Abstract
The identity of the neurotransmitters expressed by neurons has been thought to be fixed and immutable, but recent studies demonstrate that changes in electrical activity can rapidly and reversibly reconfigure the transmitters and corresponding transmitter receptors that neurons express. Induction of transmitter expression can be achieved by selective activation of afferents recruited by a physiological range of sensory input. Strikingly, neurons acquiring an additional transmitter project to appropriate targets prior to transmitter respecification in some cases, indicating the presence of reserve pools of neurons that can boost circuit function. We discuss the evidence for such reserve pools, their likely locations and ways to test for their existence, and the potential clinical value of such circuit-specific neurotransmitter respecification for treatments of neurological disorders.
Keywords: Neuronal plasticity, activity-dependent plasticity, neurotransmitter switching, dopamine and GABA coexpression, sensory stimulation, development
Introduction
The adult CNS maintains a homeostatic balance of excitatory and inhibitory input in response to perturbations of electrical activity occurring during normal physiological processes such as sensory detection of natural stimuli, adaptation to the environment, and learning new tasks. To achieve this objective, different forms of neuronal plasticity remodel the CNS at the level of synaptic strength (Turrigiano, 2008; Nelson and Turrigiano, 2008; Ibata et al., 2008), intrinsic excitability (Marder and Goaillard, 2006; Gittis and du Lac, 2006; Turrigiano, 2011) and connectivity (Engert and Bonhoeffer, 1999; Destexhe and Marder, 2004; Losonczy et al., 2008; Tye et al., 2008). Synaptic scaling and potentiation (Maffei et al., 2006; Turrigiano, 2008), plasticity of voltage-gated channel expression (LeMasson et al., 1993; Turrigiano et al., 1994; Desai et al., 1999; Luther and Birren, 2009; Nelson et al., 2005), morphological pruning and maturation of dendritic trees (Cesa et al., 2007; Gonzalez-Burgos et al., 2008; Ovtscharoff et al., 2008), regulation of activation or inactivation of spines (Yasuda et al., 2003), and modulation of receptive fields (Nudo et al., 1996; Froemke et al., 2007) are responses to the numerous perturbations of activity, both endogenous and environmental, to which the CNS is subjected. Failure of neuronal homeostasis may result in common neuropsychiatric phenotypes (Ramocki and Zoghbi, 2008).
Many studies have demonstrated that changes in electrical activity trigger the appearance of neurotransmitters in neurons that normally produce different ones, both during development (Gu and Spitzer, 1995; Brosenitsch et al., 1998; Brosenitsch and Katz, 2001, 2002; Belousov et al., 2001, 2002; Watt et al., 2000; Borodinsky et al, 2004) and in the mature nervous system (Hendry & Jones, 1988; Gutierrez, 2002, 2005; Gutierrez et al., 2003). Transmitter receptor matching occurs in parallel with activity-dependent transmitter respecification (Borodinsky and Spitzer, 2007; Cesa et al., 2008; Dulcis and Spitzer, 2008). Transmitter and receptor respecification can be triggered by experimental manipulations or by natural sensory stimuli that selectively activate a neuronal circuit (Trudeau and Gutierrez, 2007; Dulcis and Spitzer, 2008). Interestingly, recent work has identified cases in which neurons are recruited into existing networks through acquisition of an additional neurotransmitter. Because their anatomical connections are already established, these neurons are rapidly functionally integrated.
In this review we discuss the evidence for this newly recognized activity-dependent recruitment of neurons to circuits. We identify neuronal networks that allow experimental tests of circuit-activated-induction of ectopic transmitter co-expression and circuit expansion in vertebrate model systems. The ability to manipulate this form of plasticity could provide the basis for novel non-invasive treatment of disorders of transmitter and receptor metabolism, such as Parkinson’s Disease.
Reserve pool neurons: definition and properties
Reserve pool neurons are cells that share inputs and outputs with adjacent core pools of neurons but express different neurotransmitters. They are already part of active circuits, prior to the time at which activity leads to transmitter respecification. As a result, core and reserve pool neurons normally exert different effects on their common targets. Physiological stimuli change the afferent input to both the core and the reserve pool, which triggers expansion or contraction of the transmitter phenotypes of the reserve pool neurons. This neurotransmitter respecification can involve co-expression of an additional transmitter (Figure 1) or elimination of the expression of a pre-existing cotransmitter. The term “reserve” is used to indicate that these neurons are integrated into circuits, but ready to change the identity of the transmitters they express. Reserve pool neurons thus respond to changes in activity with a gain of function or loss of function generated by transmitter respecification. Core and reserve pool neurons are identifiable by shared molecular markers such as transcription factors, ion channels, or calcium-binding proteins, some of which are likely to contribute to the mechanism for this plasticity.
Figure 1.
