Abstract
Developing neurons use a combination of guidance cues to assemble a functional neural network. A variety of proteins immobilized within the extracellular matrix (ECM) provide specific binding sites for integrin receptors on neurons. Integrin receptors on growth cones associate with a number of cytosolic adaptor and signaling proteins that regulate cytoskeletal dynamics and cell adhesion. Recent evidence suggests that soluble growth factors and classic axon guidance cues may direct axon pathfinding by controlling integrin-based adhesion. Moreover, since classic axon guidance cues themselves are immobilized within the ECM and integrins modulate cellular responses to many axon guidance cues, interactions between activated receptors modulate cell signals and adhesion. Ultimately, growth cones control axon outgrowth and pathfinding behaviors by integrating distinct biochemical signals to promote the proper assembly of the nervous system. In this review, we discuss our current understanding how ECM proteins and their associated integrin receptors control neural network formation.
Keywords: axon pathfinding, actin, microtubules, laminin
Neuronal growth cones
Growth cones are sensory-motile specializations at the ends of extending axons and dendrites of developing neurons (Figure 1). The proper assembly of the nervous system depends on the ability of growth cones to detect molecular cues in their environment and respond with guided process outgrowth (Dent and Gertler, 2003; Huber et al., 2003; Lowery and Van Vactor, 2009). Growth cones express a variety of receptors at their cell surface and even at the tips of filopodia (Letourneau and Shattuck, 1989; O'Donnell et al., 2009), which bind extracellular ligands. Receptors function as adhesive contacts with the surrounding environment, but also activate biochemical signals within growth cones. Receptors activate signals that positively or negatively influence axon outgrowth and if locally generated, graded signals within growth cones promote axon turning. Coordination between biochemical signals and receptor adhesion is emerging as an important regulatory mechanism to control axon pathfinding.
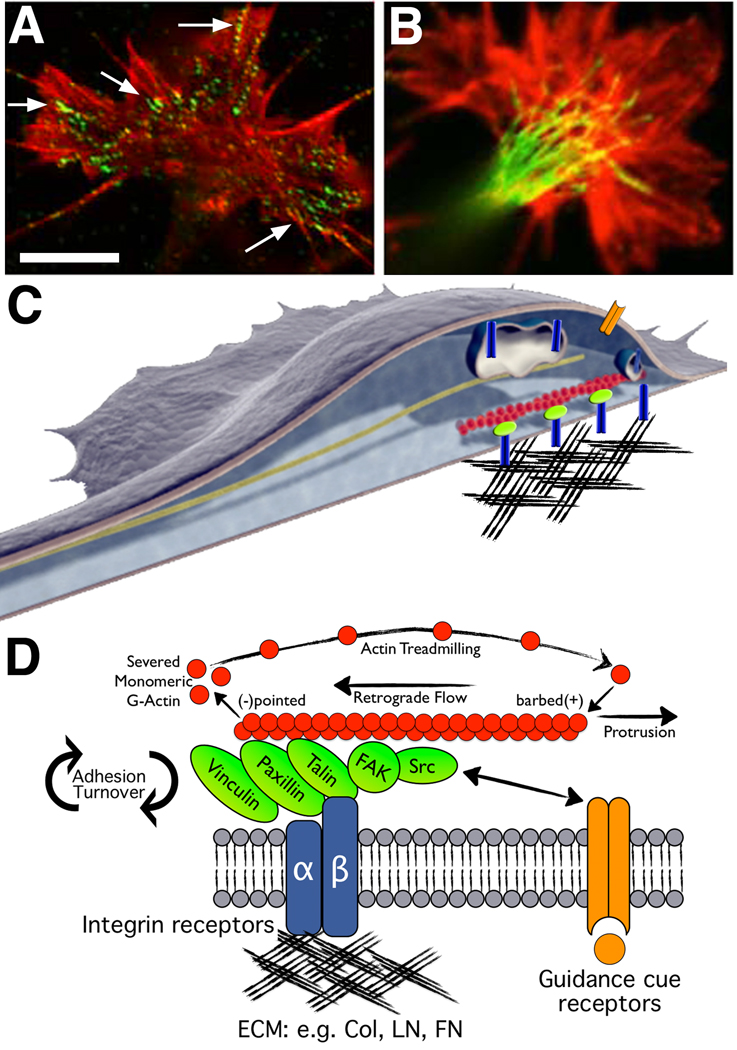
Figure 1. Growth cones assemble macromolecular adhesion complexes (point contacts) that link ECM proteins to actin filaments.
A. A Xenopus spinal neuron growth cone immuno-labelled for phospho-Tyr118-paxillin (green) and F-actin (red). Note multiple point contact adhesions along actin filaments (arrows). B. A Xenopus spinal neuron growth cone immuno-labelled for β-tubulin (green) and F-actin (red). Note that microtubules may regulate the trafficking of vesicles containing integrin and guidance cue receptors. Scale, 5 µm. C. Schematic representation of a growth cone (adapted from (Kamiguchi and Lemmon, 2000)) on the ECM with several integrin receptors (blue) linked to actin filaments through adhesion complexes (green). Integrin receptor trafficking within recycling endosomes (blue vesicles) along microtubules (dark green) may regulate axon guidance (see text for details) A guidance cue/growth factor receptor is illustrated on the apical surface (orange). D. Schematic representation of key molecular components of growth cone point contact adhesions. Integrin αβ heterodimeric receptors (dark blue lines) bind to proteins within the ECM, such as Col, LN and FN. Integrin activation leads to the assembly of multiple scaffolding proteins, such as talin, paxillin and vinculin to the cytoplasmic tail of integrins. In addition, FAK and Src are activated by clustering of integrin receptors, and they modulate the composition of adhesions through phosphorylation of key residues that allow for binding of many additional proteins (not shown). Several scaffolding proteins bind directly to actin filaments (red), which is believed to restrain retrograde flow and allow the force of actin polymerization to generate membrane protrusion. Guidance cue receptors (orange) can also regulate adhesion-associated proteins through binding and activation of FAK and Src. Cross-talk through FAK/Src signaling modulates adhesion assembly and turnover, as well as regulation of the actin cytoskeleton.
ECM composition and function
The ECM is composed of a heterogeneous mixture of glycoproteins and proteoglycans (PGs), including laminin, fibronectin, collagen, tenascin and heparan sulfate PGs (Table 1). These ECM molecules are synthesized and secreted by neurons and supporting cells into the interstitial spaces surrounding developing neurons. Large ECM proteins self-assemble to become immobilized into a semi-rigid scaffold that supports cell adhesion and traction forces. Importantly, both the mechanical and chemical properties of the ECM influence cell motility (Venstrom and Reichardt, 1993; Letourneau et al., 1994). Different ECM proteins have been shown to positively and negatively influence neurite outgrowth in vitro and in vivo. While supporting axon outgrowth, some ECM proteins are also specifically targeted by degradative proteases, providing an adaptable cellular substratum. Complicating matters further, ECM molecules can bind smaller secreted proteins and work cooperatively with soluble growth factors to influence axon outgrowth (Hynes, 2009). First we discuss our current understanding for the roles of several key ECM proteins in the regulation of growth cone motility and axon guidance. Later we discuss the integrin receptors used by developing neurons to bind ECM components. Lastly, we discuss intracellular signaling by integrins in cooperation with axon guidance cue receptors and the function of growth cone point contacts. Note that due to space limitations we do not focus on PGs here, but direct readers to recent reviews on this topic (Holt and Dickson, 2005; de Wit and Verhaagen, 2007).
Table 1. ECM proteins and associated integrin receptors involved in the morphological differentiation of neurons.
Note that in some instances non-integrin receptors are listed in parenthesis.
| ECM molecule | Integrin receptor |
Neuronal type | Function | References |
|---|---|---|---|---|
| Collagen | α1β1. | Rat sympathetic RGCs | Neurite outgrowth | (Lein et al., 1991) |
| α2β1. | DRG | Adhesion and neurite outgrowth | (Bradshaw et al., 1995) | |
| (protomer of 3 α chains) 28 Col isoforms Non fibrillar, fibrillar-forming, fibrillar associated Adapted from (Kalluri, 2003) |
α?β8. | Motor, DRG | Neurite outgrowth | (Venstrom and Reichardt, 1995) |
| (DDR1) | Cerebellar granule neurons | Neurite outgrowth | (Bhatt et al., 2000) | |
| Laminin | α1β1, α1β8 | DRG | Neurite outgrowth | (Rivas et al., 1992; Venstrom and Reichardt, 1995) |
 |
α1β1,α3β1 | PC12, DRG, Cortical | Neurite outgrowth; migration | (Tomaselli et al., 1990; Tomaselli et al., 1993; Anton et al., 1999) |
| (trimer, αβγ chains) 5α, 3β, 3γ known 15 LN isoforms Adapted from (Aumailley et al., 2005) |
α1β1, α4β1 | Neural crest | Cell adhesion | (Lallier and Bronner-Fraser, 1993) |
| α6β1 | Retinal, Olfactory; Ciliary ganglion | Neurite outgrowth and migration | (Calof et al., 1994; Weaver et al., 1995) | |
| α3β1, α7β1 | Cortical | Neuritogenesis | (Gupton and Gertler, 2010) | |
| α3β1, α6β1, α7β1 | Adult DRG | Adhesion and neurite outgrowth | (Plantman et al., 2008) | |
| α3β1, α6β1 | Retinal, Hippocampal, Thalamic, Cortical | Adhesion and neurite outgrowth | (Ivins et al., 1998) | |
| Tenascin | α8β1 | Motor, DRG | Neuritogeneis and neurite outgrowth | (Varnum-Finney et al., 1995) |
 |
α7β1 | cerebellar granule | Neurite outgrowth | (Mercado et al., 2004) |
| α9β1 | PC12, Adult DRG | Neurite outgrowth and regeneration | (Andrews et al., 2009) | |
| (hexbrachion, 190–300 kDa subunits) TN-C, TN-R, TN-W, TN-X, TN-Y Adapted from (Jones and Jones, 2000) |
(contactin) | Hippocampal | Neurite outgrowth | (Michele and Faissner, 2009) |
| Fibronectin | α4β1 | Chick DRG and sympathetic; Mouse retinal ganglion | Neurite outgrowth | (Humphries et al., 1988; Hikita et al., 2003) |
 |
α5β1 | Cortical; Striatal progenitor cells | Neuronal differentiation and migration | (Yoshida et al., 2003; Tate et al., 2004) |
| (~440 kDa dimer) 20 alternative spiced monomers Adapted from (Leiss et al., 2008) |
α8β1 | Chick DRG; Tectal | Adhesion, neurite outgrowth and migration | (Muller et al., 1995; Stettler and Galileo, 2004) |
| αvβ1; αvβ3, αvβ5 | Neural crest | Adhesion and migration | (Delannet et al., 1994) | |
|
Vitronectin 75 kDa monomer |
αvβ5 | Retinal; Cerebellar granule | Neurite outgrowth | (Neugebauer et al., 1991; Murase and Hayashi, 1998) |
DDR1, Discoidin domain receptor 1.
