Abstract
Recent advances in design of powered artificial legs have led to increased potential to allow lower limb amputees to actively recover stumbles. To achieve this goal, promptly and accurately identifying stumbles is essential. This study aimed to (1) select potential stumble detection data sources that react reliably and quickly to stumbles and can be measured from a prosthesis, and (2) investigate two different approaches based on selected data sources to detect stumbles and classify stumble types in patients with transfemoral (TF) amputations during ambulation. In the experiments, the normal gait of TF amputees was perturbed by a controllable treadmill or when they walked on an obstacle course. The results showed that the acceleration of prosthetic foot can accurately detect the tested stumbling events 140–240 ms before the critical timing of falling and precisely classify the stumble type. However, the detector based on foot acceleration produced high false alarm rates, which challenged its real application. Combining electromyographic (EMG) signals recorded from residual limb with the foot acceleration significantly reduced the false alarm rate but sacrificed the detection response time. The results of this study may lead to design of a stumble detection system for instrumented, powered artificial legs; however, continued engineering efforts are required to improve the detection performance and resolve the challenges that remain for implementing the stumble detector on prosthetic legs.
Index Terms: Stumble, detection, EMG, lower limb amputees, prosthetics
I. INTRODUCTION
The risk of falling for persons with lower limb amputations is high because of a combination of the following two factors: (1) Lower limb amputations lead to altered balance, strength, and gait pattern [1]–[2]; (2) more than half of the population with lower limb amputations is elderly people (aged 65 years or older) [2]–[3], for whom falls are one of the major causes of serious injuries [4]. It has been reported that falls caused soft tissue injury, boney injury, deterioration in balance, fear of falling, and reduced participation in activities of daily living in patients with leg amputations [1]–[2]. Therefore, solutions are demanded to prevent falls in patients with leg amputations so that they can lead active lifestyle and have improved quality of life.
One of the potential solutions is to improve the safety of lower limb prostheses. Focusing on transfemoral (TF) prostheses, current microcomputer-controlled (MCC) passive prostheses incorporate a simple mechanism, i.e. locking knee joint when a large deceleration of the prosthetic knee is sensed during the swing phase, to improve the user’s walking stability and prevent falls [5]–[6]. However, dealing with various types of unexpected perturbations during normal gait, such as slipping on a wet surface, still present a significant challenge for leg amputees when wearing the passive prostheses [7]. With the advent of powered lower limb prostheses [8]–[9], a much greater scope of capabilities is now possible to allow the leg amputees to recover stumbles in a natural way. To achieve this goal, promptly and accurately identifying stumbles elicited by different types of perturbations is essential so that the powered prosthesis can produce protective reactions corresponding to the stumble types.
Unfortunately, very limited studies have been reported on the methods to detect and classify stumbles during normal gait for artificial legs. A resent preliminary study [10] has demonstrated a design of stumble detection method based on three accelerometers, which is potentially useful for an intelligent transfemoral prosthesis. The authors reported 100% detection accuracy; however, they only tested the method on healthy subjects and studied a particular case of stumbling during the swing phase. In addition, no false alarm rate (FAR) for stumble detection was showed, while the FAR is an important parameter to evaluate the usefulness of the system for prosthesis use. Designing a stumble detection system with high accuracy and a fast time response is challenging. First, human corrective responses to perturbations depend on the perturbation type (i.e. trip or slip) [11]–[12] and when the perturbation takes place during normal gait (i.e. gait phase) [12]–[14]. Previous studies have reported that an elevating strategy of perturbed leg was performed when healthy subjects were tripped in early swing; a lowering strategy was seen for mid and late swing perturbations [13–14]. When a slip happened, healthy subjects extended the joints of perturbed leg, which contacted the ground presumably to deliver an impulse thrust to counter the backward lean of the trunk [15]. Therefore, the detection system is required to not only detect the stumbling events but also recognize different stumble types to ensure the correct stumble recovery strategy applied. Another challenge for stumble detector design is that the system must respond fast enough to allow the prosthesis to recover stumbles before a fall happens. Furthermore, the designed detection system should be practical for TF prostheses. It is desirable and practical if applied sensors can be integrated into the prosthesis or socket, and the system calibration procedure is simple.
Motivated by the need to further improve the safety of prosthetic legs, we investigated new approaches to identify stumbles, which could be used to trigger the active stumble reaction of transfemoral prostheses. The paper has two major sections. Section 1 describes examination and selection of appropriate data sources, which reliably and promptly respond to stumbles, for stumble detection. Based on these potential data sources, in Section 2, we investigated different designs of a stumble detection system for transfemoral prostheses. The designed system was evaluated on data collected from seven subjects with TF amputations when they walked on a controllable treadmill or an obstacle course. The results of this study may lead to an improved design of powered artificial legs that actively deliver stumble recovery, which in turn reduces the risk of falling in lower limb amputees.
II. Section 1: Investigation of Potential Data Sources for Stumble Detection
A. Overview
To design an accurate and responsive stumble detector, it is essential to find appropriate data sources that reliably and promptly respond to different types of stumbles. Previous studies on healthy subjects showed that perturbations during normal gait led first to passive changes in the kinematics and kinetics of the perturbed limb [13], followed by the neural response measured via surface electromyographic (EMG) signals [11, 13, 16–17], and finally to the active correction of body motions [12–13, 18]. In this study, to make the stumble detection system practical for prostheses, only the mechanical variables and neuromuscular reactions of residual limb, which are measurable from the prosthesis or prosthetic socket, were considered as the potential sources for stumble detection.
B. Methods
1) Participate and Measurements
This study was conducted with Institutional Review Board (IRB) approval at the University of Rhode Island and the Providence VA Medical Center and the informed consent of all subjects. Seven subjects with unilateral TF amputations (TF01–07) were recruited; the demographic information for these TF amputees is shown in Table 1.
TABLE I.
Summary of demographic information for seven recruited subjects with transfemoral amputations (TF01-TF07)
| Age | Weight (kg) | Height (cm) | Gender | Years post- amputation |
Residual limb length ratio* |
Prosthesis for daily use | |
|---|---|---|---|---|---|---|---|
| TF01 | 57 | 75.8 | 175.3 | M | 31 | 51% | RHEO |
| TF02 | 46 | 97.0 | 160.0 | F | 3 | 93% | C-Leg |
| TF03 | 38 | 65.7 | 162.6 | F | 29 | 68% | RHEO |
| TF04 | 48 | 63.1 | 166.4 | M | 7 | 94% | C-Leg |
| TF05 | 52 | 64.0 | 164.0 | F | 31 | 84% | RHEO |
| TF06 | 56 | 75.2 | 173.4 | M | 38 | 62% | SNS Knee |
| TF07 | 42 | 66.1 | 165.8 | F | 11 | 77% | C-Leg |
Residual limb length ratio: the ratio between the length of residual limb (measured from the ischial tuberosity to the distal end of the residual limb) to the length of the non-impaired side (measured from the ischial tuberosity to the femoral epicondyle).
Surface EMG signals from the thigh muscles surrounding the residual limb were monitored. The number of EMG electrodes (7–9) placed on the residual limb depended on the residual limb length. The subjects were instructed to perform hip movements and to imagine and execute knee flexion and extension. Bipolar EMG electrodes were placed at locations, where strong EMG signals could be recorded. The electrodes were embedded in a customized gel liner for reliable electrode-skin contact. Amputee subjects rolled on the gel liner before socket donning. A ground electrode was placed near the anterior iliac spine. A 16-Channel EMG System (Motion Lab System, US) was used to collect EMG signals from all subjects. The EMG system filtered signals between 20 Hz and 450 Hz with a pass-band gain of 1000 and then sampled at 1000 Hz. The vertical ground reaction forces were measured by a load cell (Bertec Corporation, OH, US) mounted on the prosthetic pylon and were also sampled at 1000Hz. Kinematic data were monitored by a marker-based motion capture system (Oqus, Qualisys, Sweden). Light-reflective markers were placed on the bilateral iliac crest, great trochanter, and posterior superior iliac spine to monitor the motions of pelvis. To track the movements of lower limbs, four nonaligned markers were placed on six lower limb segments (i.e. prosthetic socket, pylon, and foot on the amputated side, and thigh, shank, and foot of the unimpaired leg), respectively. The markers' positions were sampled at 100 Hz. In addition, force-sensitive insoles (Pedar-X, Novel Electronics, Germany) were placed under both feet to measure the center of pressure (COP) for an evaluation purpose. Pressure data were sampled at 100 Hz. The experimental sessions were videotaped. The video data were used to monitor the actual walking status of subjects during the experiments. All data recordings in this study were synchronized.
