Abstract
Bifunctional sensor transmitter modules of two-component systems exert both positive and negative control on the receiver domain of the cognate response regulator. In negative control, the transmitter module accelerates the rate of phospho-receiver dephosphorylation. This transmitter phosphatase reaction serves the important physiological functions of resetting response regulator phosphorylation level and suppressing cross talk. Although the biochemical reactions underlying positive control are reasonably well-understood, the mechanism for transmitter phosphatase activity has been unknown. A recent hypothesis is that the transmitter phosphatase reaction is catalyzed by a conserved Gln, Asn or Thr residue, via a hydrogen bond between the amide or hydroxyl group and the nucleophilic water molecule in acyl-phosphate hydrolysis. This hypothetical mechanism closely resembles the established mechanisms of auxiliary phosphatases such as CheZ and CheX, and may be widely conserved in two-component signal transduction. In addition to the proposed catalytic residues, transmitter phosphatase activity also requires the correct transmitter conformation and appropriate interactions with the receiver. Evidence suggests that the phosphatase-competent and autokinase-competent states are mutually exclusive, and the corresponding negative and positive activities are likely to be reciprocally regulated through dynamic control of transmitter conformations.
Introduction
Two-component signal transduction is a common signaling mechanism in bacteria. Bacterial species, on average, contain about two dozen two-component systems (Barakat et al., 2011), which exhibit versatile functions but remarkably conserved architectures. These systems regulate most aspects of bacterial physiology, including chemotaxis, nutrient uptake, stress response, central metabolism, and virulence.
Signal transduction in most two-component systems occurs between the transmitter module of a sensor protein and the receiver domain of the cognate response regulator (Stock et al., 1989, Parkinson & Kofoid, 1992). The sensor transmitter module is organized into an amino-terminal DHp (Dimerization and Histidine phosphotransfer) domain and a carboxyl-terminal CA (Catalytic and ATP-binding) domain. The DHp domain is a dimeric four-helix bundle, formed by two helical hairpin monomers (Gao & Stock, 2009). The DHp amino-terminal helix α1 features the characteristic H box sequence motif, where the phospho-accepting His resides. The monomeric CA domain adopts an α/β sandwich fold related to the Bergerat nucleotide-binding fold of the GHL (Gyrase, Hsp90, MutL) ATPase family (Gao & Stock, 2009). CA domain sequences exhibit conserved motifs, residues from which form an ATP-binding cavity and a flexible ATP lid.
A number of distinct transmitter sequence subfamilies have been distinguished according to conserved residues in both the DHp and CA domains (Grebe & Stock, 1999). The Pfam database (Finn et al., 2010) aggregates several closely-related DHp domain sequence families into the large HisKA family (pfam00512). A smaller but widespread DHp domain sequence family is denoted as HisKA_3 (pfam07730).
Receiver domains of response regulators also are structurally conserved, formed by a five-stranded β sheet surrounded by five α helices (Bourret, 2010). The majority of response regulators control output through a carboxyl-terminal regulatory domain, the most common of which bind DNA. However, approximately 17% of bacterial response regulators only contain the receiver domain (Galperin, 2006).
The sensor transmitter module exerts both positive and negative control on the receiver domain. Positive control results from transmitter autokinase activity at an invariant His residue, followed by phosphotransfer to an invariant Asp residue on the receiver domain. Receiver phosphorylation induces conformational changes that alter output, including a relative rearrangement of the receiver and regulatory domains. Dimerization or oligomerization is also commonly observed in phosphorylated response regulators (Gao & Stock, 2009).
Negative control results from transmitter-mediated receiver domain dephosphorylation, termed transmitter phosphatase activity (Song et al., 2004). Phospho-receiver domains exhibit characteristic rates of autodephosphorylation, with half-lives ranging from seconds to hours at physiological temperatures (Bourret, 2010). In many two-component systems, however, the receiver dephosphorylation rate is substantially enhanced by the cognate sensor transmitter module. Therefore, the signal-adjusted balance between positive and negative control determines output response (West & Stock, 2001, Gao & Stock, 2009).
Although a considerable body of literature exists for positive regulation, comparatively less is available on negative regulation in two-component signaling. In particular, the mechanism of transmitter phosphatase activity has been a long standing question. This missing information is important for understanding many aspects of bacterial physiology. For example, negative control is critical in systems regulating virulence or antibiotic resistance, such as PhoQ-PhoP (Chamnongpol et al., 2003), VanS-VanR (Depardieu et al., 2003), and QseC-QseB (Kostakioti et al., 2009). This review aims to highlight recent advances in the study of sensor negative function, including its physiological role and mechanism. A comparison of negative control among different sensors suggests that a common mechanism for transmitter phosphatase activity is widespread in two-component signal transduction.
Most transmitters are bifunctional
As early work on two-component systems demonstrated that phosphorylation is the underlying mechanism of signal transmission, it also was discovered that the sensor can exhibit phosphatase activity on the response regulator (Ninfa & Magasanik, 1986, Keener & Kustu, 1988, Igo et al., 1989). Since then, it has been recognized that most of the sensors studied are bifunctional, acting to both phosphorylate and dephosphorylate the cognate response regulator (Gao & Stock, 2009).
Exceptions to this are the monofunctional transmitters in chemotaxis and in multistep phosphorelays. In chemotaxis, receiver phosphorylation is catalyzed by CheA, whereas the phospho-receiver is dephosphorylated by auxiliary phosphatases such as CheZ or CheX (Silversmith, 2010). The Bacillus sporulation phosphorelay similarly employs the Rap and Spo0E auxiliary phosphatases (Perego et al., 1996, Silversmith, 2010). Since these proteins lack positive function, auxiliary phosphatase activity is distinct from the transmitter phosphatase activity exhibited by bifunctional sensors. In other multistep phosphorelays, response regulator dephosphorylation occurs through reverse signal decay as described below.
Physiological functions of transmitter phosphatase activity
Transmitter phosphatase activity counterbalances the transmitter autokinase plus phosphotransfer functions in two-component signal transduction. The ratio of these two sensor functions determines the response regulator equilibrium between phosphorylated and dephosphorylated forms, which in turn regulates output signal (West & Stock, 2001, Gao & Stock, 2009). Thus, mutants with phosphatase-defective sensors exhibit aberrant physiology. For example, NtrB (NRII) transmitter phosphatase mutants confer growth defects in minimal media (Pioszak & Ninfa, 2003a), and EnvZ transmitter phosphatase mutants exhibit a constitutive high-osmolarity phenotype (Russo & Silhavy, 1993). Systems at steady state are assumed to exhibit equal rates of positive and negative control (Russo & Silhavy, 1993). A related function presumably is to reset the baseline signaling state rapidly upon stimulus depletion (Gao & Stock, 2009).
A second function is to suppress cross talk (Fig. 1), which results from unwanted phosphorylation of the receiver by sources other than the cognate transmitter module (Stock et al., 1989, Wanner, 1992). Indeed, the absence of a bifunctional sensor is a prerequisite for in vivo cross talk and in vitro non-cognate phosphotransfer (Laub & Goulian, 2007, Siryaporn & Goulian, 2008, Groban et al., 2009). The bifunctional sensor thus counters inappropriate receiver phosphorylation by small phosphodonors and non-cognate sensors. The QseB response regulator, for example, is phosphorylated through cross talk in the absence of the bifunctional QseC sensor. Consequently, qseC null mutants exhibit pleiotropic metabolic phenotypes and are attenuated for virulence (Kostakioti et al., 2009, Hadjifrangiskou et al., 2011).
Figure 1. Cross talk suppression by transmitter phosphatase activity.