Collagen
Collagenous proteins were once viewed as simple structural and scaffolding molecules that only add tensile strength to tissues. However, there are at least 28 different collagen proteins in vertebrates that are expressed in tissue-specific patterns during development. These diverse proteins play a number of direct and indirect roles in the development of the nervous system ranging from adhesive ligands to differentiation factors to chemotropic agents.
Non-fibril-forming collagens and collagen-like proteins are widely expressed in both the developing central and peripheral nervous system (CNS and PNS) (Fox, 2008; Hubert et al., 2009), with some specific forms expressed by neurons (Sumiyoshi et al., 2001; Sund et al., 2001; Fox et al., 2007; Hubert et al., 2007). A number of studies have shown collagens are permissive substrata for neural cell migration, differentiation, and neurite outgrowth in culture (Venstrom and Reichardt, 1993; Letourneau et al., 1994; Watanabe et al., 2007). Collagens also provide cues that orient axon outgrowth to allow accurate axon pathfinding. For example, a mutation in the cle-1 gene in C. elegans, a type XV/XVIII collagen homologue expressed by motoneurons (MNs), disrupts normal cell migration and motor axon guidance (Ackley et al., 2001). The cle-1 mutation is a deletion of the COOH-terminal endostatin domain termed the NC1 domain and could be rescued by ectopic expression of the trimer-forming NC1 domain. Similarly, the drosophila multiplexin gene (dmp), which is related to collagens XV and XVIII, has also been reported to have a role in motor axon pathfinding that depends on its endostatin domain (Meyer and Moussian, 2009).
Collagen proteins have also been shown to play essential roles in axon guidance in vertebrates. For example, in Zebrafish diwanka mutants, MN growth cones fail to exit the spinal cord into the periphery (Granato et al., 1996; Zeller and Granato, 1999; Schneider and Granato, 2006). diwanka mutations were found to be in a lysyl hydroxylase protein (LH3), an enzyme with glycosyltransferase activity that modifies type XVIII collagen. Interestingly, the glycosyltransferase activity of LH3 functions within adaxial cells to chemically modify collagen XVIII that is deposited on the surface of the developing myotome. In this model, collagen XVIII is glycosylated by LH3 and secreted into the ECM where it becomes a suitable substratum to promote the exit of motor axons into the periphery. At later stages of motor axon development, loss of another collagen disrupts proper pathfinding at intermediate choice points in the peripheral myotome (Beattie et al., 2000). In the zebrafish stumpy mutant, trunk motor axons stall at their intermediate targets. Positional cloning revealed that stumpy contains a mutation in the zebrafish homolog of collagen XIXa1. Collagen XIXa1 expression was observed in a temporal and spatial pattern consistent with a role in motor axon guidance at intermediate targets (Hilario et al.).
In addition to MN pathfinding, collagens also play roles in target recognition during arborization of retino-tectal axons. In zebrafish embryos, retinal ganglion cell axons terminate in one of four main retinorecipient tectal layers. In dragnet mutants, retinal axon arbors are mistargeted within the tectum (Xiao et al., 2005; Xiao and Baier, 2007). The dragnet gene encodes the α5 chain of collagen IV, a network-forming non-fibrillar collagen. Evidence suggest that col4α5 is required for axon targeting, but not pathfinding, as axons in dragnet mutants reach appropriate retinorecipient tectal layers, but sprout promiscuously into inappropriate layers. Interestingly, col4α5 mRNA is absent from the tectal neurons, but is expressed within the epidermal cells lining the tectum. Heparan sulfate PGs normally bind collagen in the tectal basement membrane, but they are dispersed in the dragnet mutant. These findings suggest that col4α5-containing basement membrane anchors secreted factors that contribute to the proper targeting and confinement of retinal arbors to correct tectal layers.
Laminin
Laminins are heterotrimeric glycoproteins, consisting of α, β, and γ subunits, which assemble into a characteristic cruciform structure that is a major component of the ECM in the developing and mature CNS (Venstrom and Reichardt, 1993; Letourneau et al., 1994) (Table 1). Laminin (LN) is a large molecule with numerous biological functions, including regulation of cell adhesion, motility and differentiation. Moreover, different αβγ subunits assemble to form 15 unique LN isotypes with distinct functions. For example, LN-1 (α1β1γ1, LN-111) promotes robust axon outgrowth as a substratum for a variety of CNS and PNS neurons in culture. However, LN isoforms vary in their tissue distribution and ability to promote neuritogenesis (Plantman et al., 2008). In fact, some isoforms of LN, such as s-laminin, which contains the β2 subunit, actively stabilize presynaptic MN terminals and promote pre- and post-synaptic differentiation (Noakes et al., 1995; Son et al., 1999; Nishimune et al., 2008) (see review in this issue by (Singhal and Martin, 2011). LN isoforms stimulate axon extension primarily through adhesion and signaling downstream of integrin receptors (Kuhn et al., 1995; Halfter, 1996; Patton et al., 1997; Adams et al., 2005), although other receptors such as syndecans or dystroglycan may be involved in some neurons. A number of in vitro studies have demonstrated that LN-1 acts not only as a simple permissive substratum, but if provided locally, can direct growing axons (Gomez et al., 1995; Kuhn et al., 1995; Halfter, 1996; Patton et al., 1997; Kuhn et al., 1998; Adams et al., 2005; Turney and Bridgman, 2005).
While LN in isolation has potent effects on neuronal morphogenesis, it likely also functions to modulate responses to axon guidance cues in vivo, as a number of studies have now observed modulatory effects of LN in vitro. For example, LN-1 was shown to convert chemoattraction of retinal ganglion cell (RGC) neurons toward netrin into repulsion (Hopker et al., 1999), which may be due to reduced levels of cyclic AMP (cAMP) within growth cones on LN. This switch may function at the optic nerve head (ONH), where netrin-1 is enriched and axons turn sharply to exit the eye. As LN-1 is reduced within the ONH, but is high within the optic fiber layer and optic stalk surrounding the ONH (Cohen et al., 1987), netrin may transiently attract RGC axons into the ONH, but subsequently repel axons out of the retina and into the optic stalk (Mann et al., 2004). The effects of the inhibitory factor EphB is also LN-sensitive. EphB has no effect on RGC axons growing on the cell adhesion molecule L1, but when cultured on a combined LN-L1 substratum, growth cones pause, but remain dynamic in response to EphB (Suh et al., 2004). Interestingly, in the developing visual pathway, L1 is present on retinal axons (Bartsch et al., 1989; Hankin and Lagenaur, 1994; Lyckman et al., 2000), while LN is expressed within the optic disc where RGC axons converge (Hopker et al., 1999). The presence of EphB in this region may therefore promote growth cone pausing to ensure that RGC axons do not overshoot this exit point. Moreover, pausing may allow sufficient time for growth cones to detect guidance molecules, such as netrin-1 and switch their response to be repelled into the optic nerve (Deiner et al., 1997). Growth cone pausing on LN in response to EphB may also be due to transient stabilization of integrin-dependent adhesions ((Woo et al., 2009); see section on modulation of adhesion).
Similar to the studies described above, LN can also switch or nullify inhibitory axon guidance cues. For example, ephrin-A5 is an important repulsive factor toward RGC axons, which controls retinotectal topographic mapping through graded ligand and receptor expression patterns. Consistent with this role, ephrin-A5 is repulsive to retinal growth cones on the ECM protein fibronectin. However, when cultured on LN, RGCs are attracted toward a gradient of soluble ephrin-A5 (Weinl et al., 2003). Chemoattraction toward ephrin-A5 on LN likely depends on the concentration of ephrin-A5 and nasal-temporal position of RGCs (i.e. EphA expression), as higher concentrations of ephrin-A5 stall axon outgrowth by temporal RGC axons on LN (Woo et al., 2009). LN also blocks the inhibitory effects of myelin-associated glycoprotein (MAG) (David et al., 1995; Laforest et al., 2005), which is responsible in part for limited regeneration by adult mammalian CNS neurons after injury (Cafferty et al., 2010). These observations have important implications for axonal regeneration in the adult mammalian CNS.
Analysis of LN mutants in a number of model systems provides strong evidence that LN is necessary for proper axon guidance. A role for LN in axon guidance has been described in C. elegans, Drosophila, zebrafish and mouse embryos (Garcia-Alonso et al., 1996; Karlstrom et al., 1996; Forrester and Garriga, 1997; Huang et al., 2003; Paulus and Halloran, 2006; Chen et al., 2009), as well as with antibody perturbation studies in grasshopper (Bonner and O'Connor, 2001). In C. elegans, LN has been shown to modulate the guidance of two distinct neuronal populations. First, mutation in Unc-6, the worm orthologue of netrin-1 and a LN-related protein, results in guidance errors of pioneer axons along the body wall (Ishii et al., 1992). Another mutant in a LN α chain called epi-1 (epithelialization defective), was shown to regulate the axonal extension of canal-associated neurons (CANs) (Forrester and Garriga, 1997). These neurons are born in the head and migrate to the middle of the C. elegans embryo where they eventually extend ascending and descending axons. While CANs of epi-1 mutants migrate to their proper location, their axons follow aberrant pathways, suggesting that the LN α chain only has a role in axon guidance. In Drosophila, the Laminin A (LamA) gene is responsible for ocellar pioneer axons to successfully reach the brain once they exit the eye-antenna imaginal disc (Garcia-Alonso et al., 1996). In vertebrates, the role for LN in development seems to be more profound. Analysis of zebrafish bashful (bal)/LN-α1 mutants revealed that most CNS axon pathways are defective (Karlstrom et al., 1996; Paulus and Halloran, 2006; Wolman et al., 2008). Many axon types, including retinal ganglion cell axons, early forebrain axons, and hindbrain reticulospinal axons, make directional decision pathfinding errors. Additionally, axon tracts in bal mutants show defects in fasciculation, extension and increased branching. Interestingly, in contrast to CNS axons, most peripheral axons appear normal in bal mutants. Two other zebrafish mutants identified in a screen for retinal axon guidance defects, named grumpy (gup), and sleepy, were subsequently shown to be mutants in LN subunits β1, and γ1, respectively (Karlstrom et al., 1996). In mouse, conditional knock-out of γ1 subunits specifically in cortical neurons results in profound defects in neuronal migration and morphogenesis (Chen et al., 2009). While previously studies suggested that LN primarily functions in the pial basement membrane (Halfter et al., 2002), this study shows that LN also supports migration and process extension within the cortical plate. Interestingly, mutant cortical neurons even exhibit axon outgrowth defects in vitro, suggesting that these neurons secrete their own substratum to support axon extension in an autocrine manner.