2) Experimental Protocol
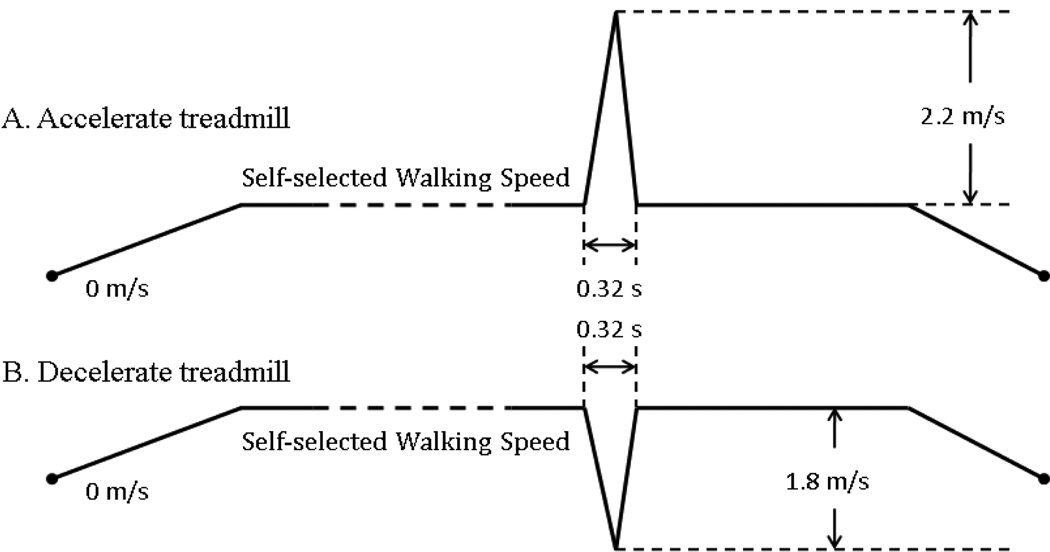
Two sets of experiments were designed and conducted. Five subjects with TF amputations (TF01–05) participated in the first experimental set. In order to design a detector capable of indentifying stumbles and classifying the stumble types, both trips and slips were induced. Various methods have been used to simulate tripping [11–13, 18–20] and slipping [7, 11, 15–16, 19, 21–22] in an effort to study the control mechanisms underlying stumble and recovery during walking. In this study, the perturbations were simulated by sudden accelerations or decelerations of a treadmill belt (ActiveStep, Simbex, US) during walking. This type of perturbation strategy (1) elicits stumble responses comparable to those occurring in daily life [23], (2) minimizes anticipatory reactions to a stumble, and (3) can be tested in a reproducible manner [19]. The treadmill speed profile was easily programmed, as shown in Fig. 1. The magnitude of acceleration or deceleration was the same for all subjects. A trigger signal, sent from the treadmill after the profile was initially executed, was used to synchronize the treadmill speed profile with the other recorded data. Five TF amputees used a hydraulic knee (Total Knee, OSSUR, Germany) and were given time prior to the experiment to acclimate to the prosthesis and achieve a smooth walking pattern. The subject wore a harness for fall protection when walking on the treadmill without any assistance. A self-selected walking speed was determined first for each subject. The average duration of swing phase was computed. Ten trials with sudden treadmill accelerations and ten trials with treadmill decelerations were tested. The perturbations involving sudden belt accelerations were introduced in the swing phase with certain delays (i.e. 20% and 65% of average duration of swing phase) after toe off; the perturbations involving belt decelerations were designed in the initial double-stance phase (10ms after heel strike). Most of the perturbations were applied to the prosthetic leg; a few were applied to the unimpaired leg. Only one perturbation was introduced in each trial in a random selected gait cycle. The trials with perturbations ended in 15 seconds after the perturbation was delivered. To reduce the subjects’ ability to anticipate a perturbation, 6 walking trials without any perturbation were included. The 6 walking trials and 20 trials with perturbations were conducted in a random order. In addition, subjects conversed with an experimenter throughout each trial in order to further distract subjects’ attention. Rest periods were allowed between trials to avoid fatigue.
Fig. 1.
Designed treadmill speed profiles.
Another two subjects (TF06–07) participated in the second experimental set, in which the subjects walked on realistic terrains without control of walking speed. The collected data were mainly used to evaluate the false alarm rate of designed stumble detector and its feasibility for real application. The recruited subjects were required to walk on an obstacle course, including a level ground walking pathway, 5-step stair, 10 feet ramp, and obstacle blocks on the level ground. No perturbation was purposely applied. The subjects were allowed to use hand railing on the stairs and ramp and a parallel bar on the level ground. In addition, an experimenter walked along with the subject to ensure the subject’s safety. A total of 15 trials were tested for each subject; in each trial the subjects walked on the obstacle course continuously for approximately 5 minutes. Rest periods were allowed during the testing.
3) Data Processing and Criteria for Data Source Selection
EMG signals from the residual thigh muscles, acceleration of prosthetic foot, vertical ground reaction force (GRF) measured by the load cell on prosthetic pylon, and prosthetic knee angular acceleration were investigated. The foot acceleration was computed by the second order time derivative of position of a marker on the prosthetic toe. The knee flexion/extension angle was derived by the Visual3D software (C-Motion Inc. US) and then low-pass filtered with the cutoff frequency at 20 Hz. The knee angular acceleration was calculated as the second order time derivative of knee angle.
Three criteria were applied to determine the potential data source for stumble detection. First, the selected data sources must react fast enough to allow the prosthesis to recover stumbles before a fall happens. A previous study [24] reported that the recovery action must occur before the center of mass (COM)-the center of pressure (COP) inclination angle exceeds 23–26 degrees of deviation from vertical; otherwise, falls might happen. The COM-COP inclination angle in anterior-posterior direction was defined as the angle formed by the intersection of the line connecting the COP and COM with the vertical line through the COP in sagittal plane [25]. Such an indicator was estimated based on the inverted pendulum model that has been used to quantify human balance [24–25] and was used to find the critical timing of falling in the present study. The COM was estimated based on a human model with 7 body segments: head-arm-trunk (HAT), 2 thighs, 2 shanks, and 2 feet [26–27]. The mass of each segment was estimated by using the modified Hanavan model described in [28]. The COP positions were computed by using the Pedar-X software (Novel Electronics, Germany). The critical timing (CT) of falling was defined as the moment, at which the COM-COP inclination angle exceeded a range of −23 to 23 degrees from vertical. Therefore, the selected data sources for stumble detection must react before this critical timing. Second, the data sources that consistently show obvious reactions to various types of perturbations were considered reliable and were preferred for accurate stumble detection. Thirdly, the data sources that can indicate the type of stumbles were selected because the reactive control strategy of artificial legs to stumbles also depends on the stumble types.