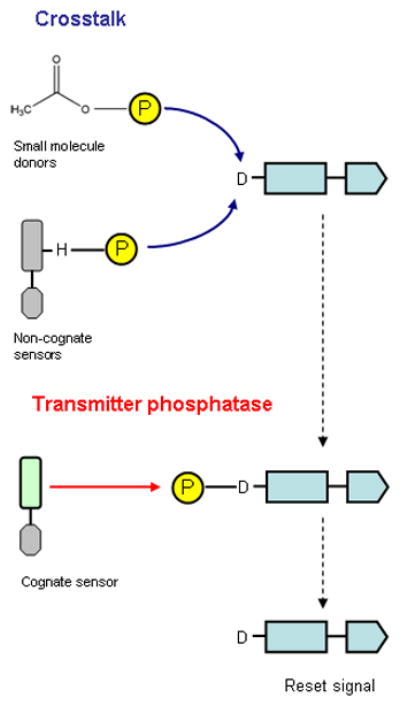
During cross talk, the receiver domain is phosphorylated by small molecule phosphodonors, illustrated here as acetyl phosphate, and by non-cognate sensors. The transmitter phosphatase function of the cognate sensor reduces cross talk noise by dephosphorylating phospho-receiver, therefore returning the receiver to the inactive state and resetting the signal.
Whereas the phosphotransfer reaction can occur in vivo between certain non-cognate sensors and response regulators, transmitter phosphatase activity appears to be specific for a cognate pair. Non-cognate transmitter phosphatase activity is negligible both in vivo and in vitro (Siryaporn & Goulian, 2008, Groban et al., 2009). However, laboratory evolution of the sensor CpxA for stronger cross talk with the non-cognate response regulator OmpR also transforms CpxA to being bifunctional on OmpR. In this case, combining CpxA missense substitutions that individually increase OmpR phosphorylation gives rise to CpxA transmitter phosphatase activity on phospho-OmpR. Additionally, the bifunctional, phosphatase-competent CpxA mutant interacts more strongly with OmpR than monofunctional mutants. These substitutions are in DHp domain residues known to determine interaction specificity. Therefore, transmitter phosphatase activity requires strong interaction between transmitter and receiver (Siryaporn et al., 2010).
Support for this notion comes from analysis of missense substitutions in the sensor NarX DHp domain. Certain of these decrease transmitter phosphatase activity toward the cognate response regulator NarL, and also eliminate phosphotransfer toward the cross-regulated response regulator NarP (Noriega et al., 2010). Therefore, these substitutions likely perturb transmitter-receiver interactions, which in turn diminish phospho-NarL transmitter phosphatase activity, while retaining substantial NarL phosphotransfer function.
Receiver domain phosphorylation and dephosphorylation
The receiver domain catalyzes its own phosphorylation, using the phosphorylated DHp domain as a high affinity substrate. The receiver active site is an acidic pocket formed by three Asp, one Lys, and one Thr or Ser residue. The phosphoryl group is covalently bound to the phospho-accepting Asp, and forms a salt bridge with the Lys and a hydrogen bond with the Thr or Ser (Stock et al., 2000, Bourret, 2010). The phosphotransfer reaction proceeds through an in-line nucleophilic attack by the phospho-accepting Asp carboxylate group on the phosphorous atom, which is thought to go through a bipyramidal, pentavalent transition state. A Mg2+ ion, required for this transition state, is coordinated by all three active site Asp residues (Stock et al., 2000, Bourret, 2010).
The phospho-receiver catalyzes acyl-phosphate hydrolysis in a mechanism similar to that of the phosphotransfer reaction (Fig. 2A). A nucleophilic water molecule is positioned in-line and attacks the phosphorous atom of the leaving phosphoryl group (Wolanin et al., 2003). In addition, receiver autophosphatase activity requires a Mg2+ ion (Lukat et al., 1990), as well as the conserved Lys residue of the active site. Whereas substitutions for this Lys have minimal effects on Mg2+ binding and the phosphotransfer reaction, they greatly diminish the rates of both autophosphatase and regulated phosphatase activities of phospho-receiver (Lukat et al., 1991, Pioszak & Ninfa, 2004).
Figure 2. Different phosphatase activities in bacterial two-component signaling.
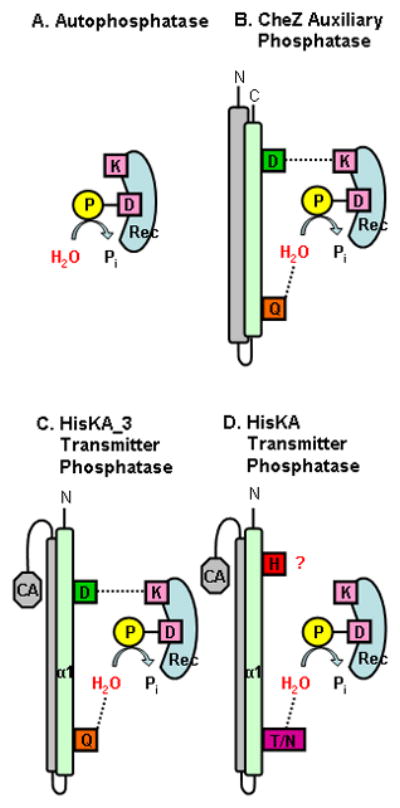
A. The phospho-receiver exhibits autophosphatase activity, during which the phosphoryl group is hydrolyzed by a nucleophilic water molecule. The invariant Lys residue participates directly in dephosporylation reactions.
B. Auxiliary phosphatases, illustrated here as CheZ, do not exhibit positive function (note the absence of a phospho-accepting His residue). The CheZ conserved Gln residue catalyzes the phosphatase reaction by aligning the nucleophilic water for phosphoryl group hydrolysis. CheZ also has a conserved Asp residue which forms a salt bridge with the receiver Lys residue.
C. The HisKA_3 transmitter phosphatase activity is hypothesized to follows a similar mechanism to auxiliary phosphatase, employing a catalytic Gln residue to coordinate water hydrolysis of the phosphoryl group and an Asp residue that interacts with the receiver Lys residue.
D. The HisKA transmitter phosphatase activity is hypothesized to employ a conserved Asn or Thr residue in an analogous mechanism to HisKA_3 transmitters. The role for the phospho-accepting His residue is uncertain.
Despite their conserved structures, different receiver domains exhibit widely variable autophosphatase rates (Bourret, 2010). This variability results in part from sequence determinants that dictate differential access of the nucleophilic water to the active site (Pazy et al., 2010).
Reverse signal decay in multistep phosphorelays is distinct from transmitter phosphatase
Phosphorelays transfer phosphoryl groups along four steps in series, in the order His(1)-Asp(1)-His(2)-Asp(2) (Fig. 3A) (Appleby et al., 1996, West & Stock, 2001). The His(1) residue lies within a conventional transmitter module, which catalyzes phosphotransfer to the Asp(1) residue in a downstream conventional receiver domain. The phosphoryl group then is passed to the His(2) residue in a His-containing phosphotransfer (HPt) domain, and finally to the Asp(2) residue in the receiver domain of the response regulator. The receiver(1) and HPt domains may be in separate polypeptides, as in the KinA-Spo0F-Spo0B-Spo0A phosphorelay for Bacillus sporulation, or may be part of a hybrid sensor. Some hybrid sensors incorporate only the receiver(1) domain, as in the Sln1-Ypd1-Skn7 and LuxN-LuxU-LuxO phosphorelays, whereas others contain both the receiver(1) and HPt domains, as in the BvgS-BvgA and ArcB-ArcA phosphorelays.
Figure 3. Phosphotransfer and reverse signal decay routes in phosphorelays.