Tenascin
The tenascin (TN) family of oligomeric glycoproteins mediate neuron-glia interactions and can exert both inhibitory and stimulatory effects on cell motility. The TN family contains five known members, TN-C, TN-R, TN-X, TN-Y and TN-W (Table 1). However, only TN-C, TN-R, TN-Y and TN-W have been detected in the developing CNS (Dorries and Schachner, 1994; Dorries et al., 1996; Xiao et al., 1996; Faissner, 1997; Gotz et al., 1997; Weber et al., 1998; Tucker et al., 1999; Treloar et al., 2009). Tenascin molecules are mainly secreted by immature and reactive astrocytes, and by subsets of radial glia cells (Brodkey et al., 1995; Kawano et al., 1995). In addition, a restricted number of immature neurons produce TN, including granule cells in the hippocampus, some MNs of the spinal cord, as well as a subset of neurons of the developing retina (Kawano et al., 1995; Bartsch, 1996). Interestingly, while TN is absent from most regions of the normal adult brain, TN persists in areas known to retain a high degree of plasticity, such as the hypothalamus and the olfactory system, indicating a role in neuronal regeneration and axonal outgrowth throughout life (Gonzalez and Silver, 1994).
Tenascin family proteins contain varying numbers of distinct functional domains that have differential effects on specific neuronal sub-types (Jones and Jones, 2000). The amino terminus is characterized by a series of cysteines and heptad repeats, which are involved in multimerization, followed by a number of Epidermal Growth Factor (EGF)-like repeats and several fibronectin type III (FN-III)-like modules. The carboxy terminus of TN displays sequences homologous to the fibrinogen β- and γ-chains. Interestingly, specific TN domains can have different effects on axon outgrowth. For example, the Epidermal Growth Factor (EGF)-like region of TN-R is anti-adhesive to cells and inhibits neurite outgrowth, while certain FN-III domains of TN promote adhesion and axon outgrowth (Meiners et al., 1999). Alternative splicing of the FN-III domains generates further diversity in TN. For example, at least 9 alternatively spliced forms of TN-C have been identified, which occur at varying frequency over development (Jones and Jones, 2000) and can have differential effects on specific neuronal sub-types (Gotz et al., 1996). TN functional domains were determined using targeted monoclonal antibodies that block neurite outgrowth but had no effect on its anti-adhesive properties (Gotz et al., 1996; Kiernan et al., 1996). The inhibitory properties of TN on axon outgrowth has been revealed using patterned substrata, where growth cones choose between TN and growth promoting substratum such as LN (Faissner and Kruse, 1990; Faissner and Steindler, 1995; Neidhardt et al., 2003; Robles and Gomez, 2006). These experimental paradigms reveal specific signals and cytoskeletal rearrangements that occur in response to contact with TN (Williamson et al., 1996; Robles and Gomez, 2006). Specific domains of TN can also differentially influence cellular sub-compartments, such as the cell body, axons and dendrites (Dorries et al., 1996).
The role of TN in vivo is less well understood, but several studies suggest that TNs may have a role in fine-tuning neuronal connections and synaptogenesis, as seen in TN-C knockout mice, which display abnormalities in motor coordination and exploratory behavior (Fukamauchi et al., 1996; Kiernan et al., 1999). Additionally, mice with mutant TN-R display decreased axonal conduction velocities in the CNS (Weber et al., 1999). In the developing optic system of zebrafish, a reduction in TN-R expression causes excessive axonal branching of the optic tract, implicating a contact-repellent function for TN-R (Becker et al., 2003). Similarly, in the mouse olfactory system, TN-C is believed to function as an inhibitory boundary molecule to restrict olfactory sensory neuron growth in the developing olfactory bulb (Treloar et al., 2009). In contrast, recent evidence suggests that TN-C in the injured spinal cord of adult zebrafish promotes axonal regrowth (Yu et al., 2011). These widely differing functional effects of TN can likely be attributed to the specific receptors expressed by neurons (Table 1). In fact, forced over-expression of α9 integrin subunits allows regeneration of adult rat DRG neurons on TN-C ((Andrews et al., 2009) and see review in this issue by (Kwok and Fawcett, 2011)).
Fibronectin
Fibronectin (FN) is widely expressed within the ECM of the CNS and PNS and functions in neuronal cell adhesion, migration and outgrowth during development and regeneration (Venstrom and Reichardt, 1993; Letourneau et al., 1994) (see review in this issue by (Gardiner, 2011). Like other ECM molecules, FN is structurally complex, containing several functional domains. The arginine-glycine-aspartic acid (RGD)-containing domain, located near the middle of the molecule is recognized by several integrins. FN is expressed in spatially and temporally dynamic patterns in regions of active morphogenesis in the spinal cord and cortical subplate (Stewart and Pearlman, 1987; Chun and Shatz, 1988). In the earliest stages of cortical development, FN is produced by cells in the ventricular zone throughout the telencephalic vesicle, where it may serve as a component of the local environment that supports cell division and determines cell fate (Pearlman and Sheppard, 1996). It is also distributed along radial glial processes in close association with preplate neurons. Additionally, FN is produced by migrating neurons that target specific cortical domains, suggesting that it may help neurons discriminate between adjacent glial guides (Pearlman and Sheppard, 1996).
A supportive role for FN in neuronal survival, differentiation and morphogenesis comes from a number of cell culture studies. For example, FN promotes neural stem cell proliferation and migration (Jacques et al., 1998), neuronal survival (Millaruelo et al., 1988), stimulates neurite outgrowth (Bozyczko and Horwitz, 1986) and promotes adhesion of sensory neurons (Muller et al., 1995; Venstrom and Reichardt, 1995). Moreover, when given a choice between FN and LN on patterned substrata, DRG and spiral ganglion neuron growth cones exhibit behavioral changes indicating a preference for one substratum over another (Gomez and Letourneau, 1994; Evans et al., 2007). FN also modulates the responses of DRG neurons to the inhibitory effects of chondroitin sulfate proteoglycan (CSPG), as growth cone response to CSPG boundaries differ on FN compared with LN (Hynds and Snow, 2001). Similarly, the response of neurons to gradients of classic guidance cues such as netrin and ephrin-A5 differ for neurons growing upon FN versus LN (Hopker et al., 1999; Weinl et al., 2003). While these results suggest that FN can have a significant influence on the guidance of axons, it is not known if similar functions for FN occur in vivo.
ECM proteolysis regulates axon guidance
An additional level of control of cell motility and axon outgrowth by the ECM occurs through targeted cleavage of ECM components. Although still poorly understood, there is good evidence that the ECM is actively remodeled to reveal or disrupt integrin binding sites. These processes occur through the activity of proteolytic enzymes such as the matrix metalloproteases (MMPs), the A Disintegrin and Metalloproteases (ADAMs) and plasminogens (reviewed in: (Yong et al., 2001; McFarlane, 2003; Rivera et al., 2010)). Invasive cells such as metastatic carcinoma cells are known to use degradative proteases to promote their migration into tissues (Poincloux et al., 2009) and similar processes are expected in developing neurons. Matrix proteases were once believed to regulate axon extension by simply degrading the surrounding matrix to create a passage for axonal outgrowth (Muir, 1994; Zuo et al., 1998; Ethell and Ethell, 2007; Gutierrez-Fernandez et al., 2009; Sarig-Nadir and Seliktar, 2010). However, more recent studies performed both in vitro and in vivo now suggest that proteolytic cleavage is directed toward specific ligands in the environment, as well as receptors on growth cones to activate or terminate motility (Fambrough et al., 1996; Schimmelpfeng et al., 2001; Webber et al., 2002; Hehr et al., 2005; Chen et al., 2007). Other evidence suggests that matrix proteases act to reveal cryptic sites in molecules that are otherwise inert in axon guidance, as well as release neurotrophic factors that may associate with the ECM (Yong et al., 2001; McFarlane, 2003). ADAMs are particular intriguing, as these transmembrane proteins contain both a metalloprotease domain and a disintegrin domain (Yang et al., 2006) and have been shown to function in axon guidance. For example, inhibition of ADAM10 activity within the neuroepithelial cells along the pathway of growing RGC axons leads to axon guidance errors (Hehr et al., 2005; Chen et al., 2007). However, as the proteolytic activity of ADAMs is directed toward both ECM proteins and axon guidance receptors, the specific cause of axon guidance defects reported in ADAM loss of function experiment can be ambiguous. Moreover, the disintegrin function of ADAMs provides further complications, as this domain has been shown to both activate and inactivate integrin receptors (Yang et al., 2006). A secreted form of ADAM referred to as ADAMTS contains a conserved thrombospondin type 1-like repeat that binds glycosaminoglycan side chains of PGs and may be involved in PG cleavage (Rivera et al., 2010). While there has been bits of tantalizing data implicating MMP activity in the regulation of axon guidance, much still remains unknown. For example, the molecular mechanisms that control the location and activity of MMPs within growth cones are poorly understood.
ECM receptors on growth cones
Integrins encode substratum specificity
Integrins are a family of heterodimeric receptors, composed of an α and β subunit, initially characterized for their role in anchoring cells to the ECM (Hynes, 1996; Hynes, 2002). Eighteen α and eight β subunits in humans have been identified to date, which assemble into 24 integrin heterodimers. The binding specificity of heterodimers is determined by the affinity of the combined subunits. Many integrin subunits are expressed at high levels in developing neurons, but most decline to low levels in the adult (Jones, 1996; Pinkstaff et al., 1999). Interestingly, some regions of the nervous system maintain expression of integrin subunits, where they regulate synaptic stability and plasticity (Schmid and Anton, 2003). Moreover, adult hippocampal progenitor cells also express multiple integrin receptors, which are necessary for their morphological differentiation (Harper et al., 2010). Manipulation of integrin expression or function indicates that integrins function in neuronal migration and axon extension in several CNS regions during development (Georges-Labouesse et al., 1998; Becker et al., 2003; Harper et al., 2010; Marchetti et al., 2010). The broad expression of multiple integrin subunits in developing neurons allows these neurons to extend axons on a variety of ECM proteins (Table 1) (Pinkstaff et al., 1999; Gupton and Gertler, 2010). Interestingly, ectopic expression of specific integrin sub-units is sufficient to allow normally insensitive neurons to polarize on certain ECM substrata. As previously described, this ability to promote axon outgrowth has been exploited in regenerating systems by expressing α9 integrin in adult neurons that do not normally express this subunit ((Andrews et al., 2009) and see review in this issue by (Kwok and Fawcett, 2011)). Significantly, this manipulation also improved regeneration in vivo. These results highlight both the importance of understanding integrin function in development and their potential as a therapeutic target after injury or disease.