C. Results
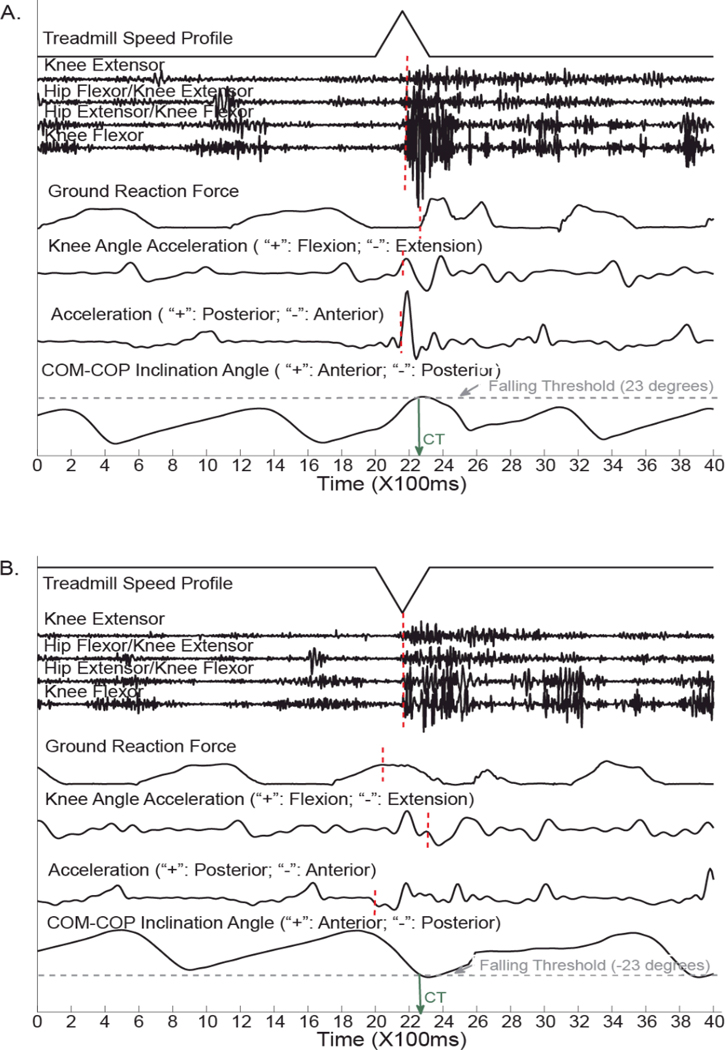
Fig. 2 shows the recorded data in two representative trials when TF01 walked on the treadmill. Studied data sources were aligned with the treadmill speed profiles and calculated COM-COP inclination angle. When a tripping was induced in a swing phase of amputated side (Fig. 2A), an obvious response of the foot acceleration in anterior-posterior direction was first observed, approximate 120 ms before the critical timing (green arrow in Fig. 2A), quickly followed by the pattern change in knee angular acceleration (~100 ms before the CT) and EMG response (~80 ms before the CT). The pattern change of vertical GRF was slightly after the CT. When a slip happened in initial double-stance phase of the prosthetic side (Fig. 2B), the foot acceleration also responded first right after the change of treadmill speed (~240ms before the CT), then followed by the GRF pattern change (~220ms before the CT) and the muscle response (~90ms before the CT). The response in knee angular acceleration was after the CT.
Fig. 2.
Examples of collected data sources aligned with treadmill speed profiles and computed COM-COP inclination angle. Representative data were recorded from TF01.
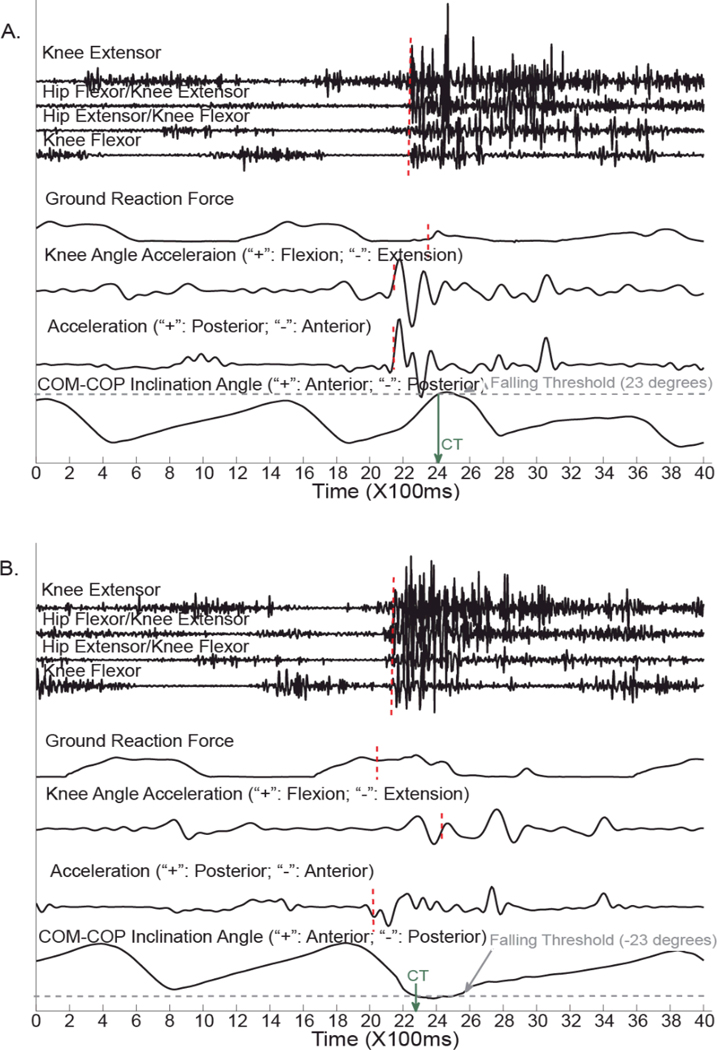
During the second set of experiments, although we did not purposely perturb the subjects’ gait, one slip occurred when TF06 descended the stair, two trips were captured when TF07 stepped over an obstacle block and performed stair ascent task, and two slips were caught when TF07 descended staircases. The slips during stair descent were caused by inadequate placement of prosthetic foot during the initial contact. TF07 was tripped by the obstacle block and staircase during the swing phase of amputated limb. Since the TF patients used railing or protected by parallel bars and experimenters, no fall happened in the experiments. Fig. 3 showed two examples of recorded data during tripping and slipping when TF07 walked on the obstacle course. During tripping (Fig. 3A), an obvious foot deceleration and deceleration in knee angle was observed around 260ms before the CT. The EMG responses were 160ms ahead of the CT. The pattern change of GRF was around 60 ms before the CT. During slipping (Fig. 3B), the foot acceleration responded fastest (~250ms before the CT). The GRF pattern change happened at ~230ms before the CT, and the EMG signals responded around ~150ms before the CT. The knee angular acceleration reacted to the perturbation after the CT.
Fig. 3.
Examples of collected data sources when TF07 walked on the obstacle course. The red dotted vertical lines highlight the timings of data reaction. The green arrow indicates the critical timing (CT) of falling.
D. Discussion
Stumbles were observed when our recruited TF amputees walked on the designed obstacle course although no perturbation was purposely applied. This observation indicates that stumbling is common in patients with lower limb amputations when they negotiate with uneven terrains. In addition, all the observed stumbles were originated from the amputated side of the limb, which either collided with the obstacle/staircase or slipped on the edge of staircase. Unfortunately, very limited study has been found in the literature that surveys lower limb amputees about the type of negotiating terrains and perturbations that cause most of stumbles in their daily lives. Understanding the most frequently occurred types of stumble in leg amputees would be extremely useful for future design of a safety system for lower limb prostheses for fall prevention.
The foot acceleration is selected as the best data source for stumble detection and classification because it satisfied all three selection criteria defined in this study. The acceleration of prosthetic foot responded fastest to all applied perturbations with an obvious change in magnitude. Additionally, its direction was associated with the stumble types. The waveform pattern changes of knee angular acceleration and vertical GRF were also observed during stumbling; however, their reaction time to the perturbations depended on the stumble types (i.e. trip or slip and when the perturbation takes place in gait cycle). For some cases, these two data sources presented obvious signal pattern changes after the defined critical timing of falling, and therefore, should not be considered for stumble detection. The residual muscles clearly showed significantly high activation level, long activation duration, and co-contraction during stumbling, consistent with those observed in able-bodied subjects [13, 19]. The timing of observed neural reactions was around 160ms after the initial treadmill perturbations and was approximate 100ms after the perturbations when the subjects walked on the realistic terrains. This difference in reaction time may be caused by the magnitude of perturbations. In addition, the neural responses of amputees in the residual thigh muscles were slower than those of able-bodied subjects (90–140ms) reported in the previous study [16], which could be partially attributed to the loss of perception in the distal limb.
The reactions of foot acceleration were approximately 100ms faster that EMG responses, although both data sources responded before the defined critical timing. Therefore, in order to detect stumbles with quick response time, foot acceleration should be preferred. One of the potential drawbacks in using foot acceleration is that a sudden acceleration or deceleration of prosthetic foot may not necessary correlated to a stumble, while co-contraction of muscles in the thigh with high activation levels may accurately indicate the protective neural response to balance disturbances. Therefore, in the next section, we considered both foot acceleration and EMG signals as the potential stumble detection sources and investigated and evaluated two approaches for stumble detection.
