revealed the potential of a subpopulation to express Pax3. Since the Pax3+ cells were adjacent to those synthesizing Wnt3a in vitro, it is possible that in vivo, the amount or distribution of Wnts within the paraxial mesoderm was inadequate to support the specification of somite cells to the skeletal muscle lineage.
Altered interactions with axial structures do not appear to be the only cause of disrupted skeletal myogenesis in chick embryos lacking Myo/Nog cells. Subpopulations of somite cells in these embryos express the cardiac markers GATA4 and cardiac troponins I and T. The presence of cardiac progenitors and cardiomyocytes in the somites of ablated embryos can be interpreted to mean that the default pathway of paraxial mesoderm cells in the absence of sufficient inducers of the skeletal muscle lineage and unabated BMP signaling is cardiomyogenesis. Alternatively, not all somite cells in ablated embryos may be competent to respond to inducers of skeletal myogenesis because they are stably specified to the cardiac lineage. We favor the latter hypothesis for the following reasons. Our cell culture conditions clearly favor skeletal myogenesis, and yet a significant number of somite cells from ablated embryos express GATA4 and differentiate into cardiomyocytes. Culturing somite cells from ablated embryos with a neural tube and notochord from a normal embryo does not reduce the percentage of cardiac cells. Furthermore, Myo/Nog cell ablation results in an expansion of the domain of BMP signaling during primitive streak formation and the initiation of gastrulation when BMPs are involved in regulating early stages of cardiomyogenesis (van Wijk et al., 2007). Finally, when stage 2 epiblast cells are cultured in the absence of Myo/Nog cells, they differentiate into cardiac instead of skeletal muscle (Gerhart et al., 2004a). Analyses of the effects of Myo/Nog cell ablation on the expression of cardiac markers in the early embryo and the development of the heart are expected to reveal a critical role for Myo/Nog cells in regulating the size of the pool of cardiac progenitor cells.
The functions of Myo/Nog cells continue to expand as we examine their behaviors in different tissues as development progresses. Regardless of their location in the embryo, these cells display the signature of their phenotype, i.e., the expression of MyoD mRNA, the G8 antigen and noggin (Gerhart et al., 2006; Gerhart et al., 2007; Gerhart et al., 2009). Their stability as a noggin delivery system in the epiblast, muscle and non-muscle tissues indicates that Myo/Nog cells represent a unique population whose function is to modulate the activities of BMPs from the blastocyst stage through the period of organogenesis. Additional behaviors of these cells were revealed in their response to apoptosis in the epiblast (present study) and wounding of the lens epithelium (Walker et al., 2010). Given the importance of regulating BMP signaling and maintaining tissue integrity, it is not surprising that Myo/Nog cells emerge and function prior to the onset of gastrulation. Myo/Nog cells may be vulnerable to teratogens or mutations in genes coding for mediators of signaling pathways that affect their viability, migration, function and/or proliferation. In this regard, it is possible that Myo/Nog cell dysfunction underlies the cause of some birth defects of unknown etiology.
Highlights.
Myo/Nog cells regulate BMP signaling in the epiblast.
Myo/Nog cells respond to cell death in the epiblast.
Embryos lacking Myo/Nog cells have severe malformations.
Myo/Nog cell ablation inhibits the emergence of skeletal muscle progenitor cells.
Cardiomyogenic cells are present in the somites of embryos lacking Myo/Nog cells.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Drs. Scott Gilbert and Charles Ordahl for insightful discussions. This study was funded by the National Institutes of Health (AR052326 to M.G.W. and K.K.) and the March of Dimes (M.G.W). We dedicate this manuscript to our friend and colleague Dr. Margaret (Peggy) Wheelock, a quintessential scientist who enthusiastically supported our research for decades.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jacquelyn Gerhart, Email: gerhartj@mlhs.org.
Victoria L. Scheinfeld, Email: Scheinfeld@mlhs.org.
Tara Milito, Email: taramarie1489@gmail.com.
Christine Neely, Email: chrissyneely@verizon.net.
Dakota Fisher-Vance, Email: dfishervan@brynmawr.edu.
Kelly Sutter, Email: kelly.sutter@gmail.com.
Mitchell Crawford, Email: Mitchell.crawford39@yahoo.com.
Karen Knudsen, Email: kknudsen95@yahoo.com.
Mindy George-Weinstein, Email: george-weinsteinm@mlhs.org.