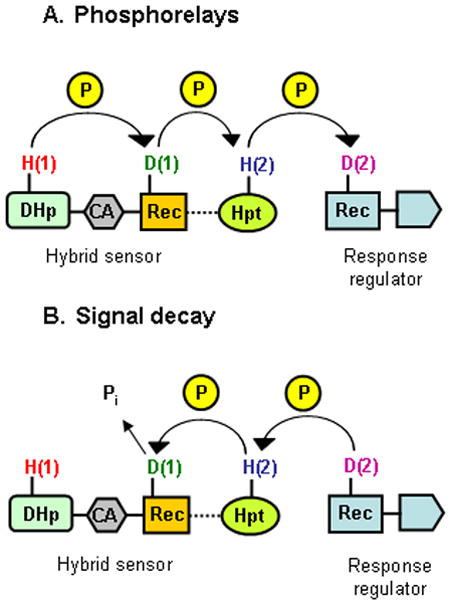
A. A generic phosphorelayis illustrated between a hybrid sensor and the receiver domain of its cognate response regulator. The phosphoryl group is transferred through the His(1) and Asp(1) residues on the sensor to the His(2) residue on the Hpt domain (which may be part of the sensor or may be separate), and subsequently to the Asp(2) residue on the response regulator receiver domain.
B. In reverse signal decay, the phosphoryl group is transferred through the Asp(2)-His(2)-Asp(1) residues, and is hydrolyzed at the Asp(1) residue. The His(1) residue on the transmitter module is not involved in this reaction.
Receiver(1) autodephosphorylation appears to underlie response regulator dephosphorylation in multistep phosphorelays. In this reverse signal decay, the phosphoryl group is transferred in reverse, in the order Asp(2)-His(2)-Asp(1), as demonstrated both in vivo and in vitro for BvgS-BvgA and ArcB-ArcA (Fig. 3B) (Georgellis et al., 1998, Peña-Sandoval et al., 2005, Uhl & Miller, 1996). Detailed kinetic analysis demonstrates that Sln1-Ypd1-Skn7 phosphotransfer is reversible between the receiver(1), HPt and receiver(2) domains (Janiak-Spens et al., 2005). By contrast, the transmitter domain is required neither for efficient dephosphorylation of the receiver(1) domain in vitro (Uhl & Miller, 1996, Ault et al., 2002), nor for negative regulation in vivo (Peña-Sandoval et al., 2005).
Thus, receiver(1) autodephosphorylation (Uhl & Miller, 1996, Ault et al., 2002) drives receiver(2) dephosphorylation by reverse signal decay through the HPt domain. This concept is supported by the function of the Rap phosphatases, which dephosphorylate Spo0F to drain phosphoryl groups, via Spo0B, from Spo0A (Perego et al., 1996). Therefore, negative regulation in phosphorelays reflects signal decay through the reverse phosphorelay, and is distinct from transmitter phosphatase activity.
Hypotheses for the mechanism of transmitter phosphatase activity
The mechanism for bifunctional sensor transmitter phosphatase activity has been elusive. It is established that this function resides in the DHp domain, because the isolated domain displays transmitter phosphatase activity both in vivo and in vitro (Jiang et al., 2000, Zhu et al., 2000, Carmany et al., 2003). An early hypothesis suggested reverse phosphotransfer to the phospho-accepting His residue, but this was later disproved. Catalytic involvement of the phospho-accepting His residue for hydrolysis also has been proposed. Finally, a recently-hypothesized mechanism, analogous to that for auxiliary phosphatases, is based on a conserved, catalytic amide or hydroxyl group near the phospho-accepting His residue (Huynh et al., 2010). A key element of this hypothesis is that, although auxiliary phosphatases and bifunctional sensors display different structures and interactions with their partner proteins, the phosphatase mechanism nevertheless is conserved (Fig. 2).
Reverse phosphotransfer does not explain transmitter phosphatase activity
Reverse phosphotransfer denotes the backward phosphorylation of the transmitter His residue by the phospho-receiver (Dutta & Inouye, 1996, Zhu et al., 2000). This proposed mechanism is not to be confused with the distinct Asp(1)-His(2) forward phosphotransfer and reverse signal decay described above for multistep phosphorelays. Instead, the reverse phosphotransfer hypothesis for transmitter phosphatase activity was prompted by analysis of an EnvZ mutant with the N347D missense substitution in the CA domain N box motif (Dutta & Inouye, 1996). This mutant lacks autokinase activity but retains strong transmitter phosphatase activity. Reverse phosphotransfer from phospho-OmpR to EnvZ(N347D) is observed only in the absence of Mg2+, and is inhibited by ADP. In the presence of Mg2+, reverse phosphotransfer is not observed, and inorganic phosphate is released from phospho-OmpR (Dutta & Inouye, 1996). Moreover, reverse phosphotransfer is not observed with wild-type EnvZ (Zhu et al., 2000), nor with CpxA (Raivio & Silhavy, 1997), PhoR (Carmany et al., 2003), or WalK (Gutu et al., 2010). Finally, at least partial transmitter phosphatase activity is retained by certain phospho-accepting His substitution mutants of EnvZ (Hsing & Silhavy, 1997, Skarphol et al., 1997), NtrB (H139N) (Atkinson & Ninfa, 1993) and PhoQ (H277A) (Chamnongpol et al., 2003). Together, these observations provide direct evidence against the reverse phosphotransfer mechanism.
Histidine hypothesis for transmitter phosphatase activity in HisKA sensors
Whereas the reverse phosphotransfer route is disproved, the phospho-accepting His residue nevertheless may be important for transmitter phosphatase function. In particular, Hsing & Silhavy (1997) suggested that the His residue acts as a general base to activate the nucleophilic water molecule. This role for the His residue recently was supported by analysis of the HK853-RR468 complex, which is thought to represent interaction of phospho-RR468 with the transmitter phosphatase conformation of HK853 (Casino et al., 2009). This structure contains a sulfate ion between the transmitter His-260 and the receiver Asp-53. One O atom from this sulfate ion is speculated to represent the nucleophilic water, coordinated by His-260 (Casino et al., 2009).
On the other hand, EnvZ, PhoQ and WalK mutants with Ala substituted for the phospho-accepting His residue retain at least partial transmitter phosphatase activity (Hsing & Silhavy, 1997, Chamnongpol et al., 2003, Gutu et al., 2010). Thus, His per se cannot be essential for activating the nucleophilic water. Perhaps the disparate effects of different substitutions (Hsing & Silhavy, 1997, Skarphol et al., 1997) result from structural perturbation. Alternatively, it is possible that the His residue plays an ancillary role in transmitter phosphatase activity.
Auxiliary phosphatase activity
A recent hypothesis, described below, is based on mechanisms for diverse auxiliary phosphatases, which employ a conserved Gln, Asn or Asp residue to orient the nucleophilic water and thereby stimulate the rate of acyl-phosphate hydrolysis (Fig. 2B) (Silversmith, 2010). The chemotaxis auxiliary phosphatases CheZ and CheX exhibit the conserved DxxxQ and ExxN motifs, respectively, and catalyze the phospho-CheY phosphatase reaction though essentially identical mechanisms. The conserved Gln and Asn residues provide an amide group to orient the nucleophilic water, whereas the Asp and Glu residues form a salt bridge with the receiver active site Lys residue (Zhao et al., 2002, Pazy et al., 2010). Similar to CheZ and CheX, the sporulation auxiliary phosphatase RapH inserts a catalytic Gln residue into the Spo0F active site, but apparently does not require a second, acidic residue for phosphatase activity (Parashar et al., 2011). On the other hand, the sporulation auxiliary phosphatase Spo0E exhibits a similar but reversed QELD motif, in which the roles of Asp and Gln residues are swapped: Asp appears to be catalytic, whereas Gln stabilizes interactions (Diaz et al., 2008). Thus, Spo0E likely coordinates the nucleophilic water molecule via a carboxyl group rather than an amide group. Overall, these auxiliary phosphatase mechanisms are reminiscent of ©-phosphate group hydrolysis by Ras-like GTPases. These α/β proteins also rely on a catalytic Gln or Asn residue, which is located on a conserved switch helix and acts in a similar planar transition state (Vetter & Wittinghofer, 2001, Li & Zhang, 2004). Therefore, an overall consensus mechanism is observed in different phosphatase families. Although occurring in different alignment geometries of the catalytic, attacking groups in the phospho-receiver active site, these phosphatase reactions are catalyzed via the coordination of the nucleophilic water by a conserved amide or carboxyl group.