III. Section 2: Design and Evaluation of Stumble Detectors
A. Architecture of the Stumble Detection System
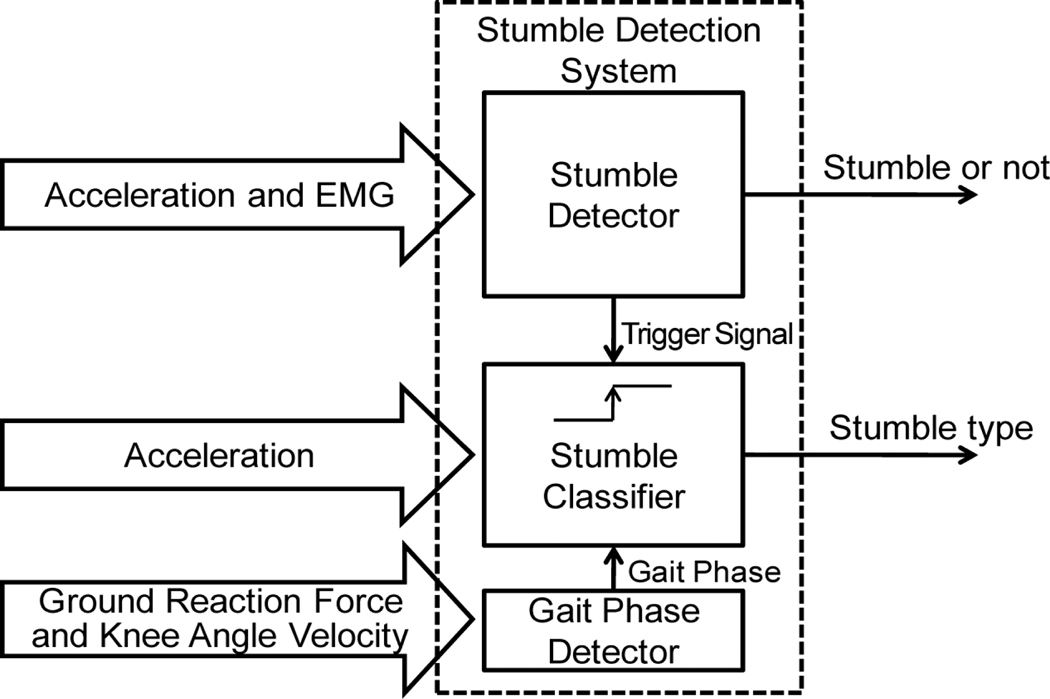
Since our ultimate goal is to design a stumble detection system that can trigger the protective reaction of artificial legs for stumble recovery, the outputs of this system should tell whether or not there is a stumble and type of the stumble (e.g. trip in early swing and slip in initial double stance). Therefore, a stumble detection system consisted of two modules: a stumble detector and stumble classifier (Fig. 4). The first output was used to initialize the stumble recovery action of artificial legs. The classified stumble type together with the state of prostheses (i.e. current joint position and external forces applied on the prosthesis) can determine the stumble recovery strategy to be applied because the stumble recovery strategy varies depending on the stumble types.
Fig. 4.
Architecture of stumble detection system.
B. Methods
1) Design of Stumble Detector
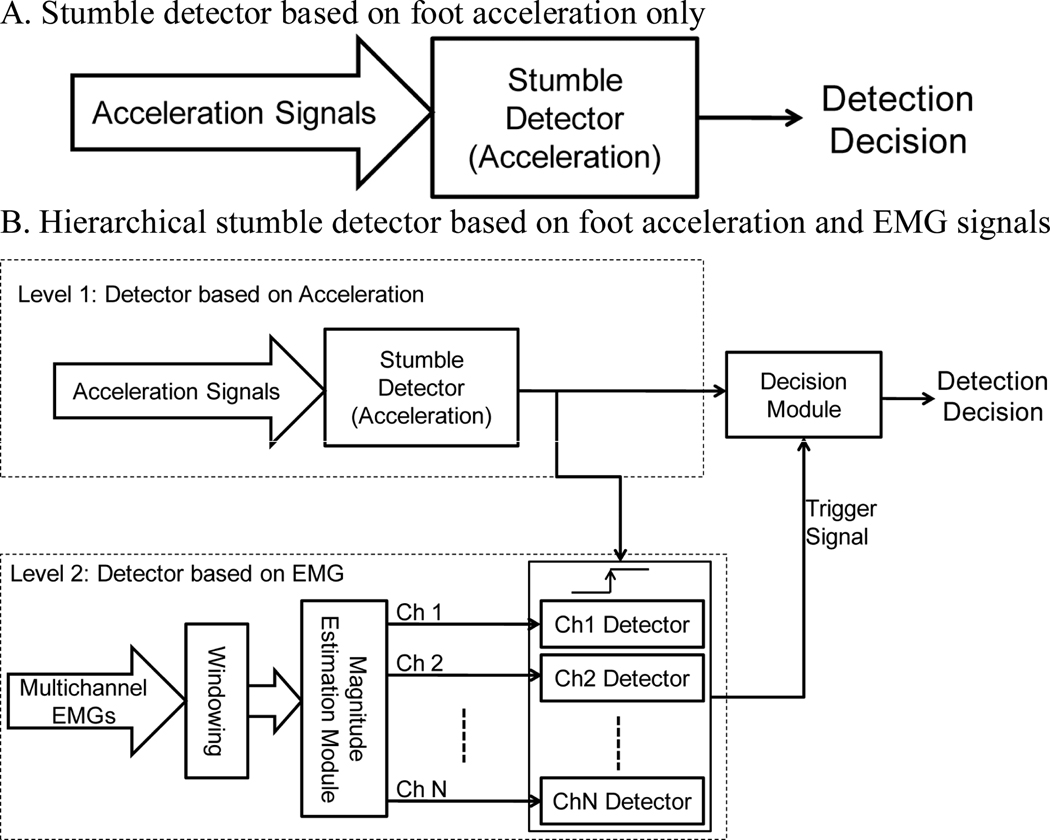
In this study, two designs of stumble detector (Fig. 5) were investigated. Since the reaction of foot acceleration was fastest among investigated data sources, the foot acceleration was considered as the primary data source for stumble detection in both designs.
Fig. 5.
Two designs of stumble detector.
The first design (Fig. 5A) was based on the absolute magnitude of foot acceleration in anterior-posterior direction. A decision was made every 10 ms based on each sampled data. In the second approach, the foot acceleration and EMG signals recorded from residual thigh muscles were fused hierarchically to detect stumbles (Fig. 5B). The acceleration-based detector was assigned as the level 1 detector and designed the same as the detector in Fig. 5A. The EMG-based detector was the secondary detector (the level 2 detector), which was activated when a gait abnormality was identified by the level 1 detector. In the level 2 detector, raw EMG inputs were first band-pass filtered between 25 and 400 Hz by an eighth-order Butterworth filter and then were segmented by overlapped sliding analysis windows (150 ms in length and 10 ms increments). Since the EMG reactions to perturbations were characterized by increased magnitude and synchronized activation across multiple muscles compared to normal gait EMGs (Fig. 2 & Fig. 3), EMG magnitude was used for stumble detection and was estimated by the root mean square (RMS) [29]. For each EMG channel, a sub-detector was designed to make a decision based the magnitude of this EMG signal in each analysis window. Next, a majority vote principle was used to determine the output of the level 2 detector. That is to say, if more than half of EMG signals presented larger magnitudes than the detection thresholds designed for individual EMG channels, the output of the level 2 detector was a decision of an abnormal gait. This was because the observed EMG reactions to perturbations were synchronized across the tested muscles in the thigh (Fig.2 & Fig. 3). Such a design can eliminate false detections caused by the abnormal signal recordings in just one or a few number of channels, unrelated to the stumbling. Since one decision was made in one analysis window, the decision of level 2 detector was updated every 10ms, aligned with the decision of level 1 detector. Finally, a stumble was detected if both level 1 and level 2 detectors identified the gait as abnormal.
The foot-acceleration-based detector and EMG sub-detectors were formulated as outlier detectors and composed of the following hypotheses: (1) the walking status is normal (H0), and (2) the status is abnormal (H1). For the design that used foot acceleration only (Fig. 5A), the detection of abnormal gait was equivalent to stumble detection. The data model for the normal gait (H0) was built first; any observation located far from the center of the data model of H0 was considered an outlier and detected as an abnormal case (H1). Mahalanobis distance [30], a widely used method for outlier detection, was employed to quantify the geometric distance between the observation (F) and the mean (μ0) of the observations in H0, and can be defined by
| (1) |
Where σ0 is the standard deviation of the observations in H0 The criterion to test detection hypothesis was
| (2) |
In this study, a single dimensional observation was used for the foot-acceleration-based detector (i.e. absolute value of foot acceleration in anterior-posterior direction) and EMG sub-detectors (i.e. RMS of an EMG signal), respectively. We investigated different detection threshold for each studied data source and selected the optimal thresholds based on the receiver operating characteristic (ROC) to minimize the detection errors (i.e. the detection missing rate and false alarm rate). In the typical approach for detecting outliers based on Mahalanobis distance, the observations are assumed to follow a normal distribution. Therefore, the square of Mahalanobis distance is compared with a threshold formulated in terms of chi-square distribution [30]. Since in this study the histogram of observations in H0 did not follow normal distribution well, the detection threshold was formulated by
| (3) |
Where Max(Mahal (F0, μ0)) is the maximum value of Mahalanobis distances derived from observations in H0. Such a maximum value has been used as the outlier detection threshold [31–32] to ensure all the data considered as H0 were within the detection boundary. T in (3) is a scale factor (T≥1). The detection threshold was optimized by adjusting the T value. It is noteworthy that the same T value was selected for individual EMG sub-detectors in all recruited subjects because customizing the optimal thresholds requires the knowledge on residual muscles’ responses to stumbles in individual patients, which are usually impractical to obtain in real application. The mean (μ0), variance (σ0), and Max(Mahal(F0, μ0)) were estimated based on the observations collected from the trials without any perturbations. Then the ROC was computed based on data collected in half of the treadmill trials with perturbations for optimal threshold (i.e. the T values) selection. Note that once the T values are determined, in real application the choice of the detection threshold only requires data collected during normal walking.
