Abstract
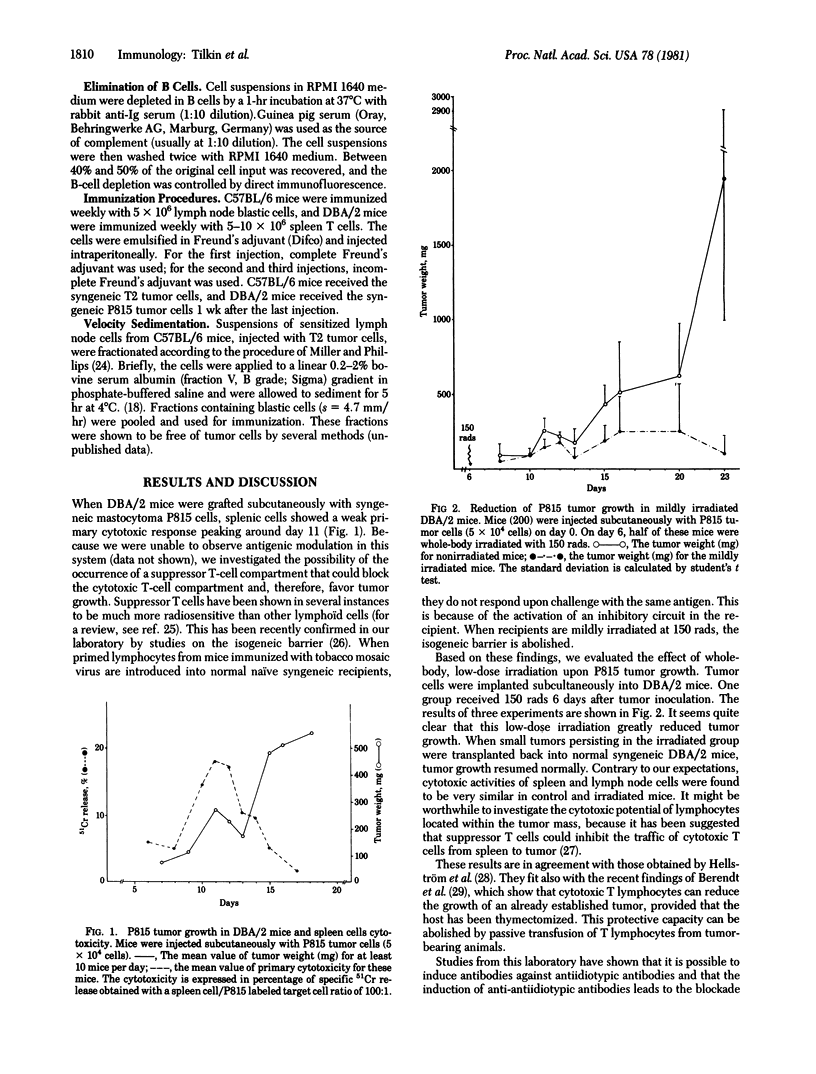
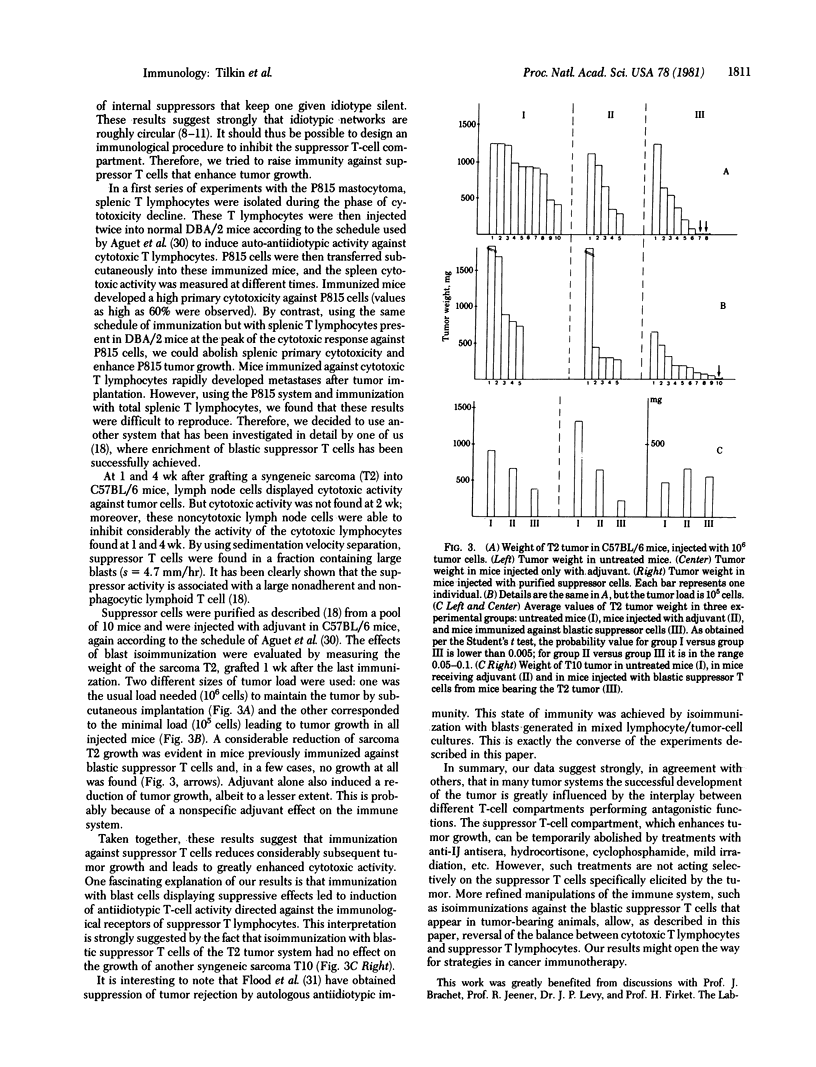
Suppressor T cells have been shown to be much more radiosensitive than other lymphoïd cells, and we have tried to reduce tumor growth by low-dose irradiation. Syngeneic DBA/2 mice received whole-body irradiation (150 rads; 1 rad = 0.01 J/kg) 6 days after P815 tumor inoculation. Tumor growth is significantly reduced in mildly irradiated mice. We also attempted to reduce syngeneic tumor growth by raising immunity against suppressor T cells in two different systems. DBA/2 mice were immunized against splenic T cells collected after disappearance of cytotoxicity and then injected with P815 tumor cells. These mice develop a very high primary cytotoxicity against P815 cells. C57BL/6 mice were immunized against blastic suppressor T cells, before injection of T2 tumor cells. Some of these mice reject the tumor and other develop smaller tumors than control mice. These results could be explained by the induction of antiidiotypic activity directed against the immunological receptors of suppressor T lymphocytes, because immunization with blastic suppressor T cells from mice bearing the T2 tumor does not modify the growth of another tumor, T10.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Andersson L. C., Andersson R., Wight E., Binz H., Wigzell H. Induction of specific immune unresponsiveness with purified mixed leukocyte culture-activated T lymphoblasts as autoimmunogen. II. An analysis of the effects measured at the cellular and serological levels. J Exp Med. 1978 Jan 1;147(1):50–61. doi: 10.1084/jem.147.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt M. J., North R. J. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med. 1980 Jan 1;151(1):69–80. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Cazenave P. A. Idiotypic-anti-idiotypic regulation of antibody synthesis in rabbits. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5122–5125. doi: 10.1073/pnas.74.11.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapdelaine J. M., Plata F., Lilly F. Tumors induced by murine sarcoma virus contain precursor cells capable of generating tumor-specific cytolytic T lymphocytes. J Exp Med. 1979 Jun 1;149(6):1531–1536. doi: 10.1084/jem.149.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B., Jacob H., Gaillard J. A., Jacob F. Antiinflammatory effects of murine malignant cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4052–4056. doi: 10.1073/pnas.71.10.