Integrin receptor activation and trafficking
Integrins are activated upon ligand engagement and receptor clustering. Although still poorly understood in nerve growth cones, inside-out signaling pathways that increase integrin ligand affinity and promote clustering have been investigated in non-neuronal cells (Moser et al., 2009) and are likely similar in growth cones. For example, an interaction with the head domain of the scaffolding protein talin is necessary for integrin activation (Tadokoro et al., 2003; Simonson et al., 2006). Loss or mutation of talin that prevents this interaction blocked activation of β1, β2, and β3-containing integrins. Structure-function studies suggest that talin-binding changes the angle of the transmembrane segment of β integrins such that the head groups of integrin heterodimers are able to bind their ligands and signal (Garcia-Alvarez et al., 2003; Wegener et al., 2007). Talin itself is a target of multiple regulatory mechanisms (Legate and Fassler, 2009), some of which are known to operate in axon guidance, such as calpain proteolysis (Robles et al., 2003). Although little is known about how talin regulates growth cone motility or axon guidance, we find that it does target to the tips of filopodia where β1 integrin also concentrates (unpublished observations and (Gomez et al., 2001)), which consistent with its role in filopodial protrusion (Sydor et al., 1996).
Integrin function in growth cones is regulated not only by ligand binding, but it also appears to be regulated by receptor trafficking (Ezratty et al., 2009). During process extension, growth cones must undergo constitutive exocytosis and endocytosis (Tojima et al., 2011). Exocytosis in growth cones involves SNARE-dependent (VAMP-2, VAMP-4, VAMP7) processes, while endocytosis involves both clathrin-dependent and independent mechanisms (Eva et al., 2010; Racchetti et al., 2010; Tojima et al., 2011) . While it is unclear if integrin receptors are specifically trafficked to the plasma membrane in response to environmental cues, recent work showed that integrins activate VAMP-7 mediated exocytosis to promote neuritogenesis by cortical neurons (Gupton and Gertler, 2010). Therefore, local activation of integrins could direct axon outgrowth through targeted VAMP7-mediated exocytosis. Similarly, asymmetric retrieval of membrane also orients axon outgrowth. Recent work showed that a gradient of myelin-associated glyocprotein (MAG) resulted in repulsive growth cone turning through local clathrin-dependent endocytosis of β1 integrin containing vesicles (Hines et al., 2010). Together these results suggest that signals activated by gradients of guidance cues may be locally amplified by directed insertion or retrieval of integrin receptors.
Integrin co-receptors signal cross-talk
While most studies focus have focused on integrin receptors, the heparan sulfate proteoglycan (HSPG) syndecan is also an important ECM receptor. Syndecans function as co-receptors with certain integrins and are essential for focal adhesion formation by fibroblasts on LN and FN (Morgan et al., 2007). Moreover, syndecans may serve a similar function with growth factors (Lee and Chien, 2004) and are necessary for normal responses to several guidance cues (Kantor et al., 2004; Lee et al., 2004; Matsumoto et al., 2007). In particular, mutation of syndecan genes or enzymatic cleavage of their heparan chains results in guidance defects to Slit in C. elegans (Rhiner et al., 2005), Drosophila (Johnson et al., 2004; Steigemann et al., 2004), and mouse (Hu, 2001; Inatani et al., 2003). These studies illustrate the complex nature of neuronal interactions with the ECM, which provides the versatility necessary for the assembly of the nervous system. For further review, see (Lee and Chien, 2004; Van Vactor et al., 2006) and the article by Broadie et al. in this issue.
Another important feature of integrin signaling is cross-talk with non-ECM receptors. In particular, interactions between signals generated by integrins and neurotrophin receptors have been recognized for years (Tucker and Mearow, 2008) and similar interactions with axon guidance cue receptors are just being revealed ((Nakamoto et al., 2004); see below). For example, embryonic DRG explants plated on FN or LN require Nerve Growth Factor (NGF) for efficient growth (Millaruelo et al., 1988), whereas LN or FN alone can induce axonogenesis from adult DRGs (Tucker et al., 2005). In embryonic and adult DRGs, subpopulations of sensory neurons have distinctive requirements for ECM and NGF (Guan et al., 2003; Tucker et al., 2006), which can be mediated by differential integrin expression ((Gardiner et al., 2005) and see review in this issue by (Gardiner, 2011)). These subpopulations serve different sensory functions and their substratum preference may be a mechanism for proper innervation. The effects of NGF on axon outgrowth are integrin-specific since a similar enhancement of outgrowth is not observed on L1, which also supports axon extension (Liu et al., 2002). The convergence of integrin and neurotrophin signaling may function to restrict sensory innervation in vivo, since LN and NGF together result in segregation of axons between embryonic DRGs, whereas FN and NGF allow inter-mingling of axons (Hari et al., 2004). One likely target that serves to integrate integrin and growth factor generated signals are the non-receptor tyrosine kinases (NTKs), FAK and Src. FAK binds to and is required for EGF stimulated cell motility on FN via its targeting to focal adhesions (Sieg et al., 2000). Similarly, FAK and Src are activated downstream of NGF and the FAK-mediated upregulation of integrin receptors is necessary for NGF-enhancement of axon growth from DRGs (Tucker et al., 2005; Tucker et al., 2008). A role for FAK downstream of growth factor receptors and integrins in both fibroblasts and neurons, together with its recently described function downstream of several axon guidance cue receptors (see below), suggests that FAK likely plays a critical role in axon pathfinding in the developing embryo.
A dynamic protein network (adhesome) links integrin receptors to the ECM
Growth cones form adhesions to ECM proteins that resemble fibroblast focal contacts
Integrin receptors physically link cells to their immediate environment at distinct contact points containing a large number of linkage and regulatory proteins. Structures such as focal contacts (FCs) and focal adhesions (FAs) are highly complex and dynamic macromolecular assemblies that link the actin cytoskeleton to ECM proteins through integrin receptors. Studies that have largely focused on fibroblasts and epithelial cells have shown that individual cells form distinct adhesion sites that serve unique functions. For example, FCs are nascent adhesions that form first just behind the leading edge of lammelipodial and filopodial protrusions and support transient sampling of the environment. In fibroblasts, some FCs mature into larger and more stable FAs that support traction forces from cell to ECM. Focal contacts and adhesions may contain upwards of 150 different proteins that are interchanged and post-translationally modified during their lifetime within the integrin adhesome (Zaidel-Bar et al., 2007).
Neuronal growth cones assemble dynamic adhesion complexes on ECM substrata similar to fibroblast FCs. Live imaging of neurons expressing paxillin-mCherry and GFP-dSH2, a fluorescent phosphotyrosine (PY) reporter, show that adhesions form within nascent filopodial and lamellipodial protrusions, which remain fixed in position during growth cone advance (Figure 2). While a subset of growth cone adhesions do stabilize and expand rearward similar to fibroblast FAs (Woo and Gomez, 2006), distinct growth cone adhesions have not been systematically classified since their morphological and kinetic features are not as clearly distinguishable. Therefore, growth cone adhesions are collectively referred to as point contacts (PCs) (Gomez et al., 1996; Renaudin et al., 1999; Robles and Gomez, 2006). Retrospective immunofluorescent staining confirms that positionally stable growth cone PCs colocalize with classic FA components such as β1-integrin, vinculin, and paxillin ((Robles and Gomez, 2006) and unpublished observations). Vinculin and paxillin are adaptor proteins that are involved in direct or indirect linking of actin filaments to the cytoplasmic tail of integrin receptors (Figure 1). Consistent with this notion, adhesions often cluster along actin filament bundles (Robles and Gomez, 2006), suggesting that PCs may mediate traction force generation in growth cones. It is important to note that PCs have only been observed in growth cones on ECM substrata and not in growth cones on non-biological substrata (glass or PDL) or neural cell adhesion molecule (NCAM, unpublished observations).
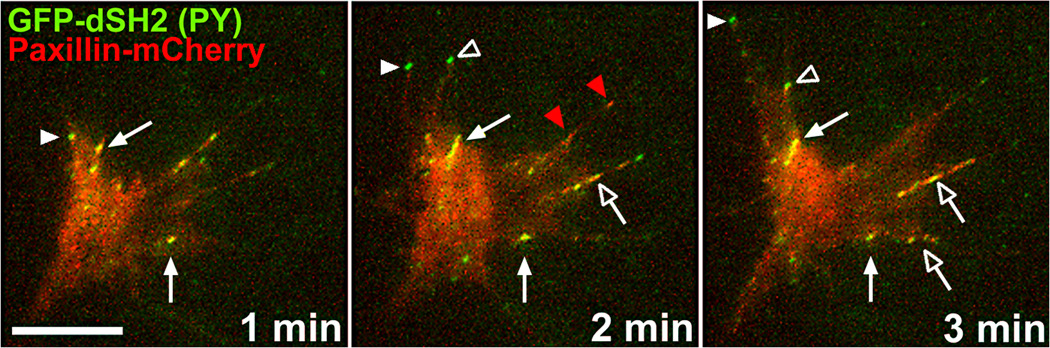
Figure 2. Two-channel total internal reflection fluorescence (TIRF) microscopy of phosphotyrosine (PY) and paxillin in a living growth cone.
This neuron is co-expressing GFP-dSH2 (green), which brightly labels regions containing concentrated tyrosine-phosphorylated proteins and paxillin-mCherry (red), which labels integrin-dependent adhesion sites. Note that only PY is concentrated at the tips of elongating filopodia (solid white arrowheads mark one extending filopodium and open white arrowheads mark a filopodium that extends then retracts), which appear green due to the absence of paxillin. On the other hand, stable adhesions that contain both paxillin and PY appear yellow throughout this time period (solid arrows). Stable adhesion likely contain many other signaling and adaptor proteins, such as FAK and α-actinin. New adhesions often form from retracting PY-positive filopodia that stabilize and cluster with paxillin (at open arrowhead at 3 min) or by apparent simultaneous clustering of paxillin and PY-proteins within nascent protrusions (open arrows). However, in some instances, paxillin appears to cluster at adhesion sites independent of PY-containing proteins (red arrowheads). Scale, 10 µm.
Growth cone PCs also contain signaling proteins that modulate adhesion assembly and disassembly and likely regulate a number of other important cellular functions. Two crucial proteins are the non-receptor tyrosine kinases (NTKs), FAK and Src, which localize to point contacts and are known to control the molecular composition of adhesion complexes in motile cells by regulating protein-protein interactions (Robles and Gomez, 2006; Woo et al., 2009). Importantly, these kinases are modulated downstream of a variety of receptors (Knoll and Drescher, 2004; Li et al., 2004; Bechara et al., 2008) and interact with multiple signaling pathways, suggesting their localization to PCs controls additional cellular functions (Huveneers and Danen, 2009). The Rho family GTPases Rac1 and RhoA also regulate the assembly and maturation of growth cone PCs downstream of integrin signaling (Figure 3). In migrating fibroblasts, FC formation at the leading edge of lamellipodia and filopodia are promoted by Rac1 activity (Rottner et al., 1999), while maturation into FAs requires RhoA activity and actomyosin contraction (Chrzanowska-Wodnicka and Burridge, 1996). Consistent with the presence of distinct adhesion complexes in growth cones, Rac1 was found to promote the assembly of transient point contacts, while RhoA was necessary to stabilize existing PCs (Woo and Gomez, 2006). It is important to note that while PCs have not yet been observed in neurons in vivo, loss of function of proteins involved in PC assembly and turnover interferes with many aspects of normal neural development (Beggs et al., 2003; Clegg et al., 2003; Rico et al., 2004; Robles and Gomez, 2006; Woo et al., 2009), suggesting these adhesion complexes also play crucial roles in developing neurons in vivo. For example, we find that midline crossing by spinal commissural interneurons and retinotopic mapping by RGC is disrupted in FAK loss of function conditions (Robles and Gomez, 2006; Woo et al., 2009).