2) Stumble Classification
In this study, three-class classifier were designed to identify (1) tripping in early swing phase, (2) tripping in late swing phase, and (3) slipping in initial double-stance phase. These three classes were studied because they were most frequently occurred and resulted in different stumbling recovery strategies in healthy subjects [16, 18].
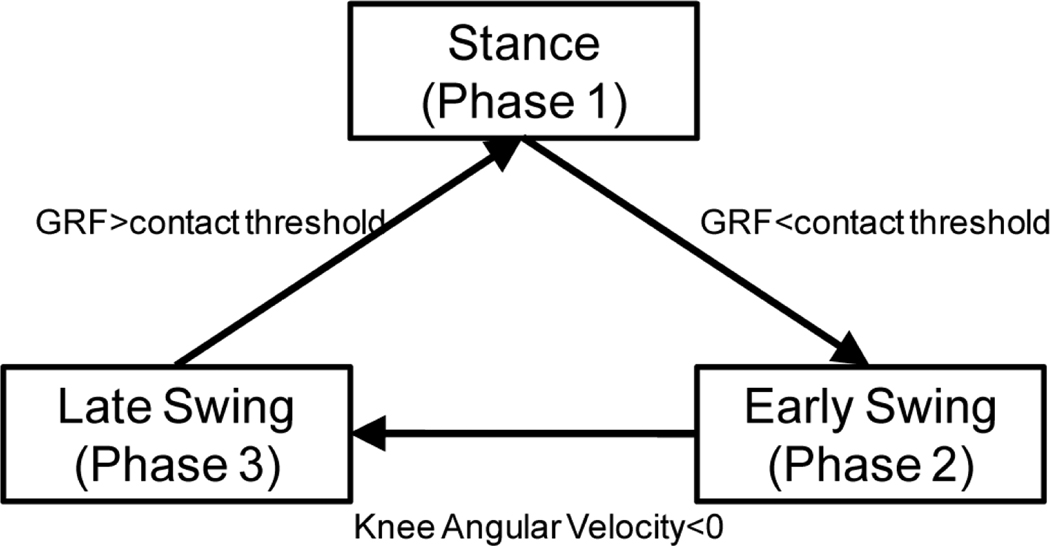
The stumble classifier was activated only when a stumble was detected. A decision tree was designed to classify the stumble types. Based on Fig. 2&3, the direction of foot acceleration was associated with tripping (sudden deceleration of foot swing) and slipping (sudden forward acceleration of the foot); therefore, the direction of foot acceleration was used at the first decision node to separate tripping, i.e. classes (1) and (2), from the slipping, i.e. the class (3). The second decision node took the instantaneous output from gait phase detector to identify the gait phase when tripping was identified; therefore, the type (1) and type (2) tripping can be separated. The gait phase detection module received inputs from vertical GRF and knee joint angle, both of which were measured in current MCC prostheses, and determined gait phase continuously. In the presented study a stride cycle was divided into three phases: stance phase, early swing phase, and late swing phase (Fig. 6). When the vertical GRF measured from prosthetic pylon was greater than a contact threshold (1% of maximum GRF), a stance phase was identified. During the swing phase, if the knee angular velocity is greater than zero, the early swing was detected. Otherwise, the output phase was the late swing. The criterion for the gait phase detection was shown in Fig. 6.
Fig. 6.
The criteria for gait phase detection. The contact threshold was 1% of maximum vertical ground reaction force measured from individual subjects.
3) Detection Performance Evaluation
The performance of stumble detector was evaluated by the detection sensitivity (SE) in (4), false alarm rate (FAR) in (5), and remaining time (RT) of stumble recovery.
| (4) |
| (5) |
The remaining time (RT) of stumble recovery was defined in (6), as the time elapse from the moment of detecting a stumble (TSD) to the critical timing of falling (TCT) that was determined by the COM-COP inclination angle. The positive RT indicated the detection of a stumble was before the critical timing, which allowed for activation of prosthesis control for stumble recovery. Therefore, the large RT was desirable.
| (6) |
In addition, when stumbles were accurately detected, the accuracy (CA) in classifying the stumble types was quantified as in (6). The actual stumble type (ground truth) was determined by experimental videos.
| (7) |
The stumble detection system was built based on the data collected from treadmill walking trials without any perturbations and designed optimal T values in (3); it was evaluated by data collected from the treadmill trials with simulated trips applied in the swing and slips applied in the initial heel contract of amputated side and the trials when the subjects walked on the obstacle course. Note that the data in the trials, used for defining the optimal T values, were not included for evaluation. Since no perturbation was purposely applied in the second experimental set, if no stumble occurred during the testing, the gait status was considered normal regardless of the type of negotiating terrains, and only FAR was quantified.
C. Results
1) Detection Threshold Selection
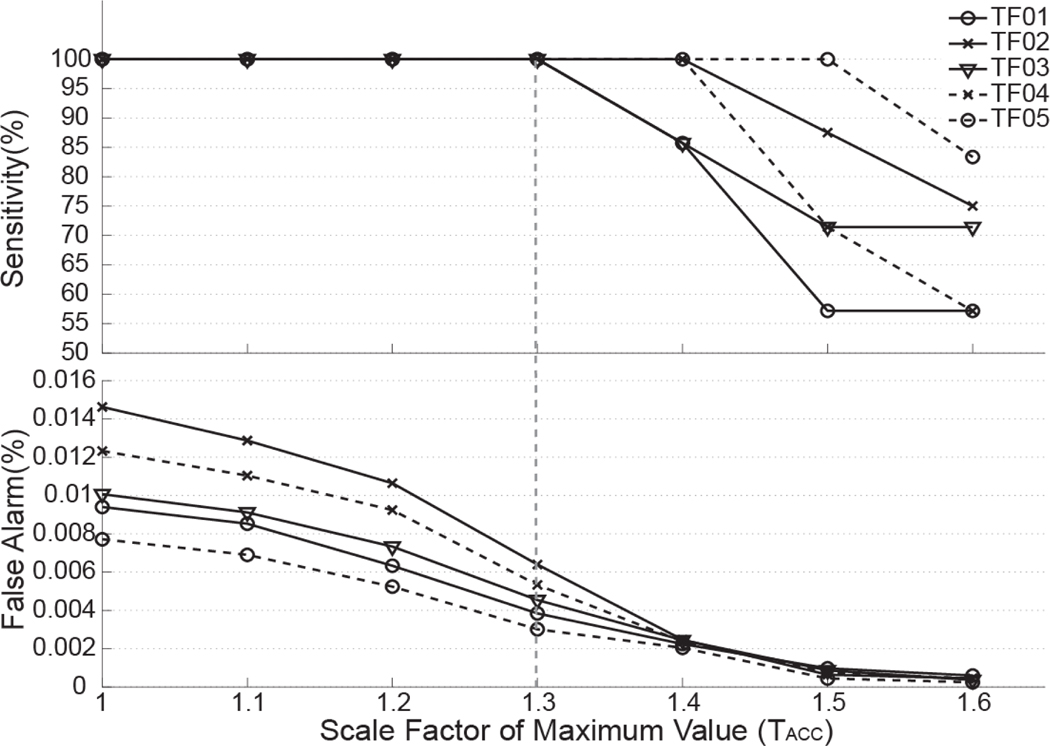
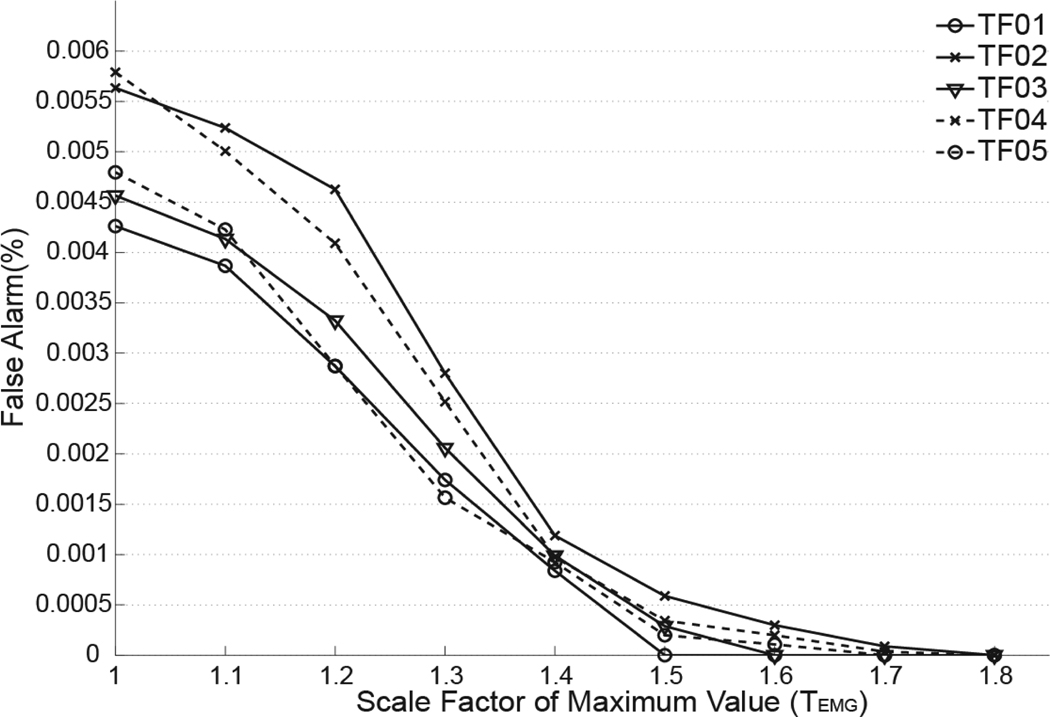
When optimizing the detection threshold (i.e. TACC value) for the foot-acceleration-based detector, we found that the detection sensitivity (SE) was 100% for TF01–05 when TACC was less than 1.3 (Fig.7). Therefore, the optimal TACC value was 1.3 for detection threshold design because it produced 100% sensitivity and a minimum false alarm rate (FAR) at the meantime. Fig. 8 shows the false alarm rates for TF01–05 when the scale factor (TEMG) of EMG sub-detectors changes. The sensitivity was not shown because the detection sensitivity was 100% when theTEMG was in the range of 1 to 1.8. The false alarm rate was reduced to 0% when the TEMG was 1.8 for all five TF subjects. Therefore, the optimal threshold was chosen when TEMG was 1.8. The optimal TACC and TEMG value were used for the following evaluation of detection performance.