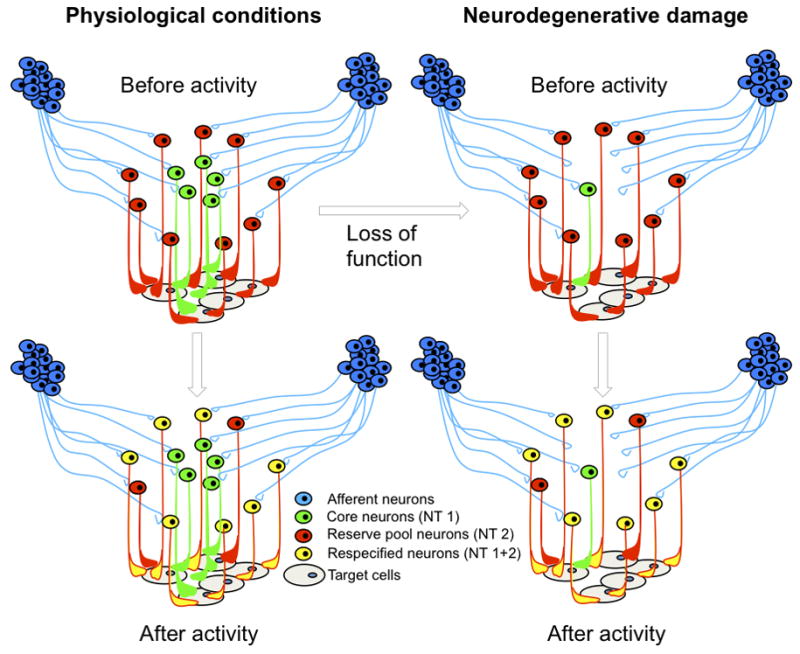
Activity-dependent neurotransmitter (NT) respecification of reserve pool neurons occurring under physiological conditions (left) and potentially following neurodegenerative damage (right). Reserve pool neurons (red) acquire the core neuron phenotype (green) following activation by afferent neurons (blue). Respecified co-expressing neurons (yellow) enhance normal function or restore lost function by acting on target cells (grey).
One can think of some reserve pool neurons as having “a day job”, and then acquiring “another day job”, or perhaps “a night job”, in addition to their “day job”. In the same way one can think of other reserve pool neurons as holding down two jobs, one of which they relinquish in response to a change in circuit activity. In the first scenario, reserve pool neurons already project to the same targets as the core neurons for which they are in reserve, but express a different transmitter than the core neurons; following a change in circuit activity they acquire the transmitter already expressed by the core neurons. In the second scenario, both reserve pool neurons and core neurons release the same transmitter at their synapses with a common target; following a change in circuit activity the reserve pool neurons lose the transmitter that they previously had in common with the core neurons. It seems unlikely that neurons lose all their transmitters through this process - resulting in “empty” neurons that do not synthesize any transmitters - unless the number of neurons is to be pruned back. Substantial evidence indicates that neurons need to be synaptically connected in order to survive.
Transmitter respecification in reserve pool neurons influences the excitability of postsynaptic elements, by altering their complement of transmitter receptors. The appearance of a new transmitter presynaptically is accompanied by the appearance of cognate transmitter receptors postsynaptically; the reverse happens when a transmitter disappears presynaptically, and the cognate transmitter receptors are downregulated postsynaptically.
The advantages of reserve pool architecture suggest the basis for evolutionary selection of this form of plasticity. First, respecified reserve pool neurons already innervate the correct targets, allowing rapid application of the newly acquired transmitter phenotype in response to environmental stimuli. Second, reserve pool neurons possess afferents whose activity evokes appropriate temporal regulation of transmitter release. Third, the target responds to the respecified transmitter by expressing the correct neurotransmitter receptors. Fourth, because neither neurogenesis nor axonal growth is involved in this form of plasticity, reversibility of respecification does not require pruning of processes or elimination of neurons by cell death.
Activity-dependent neurotransmitter respecification
It is useful to understand the extent of activity-dependent transmitter respecification before reviewing reserve pool neuron recruitment. Activity-dependent transmitter respecification has been observed in a variety of vertebrate nervous systems ranging from frogs to rodents to monkeys.
An early study of GABAergic differentiation by cultured embryonic Xenopus spinal neurons revealed a strong dependence on extracellular calcium (Spitzer et al., 1993). Identification of the natural pattern of spontaneous calcium transients led to an appreciation of the correlation between calcium spike frequency and the incidence of GABAergic neurons (Gu and Spitzer, 1995) and calcium spike frequency was shown to regulate the level of GAD67 transcripts (Watt et al., 2000). Recordings of the patterns of spontaneous calcium spikes in identified populations of spinal neurons in the embryonic Xenopus neural tube, coupled with suppression and enhancement spiking, revealed homeostatic regulation of transmitter specification. Suppression of spiking led to an increase in the number of neurons expressing excitatory transmitters and a reduction in the number of neurons expressing inhibitory transmitters. The opposite result was obtained when spiking was enhanced (Borodinsky et al., 2004).