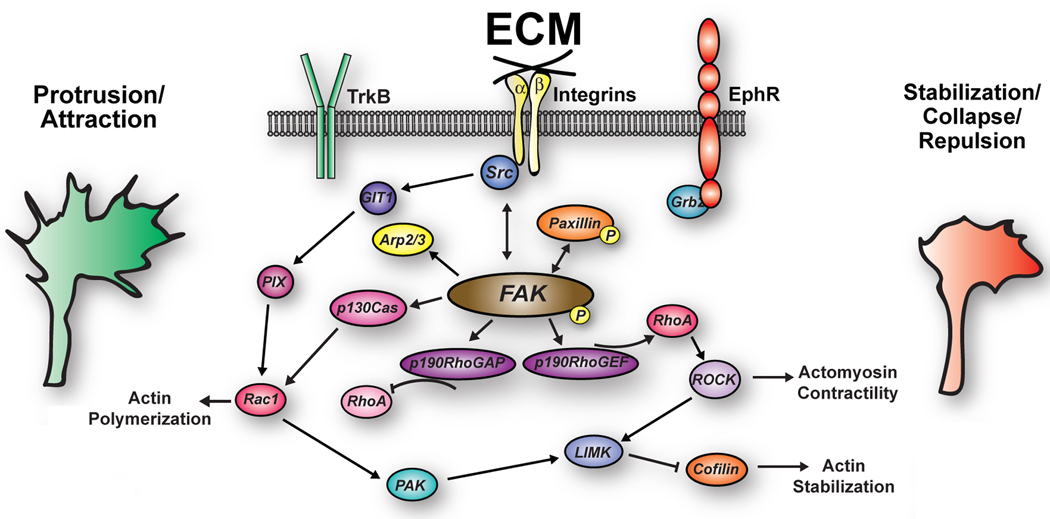
Figure 3. Cooperation between integrin-dependent signaling pathways and modulatory receptors regulate growth cone motility by balancing Rho GTPase signaling.
Integrins signal through a Src-FAK complex to promote membrane protrusion by elevating Rac1 and inhibiting RhoA. However, shifting this balance to more RhoA and less Rac1 activity leads to less actin polymerization and more actomyosin contractility. Modest RhoA signaling will stabilize adhesions to promote growth cone stalling or turning. Strong RhoA signaling will cause growth cone collapse and axon retraction. Shifting the balance of Rho GTPase signaling may occur through growth-promoting and inhibiting extracellular ligands that directly signal through Src-FAK. Alternatively, modulation of Src-FAK signals may occur as a result of re-association of FAK-associated protein complexes with modulating receptors.
As it appears that fibroblast and growth cone adhesions share many similarities, it follows that many of the activities associated with fibroblast FAs and FCs also occur at growth cone PCs. In fibroblasts and other non-neuronal cells, integrin-dependent adhesions have been shown to not simply serve as anchorage points between the ECM and the cytoskeleton. Rather, they function as active signaling centers, where a wide variety of proteins are regulated to control diverse cellular functions (Zaidel-Bar et al., 2007). For example, PCs may locally control actin polymerization by targeting factors such as Arp2/3, p130cas and various GEFs and GAPs for Rho GTPases (Figure 3) (Cary et al., 1998; Huang et al., 2007; Liu et al., 2007; Serrels et al., 2007; Tomar and Schlaepfer, 2009). FAs have also been shown to colocalize with and organize caveoli-containing lipid rafts (Gaus et al., 2006), which serve as a platform for diverse signaling events and are known to be necessary for neurite outgrowth and growth cone chemotropism (Guirland et al., 2004; Langhorst et al., 2008). In addition, vinculin, a fundamental component of FAs and growth cone PCs, associates with proteins that target mRNA and protein translation machinery to FAs (Chicurel et al., 1998; Lee et al., 2009; Willett et al., 2009). As local protein translation was found to be necessary for growth cone chemotropism (Campbell and Holt, 2001), it is possible that PCs function to locally modulate protein translation in growth cones. While PCs of growth cones share many similar functions as adhesion sites of motile non-neuronal cells, it is likely that growth cone PCs will have many additional upstream modulators and downstream activities given the profoundly complex roles growth cones serve in neural network assembly and synapse formation.
Modulation of integrin-dependent adhesion by axon guidance cues
Several studies have demonstrated that axon guidance factors such as netrins, Semaphorins, Slits and ephrins can promote or inhibit axon outgrowth by modulating integrin receptors or their associated adhesion complexes (Nakamoto et al., 2004). Some guidance cues act directly on integrin receptors, while others indirectly influence integrin-associated adhesion complex proteins. As examples of direct effects on integrin receptors, both Semaphorin 7a (Sema7a) and myelin-associated glycoprotein (MAG) contain integrin-binding RGD sequences and function as ligands to activate integrin receptors (Pasterkamp et al., 2003; Goh et al., 2008). Interestingly, both MAG and Sema7a activate FAK downstream of integrin binding, yet have opposite effects on axon outgrowth. Activation of FAK (and Src) downstream of both growth promoting and inhibiting axon guidance cues is not uncommon and is discussed further below. Briefly, activation of FAK by MAG may lead to adhesion disassembly and subsequent integrin endocytosis, whereas Sema7a may promote adhesion cycling without receptor endocytosis. A second myelin-associated protein, Nogo-A, inhibits axon outgrowth and regeneration by directly inhibiting integrin receptors through an unknown mechanism (Hu and Strittmatter, 2008). Several other guidance cues have been shown to bind various integrin heterodimers in non-neuronal cells and neurons. For example, neurotrophins (Staniszewska et al., 2008), Wnt5a (Kawasaki et al., 2007) and netrin (Yebra et al., 2003) each can bind specific integrin heterodimers.
Netrin is particularly intriguing, as it is an important secreted axon guidance cue that works in cooperation with integrin-ECM adhesion and signaling in several ways (Baker et al., 2006). First, netrin was found to support cell adhesion by directly and independently binding integrin and deleted in colorectal cancer (DCC) receptors (Yebra et al., 2003; Shekarabi et al., 2005). Interestingly, netrin colocalizes with the basal lamina ECM in vivo and binds purified ECM proteins in vitro, implying that secreted netrin may be immobilized within the ECM (Yebra et al., 2003). Moreover, cortical GABAergic interneurons expressing α3/β1 integrin receptors bind directly to netrin, which is necessary for proper migration of these neurons into the cortex (Stanco et al., 2009). However, integrin signaling also appears to modulate netrin function downstream of DCC receptors, as chemotropic turning toward netrin is sensitive to the ECM substratum (Hopker et al., 1999). Specifically, the study by Höpker et al. found that attractive turning on FN or PDL was switched to repulsion when neurons were plated on LN. Although substratum-dependent differences in cAMP/PKA/Epac signaling within growth cones have been implicated (Ming et al., 1997; Nishiyama et al., 2003; Murray et al., 2009), the exact mechanisms leading to bidirectional turning responses are unknown.
Another intriguing link between integrin and DCC receptors is their common activation of FAK and Src. The cytoplasmic tails of β1-containing integrin receptors interact with FAK in combination with several other adhesion-related adaptor proteins (Legate and Fassler, 2009), leading to FAK auto-phosphorylation at tyrosine 397 (Y397) and recruitment of Src (Figure 3). Similarly, three papers reported that netrin activates FAK and Src to promote DCC tyrosine phosphorylation and FAK binding of DCC (Li et al., 2004; Liu et al., 2004; Ren et al., 2004). It is noteworthy that both integrin adhesion proteins and DCC receptors bind within the FAT domain of FAK. However, it remains unclear whether integrin and DCC receptors utilize FAK in parallel, or if these receptors cooperate with or compete for FAK function. Future studies should consider the growth substratum when testing the effects of netrin. Lastly, integrin receptors were recently found to facilitate cell migration toward netrin in C. elegans by targeting UNC-40 (DCC) receptors to the plasma membrane (Hagedorn et al., 2009). This result suggests that integrins may associate with DCC in cis.
Semaphorins comprise a large family of secreted and membrane associated guidance cues that influence both axon extension and cell motility by regulating integrin receptors and their associated adhesion complexes. Semaphorins have diverse effects on cell motility, likely owing to the wide variety of receptors and co-receptors that bind semaphorins. The primary ligand-binding receptors for semaphorins are the plexins and neuropilins. However, a number of associated receptors appear necessary for signal transduction and specific cellular responses (Franco and Tamagnone, 2008). For example, Sema3A stimulates integrin-dependent extension of hippocampal neuron dendrites by activating FAK (Schlomann et al., 2009). FAK is not activated by Sema3A if integrins are not engaged, suggesting that active FAK is associated with and may influence integrin adhesion complexes. Conversely, growth cone collapse and the repulsive effects of semaphorins on hippocampal axons do not appear to require integrin engagement, since the effects of semaphorins could occur on non-integrin binding substrata (Song et al., 1998; Schlomann et al., 2009). While modulation of integrin receptors may not be necessary for the collapsing or repulsive effects of semaphorins, it is still possible that integrins are involved in the normal inhibitory effects of semaphorins that occur within cells on ECM proteins or in vivo. This possibility is supported by observations that paxillin-containing PCs that form within growth cones on LN are rapidly disassembled by soluble Sema3A (Woo and Gomez, 2006). Moreover, FAK is necessary for Sema3A-induced collapse of cortical neurons, which depends on cis interactions between the cell adhesion molecule L1 and neuropilin receptors and disassembly of paxillin containing adhesions (Bechara et al., 2008).