Fig. 7.
Influence of hypothesis testing threshold (represented as the value of scale factor TACC) on sensitivity and false alarm rates derived from the acceleration-based detector. Results were derived from data collected from 5 TF amputees (TF01–TF05) when they walked on a treadmill.
Fig. 8.
Influence of hypothesis testing threshold (represented as the value of scale factor TEMG) on the false alarm rates (FAR) derived from the level 2 detector with multiple EMG subdetectors. Results were derived from data collected from 5 TF amputees (TF01–TF05) when they walked on a treadmill.
2) Performance of Stumble Detection and Classification
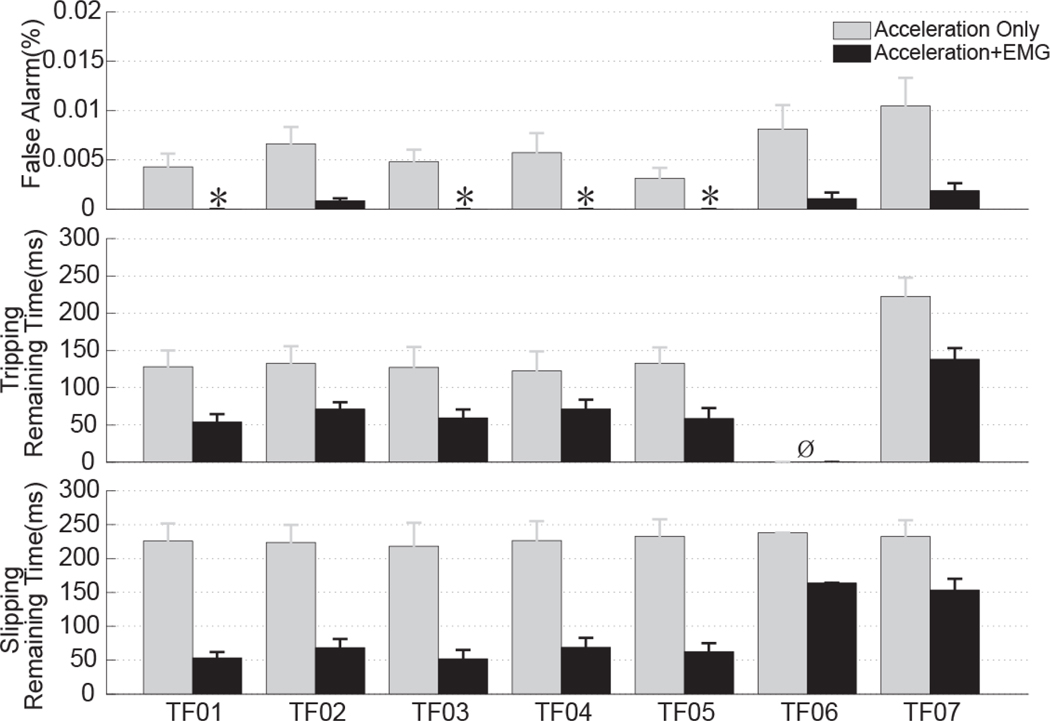
Fig. 9 shows the performance of designed stumble detectors. The SE and CA derived from two designs were not shown because they were 100%, which means the tested stumbles were correctly detected and classified for all the subjects. For the tests on the treadmill (TF01–TF05), when using both the foot acceleration and the EMG signals (black bars in Fig. 9), the stumble detector produced 0%–0.0009% FAR (i.e. from no false stumble detection to one false decision in 18.5 minutes), which was significantly lower than 0.0035%–0.0085% FAR derived from the detector based on the acceleration only (gray bars in Fig. 9); however, the remaining time for stumble recovery based on multiple data sources was 70–180 ms shorter than that derived from the detector based on acceleration alone. The response of foot acceleration to slips was around 230ms before the critical timing, while the response to trips was 140ms before the CT. This difference in reaction time was because the perturbation simulating slips was directly applied to the prosthetic foot, while the perturbation simulating trips was applied to the unimpaired foot on the treadmill. For the tests on the obstacle course (TF06-TF07), the results again showed that integrating the detection decisions from both data sources significantly reduced the FAR but sacrificed remaining time of stumble recovery by approximate 80ms. Compared to the results derived when the subjects walked on the treadmill, the results derived when the subjects walked on an obstacle course demonstrated (1) high false alarm rate, (2) early detection of trips for both detector designs, and (3) early stumble detection when both EMG signals and foot acceleration were used.
Fig. 9.
Comparison of the performance of the first design of the detector (based on the foot acceleration only) and the second design (based on both acceleration and EMG signals) for transfemoral amputees (TF01–TF07). *denotes FAR equals 0. Ø means no stumbling was captured.
D. Discussion
Acceleration of prosthetic foot was sufficient to detect the stumbles captured in this study with fast time response. If combined with the identified gait phase detected based on vertical GRF and knee angle, the foot acceleration can be also used to accurately classify trips in the early swing, trips in the last swing, and slips at the initial heel contact. However, the foot-acceleration-based stumble detector produced high false alarm rate, which might challenge its real application. For example, the worst FAR of acceleration-based detector in this study was ~0.01% for TF07 (Fig. 9). Since the decision was made every 10ms, that means every 1.6 minutes there may be one false detection decision. If such false decisions directly trigger the stumble reaction in prostheses, the designed stumble detection system will actually disturb the normal walking instead of improving the walking safety of leg amputees. The high false alarm rate partly resulted from the fact that the detector was formulated as an outlier detection task. The benefit of such a design is that the initial calibration of detection system (i.e. the procedure to determine the hypothesis testing threshold in (2)) is independent from the data collected during stumbling. That is to say, to find the detection thresholds, only the data collected from normal walking are needed, which makes the calibration procedure simple and practical. The disadvantage of outlier-based detection is that it produced high FAR because the outliers of foot acceleration may be elicited by situations other than balance perturbations. For example, large decelerations of prosthetic foot were observed during the weight acceptance when TF amputees stepped over an obstacle, which caused false detection of stumbles.