Amide hypothesis for transmitter phosphatase activity in HisKA_3 sensors
Recent work identified a conserved DxxxQ motif on the DHp helix α1, immediately adjacent to the phospho-accepting His residue (Fig. 4A). The DxxxQ motif defines the active site for CheZ auxiliary phosphatase activity (Zhao et al., 2002), as summarized above. In the DesK X-ray structures, the invariant Asp and highly conserved Gln residues from this motif are separated by one helical turn on the DHp-receiver interaction surface (Albanesi et al., 2009). Analysis of NarX revealed that the Gln residue is essential for transmitter phosphatase activity both in vivo and in vitro (Huynh et al., 2010). Mutants in which the Gln residue is substituted with Ala, Glu or His retain autokinase and phosphotransfer activities, yet are severely defective for transmitter phosphatase activity. By contrast, the Asn substitution mutant retains substantial transmitter phosphatase activity, consistent with the notion that an amide group is important for catalysis.
Figure 4. HisKA_3 and HisKA DHp domains showing the catalytic residues. The monomeric DHp domains of DesK and HK853 are shown with the phospho-accepting His residue (red) and the conserved residues hypothesized to be involved in the transmitter phosphatase function.
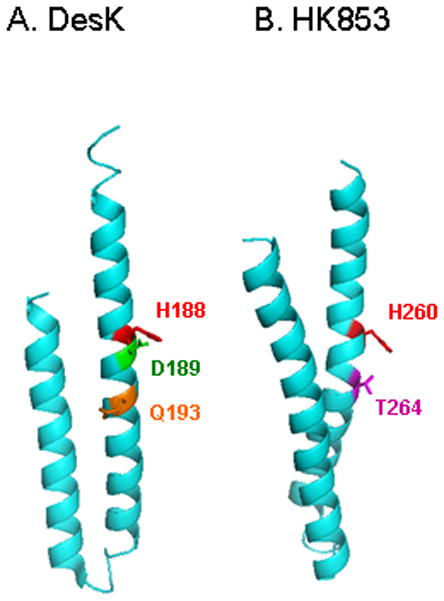
A. The DesK Asp (D189, green) and Gln (Q193, orange) correspond to the HisKA_3 DxxxQ motif. The structure is the phosphatase-competent H188Vb form (PDB accession 3EHH).
B. The HK853 Thr (T264, magenta) residue corresponds to the HisKA conserved Asn/Thr residue in the E/DxxN/T motif. The structure is from the HK853-RR468 cocrystal (PDB accession 3GL9).
Thus, by analogy with CheZ, it is hypothesized that the Gln residue catalyzes the phosphatase reaction by providing an amide group for the correct alignment of the nucleophilic water molecule in phosphoryl group hydrolysis, and the Asp plays an ancillary role by stabilizing the Lys residue of the receiver active site, perhaps via a hydrogen bond (Fig. 2C). However, the Asp residue is essential for autokinase activity (Huynh et al., 2010), and also participates in DHp-CA domain interactions (Trajtenberg et al., 2010).
Analysis suggests that the phospho-accepting His residue is irrelevant for the transmitter phosphatase activity of HisKA_3 family sensors. Strong activity is retained in most missense mutants examined, including DesK (H188V) (Albanesi et al., 2004), AbsA1 (H202A) (Sheeler et al., 2005), and NarX (H399Q) (Huynh et al., 2010). Moreover, the DesK X-ray structures, representing the HisKA_3 family, suggest that the phospho-accepting His residue lies within the DHp coiled-coil interior in the transmitter phosphatase conformation (Albanesi et al., 2009).
Currently, transmitter-receiver structural models exist only for the HisKA family (Casino et al., 2009, Yamada et al., 2009), and the DesK transmitter structures display distinct conformational differences in comparison to HisKA family structures (Stewart, 2010). Therefore, it presently is not possible to evaluate the HisKA_3 amide hypothesis by examining a cocrystal structure.
About 30% of HisKA_3 sequences have His in place of Gln in the DxxxQ motif, so the His side-chain must provide the nucleophilic water-orienting function in this proposed mechanism. However, the NarX Q404H mutant is severely defective for transmitter phosphatase activity (Huynh et al., 2010) although the AbsA1 sensor, which has the DxxxH sequence, displays strong transmitter phosphatase activity (Sheeler et al., 2005). Thus, future experiments with diverse HisKA_3 family sensors are necessary to establish the roles for individual residues in transmitter phosphatase activity.
Hydroxyl/amide hypothesis for transmitter phosphatase activity in HisKA sensors
The HisKA family constitutes the majority of sensors. The HisKA DHp domain sequences exhibit a conserved E/DxxN/T motif, immediately adjacent to the phospho-accepting His residue. This E/DxxN/T motif is reminiscent of the CheX ExxN motif, which acts in a virtually identical mechanism to the CheZ DxxxQ motif (Pazy et al., 2010). The conserved Asn/Thr of the E/DxxN/T motif is critical for phosphatase function (Fig. 2D and 4B). Among the substitutions for the EnvZ Thr-247 residue, only Ser and Asn retain activity whereas seven others do not (Dutta et al., 2000). Furthermore, the permissibility of the Thr to Asn substitution underlines the interchangeability between amide and hydroxyl at this position, consistent with the presence of Asn at this position in many HisKA family sensors including NtrB and ThkA. Substitutions for the analogous, conserved CpxA Thr-253, PhoR Thr-220 and VanS Thr-238 residues also abolish transmitter phosphatase activity, although the Asn substitution is not permissible in PhoR (Yamada et al., 1989, Baptista et al., 1997, Raivio & Silhavy, 1997).
Structural analysis is consistent with the hypothesis, that the conserved Thr or Asn residue in the E/DxxN/T motif coordinates the nucleophilic water for transmitter phosphatase activity. Yamada et al. (2009) noted that the modeled ThkA-TrrA complex has ThkA residue Asn-551 in close proximity to TrrA residue Asp-57, similar to CheZ and CheY residues Gln-147 and Asp-57, respectively. Casino et al. (2010) superimposed the CheX-CheY and HK853-RR468 cocrystal structures, revealing that HK853 residue Thr-264 is positioned similarly to the CheX catalytic residue Asn-99.
Although the similarity of the CheX ExxN and HisKA E/DxxN/T motifs is striking, HisKA transmitter phosphatase activity may not involve the Asp/Glu residue (Huynh et al., 2010). First, the NtrB E140A and E140Q mutants retain transmitter phosphatase activity but are defective for autokinase activity (Atkinson & Ninfa, 1993). Second, in the HK853-RR468 complex (Casino et al., 2009), HK853 residue Glu-261 is turned away from the receiver active site and seems unlikely to interact with the conserved Lys in the receiver domain. Instead, residue Arg-263 (also highly conserved in HisKA sequences) makes a hydrogen bond to RR468 residue Lys-105. Together, these observations suggest that the conserved Glu/Asp residue in HisKA family transmitters does not function like CheX residue Glu-96 to coordinate the receiver domain Lys residue (Pazy et al., 2010).