The Eph/ephrins are probably the best characterized and most complex system of receptors/ligands that regulate a number of important developmental functions through the control of cell adhesion. Ephs and ephrins are cell surface molecules that function as both signaling ligands and receptors and work cooperatively with integrin receptors. Eph proteins are transmembrane receptor tyrosine kinases activated by ephrin ligands in forward signaling, while ephrins are either transmembrane or GPI-anchored receptors reciprocally activated by Eph ligands in reverse signaling (Pasquale, 2005). In non-neuronal cells, forward signaling through Eph receptors has been shown to both activate and inhibit integrin-mediated cell spreading and adhesion, suggesting that complex cellular conditions regulate Eph receptor output (Miao et al., 2000; de Saint-Vis et al., 2003; Deroanne et al., 2003; Miao et al., 2005; Prevost et al., 2005; Sharfe et al., 2008; Noren et al., 2009). The diversity of cellular effects of ephrins likely result from activation of multiple possible signaling pathways affecting the actin cytoskeleton and adhesion (Pasquale, 2008). Many Eph receptors activate Ras/Rho family GTPases. For example, EphA receptors activate RhoA signaling through the Rho GEF Ephexin (Shamah et al., 2001). More recently, NTK signaling has been identified as a common pathway of both forward and reverse signaling. Src family kinases interact with EphA receptors, phosphorylate Ephexin (Sahin et al., 2005) and are required for retinal axons to respond to ephrin-A (Knoll and Drescher, 2004). In addition, several studies have found that FAK can have diverse effects downstream of ephrins. EphrinA was shown to increase FAK phosphorylation and promote cell migration or retraction in certain cell types (Carter et al., 2002; Parri et al., 2007), but reduce FAK phosphorylation and decrease cell adhesion and motility in other cells (Miao et al., 2000; Bourgin et al., 2007). In retinal ganglion cell (RGC) growth cones, ephrin-A1 activates FAK and Src (Woo et al., 2009) and results in stabilization of integrin adhesions and stalled outgrowth. Interestingly, by comparing active FAK in nasal versus temporal RGC growth cones, it appears that moderate levels of active FAK correlate with maximal rates of outgrowth and changes above or below this optimal set-point inhibit outgrowth. This may explain how ephrinA inhibits outgrowth by temporal RGC axons, yet promotes outgrowth by nasal RGCs (Hansen et al., 2004; Woo et al., 2009). It is also noteworthy that Eph receptors can physically interact with FAK and other known integrin-associated adaptor proteins and localize to cellular focal adhesions (Miao et al., 2000), suggesting these receptors may associate within a common protein complex (Figure 3). Adding to this molecular complexity, forward and reverse Eph/ephrin signaling within individual cells may function antagonistically toward integrin adhesion (Sharfe et al., 2008).
The NTKs FAK and Src are necessary downstream of many growth promoting and inhibiting axon guidance cues. How can these signaling kinases transduce opposing cellular behaviors from different axon guidance cues? In the context of modulation of integrin-dependent adhesion, several possible mechanisms could explain varying effects on cell motility. First, the molecular targets of FAK/Src may depend on receptor activation. FAK/Src normally target to and regulate integrin-based adhesion sites of cells on ECM substrata. However, upon stimulation with guidance cues, FAK and Src may associate with guidance cue receptors (Li et al., 2004; Liu et al., 2004; Ren et al., 2004; Bechara et al., 2008; Shi et al., 2009). As NTKs can activate a number of different targets, including RhoA GEF and GAP proteins (Tomar and Schlaepfer, 2009), NTK target specificity could be determined by protein associations driven by receptor binding. In support of this possibility, treatment of hippocampal neurons with ephrin B2 led to FAK dissociation from β3-containing integrins and association with EphB2 receptors in dendritic spines (Shi et al., 2009). In this model, activation of guidance cue receptors could further affect cell motility by drawing FAK/Src away from integrin receptors. Alternatively, parallel signals activated by guidance cue receptors may modulate permissive integrin-based adhesion dynamics. For example, increased RhoA signaling by Ephexin may activate actomyosin contractility, which stabilizes integrin adhesions (Lim et al., 2008).
Closing remarks
Cells navigate within their environment through coordination of leading edge membrane protrusion/retraction and associated adhesion/de-adhesion of new protrusions. Extensive research has focused on the molecular mechanisms that control membrane protrusion, but far less is known about how adhesion influences cell motility. This is especially true for neuronal growth cones, which are known to interact with a wide variety of ECM proteins, cell adhesion molecules and soluble guidance factors. Signals activated by soluble or cell-associated guidance cues likely influence growth cone motility by modulating adhesion to the ECM and other cells. Cell adhesion can be controlled in a number of ways, such as through receptor activation and clustering, or by modulating linkage with the cytoskeleton. In addition, signals generated by integrin receptors likely reciprocally influence responses to other axon guidance cues. This cross-talk between signals generated by adhesive integrin receptors and axon guidance cue receptors can provide great versatility for pathway selection by developing neurons. Further, adhesion to the ECM may not only stabilize leading edge protrusions, but may provide tissue-specific mechanical signals to growth cones (see review in this issue by (Moore and Sheetz, 2011)). Mechanical forces can modulate ion channels and enzyme activity within cells. Accordingly, cell motility and axon outgrowth are influenced by the elastic properties of the cell substratum (Lo et al., 2000; Kostic et al., 2007). Since the elastic properties of tissues through which axons grow can vary significantly (Discher et al., 2005), adhesion-dependent mechanosensors may provide specific signals that guide axons. Future studies should focus on how guidance cues influence axon outgrowth by modulating cell adhesion and examine the roles of integrin adhesion dynamics, as well as mechanical signals in vivo.
Acknowledgments
We thank Erik Dent for assistance with artwork and members of the Gomez laboratory for comments on the manuscript. This work was supported by NIH NS41564 to T.M.G.
Bibliography
- Ackley BD, Crew JR, Elamaa H, Pihlajaniemi T, Kuo CJ, Kramer JM. The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J Cell Biol. 2001;152:1219–1232. doi: 10.1083/jcb.152.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DN, Kao EY, Hypolite CL, Distefano MD, Hu WS, Letourneau PC. Growth cones turn and migrate up an immobilized gradient of the laminin IKVAV peptide. J Neurobiol. 2005;62:134–147. doi: 10.1002/neu.20075. [DOI] [PubMed] [Google Scholar]
- Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, Gage FH, ffrench-Constant C, Fawcett JW. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci. 2009;29:5546–5557. doi: 10.1523/JNEUROSCI.0759-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Baker KA, Moore SW, Jarjour AA, Kennedy TE. When a diffusible axon guidance cue stops diffusing: roles for netrins in adhesion and morphogenesis. Curr Opin Neurobiol. 2006;16:529–534. doi: 10.1016/j.conb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Bartsch U. The extracellular matrix molecule tenascin-C: expression in vivo and functional characterization in vitro. Prog Neurobiol. 1996;49:145–168. doi: 10.1016/0301-0082(96)00014-7. [DOI] [PubMed] [Google Scholar]
- Bartsch U, Kirchhoff F, Schachner M. Immunohistological localization of the adhesion molecules L1, N-CAM, and MAG in the developing and adult optic nerve of mice. J Comp Neurol. 1989;284:451–462. doi: 10.1002/cne.902840310. [DOI] [PubMed] [Google Scholar]
- Beattie CE, Melancon E, Eisen JS. Mutations in the stumpy gene reveal intermediate targets for zebrafish motor axons. Development. 2000;127:2653–2662. doi: 10.1242/dev.127.12.2653. [DOI] [PubMed] [Google Scholar]
- Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, Abouzid K, Guan JL, Tessier-Lavigne M, Lemmon V, Castellani V. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO. 2008;27:1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Schweitzer J, Feldner J, Becker T, Schachner M. Tenascin-R as a repellent guidance molecule for developing optic axons in zebrafish. J Neurosci. 2003;23:6232–6237. doi: 10.1523/JNEUROSCI.23-15-06232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, McLane MA, Becker CG. Integrin antagonists affect growth and pathfinding of ventral motor nerves in the trunk of embryonic zebrafish. Mol Cell Neurosci. 2003;23:54–68. doi: 10.1016/s1044-7431(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt RS, Tomoda T, Fang Y, Hatten ME. Discoidin domain receptor 1 functions in axon extension of cerebellar granule neurons. Genes Dev. 2000;14:2216–2228. doi: 10.1101/gad.821600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J, O'Connor TP. The permissive cue laminin is essential for growth cone turning in vivo. J Neurosci. 2001;21:9782–9791. doi: 10.1523/JNEUROSCI.21-24-09782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J Cell Biol. 2007;178:1295–1307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyczko D, Horwitz AF. The participation of a putative cell surface receptor for laminin and fibronectin in peripheral neurite extension. J Neurosci. 1986;6:1241–1251. doi: 10.1523/JNEUROSCI.06-05-01241.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, McNagny KM, Gervin DB, Cann GM, Graf T, Clegg DO. Integrin alpha 2 beta 1 mediates interactions between developing embryonic retinal cells and collagen. Development. 1995;121:3593–3602. doi: 10.1242/dev.121.11.3593. [DOI] [PubMed] [Google Scholar]
- Brodkey JA, Laywell ED, O'Brien TF, Faissner A, Stefansson K, Dorries HU, Schachner M, Steindler DA. Focal brain injury and upregulation of a developmentally regulated extracellular matrix protein. J Neurosurg. 1995;82:106–112. doi: 10.3171/jns.1995.82.1.0106. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calof AL, Campanero MR, O'Rear JJ, Yurchenco PD, Lander AD. Domain-specific activation of neuronal migration and neurite outgrowth-promoting activities of laminin. Neuron. 1994;13:117–130. doi: 10.1016/0896-6273(94)90463-4. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas) Nat Cell Biol. 2002;4:565–573. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Hehr CL, Atkinson-Leadbeater K, Hocking JC, McFarlane S. Targeting of retinal axons requires the metalloproteinase ADAM10. J Neurosci. 2007;27:8448–8456. doi: 10.1523/JNEUROSCI.1841-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Haegeli V, Yu H, Strickland S. Cortical deficiency of laminin gamma1 impairs the AKT/GSK-3beta signaling pathway and leads to defects in neurite outgrowth and neuronal migration. Dev Biol. 2009;327:158–168. doi: 10.1016/j.ydbio.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Singer RH, Meyer CJ, Ingber DE. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature. 1998;392:730–733. doi: 10.1038/33719. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JJ, Shatz CJ. A fibronectin-like molecule is present in the developing cat cerebral cortex and is correlated with subplate neurons. J Cell Biol. 1988;106:857–872. doi: 10.1083/jcb.106.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DO, Wingerd KL, Hikita ST, Tolhurst EC. Integrins in the development, function and dysfunction of the nervous system. Front Biosci. 2003;8:d723–d750. doi: 10.2741/1020. [DOI] [PubMed] [Google Scholar]