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood P. M., Kripke M. L., Rowley D. A., Schreiber H. Suppression of tumor rejection by autologous anti-idiotypic immunity. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2209–2213. doi: 10.1073/pnas.77.4.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Matsuzawa T., Nakagawa K., Tada T. Cellular interaction between cytotoxic and suppressor T cells against syngeneic tumors in the mouse. Cell Immunol. 1978 Jul;38(2):378–387. doi: 10.1016/0008-8749(78)90068-0. [DOI] [PubMed] [Google Scholar]
- Glaser M. Regulation of specific cell-mediated cytotoxic response against SV40-induced tumor associated antigens by depletion of suppressor T cells with cyclophosphamide in mice. J Exp Med. 1979 Mar 1;149(3):774–779. doi: 10.1084/jem.149.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I., Kant J. A., Tamerius J. D. Regression and inhibition of sarcoma growth by interference with a radiosensitive T-cell population. J Exp Med. 1978 Sep 1;148(3):799–804. doi: 10.1084/jem.148.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlyn M., Steplewski Z., Herlyn D., Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1438–1442. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Edidin M. The biological function of the major histocompatibility complex: hypotheses. Contemp Top Immunobiol. 1980;9:231–253. doi: 10.1007/978-1-4615-9131-3_9. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Perry L. L., Benacerraf B., McCluskey R. T., Greene M. I. Enhanced syngeneic tumor destruction by in vivo inhibition of suppressor T cells using anti-I-J alloantiserum. Am J Pathol. 1978 Aug;92(2):491–506. [PMC free article] [PubMed] [Google Scholar]
- Pfreundschuh M., Shiku H., Takahashi T., Ueda R., Ransohoff J., Oettgen H. F., Old L. J. Serological analysis of cell surface antigens of malignant human brain tumors. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5122–5126. doi: 10.1073/pnas.75.10.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata F., MacDonald H. R., Shain B. Suppressor T cells regulate the cytolytic T lymphocyte response to syngeneic tumors induced by murine sarcoma virus (MSV) in the mouse. J Immunol. 1979 Aug;123(2):852–860. [PubMed] [Google Scholar]