Transmitter-receiver interaction
Transmitter phosphatase activity involves interactions between the DHp and receiver domains. In the HK853-RR468 cocrystal structure and ThkA-TrrA modeled complex, the receiver domain inserts its helix α1 between DHp helices α1 and α2. Since each sensor dimer interacts with two response regulator molecules, this interaction creates a six-helix bundle structure, stabilized by the further insertion of the receiver β5-α5 loop in the DHp domain (Casino et al., 2010, Szurmant & Hoch, 2010). Similar interactions are present in the analogous Spo0F-Spo0B cocrystal (Szurmant & Hoch, 2010).
Besides its major interactions with the DHp domain, the receiver also makes additional contacts with the CA domain and the DHp-CA domain linker. The receiver β4-α4 loop, besides interacting with the C-terminus of DHp helix α2, also makes lateral contacts with the CA domain (Zapf et al., 2000, Casino et al., 2009). Furthermore, RR468 blocks the ATP lid of the ATP-bound HK853 CA domain (Casino et al., 2009).
Mutational and covariance analyses independently have shown that specificity determinants for cognate transmitter-receiver interactions are localized toward the carboxyl-terminal end of the DHp domain α1 helix for sensors of both the HisKA family (Tomomori et al., 1999, Skerker et al., 2008, Capra et al., 2010) and the HisKA_3 family (Noriega et al., 2010). Replacing these amino acids on the EnvZ DHp domain with those of a different sensor switches the transmitter specificity for the corresponding receiver (Skerker et al., 2008). Accordingly, the co-varying amino acids on the receiver domain are located mostly on helix α1 (Capra et al., 2010), congruent with conclusions made from analysis of sensor-response regulator co-crystal structures as described immediately above.
EnvZ, NtrB, and NarX kinase-active, phosphatase-defective mutants have alterations in both DHp helices α1 and α2, which likely affect interaction specificity and DHp domain conformation (Hsing et al., 1998, Jiang et al., 2000, Pioszak & Ninfa, 2003a, Noriega et al., 2010). Conversely, CpxA mutations that enhance DHp-receiver interactions can give rise to transmitter phosphatase activity toward the non-cognate response regulator OmpR (Siryaporn et al., 2010).
Whereas the DHp domain alone encompasses the transmitter phosphatase activity, this function is enhanced by interaction with the CA domain, as demonstrated in vitro with EnvZ and NtrB (Jiang et al., 2000, Zhu et al., 2000). Moreover, EnvZ, NtrB, NarX and VanB transmitter phosphatase mutants have been isolated with alterations in the CA domain (Hsing et al., 1998, Depardieu et al., 2003, Pioszak & Ninfa, 2003a, Noriega et al., 2010). Finally, the in vitro transmitter phosphatase function of many sensors is accelerated by ATP, ADP and non-cleavable ATP analogs (Table 1). For these ATP-responsive sensors, nucleotide binding to the CA domain likely causes a conformational change that is distinct from the kinase-competent state, since the γ-phosphate group need not be hydrolyzed. Moreover, nucleotide-enhancement of transmitter phosphatase activity requires the presence of the CA domain (Jiang et al., 2000, Zhu et al., 2000). Therefore, the nucleotide-bound CA domain probably stabilizes the transmitter-receiver interactions during the phosphatase reaction. Note however that phosphatase-deficient CA domain alterations have been isolated even for sensors such as VanS and NarX for which transmitter phosphatase activity is not stimulated by nucleotides, suggesting that these sensors also employ CA-receiver interactions for this reaction. Finally, it is suggested that ATP-lid closure, resulting from receiver-CA domain interaction, is also conducive for transmitter phosphatase, since the transmitter autokinase reaction is inhibited (Casino et al., 2009).
Table 1.
Influence of nucleotides on stimulation of transmitter phosphatase activity.
| Sensor | HPK Subfamilya | Stimulation by
|
Phospho-RR half-life (min)b | Reference | ||
|---|---|---|---|---|---|---|
| ATP | ADP | ATP Analog | ||||
| MtrB | 1a | —c | Yes | — | — | (Möker et al., 2007) |
| VanS | 1a | No | No | — | 860d | (Wright et al., 1993) |
| WalK/YycG | 1a | Yes | Yes | Yese | 1,400 | (Gutu et al., 2010) |
| CpxA | 2b | Yes | — | — | — | (Raivio & Silhavy, 1997) |
| EnvZ | 2b | Yes | Yes | Yesf | 350d | (Igo et al., 1989) |
| Yes | Yes | Yesg | 350d | (Zhu et al., 2000) | ||
| PhoQ | 3a | — | Yes | Yesg | — | (Sanowar & Le Moual, 2005) |
| FixL | 4 | No | — | — | 140d | (Lois et al., 1993) |
| NtrB/NRII | 4 | Yes | No | — | 20d | (Keener & Kustu, 1988) |
| Yes | No | Yesg | 20d | (Jiang et al., 2000) | ||
| DegS | 7h | Yes | — | — | 90d | (Dahl et al., 1992) |
| NarX | 7h | No | No | Nog | 140 | C. E. Noriega & V. Stewart, unpublished |
Classification of Grebe & Stock (1999). All are in the Pfam HisKA subfamily except as noted.
Approximate half-life for the phosphorylated cognate response regulator.
—, not tested or reported
Value taken from (Thomas et al., 2008).
ATPγS
AMP-PCP
AMP-PNP
Pfam HisKA_3 subfamily
It is not obvious why nucleotide stimulates transmitter phosphatase activity for some sensors but not others. There is no apparent correlation with DHp domain sequence subfamily or with the phosphorylation half-life for the cognate response regulator (Table 1). It remains to be seen if the differential dependence on nucleotide results from structural, mechanistic or regulatory differences.
Another important structural element in transmitter phosphatase is the Per-ARNT-Sim (PAS) domain, present in approximately one-third of sensors (Gao & Stock, 2009). The PAS domain can function either in ligand binding or in signal conduction (Möglich et al., 2009b). Recent structural and mutational analyses of WalK and ThkA indicate that deletion of the PAS domain greatly diminishes transmitter phosphatase activity while leaving the autokinase and phosphotransfer functions mostly undisturbed (Yamada et al., 2009, Gutu et al., 2010). In the ThkA-TrrA complex, the requirement of the PAS domain results from its interaction with the receiver helix α4 (Yamada et al., 2009).
Control of transmitter phosphatase activity
In order to mediate environmental response, two-component sensors must differentially regulate their autokinase and transmitter phosphatase activities. Theoretical analysis shows that a population of bifunctional sensors is in “on/off” equilibrium between a single on-state (autokinase) and a single off-state (transmitter phosphatase) (Bornhorst & Falke, 2003). Output is controlled over a range of input stimulus through adjusting the ratio between these mutually-exclusive states (Russo & Silhavy, 1991). Recent analysis of sensor crystal structures (Stewart, 2010) supports the hypothesis, that the two states represent different conformations in which the autophosphorylation site is either exposed or hidden (Pratt & Silhavy, 1995). The structures suggest that rotational movement within the DHp domain helical bundle influences both accessibility of the phospho-accepting His residue as well as interactions with the CA domain (Albanesi et al., 2009, Casino et al., 2009). These motions likely also control accessibility of the hypothesized transmitter phosphatase active residues in the DxxxQ or E/DxxT/N motifs (Fig. 4). Thus, autokinase and transmitter phosphatase are reciprocal activities resulting from mutually-exclusive transmitter conformations.