REFERENCES
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Bellairs R. The primitive streak. Anat Embryol (Berl) 1986;174:1–14. doi: 10.1007/BF00318331. [DOI] [PubMed] [Google Scholar]
- Borycki AG, Mendham L, Emerson CP., Jr Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development. 1998;125:777–790. doi: 10.1242/dev.125.4.777. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Johnson RL. Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol. 1998;197:205–217. doi: 10.1006/dbio.1997.8824. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Schubert FR, Schoenwolf GC, Lumsden A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev Biol. 2002;245:187–199. doi: 10.1006/dbio.2002.0641. [DOI] [PubMed] [Google Scholar]
- Choi M, Stottmann RW, Yang YP, Meyers EN, Klingensmith J. The bone morphogenetic protein antagonist noggin regulates mammalian cardiac morphogenesis. Circ Res. 2007;100:220–228. doi: 10.1161/01.RES.0000257780.60484.6a. [DOI] [PubMed] [Google Scholar]
- Christ BaHR., editor. Wnts in Muscle Development. New York: Plenum Publishers; 2003. [Google Scholar]
- Dechesne CA, Wei Q, Eldridge J, Gannoun-Zaki L, Millasseau P, Bougueleret L, Caterina D, Paterson BM. E-box- and MEF-2-independent muscle-specific expression, positive autoregulation, and cross-activation of the chicken MyoD (CMD1) promoter reveal an indirect regulatory pathway. Mol Cell Biol. 1994;14:5474–5486. doi: 10.1128/mcb.14.8.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhoot GK, Gell PG, Perry SV. The localization of the different forms of troponin I in skeletal and cardiac muscle cells. Exp Cell Res. 1978;117:357–370. doi: 10.1016/0014-4827(78)90149-0. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H, Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol. 1976;49:321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Fan CM, Lee CS, Tessier-Lavigne M. A role for WNT proteins in induction of dermomyotome. Dev Biol. 1997;191:160–165. doi: 10.1006/dbio.1997.8713. [DOI] [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- Frisch A, Wright CV. XBMPRII, a novel Xenopus type II receptor mediating BMP signaling in embryonic tissues. Development. 1998;125:431–442. doi: 10.1242/dev.125.3.431. [DOI] [PubMed] [Google Scholar]
- Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M, Gerhart JV, Foti GJ, Lash JW. Maturation of myogenic and chondrogenic cells in the presomitic mesoderm of the chick embryo. Exp Cell Res. 1994;211:263–274. doi: 10.1006/excr.1994.1086. [DOI] [PubMed] [Google Scholar]
- Gerhart J, Bast B, Neely C, Iem S, Amegbe P, Niewenhuis R, Miklasz S, Cheng PF, George-Weinstein M. MyoD-positive myoblasts are present in mature fetal organs lacking skeletal muscle. J Cell Biol. 2001;155:381–392. doi: 10.1083/jcb.200105139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Baytion M, DeLuca S, Getts R, Lopez C, Niewenhuis R, Nilsen T, Olex S, Weintraub H, George-Weinstein M. DNA dendrimers localize MyoD mRNA in presomitic tissues of the chick embryo. J Cell Biol. 2000;149:825–834. doi: 10.1083/jcb.149.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Baytion M, Perlman J, Neely C, Hearon B, Nilsen T, Getts R, Kadushin J, George-Weinstein M. Visualizing the Needle in the Haystack: In Situ Hybridization With Fluorescent Dendrimers. Biol Proced Online. 2004b;6:149–156. doi: 10.1251/bpo84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Elder J, Neely C, Schure J, Kvist T, Knudsen K, George-Weinstein M. MyoD-positive epiblast cells regulate skeletal muscle differentiation in the embryo. J Cell Biol. 2006;175:283–292. doi: 10.1083/jcb.200605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Neely C, Elder J, Pfautz J, Perlman J, Narciso L, Linask KK, Knudsen K, George-Weinstein M. Cells that express MyoD mRNA in the epiblast are stably committed to the skeletal muscle lineage. J Cell Biol. 2007;178:649–660. doi: 10.1083/jcb.200703060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Neely C, Pfautz J, George-Weinstein M. Tracking and ablating subpopulations of epiblast cells in the chick embryo. Biol. Proced. Online. 2008;10:74–82. doi: 10.1251/bpo145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Neely C, Stewart B, Perlman J, Beckmann D, Wallon M, Knudsen K, George-Weinstein M. Epiblast cells that express MyoD recruit pluripotent cells to the skeletal muscle lineage. J Cell Biol. 2004;164:739–746. doi: 10.1083/jcb.200309152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Neely C, Stewart B, Perlman J, Beckmann D, Wallon M, Knudsen K, George-Weinstein M. Epiblast cells that express MyoD recruit pluripotent cells to the skeletal muscle lineage. J Cell Biol. 2004a;164:739–746. doi: 10.1083/jcb.200309152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Pfautz J, Neely C, Elder J, DuPrey K, Menko AS, Knudsen K, George-Weinstein M. Noggin producing, MyoD-positive cells are crucial for eye development. Dev Biol. 2009;336:30–41. doi: 10.1016/j.ydbio.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. Sunderland, MA: Sinauer Associates, Inc.; 2010. [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Graff JM, Thies RS, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Hansen CS, Marion CD, Steele K, George S, Smith WC. Direct neural induction and selective inhibition of mesoderm and epidermis inducers by Xnr3. Development. 1997;124:483–492. doi: 10.1242/dev.124.2.483. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Hartley KO, Hardcastle Z, Friday RV, Amaya E, Papalopulu N. Transgenic Xenopus embryos reveal that anterior neural development requires continued suppression of BMP signaling after gastrulation. Dev Biol. 2001;238:168–184. doi: 10.1006/dbio.2001.0398. [DOI] [PubMed] [Google Scholar]
- Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Heikinheimo M, Scandrett JM, Wilson DB. Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Dev Biol. 1994;164:361–373. doi: 10.1006/dbio.1994.1206. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann's organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Ang SL, Bachiller D, Rossant J. Neural induction and patterning in the mouse in the absence of the node and its derivatives. Dev Biol. 1999;216:535–549. doi: 10.1006/dbio.1999.9525. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–5681. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- Saggin L, Gorza L, Ausoni S, Schiaffino S. Troponin I switching in the developing heart. J Biol Chem. 1989;264:16299–16302. [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Stern CD. Evolution of the mechanisms that establish the embryonic axes. Curr Opin Genet Dev. 2006;16:413–418. doi: 10.1016/j.gde.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Stern HM, Brown AM, Hauschka SD. Myogenesis in paraxial mesoderm: preferential induction by dorsal neural tube and by cells expressing Wnt-1. Development. 1995;121:3675–3686. doi: 10.1242/dev.121.11.3675. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Berrong M, Matta K, Choi M, Klingensmith J. The BMP antagonist Noggin promotes cranial and spinal neurulation by distinct mechanisms. Dev Biol. 2006;295:647–663. doi: 10.1016/j.ydbio.2006.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- Streit A, Sockanathan S, Perez L, Rex M, Scotting PJ, Sharpe PT, Lovell-Badge R, Stern CD. Preventing the loss of competence for neural induction: HGF/SF, L5 and Sox-2. Development. 1997;124:1191–1202. doi: 10.1242/dev.124.6.1191. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev. 1999a;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech Dev. 1999b;85:85–96. doi: 10.1016/s0925-4773(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Strony R, Gerhart J, Tornambe D, Perlman J, Neely C, Dare J, Stewart B, George-Weinstein M. NeuroM and MyoD are expressed in separate subpopulations of cells in the pregastrulating epiblast. Gene Expr Patterns. 2005;5:387–395. doi: 10.1016/j.modgep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Chang C, Yingling JM, Wang XF, Hemmati-Brivanlou A. Smad5 induces ventral fates in Xenopus embryo. Dev Biol. 1997;184:402–405. doi: 10.1006/dbio.1997.8548. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Borello U, Vivarelli E, Kelly R, Papkoff J, Duprez D, Buckingham M, Cossu G. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- Tonegawa A, Takahashi Y. Somitogenesis controlled by Noggin. Dev Biol. 1998;202:172–182. doi: 10.1006/dbio.1998.8895. [DOI] [PubMed] [Google Scholar]
- Toyota N, Shimada Y. Differentiation of troponin in cardiac and skeletal muscles in chicken embryos as studied by immunofluorescence microscopy. J Cell Biol. 1981;91:497–504. doi: 10.1083/jcb.91.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Venters SJ, Argent RE, Deegan FM, Perez-Baron G, Wong TS, Tidyman WE, Denetclaw WF, Jr, Marcelle C, Bronner-Fraser M, Ordahl CP. Precocious terminal differentiation of premigratory limb muscle precursor cells requires positive signalling. Dev Dyn. 2004;229:591–599. doi: 10.1002/dvdy.20016. [DOI] [PubMed] [Google Scholar]
- Wagner J, Schmidt C, Nikowits W, Jr, Christ B. Compartmentalization of the somite and myogenesis in chick embryos are influenced by wnt expression. Dev Biol. 2000;228:86–94. doi: 10.1006/dbio.2000.9921. [DOI] [PubMed] [Google Scholar]
- Walker JL, Zhai N, Zhang L, Bleaken BM, Wolff I, Gerhart J, George-Weinstein M, Menko AS. Unique precursors for the mesenchymal cells involved in injury response and fibrosis. Proc Natl Acad Sci U S A. 2010;107:13730–13735. doi: 10.1073/pnas.0910382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol. 2003;258:169–184. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Wessely O, Agius E, Oelgeschlager M, Pera EM, De Robertis EM. Neural induction in the absence of mesoderm: beta-catenin-dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev Biol. 2001;234:161–173. doi: 10.1006/dbio.2001.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills AE, Choi VM, Bennett MJ, Khokha MK, Harland RM. BMP antagonists and FGF signaling contribute to different domains of the neural plate in Xenopus. Dev Biol. 2010;337:335–350. doi: 10.1016/j.ydbio.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.