Regulation of the numbers of dopamine neurons by exogenous perturbations of activity is a general feature of the dopaminergic nervous system in developing Xenopus laevis, which displays characteristic patterns of calcium-spike activity in different subpopulations of dopaminergic precursors before and after dopamine expression takes place (Velazquez-Ulloa et al., 2010). Changes in endogenous calcium spiking activity during acquisition of dopamine by the reserve pool annular neurons of the suprachiasmatic nucleus raise the possibility that activity-induced transmitter respecification occurs via a concommitant change in excitability of the neurons. The precursors of dopaminergic neurons in both core and the annulus of the ventral suprachiasmatic nucleus share expression of transcription factor Lim1,2 (Dulcis and Spitzer, 2008), but reserve pool neurons can be identified by the transcription factor Nurr1 before they express the neuropeptide Y (NPY) phenotype (Velazquez-Ulloa et al., 2010). Nurr1 is associated with differentiation of dopamine progenitors (Goridis and Rohrer, 2002; Andersson et al., 2006), and enables identification of these reserve pool neurons before activity-induced respecification takes place.
Tottering mice have a mutation in the P/Q-type calcium channel α1A subunit gene and display a marked reduction of calcium channel currents in their Purkinje neurons during development (Wakamori et al., 1998) associated with expression of tyrosine hydroxylase (TH) mRNA and protein (Hess & Wilson, 1991). Ectopic TH expression by normally GABAergic Purkinje cells in adult mice is not caused by a switch in neurotransmitter expression but occurs by coexpression of GABA and TH. Co-expression persists in adult mutant mice and is associated with ataxia, seizures, and absence epilepsy resembling petit mal epilepsy in humans (Fletcher et al., 1996).
Differentiation of the dopaminergic phenotype in rat cultured postnatal sensory ganglion neurons also depends on electrical activity (Brosenitsch et al., 1998; Brosenitsch & Katz, 2001). Calcium entry achieved by depolarization with potassium chloride or electrical stimulation at physiological frequencies increased the incidence of expression of tyrosine hydroxylase in neurons expressing the PhoxB2 transcription factor, consistent with the partnership between the genetic program and activity in transmitter specification. High frequency stimulation and enhanced calcium entry confirm the existence of putative reserve pool neurons that are able to acquire an additional transmitter phenotype in response to altered activity.
A parallel example of transmitter respecification by changes in signaling in neuronal circuits during development has been described for glutamatergic neurons of the rat hypothalamus (Liu et al., 2008). The cholinergic phenotype was induced in a subset of glutamatergic neurons following chronic in vivo administration of the NMDA receptor blocker, MK-801. Once again, the acquired ACh expression was a partial phenoptypic switch as ACh and glutamate were coreleased by the affected neurons.
Transmitter specification is also modulated by activity in the adult nervous system. Suppression of retinal activity with tetrodotoxin or eyelid suture in young adult monkeys leads to reversible reduction in the number of GABAergic neurons in the visual cortex (Hendry and Jones, 1988). High frequency stimulation causes the appearance of the GABAergic phenotype in normally glutamatergic rat hippocampal granule neurons that project to CA3 neurons (Gutierrez, 2002).
Activity-dependent transmitter specification involves neither neurogenesis nor apoptosis, in cases in which they have been examined, leading to the conclusion that it represents a transfating or repurposing of the neurons in which it occurs. Because neuronal identity is determined by a constellation of properties, including morphology, ion channel expression and a host of molecular markers, transmitter respecification does not entail a change in identity. Examination of the expression of other molecular markers confirmed the retention of identity following transmitter respecification in the spinal cord and hypothalamus (Borodinsky et al., 2004; Dulcis and Spitzer, 2008).
Recruitment of reserve pool neurons
Physiological levels of environmental illumination dynamically regulate the number of dopaminergic neurons in the ventral suprachiasmatic nucleus innervating the melanotrope cells that control pigmentation in Xenopus laevis (Dulcis and Spitzer, 2008). Expansion of the dopaminergic phenotype occurs following two-hour exposure of dark-raised larvae to a white background or to light, via recruitment of NPY-expressing neurons that already innervate the melanotrope cells. NPY neurons respond to increased activity of the retinohypothalamic projection by co-expressing dopamine as an additional neurotransmitter, along with TH, dopamine transporter and vesicular monoamine transporter proteins as well as TH transcripts, all of which were undetected in controls or in dark-raised animals. The functional consequence of this parallel circuit recruitment is that reserve pool neurons are able to restore the camouflage behavior when natively dopaminergic neurons are ablated. When larvae exposed to light are placed back in the dark, NPY-immunoreactive neurons that have been induced to express dopamine return to their default expression of NPY alone, indicating that neurotransmitter respecification is a reversible process.
Activity-dependent recruitment of cholinergic neurons occurs similarly during development of the cervical spinal cord in cat (Chakrabarty et al., 2009; Chakrabarty and Martin, 2010). In this system, activity of the corticospinal tract induces a rapid increase in the number of ChAT-positive neurons during a two-week period. The newly cholinergic neurons are recruited from a reserve pool of spinal neurons that receive the descending cortical input. Unilateral pharmacological blockade of activity during this critical period results in lack of ChAT neuron refinement in the affected spinal cord that resembles the immature pattern of neurotransmitter expression. This study shows that the active connections between spinal neurons during development are the means by which the original population of cells expressing a given neurotransmitter is restructured to establish motor function in the spinal cord.