- Cohen J, Burne JF, McKinlay C, Winter J. The role of laminin and the laminin/fibronectin receptor complex in the outgrowth of retinal ganglion cell axons. Dev Biol. 1987;122:407–418. doi: 10.1016/0012-1606(87)90305-8. [DOI] [PubMed] [Google Scholar]
- David S, Braun PE, Jackson DL, Kottis V, McKerracher L. Laminin overrides the inhibitory effects of peripheral nervous system and central nervous system myelin-derived inhibitors of neurite growth. J Neurosci Res. 1995;42:594–602. doi: 10.1002/jnr.490420417. [DOI] [PubMed] [Google Scholar]
- de Saint-Vis B, Bouchet C, Gautier G, Valladeau J, Caux C, Garrone P. Human dendritic cells express neuronal Eph receptor tyrosine kinases: role of EphA2 in regulating adhesion to fibronectin. Blood. 2003;102:4431–4440. doi: 10.1182/blood-2003-02-0500. [DOI] [PubMed] [Google Scholar]
- de Wit J, Verhaagen J. Proteoglycans as modulators of axon guidance cue function. Adv Exp Med Biol. 2007;600:73–89. doi: 10.1007/978-0-387-70956-7_7. [DOI] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Delannet M, Martin F, Bossy B, Cheresh DA, Reichardt LF, Duband JL. Specific roles of the alpha V beta 1, alpha V beta 3 and alpha V beta 5 integrins in avian neural crest cell adhesion and migration on vitronectin. Development. 1994;120:2687–2702. doi: 10.1242/dev.120.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Deroanne C, Vouret-Craviari V, Wang B, Pouyssegur J. EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J Cell Sci. 2003;116:1367–1376. doi: 10.1242/jcs.00308. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Dorries U, Schachner M. Tenascin mRNA isoforms in the developing mouse brain. J Neurosci Res. 1994;37:336–347. doi: 10.1002/jnr.490370306. [DOI] [PubMed] [Google Scholar]
- Dorries U, Taylor J, Xiao Z, Lochter A, Montag D, Schachner M. Distinct effects of recombinant tenascin-C domains on neuronal cell adhesion, growth cone guidance, and neuronal polarity. J Neurosci Res. 1996;43:420–438. doi: 10.1002/(SICI)1097-4547(19960215)43:4<420::AID-JNR4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Eva R, Dassie E, Caswell PT, Dick G, ffrench-Constant C, Norman JC, Fawcett JW. Rab11 and its effector Rab coupling protein contribute to the trafficking of beta 1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. J Neurosci. 2010;30:11654–11669. doi: 10.1523/JNEUROSCI.2425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Euteneuer S, Chavez E, Mullen LM, Hui EE, Bhatia SN, Ryan AF. Laminin and fibronectin modulate inner ear spiral ganglion neurite outgrowth in an in vitro alternate choice assay. Dev Neurobiol. 2007;67:1721–1730. doi: 10.1002/dneu.20540. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner A. The tenascin gene family in axon growth and guidance. Cell Tissue Res. 1997;290:331–341. doi: 10.1007/s004410050938. [DOI] [PubMed] [Google Scholar]
- Faissner A, Kruse J. J1/tenascin is a repulsive substrate for central nervous system neurons. Neuron. 1990;5:627–637. doi: 10.1016/0896-6273(90)90217-4. [DOI] [PubMed] [Google Scholar]
- Faissner A, Steindler D. Boundaries and inhibitory molecules in developing neural tissues. Glia. 1995;13:233–254. doi: 10.1002/glia.440130402. [DOI] [PubMed] [Google Scholar]
- Fambrough D, Pan D, Rubin GM, Goodman CS. The cell surface metalloprotease/disintegrin Kuzbanian is required for axonal extension in Drosophila. Proc Natl Acad Sci U S A. 1996;93:13233–13238. doi: 10.1073/pnas.93.23.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester WC, Garriga G. Genes necessary for C. elegans cell and growth cone migrations. Development. 1997;124:1831–1843. doi: 10.1242/dev.124.9.1831. [DOI] [PubMed] [Google Scholar]
- Fox MA. Novel roles for collagens in wiring the vertebrate nervous system. Curr Opin Cell Biol. 2008;20:508–513. doi: 10.1016/j.ceb.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fassler R, Hudson BG, John SW, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129:179–193. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep. 2008;9:865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamauchi F, Mataga N, Wang YJ, Sato S, Youshiki A, Kusakabe M. Abnormal behavior and neurotransmissions of tenascin gene knockout mouse. Biochem Biophys Res Commun. 1996;221:151–156. doi: 10.1006/bbrc.1996.0561. [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso L, Fetter RD, Goodman CS. Genetic analysis of Laminin A in Drosophila: extracellular matrix containing laminin A is required for ocellar axon pathfinding. Development. 1996;122:2611–2621. doi: 10.1242/dev.122.9.2611. [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC. Structural determinants of integrin recognition by talin. Mol Cell. 2003;11:49–58. doi: 10.1016/s1097-2765(02)00823-7. [DOI] [PubMed] [Google Scholar]
- Gardiner N. Extracellular matrix and integrin receptors in development and regeneration of the peripheral sensory nervous system. Dev Neurobiol. 2011 in press. [Google Scholar]
- Gardiner NJ, Fernyhough P, Tomlinson DR, Mayer U, von der Mark H, Streuli CH. Alpha7 integrin mediates neurite outgrowth of distinct populations of adult sensory neurons. Mol Cell Neurosci. 2005;28:229–240. doi: 10.1016/j.mcn.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol. 2006;174:725–734. doi: 10.1083/jcb.200603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Goh EL, Young JK, Kuwako K, Tessier-Lavigne M, He Z, Griffin JW, Ming GL. beta 1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol Brain. 2008;1:10. doi: 10.1186/1756-6606-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Letourneau PC. Filopodia initiate choices made by sensory neuron growth cones at laminin/fibronectin borders in vitro. J Neurosci. 1994;14:5959–5972. doi: 10.1523/JNEUROSCI.14-10-05959.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Roche FK, Letourneau PC. Chick sensory neuronal growth cones distinguish fibronectin from laminin by making substratum contacts that resemble focal contacts. J Neurobiol. 1996;29:18–34. doi: 10.1002/(SICI)1097-4695(199601)29:1<18::AID-NEU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Snow DM, Letourneau PC. Characterization of spontaneous calcium transients in nerve growth cones and their effect on growth cone migration. Neuron. 1995;14:1233–1246. doi: 10.1016/0896-6273(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez ML, Silver J. Axon-glia interactions regulate ECM patterning in the postnatal rat olfactory bulb. J Neurosci. 1994;14:6121–6131. doi: 10.1523/JNEUROSCI.14-10-06121.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz B, Scholze A, Clement A, Joester A, Schutte K, Wigger F, Frank R, Spiess E, Ekblom P, Faissner A. Tenascin-C contains distinct adhesive, anti-adhesive, and neurite outgrowth promoting sites for neurons. J Cell Biol. 1996;132:681–699. doi: 10.1083/jcb.132.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Bolz J, Joester A, Faissner A. Tenascin-C synthesis and influence on axonal growth during rat cortical development. Eur J Neurosci. 1997;9:496–506. doi: 10.1111/j.1460-9568.1997.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Guan W, Puthenveedu MA, Condic ML. Sensory neuron subtypes have unique substratum preference and receptor expression before target innervation. J Neurosci. 2003;23:1781–1791. doi: 10.1523/JNEUROSCI.23-05-01781.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirland C, Suzuki S, Kojima M, Lu B, Zheng JQ. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell. 2010;18:725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A, Gingles NA, Bai H, Castellino FJ, Parmer RJ, Miles LA. Plasminogen enhances neuritogenesis on laminin-1. J Neurosci. 2009;29:12393–12400. doi: 10.1523/JNEUROSCI.3553-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell's invasive membrane in C. elegans. Dev Cell. 2009;17:187–198. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W. The behavior of optic axons on substrate gradients of retinal basal lamina proteins and merosin. J Neurosci. 1996;16:4389–4401. doi: 10.1523/JNEUROSCI.16-14-04389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin MH, Lagenaur CF. Cell adhesion molecules in the early developing mouse retina: retinal neurons show preferential outgrowth in vitro on L1 but not N-CAM. J Neurobiol. 1994;25:472–487. doi: 10.1002/neu.480250503. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Dallal GE, Flanagan JG. Retinal axon response to ephrin-as shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron. 2004;42:717–730. doi: 10.1016/j.neuron.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Hari A, Djohar B, Skutella T, Montazeri S. Neurotrophins and extracellular matrix molecules modulate sensory axon outgrowth. Int J Dev Neurosci. 2004;22:113–117. doi: 10.1016/j.ijdevneu.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Harper MM, Ye EA, Blong CC, Jacobson ML, Sakaguchi DS. Integrins contribute to initial morphological development and process outgrowth in rat adult hippocampal progenitor cells. J Mol Neurosci. 2010;40:269–283. doi: 10.1007/s12031-009-9211-x. [DOI] [PubMed] [Google Scholar]
- Hehr CL, Hocking JC, McFarlane S. Matrix metalloproteinases are required for retinal ganglion cell axon guidance at select decision points. Development. 2005;132:3371–3379. doi: 10.1242/dev.01908. [DOI] [PubMed] [Google Scholar]
- Hikita ST, Cann GM, Wingerd KL, Mullick LH, Wayne WC, Webb SW, Clegg DO. Integrin alpha4beta1 (VLA-4) expression and activity in retinal and peripheral neurons. Mol Cell Neurosci. 2003;23:427–439. doi: 10.1016/s1044-7431(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Hilario JD, Wang C, Beattie CE. Collagen XIXa1 is crucial for motor axon navigation at intermediate targets. Development. 137:4261–4269. doi: 10.1242/dev.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Abu-Rub M, Henley JR. Asymmetric endocytosis and remodeling of beta1-integrin adhesions during growth cone chemorepulsion by MAG. Nat Neurosci. 2010;13:829–837. doi: 10.1038/nn.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Dickson BJ. Sugar codes for axons? Neuron. 2005;46:169–172. doi: 10.1016/j.neuron.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci. 2008;28:1262–1269. doi: 10.1523/JNEUROSCI.1068-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci. 2001;4:695–701. doi: 10.1038/89482. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hall DH, Hedgecock EM, Kao G, Karantza V, Vogel BE, Hutter H, Chisholm AD, Yurchenco PD, Wadsworth WG. Laminin alpha subunits and their role in C. elegans development. Development. 2003;130:3343–3358. doi: 10.1242/dev.00481. [DOI] [PubMed] [Google Scholar]