There are several solutions to reduce the false alarm rate. In this study, we presented one way to decrease FAR of acceleration-based stumble detector by fusing a secondary detection decision from another data source (i.e. EMG signals) that reliably responds to stumbles. This is because simultaneously detecting outliers from multiple data sources sensitive to stumbles increases the possibility that normal gait has been interrupted by balance perturbations. The stumble detector based on both acceleration and EMG signals produced no false decision for the data collected from TF01, TF03, TF04, and TF05 and FARs equivalent to one false decision in every 18.5, 12.8, and 8.3 minutes’ walking for TF02, TF06, and TF07, respectively. The FARs observed in the latter three subjects may still challenge the practical value of designed stumble detectors; additional methods might be considered to further decrease the FAR. One solution is to include data from different ambulation activities beyond level-ground walking when modeling normal gait (H0) for the stumble detector. In addition, other outlier detection algorithms and additional mechanical measurements from the prosthesis, which present quick passive responses to stumbles, can be investigated to further minimize the false detection rate.
Although the stumble detector based on both EMG signals and acceleration reduced the false alarm rate, it sacrificed the remaining time of stumble recovery by 70–150 ms, compared to the detector based on acceleration only. This is because the neural reactions observed were slower than the passive mechanical responses. In addition, the EMG response of amputees is slower than that in healthy subjects, partly due to the peripheral sensory loss. It would be interesting to investigate whether or not the artificial sensory feedback can quicken the neural response in leg amputees and detection of stumbles. For both the tripping and slipping trials, the delayed response was only around 50ms before the critical timing of falling defined based on a simple inverted pendulum model. Whether or not this response is fast enough to activate protective responses in prostheses and arrest a potential fall is unclear. In addition, we utilized a simple mechanical model to define the critical timing, which may not be accurate to quantify the human walking balance. For instance, when computing the COM, we ignored the function of arms. Actually, the upper limbs have been reported to play an important role for maintaining the balance during stumbling [33]. Therefore, further studies might be necessary to (1) accurately define the required response time for stumble detection based on other complex mechanical models, such as the feedback model [34] or the neurobiological model [35], or parameters for quantifying the walking balance, such as whole-body angular momentum [36], and (2) quantify the duration necessary for the prosthesis to build joint torque and react to stumbles.
Another problem in using the acceleration of prosthetic foot as the primary source for stumble detection is that the detector may miss the stumbles when the passive mechanical changes are not presented on the prosthetic side of legs. In this study only three types of perturbations were studied and were believed to be the often occurred perturbations for stumbling in leg amputees. These types of perturbations led to obvious and quick passive mechanical responses in foot acceleration. However, in the real situation, there are possibilities that perturbations are applied to the unimpaired leg. For instance, the sound leg may be tripped by an obstacle. In this case, fast passive foot deceleration will not be observed at the amputated side; therefore, the design based on the acceleration of prosthetic foot may miss the detection of this stumble or misclassify the stumble type. One possible solution is place an accelerometer on unimpaired foot, which, however, requires users to wear an additional sensor on the unimpaired leg and increases the system complexity. Interestingly, the perturbations induced by the treadmill applied on the unimpaired leg also clearly elicited the reactions in muscles of amputated leg; the remaining time remaining of stumble recovery was ~160ms, similar with that observed in the trials when the perturbations were applied to the amputated side, which implies that the observed protective neural response may be elicited by the long-loop reflex. Therefore, if the EMG responses are sufficiently fast for prostheses to react, the EMG signals may be the potential data sources for stumble detection when perturbations happen on the unimpaired leg. Although relying on the EMG signals only may be inadequate for identifying the stumble type (trip or slip), it can at least activate the artificial leg to execute a certain action (e.g. increasing the knee joint impedance during the stance phase) to stabilize the user's balance. In addition, it is interesting to study the minimum number of EMG signals required for maintaining the detection accuracy and reaction time.
There are several limitations in using the treadmill to simulation trips and slips for stumble detection design. First, in simulation of tripping, the perturbation was applied to the stance leg instead of the swing leg. Therefore, although the passive reaction of acceleration of prosthetic foot was observed when simulated trips happened in the swing of unimpaired leg, this reaction may not be presented in the real situation. Secondly, the EMG responses to stumbles observed in the tests on the treadmill were 60ms slower than the responses in the tests on the obstacle course. This observation may imply that the applied sudden treadmill accelerations/decelerations are not strong enough to simulate the perturbations in real world. Thirdly, during the normal walking, the speed of the treadmill was well controlled, and the recorded normal walking data were relatively consistent. Therefore, much lower FAR was observed than the FAR derived from the tests conducted on the obstacle course. The performance of designed stumble detection should be evaluated on TF amputees in the future when different magnitudes of treadmill perturbations are simulated. Additionally, an improved experimental design should be considered to elicit more realistic balance perturbations.
In the presented study we investigated potential data sources and designs for accurate and prompt stumble detection that not only detects stumbling events but classifies trips and slips happened in different gait phases. The results can advance the further design of a stumble detection system that can be integrated into self-contained, powered artificial legs, activate the protective control of prostheses for stumble recovery, and therefore, improve the safety of patients with lower limb amputations during ambulation. For example, when a trip of prosthetic leg in early swing is identified, and the prosthetic leg is currently swinging, the prosthesis control can further flex the knee joint to clear the prosthetic foot from the tripping object (i.e. elevating strategy). Obviously, this stumble recovery involves the posture control of amputees (e.g. hip flexion) and prosthesis reaction. Hence, investigating the stumble recovery strategies of leg amputees, designing recovery strategies for prosthesis control, and developing effective therapeutic training are demanded in the future to further reduce the risk of falling of lower limb amputees.
IV. Conclusion
This study demonstrated the investigation towards design of a stumble detection system for powered artificial legs, which might permit the active reaction of prosthetics for stumble recovery and, therefore, reduce the risk of falling in leg amputees. We first studied and selected potential stumble detection data sources that react reliably and quickly to stumbles and can be measured from a prosthesis. The results demonstrated the acceleration of prosthetic foot was most responsive, and EMG signals from residual limb reacted significantly and consistently regardless the type of the perturbations. Then, two approaches based on these two data sources were used for identifying stumbles. The results showed that foot acceleration was sufficient to detect all the stumbling events applied to the amputated side accurately and responsively. Fusing EMG signals into the foot-acceleration-based detection significantly reduced the detection false alarm, but sacrificed the remaining time of stumble recovery. The results of this study can guide the further optimization of stumble detection design for power prosthetic legs.
ACKNOWLEDGEMENTS
The authors thank Todd Kuiken, M.D., Ph.D., at the Rehabilitation Institute of Chicago for his suggestions, Andrew Burke and Fabian Sierra at the University of Rhode Island for their assistant in this project, and Aimee Schultz for editing the manuscript.
This work was partly supported by DoD/TATRC #W81XWH-09-2-0020, NIH#5R21HD064968-02, and NSF#0931820.
Contributor Information
Fan Zhang, Department of Electrical, Computer, and Biomedical Engineering, University of Rhode Island, Kingston, RI, 02881, USA.
Susan E. D’Andrea, Center for Restorative and Regenerative Medicine, the Providence VA Medical Center, Providence, RI, USA, 02906, She is also with the Department of Orthopaedics, Brown University, Alpert Medical School, Providence, RI, USA, 02903
Michael J. Nunnery, Nunnery Orthotics and Prosthetics Technologies, LLC, North Kingstown, RI, USA, 02852
Steven M. Kay, Department of Electrical, Computer, and Biomedical Engineering, University of Rhode Island, Kingston, RI, 02881, USA.
He Huang, Department of Electrical, Computer, and Biomedical Engineering, University of Rhode Island, Kingston, RI, 02881, USA.