The mechanism of release of the newly expressed neurotransmitter by the respecified neurons remains to be investigated. However, recent evidence has demonstrated that cotransmission by sympathetic neurons that are environmentally induced to co-express norepinephrine and acetylcholine is not achieved by co-storage and co-release from single varicosities at the same synapses but via concurrent release of transmitters in separated synapses of individual neurons (Vega et al., 2010). Interestingly, the same study showed that the level of segregation between endogenous and induced transmitter release is also modulated by environmental conditions.
Investigation of the plasticity of neurotransmitter expression in neurons in the raphe of the hindbrain reveals activity-dependent recruitment of serotonergic neurons from the region immediately adjacent to the core of this nucleus (Demarque and Spitzer, 2010). Overexpression of voltage-gated sodium or potassium channels to generate global increases and decreases in calcium spiking activity generates changes in the number of serotonergic neurons only in the region of the raphe. Because the axons from newly serotonergic neurons fasciculate with axons of natively serotonergic neurons, they may project to the same targets.
Striatal TH-positive neurons increase in number in mouse and macaque models of Parkinson’s disease when the dopaminergic substantia nigra pars compacta is lesioned (Auman et al., 2008; Tandé et al., 2006). The partial recovery of dopaminergic neurons observed following MPTP- and 6-OHDA-treatments of mice results from phenotypic respecification of neurons intrinsic to the striatum that depends on the activity of Ca2+-activated K+ (SK) channels. Newly TH-immunoreactive neurons in the macaque striatum express both the dopamine transporter and glutamic acid decarboxylase, suggesting that they were recruited from a population of GABAergic interneurons (Tandé et al., 2006). Whether newly TH-positive neurons are functionally integrated into brain circuits remains to be tested.
Recruitment of neurons from reserve pools near lesioned brain regions suggests that the brain may utilize plasticity that evolved to meet a normal range of physiological demands in order to achieve repair in response to damage. The likelihood of achieving successful repair would be enhanced by the proximity of reserve pool neurons to the damaged circuit. The process of respecification involves an activity-dependent trigger and restriction of transmitter co-expression to the neurons that display correct circuit integration for the function that is to be restored. However, if the brain is capable of this form of self-repair, why do Parkinson’s disease models exhibit symptoms and why do neurological disorders exist? Reserve pools may not be available for recruitment, the reserve circuit may not receive an adequate level of activity to drive respecification, or the damage to the circuit may be so great that it can no longer be compensated by reserve pool recruitment. Reserve pool neuron transmitter respecification may also generate new circuit integration errors, leading to co-release of neurotransmitters at inappropriate synapses. Such a process could explain how respecification causes new neurological symptoms, such as the ataxia phenotype observed in tottering mice with dopaminergic/GABAergic Purkinje cells (Fletcher et al., 1996).
Reserve pool candidates in the CNS
Neuronal networks in the CNS are now identifiable by their neuronal composition, anatomical circuit connectivity, physiological input and output, and molecular signatures of transcription factors, ion channels and neurotransmitters, both during development and in the adult. Sufficient information has been obtained to design experiments to test the existence of additional reserve pools in the CNS. Since recruitment of reserve pool neurons is activity-dependent, we highlight some brain nuclei where this form of plasticity can be tested by activation of sensory circuits.
The basal ganglia and substantia nigra have high clinical relevance and have been among the most intensively studied neural networks in the rodent and primate brain (Parent and Hazrati, 1995; Joel and Weiner; 2000; Kreitzer and Malenka, 2008). When massive cell death occurs in the neuromelanin-pigmented and dopamine-containing substantia nigra, the lack of dopamine release in the striatum causes a number of neurological symptoms leading to Parkinson’s disease (Hirsh et al., 1988). Fortunately the dopaminergic neurons of the substantia nigra pars compacta are not the only neurons projecting to the striatum (Grofova et al., 1982).
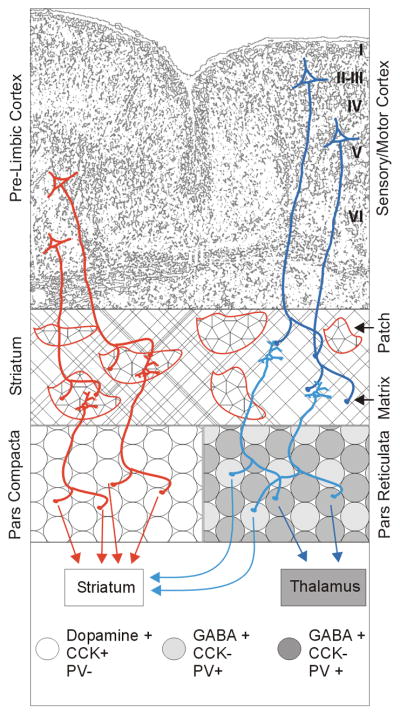
The rat substantia nigra is composed of the pars compacta, which is solely dopaminergic, and the pars reticulata, organized in large clusters of GABAergic neurons with thin intermingled stripes of dopaminergic neurons (Gonzalez-Hernandez and Rodriguez, 2000). Remarkably, the pars reticulata remains intact in Parkinson’s model systems (Patt et al., 1991), and the combination of retrograde labeling and immunocytochemistry has shown that dorsoventrally elongated clusters of the GABAergic neurons of the pars reticulata already project to the caudate nucleus in cat (Hontanilla et al, 1996) and rat (Gonzalez-Hernandez and Rodriguez, 2000). The existence of this nondopaminergic nigrostriatal pathway was elegantly confirmed by Rodriguez and Gonzalez-Hernandez (1999) by antidromic responses to striatal stimulation, retrograde HRP labeling and resistance to 6-OHDA. These GABAergic, parvalbumin-expressing neurons represent more than 80% of nigrostriatal neurons in Parkinson-induced rats (see circuit scheme in Figure 2).