In some cases, on/off equilibrium is mediated by auxiliary regulators (Buelow & Raivio, 2010). For example, NtrB transmitter phosphatase is stimulated by PII (GlnB) protein, which senses 2-oxoglutarate and adenylate energy charge. PII binds to both the DHp and CA domains, and presumably mediates the conformational shifts described above (Pioszak & Ninfa, 2003b, Jiang & Ninfa, 2009). However, most two-component systems do not involve auxiliary regulators, and so conformational control must result from coiled-coil dynamics in response to signal input (Albanesi et al., 2009, Möglich et al., 2009a, Stewart & Chen, 2010). Environmental stimuli can be either positive or negative, depending on how the input and signal conversion modules are tuned. For example, stimulus shifts NarX sensor equilibrium to the on-state (Williams & Stewart, 1997), whereas it shifts PhoQ sensor equilibrium to the off-state (Chamnongpol et al., 2003).
For some sensors, it is reported that only autokinase or phosphatase is regulated, with the opposite activity being constitutive (Stock et al., 2000, Gao & Stock, 2009, Stewart, 2010). This can pertain to sensors, such as NtrB and KdpD, for which auxiliary regulators shift the on/off equilibrium. In other cases, available information favors reciprocal regulation of a two-state on/off equilibrium (Raivio & Silhavy, 1997, Chamnongpol et al., 2003). Finally, the signal-insensitive “phosphatase” described for the LuxN and LuxPQ sensors refers to signal decay through reverse phosphorelay (Fig. 3B) rather than transmitter phosphatase activity (Long et al., 2009). In this case, signal decay likely represents the favored phosphotransfer equilibrium in the absence of signal-responsive transmitter autokinase activity.
Concluding remarks
The search for unity, a powerful impetus in biology, can impede a proper appreciation of life’s diversity (Thaler & Stahl, 1988)
Negative regulation through transmitter phosphatase activity is a widespread mechanism in two-component signal transduction, and is essential for proper function within and between signaling pathways. Current hypotheses suggest that sensors provide a catalyst for the receiver autodephosphorylation reaction, by coordinating inline attack by the nucleophilic water molecule. The DHp domain active sites for autokinase, phosphotransfer and transmitter phosphatase activities overlap substantially, and therefore individual residues likely serve multiple functions. Nevertheless, plausible mechanistic hypotheses help to stimulate attention on transmitter phosphatase activity control, physiological roles in two-component signaling, and relationship to transmitter-receiver specificity.
The hypothesized mechanism for transmitter phosphatase activity provides a unifying basis for thinking about this function and for designing future experiments (Huynh et al., 2010). Yet, it also incorporates considerable diversity, postulating amide, hydroxyl and even imidazole side-chains as water-orienting catalysts. This diversity, together with the large number of distinct sensor sequences, seemingly assures that variations in mechanism will be encountered. Hints of this already are apparent from the different effects of nucleotides and of substitutions for the phospho-accepting His residue.
Acknowledgments
We thank Li-Ling Chen, Hsia-Yin Lin and Chris Noriega for their many contributions to our thinking and experiments on transmitter phospatase activity, and Sydney Kustu and Bob Bourret for helpful discussions. Two anonymous referees provided extremely valuable constructive critique on the earlier version of this paper.
Research on two-component regulation in our laboratory was supported by U. S. Public Health Service Grant GM036877 from the National Institute of General Medical Sciences.
References
- Albanesi D, Mansilla MC, de Mendoza D. The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator. J Bacteriol. 2004;186:2655–2663. doi: 10.1128/JB.186.9.2655-2663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanesi D, Martín M, Trajtenberg F, Mansilla MC, Haouz A, Alzari PM, de Mendoza D, Buschiazzo A. Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc Natl Acad Sci U S A. 2009;106:16185–16190. doi: 10.1073/pnas.0906699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multistep phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- Atkinson MR, Ninfa AJ. Mutational analysis of the bacterial signal-transducing protein kinase/phosphatase nitrogen regulator II (NRII or NtrB) J Bacteriol. 1993;175:7016–7023. doi: 10.1128/jb.175.21.7016-7023.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault AD, Fassler JS, Deschenes RJ. Altered phosphotransfer in an activated mutant of the Saccharomyces cerevisiae two-component osmosensor Sln1p. Eukary Cell. 2002;1:174–180. doi: 10.1128/EC.1.2.174-180.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista M, Depardieu F, Reynolds P, Courvalin P, Arthur M. Mutations leading to increased levels of resistance to glycopeptide antibiotics in VanB-type enterococci. Mol Microbiol. 1997;25:93–105. doi: 10.1046/j.1365-2958.1997.4401812.x. [DOI] [PubMed] [Google Scholar]
- Barakat M, Ortet P, Whitworth DE. P2CS: a database of prokaryotic two-component systems. Nucleic Acids Res. 2011;39:D771–776. doi: 10.1093/nar/gkq1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhorst JA, Falke JJ. Quantitative analysis of aspartate receptor signaling complex reveals that the homogeneous two-state model is inadequate: development of a heterogeneous two-state model. J Mol Biol. 2003;326:1597–1614. doi: 10.1016/s0022-2836(03)00026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret RB. Receiver domain structure and function in response regulator proteins. Curr Opin Microbiol. 2010;13:142–149. doi: 10.1016/j.mib.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow DR, Raivio TL. Three (and more) component regulatory systems -auxiliary regulators of bacterial histidine kinases. Mol Microbiol. 2010;75:547–566. doi: 10.1111/j.1365-2958.2009.06982.x. [DOI] [PubMed] [Google Scholar]
- Capra EJ, Perchuk BS, Lubin EA, Ashenberg O, Skerker JM, Laub MT. Systematic dissection and trajectory-scanning mutagenesis of the molecular interface that ensures specificity of two-component signaling pathways. PLoS Genet. 2010;6:e1001220. doi: 10.1371/journal.pgen.1001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmany DO, Hollingsworth K, McCleary WR. Genetic and biochemical studies of phosphatase activity of PhoR. J Bacteriol. 2003;185:1112–1115. doi: 10.1128/JB.185.3.1112-1115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casino P, Rubio V, Marina A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell. 2009;139:325–336. doi: 10.1016/j.cell.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Casino P, Rubio V, Marina A. The mechanism of signal transduction by two-component systems. Curr Opin Struct Biol. 2010;20:763–771. doi: 10.1016/j.sbi.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Chamnongpol S, Cromie M, Groisman EA. Mg2+ sensing by the Mg2+ sensor PhoQ of Salmonella enterica. J Mol Biol. 2003;325:795–807. doi: 10.1016/s0022-2836(02)01268-8. [DOI] [PubMed] [Google Scholar]
- Dahl MK, Msadek T, Kunst F, Rapoport G. The phosphorylation state of the DegU response regulator acts as a molecular switch allowing either degradative enzyme synthesis or expression of genetic competence in Bacillus subtilis. J Biol Chem. 1992;267:14509–14514. [PubMed] [Google Scholar]
- Depardieu F, Courvalin P, Msadek T. A six amino acid deletion, partially overlapping the VanSB G2 ATP-binding motif, leads to constitutive glycopeptide resistance in VanB-type Enterococcus faecium. Mol Microbiol. 2003;50:1069–1083. doi: 10.1046/j.1365-2958.2003.03771.x. [DOI] [PubMed] [Google Scholar]