- Russel J. H., Hale A. H., Inbar D., Eisen H. N. Loss of reactivity of a BALB/c myeoloma tumor with allogeneic and syngeneic cytotoxic T lymphocytes. Eur J Immunol. 1978 Sep;8(9):640–645. doi: 10.1002/eji.1830080907. [DOI] [PubMed] [Google Scholar]
- Schaaf-Lafontaine N. Separation of lymphoid cells with a suppressor effect on the activity of cytotoxic cells in vitro during the growth of a syngeneic mouse tumour. Int J Cancer. 1978 Mar 15;21(3):329–333. doi: 10.1002/ijc.2910210313. [DOI] [PubMed] [Google Scholar]
- Schechter B., Feldman M. Suppressor cells prevent host resistance to tumor growth. Naturwissenschaften. 1979 Mar;66(3):140–146. doi: 10.1007/BF00368707. [DOI] [PubMed] [Google Scholar]
- Takei F., Levy J. G., Kilburn D. G. Characterization of suppressor cells in mice bearing syngeneic mastocytoma. J Immunol. 1977 Feb;118(2):412–417. [PubMed] [Google Scholar]
- Ueda R., Shiku H., Pfreundschuh M., Takahashi T., Li L. T., Whitmore W. F., Oettgen H. F., Old L. J. Cell surface antigens of human renal cancer defined by autologous typing. J Exp Med. 1979 Sep 19;150(3):564–579. doi: 10.1084/jem.150.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain J., Collignon C., Franssen J. D., Mariamé B., Léo O., Urbain-Vansanten G., Van de Walle P., Wikler M., Wuilmart C. Idiotypic networks and self-recognition in the immune system. Ann Immunol (Paris) 1979 Mar-Apr;130(2):281–291. [PubMed] [Google Scholar]
- Urbain J., Wikler M., Franssen J. D., Collignon C. Idiotypic regulation of the immune system by the induction of antibodies against anti-idiotypic antibodies. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5126–5130. doi: 10.1073/pnas.74.11.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Lafferty K. J. A high frequency of cytotoxic precursor cells for a syngeneic tumour. Scand J Immunol. 1979;10(4):349–352. doi: 10.1111/j.1365-3083.1979.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Winkler M., Franssen J. D., Collignon C., Leo O., Mariamé B., van de Walle P., de Groote D., Urbain J. Idiotypic regulation of the immune system. Common idiotypic specificities between idiotypes and antibodies raised against anti-idiotypic antibodies in rabbits. J Exp Med. 1979 Jul 1;150(1):184–195. doi: 10.1084/jem.150.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Bach F. H. Continuous culture of T cells cytotoxic for autologous human leukaemia cells. Nature. 1979 Aug 23;280(5724):685–688. doi: 10.1038/280685a0. [DOI] [PubMed] [Google Scholar]