Figure 2.
The neuronal network activating the substantia nigra in the mammalian brain. In red, the cortico-nigral pathway that includes pyramidal neurons of layers V–VI of the pre-limbic cortex, striatal neurons of the patches, and their target dopaminergic neurons in the substantia nigra pars compacta (white circles) that project to the striatum. In blue, the cortico-nigral pathway that includes pyramidal neurons from layers I–III and V of the sensory/motor cortex, striatal neurons of the matrix, and their two target classes of nigral pars reticulata GABA-ergic neurons that project to the thalamus (dark gray circles) and the striatum (light gray circles), respectively.
Consistent with their potential role as a reserve pool that can rescue the loss of dopaminergic neurons, nondopaminergic nigrostriatal neurons share afferents with dopaminergic neurons in addition to sharing the same target, the striatum (Gerfen 1984; 1985; Gerfen et al., 1985; 1987). Whether specific circuit activity can induce the nondopaminergic nigrostriatal reserve pool to acquire the dopaminergic phenotype and reverse behavioral deficits remains to be tested.
The olfactory system in rodents also appears wired for this form of plasticity (Serguera et al., 2008). The main olfactory epithelium that is specialized for detection of odorants projects fibers to the juxtaglomerular dopaminergic internurons in the main olfactory bulb. These interneurons process odorant signals and dopamine regulates odor discrimination and some forms of olfactory learning (Coopersmith et al., 1991; Yue et al., 2004). Investigation of the effect of circuit-specific activation will determine whether neurotransmitter respecification can be achieved via recruitment of reserve pool interneurons in the main olfactory bulb specifically in response to exposure to relevant odorants, leading to changes in odorant-evoked behavior.
Potential clinical benefits
Current clinical treatments of neurotransmitter-related disorders include pharmacology to restore appropriate levels of transmitter or modulate transmitter receptor activation, embryonic stem cell transplantation to replace degenerated cells, surgical removal of brain nuclei to reduce excitation in epilepsy, electroconvulsive therapies, deep brain stimulation (DBS), and transcranial magnetic stimulation (TMS).
Restoration of neuronal circuit function by transmitter respecification in reserve pool neurons is an attractive prospect for several reasons. Manipulation of activity differs from other treatments in several significant regards. The first is that both application and onset of detectable effects can be achieved in a relatively short period of time. Respecification of NPY/GABA annular neurons to include a dopaminergic phenotype is induced by two hours of light exposure and is sufficient to rescue camouflage behavior in MPTP-treated Xenopus laevis larvae (Dulcis and Spitzer, 2008). Transmitter respecification hijacks the existing circuitry and appears to be relatively rapid.
A second appealing aspect of transmitter respecification in reserve pool neurons is the prospect for non-invasive treatments for neurological disorders. Stimulation of sensory nerves or focal repetitive transcranial magnetic stimulation (rTMS) are two steps toward this goal. Vagus nerve stimulation therapy is approved by FDA for treatment of medication-resistant depression and for epilepsy. Vagus nerve stimulation alters concentrations of GABA, serotonin, norepinephrine, and glutamate in the brain, suggesting an activity-dependent correction of dysfunctional neurotransmitter modulatory circuits (Ressler and Mayberg, 2007). Specific human behaviors can be altered by rTMS applied to selected brain regions (Yoo et al., 2008). High frequency rTMS modulation of the motor cortex led to rapid enhancement of motor performance and increased sensory threshold that were confirmed by functional MRI. Transmitter respecification in reserve pool neurons may provide a mechanistic understanding of effectiveness of some of the current techniques involving activity manipulation.
Finally, it is becoming clear that selective circuit-activation can induce striking results in restoring function to diseased circuits. Optogenetics has been used to achieve optical DBS of afferent axons projecting to the subthalamic nucleus of hemiparkinsonian rats injected with 6-OHDA (Gradinaru et al., 2009). Although this method is invasive, these experiments demonstrate that circuit-specific activation can lead to enhanced therapeutic effects in the adult mammalian brain with fewer nonspecific effects than obtained with classical DBS. In primates, mesencephalic dopaminergic neurons project to both basal ganglia and frontal cortex, and motor learning during training is coupled to changes in dopamine release in frontal cortical areas in healthy human subjects (Garraux et al., 2007). These results suggest that task-activated circuitry can achieve circuit activation to direct local neurotransmitter specification, expression and release. We conjecture that therapies that train a subject’s mind to control selective circuit activation across distant regions in the brain without external manipulation of activity engage transmitter respecification in reserve pool neurons.