- Diaz AR, Stephenson S, Green JM, Levdikov VM, Wilkinson AJ, Perego M. Functional role for a conserved aspartate in the Spo0E signature motif involved in the dephosphorylation of the Bacillus subtilis sporulation regulator Spo0A. J Biol Chem. 2008;283:2962–2972. doi: 10.1074/jbc.M709032200. [DOI] [PubMed] [Google Scholar]
- Dutta R, Inouye M. Reverse phosphotransfer from OmpR to EnvZ in a kinase-/phosphatase+ mutant of EnvZ (EnvZ.N347D), a bifunctional signal transducer of Escherichia coli. J Biol Chem. 1996;271:1424–1429. doi: 10.1074/jbc.271.3.1424. [DOI] [PubMed] [Google Scholar]
- Dutta R, Yoshida T, Inouye M. The critical role of the conserved Thr247 residue in the functioning of the osmosensor EnvZ, a histidine Kinase/Phosphatase, in Escherichia coli. J Biol Chem. 2000;275:38645–38653. doi: 10.1074/jbc.M005872200. [DOI] [PubMed] [Google Scholar]
- Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunasekaran P, Ceric G, Forslund K, Holm L, Sonnhammer EL, Eddy SR, Bateman A. The Pfam protein families database. Nucleic Acids Res. 2010;38:D211–222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY. Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J Bacteriol. 2006;188:4169–4182. doi: 10.1128/JB.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgellis D, Kwon O, De Wulf P, Lin ECC. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J Biol Chem. 1998;273:32864–32869. doi: 10.1074/jbc.273.49.32864. [DOI] [PubMed] [Google Scholar]
- Grebe TW, Stock JB. The histidine protein kinase superfamily. Adv Microb Physiol. 1999;41:139–227. doi: 10.1016/s0065-2911(08)60167-8. [DOI] [PubMed] [Google Scholar]
- Groban ES, Clarke EJ, Salis HM, Miller SM, Voigt CA. Kinetic buffering of cross talk between bacterial two-component sensors. J Mol Biol. 2009;390:380–393. doi: 10.1016/j.jmb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutu AD, Wayne KJ, Sham LT, Winkler ME. Kinetic characterization of the WalRKSpn (VicRK) two-component system of Streptococcus pneumoniae: dependence of WalKSpn (VicK) phosphatase activity on its PAS domain. J Bacteriol. 2010;192:2346–2358. doi: 10.1128/JB.01690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjifrangiskou M, Kostakioti M, Chen SL, Henderson JP, Greene SE, Hultgren SJ. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Mol Microbiol. 2011 doi: 10.1111/j.1365-2958.2011.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing W, Russo FD, Bernd KK, Silhavy TJ. Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. J Bacteriol. 1998;180:4538–4546. doi: 10.1128/jb.180.17.4538-4546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing W, Silhavy TJ. Function of conserved histidine-243 in phosphatase activity of EnvZ, the sensor for porin osmoregulation in Escherichia coli. J Bacteriol. 1997;179:3729–3735. doi: 10.1128/jb.179.11.3729-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh TN, Noriega CE, Stewart V. Conserved mechanism for sensor phosphatase control of two-component signaling revealed in the nitrate sensor NarX. Proc Natl Acad Sci U S A. 2010;107:21140–21145. doi: 10.1073/pnas.1013081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo MM, Ninfa AJ, Stock JB, Silhavy TJ. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Janiak-Spens F, Cook PF, West AH. Kinetic analysis of YPD1-dependent phosphotransfer reactions in the yeast osmoregulatory phosphorelay system. Biochemistry. 2005;44:377–386. doi: 10.1021/bi048433s. [DOI] [PubMed] [Google Scholar]
- Jiang P, Atkinson MR, Srisawat C, Sun Q, Ninfa AJ. Functional dissection of the dimerization and enzymatic activities of Escherichia coli nitrogen regulator II and their regulation by the PII protein. Biochemistry. 2000;39:13433–13449. doi: 10.1021/bi000794u. [DOI] [PubMed] [Google Scholar]
- Jiang P, Ninfa AJ. Alpha-ketoglutarate controls the ability of the Escherichia coli PII signal transduction protein to regulate the activities of NRII (NrB but does not control the binding of PII to NRII. Biochemistry. 2009;48:11514–11521. doi: 10.1021/bi901158h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener J, Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci U S A. 1988;85:4976–4980. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostakioti M, Hadjifrangiskou M, Pinkner JS, Hultgren SJ. QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol Microbiol. 2009;73:1020–1031. doi: 10.1111/j.1365-2958.2009.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang XC. GTP hydrolysis mechanism of Ras-like GTPases. J Mol Biol. 2004;340:921–932. doi: 10.1016/j.jmb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lois AF, Weinstein M, Ditta GS, Helinski DR. Autophosphorylation and phosphatase activities of the oxygen-sensing protein FixL of Rhizobium meliloti are coordinately regulated by oxygen. J Biol Chem. 1993;268:4370–4375. [PubMed] [Google Scholar]
- Long T, Tu KC, Wang Y, Mehta P, Ong NP, Bassler BL, Wingreen NS. Quantifying the integration of quorum-sensing signals with single-cell resolution. PLoS Biol. 2009;7:e68. doi: 10.1371/journal.pbio.1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukat GS, Lee BH, Mottonen JM, Stock AM, Stock JB. Roles of the highly conserved aspartate and lysine residues in the response regulator of bacterial chemotaxis. J Biol Chem. 1991;266:8348–8354. [PubMed] [Google Scholar]
- Lukat GS, Stock AM, Stock JB. Divalent metal ion binding to the CheY protein and its significance to phosphotransfer in bacterial chemotaxis. Biochemistry. 1990;29:5436–5442. doi: 10.1021/bi00475a004. [DOI] [PubMed] [Google Scholar]
- Möglich A, Ayers RA, Moffat K. Design and signaling mechanism of light-regulated histidine kinases. J Mol Biol. 2009a;385:1433–1444. doi: 10.1016/j.jmb.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möglich A, Ayers RA, Moffat K. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure. 2009b;17:1282–1294. doi: 10.1016/j.str.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möker N, Krämer J, Unden G, Krämer R, Morbach S. In vitro analysis of the two-component system MtrB-MtrA from Corynebacterium glutamicum. J Bacteriol. 2007;189:3645–3649. doi: 10.1128/JB.01920-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa AJ, Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986;83:5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega CE, Lin HY, Chen LL, Williams SB, Stewart V. Asymmetric cross-regulation between the nitrate-responsive NarX-NarL and NarQ-NarP two-component regulatory systems from Escherichia coli K-12. Mol Microbiol. 2010;75:394–412. doi: 10.1111/j.1365-2958.2009.06987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar V, Mirouze N, Dubnau DA, Neiditch MB. Structural basis of response regulator dephosphorylation by Rap phosphatases. PLoS Biol. 2011;9:e1000589. doi: 10.1371/journal.pbio.1000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JS, Kofoid EC. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- Pazy Y, Motaleb MA, Guarnieri MT, Charon NW, Zhao R, Silversmith RE. Identical phosphatase mechanisms achieved through distinct modes of binding phosphoprotein substrate. Proc Natl Acad Sci U S A. 2010;107:1924–1929. doi: 10.1073/pnas.0911185107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Sandoval GR, Kwon O, Georgellis D. Requirement of the receiver and phosphotransfer domains of ArcB for efficient dephosphorylation of phosphorylated ArcA in vivo. J Bacteriol. 2005;187:3267–3272. doi: 10.1128/JB.187.9.3267-3272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M, Glaser P, Hoch JA. Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol Microbiol. 1996;19:1151–1157. doi: 10.1111/j.1365-2958.1996.tb02460.x. [DOI] [PubMed] [Google Scholar]