REFERENCES
- 1.Miller WC, Speechley M, Deathe B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch Phys Med Rehabil. 2001;vol. 82:1031–1037. doi: 10.1053/apmr.2001.24295. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni J, Wright S, Toole C, Morris J, Hirons R. Falls in patients with lower limb amputations: prevalence and contributing factors. Physiotherapy. 1996;vol. 82:130–136. [Google Scholar]
- 3.Lusardi MM, Nielsen CC. Orthotics and Prosthetics in Rehabilitation. Boston: Butterworth-Heinemann Publications; 2000. [Google Scholar]
- 4.Campbell AJ, Reinken J, Allan BC, Martinez GS. Falls in old age: a study of frequency and related clinical factors. Age Ageing. 1981;vol. 10:264–270. doi: 10.1093/ageing/10.4.264. [DOI] [PubMed] [Google Scholar]
- 5.I. Otto Bock Orthopedic Industry. Manual for the 3c100 Otto Bock C-LEG. Duderstadt, Germany: 1998. [Google Scholar]
- 6.Herr H, Wilkenfeld A. User-adaptive control of a magnetorheological prosthetic knee. Industrial Robot: An International Journal. 2003;vol. 30:42–55. [Google Scholar]
- 7.Yang J, Jin D, Ji L, Wang R, Zhang J, Fang X, Zhou D, Wu M. The reaction strategy of lower extremity muscles when slips occur to individuals with trans-femoral amputation. J Electromyogr Kinesiol. 2007;vol. 17:228–240. doi: 10.1016/j.jelekin.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Sup F, Varol HA, Mitchell J, Withrow TJ, Goldfarb M. Preliminary Evaluations of a Self-Contained Anthropomorphic Transfemoral Prosthesis. IEEE ASME Trans Mechatron. 2009;vol. 14:667–676. doi: 10.1109/TMECH.2009.2032688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Villalpando EC, Herr H. Agonist-antagonist active knee prosthesis: a preliminary study in level-ground walking. J Rehabil Res Dev. 2009;vol. 46:361–373. [PubMed] [Google Scholar]
- 10.Lawson BE, Atakan Varol H, Sup F, Goldfarb M. Stumble detection and classification for an intelligent transfemoral prosthesis; Conf Proc IEEE Eng Med Biol Soc; 2010. pp. 511–514. [DOI] [PubMed] [Google Scholar]
- 11.Dietz V, Quintern J, Berger W. Corrective reactions to stumbling in man: functional significance of spinal and transcortical reflexes. Neurosci Lett. 1984;vol. 44:131–135. doi: 10.1016/0304-3940(84)90070-3. [DOI] [PubMed] [Google Scholar]
- 12.Eng JJ, Winter DA, Patla AE. Intralimb dynamics simplify reactive control strategies during locomotion. Journal of Biomechanics. 1997;vol. 30:581–588. doi: 10.1016/s0021-9290(97)84507-1. [DOI] [PubMed] [Google Scholar]
- 13.Schillings AM, van Wezel BM, Mulder T, Duysens J. Muscular responses and movement strategies during stumbling over obstacles. J Neurophysiol. 2000;vol. 83:2093–2102. doi: 10.1152/jn.2000.83.4.2093. [DOI] [PubMed] [Google Scholar]
- 14.Forner Cordero A, Koopman HF, van der Helm FC. Multiple-step strategies to recover from stumbling perturbations. Gait Posture. 2003;vol. 18:47–59. doi: 10.1016/s0966-6362(02)00160-1. [DOI] [PubMed] [Google Scholar]
- 15.Vilensky JA, Cook JA, Cooper JL. Stumbling corrective responses in healthy human subjects to rapid reversal of treadmill direction. J Electromyogr Kinesiol. 1999;vol. 9:161–171. doi: 10.1016/s1050-6411(98)00027-3. [DOI] [PubMed] [Google Scholar]
- 16.Tang PF, Woollacott MH, Chong RK. Control of reactive balance adjustments in perturbed human walking: roles of proximal and distal postural muscle activity. Experimental Brain Research. 1998;vol. 119:141–152. doi: 10.1007/s002210050327. [DOI] [PubMed] [Google Scholar]
- 17.Chambers AJ, Cham R. Slip-related muscle activation patterns in the stance leg during walking. Gait Posture. 2007;vol. 25:565–572. doi: 10.1016/j.gaitpost.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Grabiner MD, Koh TJ, Lundin TM, Jahnigen DW. Kinematics of Recovery from a Stumble. Journals of Gerontology. 1993;vol. 48:M97–M102. doi: 10.1093/geronj/48.3.m97. [DOI] [PubMed] [Google Scholar]
- 19.Berger W, Dietz V, Quintern J. Corrective reactions to stumbling in man: neuronal co-ordination of bilateral leg muscle activity during gait. J Physiol. 1984;vol. 357:109–125. doi: 10.1113/jphysiol.1984.sp015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pijnappels M, Bobbert MF, van Dieen JH. How early reactions in the support limb contribute to balance recovery after tripping. Journal of Biomechanics. 2005;vol. 38:627–634. doi: 10.1016/j.jbiomech.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Troy KL, Grabiner MD. Recovery responses to surrogate slipping tasks differ from responses to actual slips. Gait Posture. 2006;vol. 24:441–447. doi: 10.1016/j.gaitpost.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Lockhart TE, Spaulding JM, Park SH. Age-related slip avoidance strategy while walking over a known slippery floor surface. Gait Posture. 2007;vol. 26:142–149. doi: 10.1016/j.gaitpost.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owings TM, Pavol MJ, Grabiner MD. Mechanisms of failed recovery following postural perturbations on a motorized treadmill mimic those associated with an actual forward trip. Clin Biomech (Bristol, Avon) 2001;vol. 16:813–819. doi: 10.1016/s0268-0033(01)00077-8. [DOI] [PubMed] [Google Scholar]
- 24.van den Bogert AJ, Pavol MJ, Grabiner MD. Response time is more important than walking speed for the ability of older adults to avoid a fall after a trip. Journal of Biomechanics. 2002;vol. 35:199–205. doi: 10.1016/s0021-9290(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Chou LS. Detection of gait instability using the center of mass and center of pressure inclination angles. Arch Phys Med Rehabil. 2006;vol. 87:569–575. doi: 10.1016/j.apmr.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Iqbal K, Pai YC. Predicted region of stability for balance recovery: motion at the knee joint can improve termination of forward movement. Journal of Biomechanics. 2000;vol. 33:1619–1627. doi: 10.1016/s0021-9290(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 27.Detrembleur C, Dierick F, Stoquart G, Chantraine F, Lejeune T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait & Posture. 2003;vol. 18:47–55. doi: 10.1016/s0966-6362(02)00193-5. [DOI] [PubMed] [Google Scholar]
- 28.Hanavan EP., Jr A Mathematical Model of the Human Body. Amrl-Tr-64-102. AMRL TR. 1964:1–149. [PubMed] [Google Scholar]
- 29.Clancy EA, Bouchard S, Rancourt D. Estimation and application of EMG amplitude during dynamic contractions. IEEE Eng Med Biol Mag. 2001;vol. 20:47–54. doi: 10.1109/51.982275. [DOI] [PubMed] [Google Scholar]
- 30.Kay SM. Fundamentals of statistical signal processing. Englewood Cliffs, N.J.: PTR Prentice-Hall; 1993. [Google Scholar]
- 31.Filzmoser P, Garrett RG, Reimann C. Multivariate outlier detection in exploration geochemistry. Computers & Geosciences. 2005;vol. 31:579–587. [Google Scholar]
- 32.Gath EG, Hayes K. Bounds for the largest Mahalanobis distance. Linear Algebra and Its Applications. 2006;vol. 419:93–106. [Google Scholar]
- 33.Marigold DS, Bethune AJ, Patla AE. Role of the unperturbed limb and arms in the reactive recovery response to an unexpected slip during locomotion. J Neurophysiol. 2003;vol. 89:1727–1737. doi: 10.1152/jn.00683.2002. [DOI] [PubMed] [Google Scholar]
- 34.Welch TD, Ting LH. A feedback model explains the differential scaling of human postural responses to perturbation acceleration and velocity. J Neurophysiol. 2009;vol. 101:3294–3309. doi: 10.1152/jn.90775.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jo S. A neurobiological model of the recovery strategies from perturbed walking. Biosystems. 2007;vol. 90:750–768. doi: 10.1016/j.biosystems.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Neptune RR, McGowan CP. Muscle contributions to whole-body sagittal plane angular momentum during walking. J Biomech. 2011;vol. 44:6–12. doi: 10.1016/j.jbiomech.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]