Selective activation of brain circuits eliciting transmitter respecification in reserve pool neurons may contribute to activity-dependent plasticity in damaged circuits. In the postembryonic nervous system, where neurogenesis is restricted to a few regions in the brain and the extracellular environment no longer provides appropriate cues to guide axonal pathfinding of ectopically and surgically implanted embryonic stem cells, transmitter respecification in reserve pool neurons may achieve transmitter replacement in damaged neural circuits. Activity-dependent replacement of neurotransmitters in adult neuronal circuits that share the same target with the diseased circuits would have clinical impact on neurodegenerative disorders such as Parkinson’s and Alzheimer’s, movement disorders such Huntington’s disease, and restoration of function lost due to neural tissue damage caused by stroke or other small brain lesions. This process could be particularly relevant in mood disorders such as depression and schizophrenia and developmental disorders in establishing brain wiring. Although there is potential for therapeutic intervention, neurodegenerative disorders with significant psychological and neurological impairment involve extensive damage that could limit the efficacy of neurotransmitter respecification via circuit activation. However, this plasticity may be induced following minor stroke damage or as a preventive approach to keeping healthy individuals “healthy” when the ability of reserve pool neurons to provide therapeutic benefit is still intact.
Conclusions and Questions
Activity-dependent homeostatic plasticity in the CNS has been analyzed in detail and shown to involve changes in synaptic strength, number of synapses, or neuronal excitability (Nelson and Turrigiano, 2008) (Figure 3). A common feature of these classical forms of plasticity is that they all affect postsynaptic firing leading to changes in neurotransmitter release by the “plastic” neuron. In this way, activity exerts spatial and temporal control of synaptic communication within the same circuit.
Figure 3.
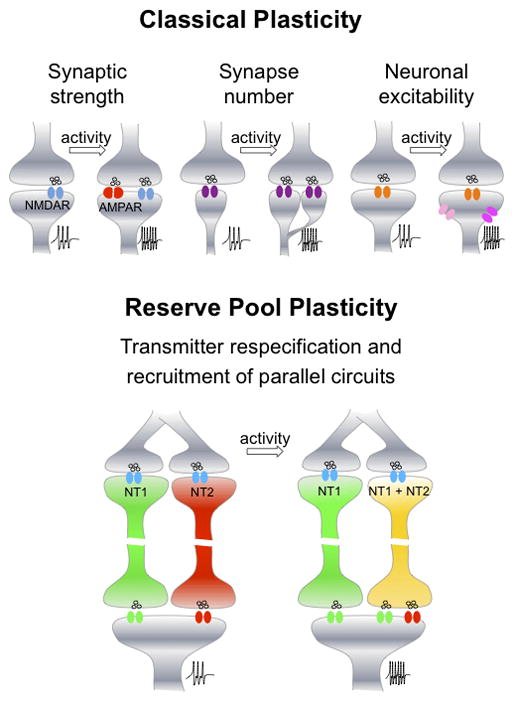
Comparison of classical plasticity and reserve pool plasticity. Increases in synaptic strength, synapse number and neuronal excitability are forms of activity-dependent plasticity that affect the level of firing of the postsynaptic neuron (indicated as action potentials at higher frequency). Activity-dependent neurotransmitter respecification in reserve pool neurons entails a change in the expression of neurotransmitters in a parallel circuit innervating the same target cells; summation of the input from the core circuit and the parallel reserve pool circuit affects the level of firing of the target cells. Both classical and reserve pool plasticity affect the amount of neurotransmitter released on the postsynaptic cell.
Reserve pool plasticity, however, allows changes in transmitter identity by induction or elimination of coexpression that affects the output of a parallel circuit innervating the same target cells. This fast functional switching of transmitter release in shared target brain regions provides additional flexibility to achieve experience-dependent adaptation and homeostatic regulation of circuit function.
New research technologies and newly identified neural circuits can now be used to test the the existence of reserve pool neuron recruitment in circuits in the young and adult brain. What is the generality of this novel form of plasticity? Is this kind of circuit reconfiguration achievable everywhere in the brain (all neurons are part of a “reserve” pool for some circuit)? Or is it restricted to particular regions of the brain or particular circuits? It is possible that some circuits cannot rely on “spare” cells in the network that display this capacity. What is the molecular mechanism of this novel form of plasticity? We hypothesize that reserve pool neurons either have or lack particular molecular machinery that allows them to gain or lose a neurotransmitter in response to circuit activity. It would be of great interest to identify such machinery, which could be used to introduce this plasticity into circuits that lack it.
Acknowledgments
We thank the members of our lab for productive discussions. Supported by NIH NS15918 and MH74702 to N.C.S.