- Pioszak AA, Ninfa AJ. Genetic and biochemical analysis of phosphatase activity of Escherichia coli NRII (NtrB) and its regulation by the PII signal transduction protein. J Bacteriol. 2003a;185:1299–1315. doi: 10.1128/JB.185.4.1299-1315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak AA, Ninfa AJ. Mechanism of the PII-activated phosphatase activity of Escherichia coli NRII (NtrB): how the different domains of NRII collaborate to act as a phosphatase. Biochemistry. 2003b;42:8885–8899. doi: 10.1021/bi030065p. [DOI] [PubMed] [Google Scholar]
- Pioszak AA, Ninfa AJ. Mutations altering the N-terminal receiver domain of NRI (NtrC) That prevent dephosphorylation by the NRII-PII complex in Escherichia coli. J Bacteriol. 2004;186:5730–5740. doi: 10.1128/JB.186.17.5730-5740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA, Silhavy TJ. Porin regulation of Escherichia coli. In: Hoch JA, Silhavy TJ, editors. Two-component signal transduction. Washington D.C: ASM Press; 1995. pp. 105–127. [Google Scholar]
- Raivio TL, Silhavy TJ. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol. 1997;179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo FD, Silhavy TJ. EnvZ controls the concentration of phosphorylated OmpR to mediate osmoregulation of the porin genes. J Mol Biol. 1991;222:567–580. doi: 10.1016/0022-2836(91)90497-t. [DOI] [PubMed] [Google Scholar]
- Russo FD, Silhavy TJ. The essential tension: opposed reactions in bacterial two-component regulatory systems. Trends Microbiol. 1993;1:306–310. doi: 10.1016/0966-842x(93)90007-e. [DOI] [PubMed] [Google Scholar]
- Sanowar S, Le Moual H. Functional reconstitution of the Salmonella typhimurium PhoQ histidine kinase sensor in proteoliposomes. Biochem J. 2005;390:769–776. doi: 10.1042/BJ20050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeler NL, MacMillan SV, Nodwell JR. Biochemical activities of the absA two-component system of Streptomyces coelicolor. J Bacteriol. 2005;187:687–696. doi: 10.1128/JB.187.2.687-696.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silversmith RE. Auxiliary phosphatases in two-component signal transduction. Curr Opin Microbiol. 2010;13:177–183. doi: 10.1016/j.mib.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siryaporn A, Goulian M. Cross-talk suppression between the CpxA-CpxR and EnvZ-OmpR two-component systems in E. coli. Mol Microbiol. 2008;70:494–506. doi: 10.1111/j.1365-2958.2008.06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siryaporn A, Perchuk BS, Laub MT, Goulian M. Evolving a robust signal transduction pathway from weak cross-talk. Mol Syst Biol. 2010;6:452. doi: 10.1038/msb.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarphol K, Waukau J, Forst SA. Role of His243 in the phosphatase activity of EnvZ in Escherichia coli. J Bacteriol. 1997;179:1413–1416. doi: 10.1128/jb.179.4.1413-1416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerker JM, Perchuk BS, Siryaporn A, Lubin EA, Ashenberg O, Goulian M, Laub MT. Rewiring the specificity of two-component signal transduction systems. Cell. 2008;133:1043–1054. doi: 10.1016/j.cell.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Peisach D, Pioszak AA, Xu Z, Ninfa AJ. Crystal structure of the C-terminal domain of the two-component system transmitter protein nitrogen regulator II (NRII; NtrB), regulator of nitrogen assimilation in Escherichia coli. Biochemistry. 2004;43:6670–6678. doi: 10.1021/bi049474r. [DOI] [PubMed] [Google Scholar]
- Stewart RC. Protein histidine kinases: assembly of active sites and their regulation in signaling pathways. Curr Opin Microbiol. 2010;13:133–141. doi: 10.1016/j.mib.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V, Chen L-L. The S helix mediates signal transmission as a HAMP domain coiled-coil extension in the NarX nitrate sensor from Escherichia coli K-12. J Bacteriol. 2010;192:734–745. doi: 10.1128/JB.00172-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AM, V, Robinson L, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Stock JB, Ninfa AJ, Stock AM. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurmant H, Hoch JA. Interaction fidelity in two-component signaling. Curr Opin Microbiol. 2010;13:190–197. doi: 10.1016/j.mib.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler DS, Stahl FW. DNA double-chain breaks in recombination of phage lambda and of yeast. Annu Rev Genet. 1988;22:169–197. doi: 10.1146/annurev.ge.22.120188.001125. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Brewster JA, Bourret RB. Two variable active site residues modulate response regulator phosphoryl group stability. Mol Microbiol. 2008;69:453–465. doi: 10.1111/j.1365-2958.2008.06296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomomori C, Tanaka T, Dutta R, Park H, Saha SK, Zhu Y, Ishima R, Liu D, Tong KI, Kurokawa H, Qian H, Inouye M, Ikura M. Solution structure of the homodimeric core domain of Escherichia coli histidine kinase EnvZ. Nat Struct Biol. 1999;6:729–734. doi: 10.1038/11495. [DOI] [PubMed] [Google Scholar]
- Trajtenberg F, Graña M, Ruétalo N, Botti H, Buschiazzo A. Structural and enzymatic insights into the ATP binding and autophosphorylation mechanism of a sensor histidine kinase. J Biol Chem. 2010;285:24892–24903. doi: 10.1074/jbc.M110.147843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl MA, Miller JF. Central role of the BvgS receiver as a phosphorylated intermediate in a complex two-component phosphorelay. J Biol Chem. 1996;271:33176–33180. doi: 10.1074/jbc.271.52.33176. [DOI] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- Wanner BL. Is cross regulation by phosphorylation of two-component response regulator proteins important in bacteria? J Bacteriol. 1992;174:2053–2058. doi: 10.1128/jb.174.7.2053-2058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci. 2001;26:369–376. doi: 10.1016/s0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- Williams SB, Stewart V. Nitrate- and nitrite-sensing protein NarX of Escherichia coli K-12: mutational analysis of the amino-terminal tail and first transmembrane segment. J Bacteriol. 1997;179:721–729. doi: 10.1128/jb.179.3.721-729.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolanin PM, Webre DJ, Stock JB. Mechanism of phosphatase activity in the chemotaxis response regulator CheY. Biochemistry. 2003;42:14075–14082. doi: 10.1021/bi034883t. [DOI] [PubMed] [Google Scholar]
- Wright GD, Holman TR, Walsh CT. Purification and characterization of VanR and the cytosolic domain of VanS: a two-component regulatory system required for vancomycin resistance in Enterococcus faecium BM4147. Biochemistry. 1993;32:5057–5063. doi: 10.1021/bi00070a013. [DOI] [PubMed] [Google Scholar]
- Yamada M, Makino K, Amemura M, Shinagawa H, Nakata A. Regulation of the phosphate regulon of Escherichia coli: analysis of mutant phoB and phoR genes causing different phenotypes. J Bacteriol. 1989;171:5601–5606. doi: 10.1128/jb.171.10.5601-5606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Sugimoto H, Kobayashi M, Ohno A, Nakamura H, Shiro Y. Structure of PAS-linked histidine kinase and the response regulator complex. Structure. 2009;17:1333–1344. doi: 10.1016/j.str.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Zapf J, Sen U, Madhusudan, Hoch JA, Varughese KI. A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Structure. 2000;8:851–862. doi: 10.1016/s0969-2126(00)00174-x. [DOI] [PubMed] [Google Scholar]
- Zhao R, Collins EJ, Bourret RB, Silversmith RE. Structure and catalytic mechanism of the E. coli chemotaxis phosphatase CheZ. Nat Struct Biol. 2002;9:570–575. doi: 10.1038/nsb816. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Qin L, Yoshida T, Inouye M. Phosphatase activity of histidine kinase EnvZ without kinase catalytic domain. Proc Natl Acad Sci U S A. 2000;97:7808–7813. doi: 10.1073/pnas.97.14.7808. [DOI] [PMC free article] [PubMed] [Google Scholar]