References
- 1.Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 2.Aumann TD, Gantois I, Egan K, Vais A, Tomas D, Drago J, Horne MK. SK channel function regulates the dopamine phenotype of neurons in the substantia nigra pars compacta. Experimental Neurology. 2008;213:419–430. doi: 10.1016/j.expneurol.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Belousov AB, O’Hara BF, Denisova JV. Acetylcholine becomes the major excitatory neurotransmitter in the hypothalamus in vitro in the absence of glutamate excitation. J Neurosci. 2001;21:2015–2027. doi: 10.1523/JNEUROSCI.21-06-02015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belousov AB, Hunt ND, Raju RP, Denisova JV. Calcium-dependent regulation of cholinergic cell phenotype in the hypothalamus in vitro. J Neurophysiol. 2002;88:1352–1362. doi: 10.1152/jn.2002.88.3.1352. [DOI] [PubMed] [Google Scholar]
- 5.Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu XN, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- 6.Borodinsky LN, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3667–3667. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brosenitsch TA, Salgado-Commissariat D, Kunze DL, Katz DM. A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons. Journal of Neuroscience. 1998;18:1047–1055. doi: 10.1523/JNEUROSCI.18-03-01047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosenitsch TA, Katz DM. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. Journal of Neuroscience. 2001;21:2571–2579. doi: 10.1523/JNEUROSCI.21-08-02571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brosenitsch TA, Katz DM. Expression of Phox2 transcription factors and induction of the dopaminergic phenotype in primary sensory neurons. Molecular and Cellular Neuroscience. 2002;20:447–457. doi: 10.1006/mcne.2002.1135. [DOI] [PubMed] [Google Scholar]
- 10.Cesa R, Scelfo B, Strata P. Activity-dependent presynaptic and postsynaptic structural plasticity in the mature cerebellum. Journal of Neuroscience. 2007;27:4603–4611. doi: 10.1523/JNEUROSCI.5617-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cesa R, Morando L, Strata P. Transmitter-receptor mismatch in GABAergic synapses in the absence of activity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18988–18993. doi: 10.1073/pnas.0806979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarty S, Shulman B, Martin JH. Activity-dependent codevelopment of the corticospinal system and target interneurons in the cervical spinal cord. Journal of Neurosciene. 2009;27:8816–8827. doi: 10.1523/JNEUROSCI.0735-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarty S, Martin JH. Postnatal development of a segmental switch enables corticospinal tract transmission to spinal forelimb motor circuits. Journal of Neuroscience. 2010;6:2277–2288. doi: 10.1523/JNEUROSCI.5286-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coopersmith R, Weihmuller FB, Kirstein CL, Marshall JF, Leon M. Extracellular dopamine increases in the neonatal olfactory-bulb during odor preference training. Brain Research. 1991;564:149–153. doi: 10.1016/0006-8993(91)91365-8. [DOI] [PubMed] [Google Scholar]
- 15.Demarque M, Spitzer NC. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron. 2010;67:321–34. doi: 10.1016/j.neuron.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- 17.Destexhe A, Marder E. Plasticity in single neuron and circuit computations. Nature. 2004;431:789–795. doi: 10.1038/nature03011. [DOI] [PubMed] [Google Scholar]
- 18.Dulcis D, Spitzer NC. Illumination controls differentiation of dopamine neurons regulating behaviour. Nature. 2008;456:195–U120. doi: 10.1038/nature07569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher CF, Lutz CM, Osullivan TN, Shaughnessy JD, Hawkes R, Frankel WN, Copeland NG, Jenkins NA. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- 21.Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- 22.Garraux G, Peigneux P, Carson RE, Hallett M. Task-related interaction between basal ganglia and cortical dopamine release. Journal of Neuroscience. 2007;27:14434–14441. doi: 10.1523/JNEUROSCI.1595-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerfen CR. The neostriatal mosaic - compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- 24.Gerfen CR. The neostriatal mosaic 1. Compartmental organization of projections from the striatum to the substantia nigra in the rat. Journal of Comparative Neurology. 1985;236:454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- 25.Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic - Compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic 2. Patch-directed and matrix-directed mesostriatal dopaminergic and nondopaminergic systems. Journal of Neuroscience. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Current Opinion in Neurobiology. 2006;16:385–390. doi: 10.1016/j.conb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cerebral Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Hernandez T, Rodriguez M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. Journal of Comparative Neurology. 2000;421:107–135. doi: 10.1002/(sici)1096-9861(20000522)421:1<107::aid-cne7>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nature Reviews Neuroscience. 2002;3:531–541. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- 31.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of Parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grofova I, Deniau JM, Kitai ST. Morphology of the substantia nigra pars reticulata projection neurons intracellularly labeled with HRP. Journal of Comparative Neurology. 1982;208:352–368. doi: 10.1002/cne.902080406. [DOI] [PubMed] [Google Scholar]
- 33.Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–7. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 34.Gutiérrez R. Activity-dependent expression of simultaneous glutamatergic and GABAergic neurotransmission from the mossy fibers in vitro. J Neurophysiol. 2002;87:2562–2570. doi: 10.1152/jn.2002.87.5.2562. [DOI] [PubMed] [Google Scholar]
- 35.Gutiérrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Gutiérrez R, Romo-Parra H, Maqueda J, Vivar C, Ramìrez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendry SH, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1:701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- 38.Hess EJ, Wilson MC. Tottering and leaner mutations perturb transient developmental expression of tyrosine-hydroxylase in embryologically distinct Purkinje-cells. Neuron. 1991;6:123–132. doi: 10.1016/0896-6273(91)90127-l. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s-disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 40.Hontanilla B, delasHeras S, GimenezAmaya JM. A topographic re-evaluation of the nigrostriatal projections to the caudate nucleus in the cat with multiple retrograde tracers. Neuroscience. 1996;72:485–503. doi: 10.1016/0306-4522(95)00547-1. [DOI] [PubMed] [Google Scholar]
- 41.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 42.Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: An analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 43.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Masson G, Marder E, Abbott LF. Activity-dependent regulation of conductances in model neurons. Science. 1993;259:1915–1917. doi: 10.1126/science.8456317. [DOI] [PubMed] [Google Scholar]
- 45.Liu XH, Popescu IR, Denisova JV, Neve RL, Corriveau RA, Belousov AB. Regulation of cholinergic phenotype in developing neurons. Journal of Neurophysiology. 2008;99:2443–2455. doi: 10.1152/jn.00762.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–U433. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 47.Luther JA, Birren SJ. p75 and TrkA signaling regulates sympathetic neuronal firing patterns via differential modulation of voltage-gated currents. Journal Neuroscience. 2009;29:5411–5424. doi: 10.1523/JNEUROSCI.3503-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 49.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nature Review Neuroscience. 2006;7:563–74. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 50.Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46:623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. Journal of Neuroscience. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ovtscharoff W, Segal M, Goldin M, Helmeke C, Kreher U, Greenberger V, Herzog A, Michaelis B, Braun K. Electron microscopic 3D-reconstruction of dendritic spines in cultured hippocampal neurons undergoing synaptic plasticity. Developmental Neurobiology. 2008;68:870–876. doi: 10.1002/dneu.20627. [DOI] [PubMed] [Google Scholar]
- 54.Parent A, Hazrati LN. Functional-anatomy of the basal ganglia 1. The cortico-basal ganglia-thalamo-cortical loop. Brain Research Reviews. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 55.Patt S, Gertz HJ, Gerhard L, Cervosnavarro J. Pathological changes in dendrites of substantia nigra neurons in Parkinson’s disease - a Golgi study. Histology and Histopathology. 1991;6:373–380. [PubMed] [Google Scholar]
- 56.Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neuroscience. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez M, Gonzalez-Hernandez T. Electrophysiological and morphological evidence for a GABAergic nigrostriatal pathway. Journal of Neuroscience. 1999;19:4682–4694. doi: 10.1523/JNEUROSCI.19-11-04682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serguera C, Triaca V, Kelly-Barrett J, Al Banchaabouchi M, Minichiello L. Increased dopamine after mating impairs olfaction and prevents odor interference with pregnancy. Nature Neuroscience. 2008;11:949–956. doi: 10.1038/nn.2154. [DOI] [PubMed] [Google Scholar]
- 60.Spitzer NC, Debaca RC, Allen KA, Holliday J. Calcium dependence of differentiation of GABA immunoreactivity in spinal neurons. J Comp Neurol. 1993;337:168–175. doi: 10.1002/cne.903370111. [DOI] [PubMed] [Google Scholar]
- 61.Tandé D, Hoglinger G, Debeir T, Freundlieb N, Hirsch EC, Francois C. New striatal dopamine neurons in MPTP-treated macaques result from a phenotypic shift and not neurogenesis. Brain. 2006;129:1194–1200. doi: 10.1093/brain/awl041. [DOI] [PubMed] [Google Scholar]
- 62.Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in intrinsic electrical properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- 63.Turrigiano GG. The self tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turrigiano GG. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 65.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–U1256. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velázquez-Ulloa NA, Spitzer NC, Dulcis D. Contexts for dopamine specification by calcium spike activity in the CNS. Journal Neuroscience. 2011;31:78–88. doi: 10.1523/JNEUROSCI.3542-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wakamori M, Yamazaki K, Matsunodaira H, Teramoto T, Tanaka I, Niidome T, Sawada K, Nishizawa Y, Sekiguchi N, Mori E. Single tottering mutations responsible for the neuropathic phenotype of the P-type calcium channel. Journal of Biological Chemistry. 1998;273:34857–34867. doi: 10.1074/jbc.273.52.34857. [DOI] [PubMed] [Google Scholar]
- 68.Watt SD, Gu X, Smith RD, Spitzer NC. Specific frequencies of spontaneous Ca2+ transients upregulate GAD 67 transcripts in embryonic spinal neurons. Mol Cell Neurosc. 2000;16:376–387. doi: 10.1006/mcne.2000.0871. [DOI] [PubMed] [Google Scholar]
- 69.Yasuda R, Sabatini BL, Svoboda K. Plasticity of calcium channels in dendritic spines. Nature Neuroscience. 2003;6:948–955. doi: 10.1038/nn1112. [DOI] [PubMed] [Google Scholar]
- 70.Yoo WK, You SH, Ko MH, Kim ST, Park CH, Park JW, Ohn SH, Hallett M, Kimg YH. High frequency rTMS modulation of the sensorimotor networks: Behavioral changes and fMRI correlates. Neuroimage. 2008;39:1886–1895. doi: 10.1016/j.neuroimage.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 71.Yue EL, Cleland TA, Pavlis M, Linster C. Opposing effects of D-1 and D-2 receptor activation on odor discrimination learning. Behavioral Neuroscience. 2004;118:184–190. doi: 10.1037/0735-7044.118.1.184. [DOI] [PubMed] [Google Scholar]