- Huang Z, Yazdani U, Thompson-Peer KL, Kolodkin AL, Terman JR. Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development. 2007;134:2337–2347. doi: 10.1242/dev.004242. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Hubert T, Grimal S, Carroll P, Fichard-Carroll A. Collagens in the developing and diseased nervous system. Cell Mol Life Sci. 2009;66:1223–1238. doi: 10.1007/s00018-008-8561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert T, Grimal S, Ratzinger S, Mechaly I, Grassel S, Fichard-Carroll A. Collagen XVI is a neural component of the developing and regenerating dorsal root ganglia extracellular matrix. Matrix Biol. 2007;26:206–210. doi: 10.1016/j.matbio.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yamada KM. Neurite extension of chicken peripheral nervous system neurons on fibronectin: relative importance of specific adhesion sites in the central cell-binding domain and the alternatively spliced type III connecting segment. J Cell Biol. 1988;106:1289–1297. doi: 10.1083/jcb.106.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- Hynds DL, Snow DM. Fibronectin and laminin elicit differential behaviors from SH-SY5Y growth cones contacting inhibitory chondroitin sulfate proteoglycans. J Neurosci Res. 2001;66:630–642. doi: 10.1002/jnr.10020. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Targeted mutations in cell adhesion genes: what have we learned from them? Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M, Irie F, Plump AS, Tessier-Lavigne M, Yamaguchi Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302:1044–1046. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–881. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- Ivins JK, Colognato H, Kreidberg JA, Yurchenco PD, Lander AD. Neuronal receptors mediating responses to antibodyactivated laminin-1. J Neurosci. 1998;18:9703–9715. doi: 10.1523/JNEUROSCI.18-23-09703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques TS, Relvas JB, Nishimura S, Pytela R, Edwards GM, Streuli CH, ffrench-Constant C. Neural precursor cell chain migration and division are regulated through different beta1 integrins. Development. 1998;125:3167–3177. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O'Connor MB, Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Jones LS. Integrins: possible functions in the adult CNS. Trends Neurosci. 1996;19:68–72. doi: 10.1016/0166-2236(96)89623-8. [DOI] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. Recycling of the cell adhesion molecule L1 in axonal growth cones. J Neurosci. 2000;20:3676–3686. doi: 10.1523/JNEUROSCI.20-10-03676.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Karlstrom RO, Trowe T, Klostermann S, Baier H, Brand M, Crawford AD, Grunewald B, Haffter P, Hoffmann H, Meyer SU, Muller BK, Richter S, van Eeden FJ, Nusslein-Volhard C, Bonhoeffer F. Zebrafish mutations affecting retinotectal axon pathfinding. Development. 1996;123:427–438. doi: 10.1242/dev.123.1.427. [DOI] [PubMed] [Google Scholar]
- Kawano H, Ohyama K, Kawamura K, Nagatsu I. Migration of dopaminergic neurons in the embryonic mesencephalon of mice. Brain Res Dev Brain Res. 1995;86:101–113. doi: 10.1016/0165-3806(95)00018-9. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Torii K, Yamashita Y, Nishizawa K, Kanekura K, Katada M, Ito M, Nishimoto I, Terashita K, Aiso S, Matsuoka M. Wnt5a promotes adhesion of human dermal fibroblasts by triggering a phosphatidylinositol-3 kinase/Akt signal. Cell Signal. 2007;19:2498–2506. doi: 10.1016/j.cellsig.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Kiernan BW, Garcion E, Ferguson J, Frost EE, Torres EM, Dunnett SB, Saga Y, Aizawa S, Faissner A, Kaur R, Franklin RJ, ffrench-Constant C. Myelination and behaviour of tenascin-C null transgenic mice. Eur J Neurosci. 1999;11:3082–3092. doi: 10.1046/j.1460-9568.1999.00729.x. [DOI] [PubMed] [Google Scholar]
- Kiernan BW, Gotz B, Faissner A, ffrench-Constant C. Tenascin-C inhibits oligodendrocyte precursor cell migration by both adhesion-dependent and adhesion-independent mechanisms. Mol Cell Neurosci. 1996;7:322–335. doi: 10.1006/mcne.1996.0024. [DOI] [PubMed] [Google Scholar]
- Knoll B, Drescher U. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J Neurosci. 2004;24:6248–6257. doi: 10.1523/JNEUROSCI.0985-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic A, Sap J, Sheetz MP. RPTPalpha is required for rigidity-dependent inhibition of extension and differentiation of hippocampal neurons. J Cell Sci. 2007;120:3895–3904. doi: 10.1242/jcs.009852. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Schmidt MF, Kater SB. Laminin and fibronectin guideposts signal sustained but opposite effects to passing growth cones. Neuron. 1995;14:275–285. doi: 10.1016/0896-6273(95)90285-6. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Williams CV, Dou P, Kater SB. Laminin directs growth cone navigation via two temporally and functionally distinct calcium signals. J Neurosci. 1998;18:184–194. doi: 10.1523/JNEUROSCI.18-01-00184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok J, Fawcett J. XXXSpecial-Issue: regeneration. Dev Neurobiol. 2011 in press. [Google Scholar]
- Laforest S, Milanini J, Parat F, Thimonier J, Lehmann M. Evidences that beta1 integrin and Rac1 are involved in the overriding effect of laminin on myelin-associated glycoprotein inhibitory activity on neuronal cells. Mol Cell Neurosci. 2005;30:418–428. doi: 10.1016/j.mcn.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Lallier T, Bronner-Fraser M. Inhibition of neural crest cell attachment by integrin antisense oligonucleotides. Science. 1993;259:692–695. doi: 10.1126/science.8430321. [DOI] [PubMed] [Google Scholar]
- Langhorst MF, Jaeger FA, Mueller S, Sven Hartmann L, Luxenhofer G, Stuermer CA. Reggies/flotillins regulate cytoskeletal remodeling during neuronal differentiation via CAP/ponsin and Rho GTPases. Eur J Cell Biol. 2008;87:921–931. doi: 10.1016/j.ejcb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Lee JH, Rangarajan ES, Yogesha SD, Izard T. Raver1 interactions with vinculin and RNA suggest a feed-forward pathway in directing mRNA to focal adhesions. Structure. 2009;17:833–842. doi: 10.1016/j.str.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Chien CB. When sugars guide axons: insights from heparan sulphate proteoglycan mutants. Nat Rev Genet. 2004;5:923–935. doi: 10.1038/nrg1490. [DOI] [PubMed] [Google Scholar]
- Lee JS, von der Hardt S, Rusch MA, Stringer SE, Stickney HL, Talbot WS, Geisler R, Nusslein-Volhard C, Selleck SB, Chien CB, Roehl H. Axon sorting in the optic tract requires HSPG synthesis by ext2 (dackel) and extl3 (boxer) Neuron. 2004;44:947–960. doi: 10.1016/j.neuron.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Higgins D, Turner DC, Flier LA, Terranova VP. The NC1 domain of type IV collagen promotes axonal growth in sympathetic neurons through interaction with the alpha 1 beta 1 integrin. J Cell Biol. 1991;113:417–428. doi: 10.1083/jcb.113.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiss M, Beckmann K, Giros A, Costell M, Fassler R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr Opin Cell Biol. 2008;20:502–507. doi: 10.1016/j.ceb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Letourneau PC, Condic ML, Snow DM. Interactions of developing neurons with the extracellular matrix. J Neurosci. 1994;14:915–928. doi: 10.1523/JNEUROSCI.14-03-00915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Shattuck TA. Distribution and possible interactions of actin-associated proteins and cell adhesion molecules of nerve growth cones. Development. 1989;105:505–519. doi: 10.1242/dev.105.3.505. [DOI] [PubMed] [Google Scholar]
- Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Li W, Gao X, Li X, Jurgensen C, Park HT, Shin NY, Yu J, He ML, Hanks SK, Wu JY, Guan KL, Rao Y. p130CAS is required for netrin signaling and commissural axon guidance. J Neurosci. 2007;27:957–968. doi: 10.1523/JNEUROSCI.4616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Schmid RS, Snider WD, Maness PF. NGF enhances sensory axon growth induced by laminin but not by the L1 cell adhesion molecule. Mol Cell Neurosci. 2002;20:2–12. doi: 10.1006/mcne.2002.1107. [DOI] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyckman AW, Moya KL, Confaloni A, Jhaveri S. Early postnatal expression of L1 by retinal fibers in the optic tract and synaptic targets of the Syrian hamster. J Comp Neurol. 2000;423:40–51. [PubMed] [Google Scholar]
- Mann F, Harris WA, Holt CE. New views on retinal axon development: a navigation guide. Int J Dev Biol. 2004;48:957–964. doi: 10.1387/ijdb.041899fm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti G, Escuin S, van der Flier A, De Arcangelis A, Hynes RO, Georges-Labouesse E. Integrin alpha5beta1 is necessary for regulation of radial migration of cortical neurons during mouse brain development. Eur J Neurosci. 2010;31:399–409. doi: 10.1111/j.1460-9568.2009.07072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Irie F, Inatani M, Tessier-Lavigne M, Yamaguchi Y. Netrin-1/DCC signaling in commissural axon guidance requires cell-autonomous expression of heparan sulfate. J Neurosci. 2007;27:4342–4350. doi: 10.1523/JNEUROSCI.0700-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane S. Metalloproteases: carving out a role in axon guidance. Neuron. 2003;37:559–562. doi: 10.1016/s0896-6273(03)00089-8. [DOI] [PubMed] [Google Scholar]
- Meiners S, Mercado ML, Nur-e-Kamal MS, Geller HM. Tenascin-C contains domains that independently regulate neurite outgrowth and neurite guidance. J Neurosci. 1999;19:8443–8453. doi: 10.1523/JNEUROSCI.19-19-08443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado ML, Nur-e-Kamal A, Liu HY, Gross SR, Movahed R, Meiners S. Neurite outgrowth by the alternatively spliced region of human tenascin-C is mediated by neuronal alpha7beta1 integrin. J Neurosci. 2004;24:238–247. doi: 10.1523/JNEUROSCI.4519-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Moussian B. Drosophila multiplexin (Dmp) modulates motor axon pathfinding accuracy. Dev Growth Differ. 2009;51:483–498. doi: 10.1111/j.1440-169X.2009.01111.x. [DOI] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL, Wang B. Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase. Role of Rho family small GTPases. J Biol Chem. 2005;280:923–932. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- Michele M, Faissner A. Tenascin-C stimulates contactin-dependent neurite outgrowth via activation of phospholipase C. Mol Cell Neurosci. 2009;41:397–408. doi: 10.1016/j.mcn.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Millaruelo AI, Nieto-Sampedro M, Cotman CW. Cooperation between nerve growth factor and laminin or fibronectin in promoting sensory neuron survival and neurite outgrowth. Brain Res. 1988;466:219–228. doi: 10.1016/0165-3806(88)90047-8. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Moore S, Sheetz M. Biophysics of substrate interaction: influence on neural motility, differentiation and repair. Dev Neurobiol. 2011 doi: 10.1002/dneu.20947. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- Muir D. Metalloproteinase-dependent neurite outgrowth within a synthetic extracellular matrix is induced by nerve growth factor. Exp Cell Res. 1994;210:243–252. doi: 10.1006/excr.1994.1036. [DOI] [PubMed] [Google Scholar]
- Muller U, Bossy B, Venstrom K, Reichardt LF. Integrin alpha 8 beta 1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol Biol Cell. 1995;6:433–448. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Hayashi Y. Concomitant expression of genes encoding integrin alpha v beta 5 heterodimer and vitronectin in growing parallel fibers of postnatal rat cerebellum: a possible role as mediators of parallel fiber elongation. J Comp Neurol. 1998;397:199–212. [PubMed] [Google Scholar]
- Murray AJ, Tucker SJ, Shewan DA. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J Neurosci. 2009;29:15434–15444. doi: 10.1523/JNEUROSCI.3071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto T, Kain KH, Ginsberg MH. Neurobiology: New connections between integrins and axon guidance. Curr Biol. 2004;14:R121–R123. [PubMed] [Google Scholar]
- Neidhardt J, Fehr S, Kutsche M, Lohler J, Schachner M. Tenascin-N: characterization of a novel member of the tenascin family that mediates neurite repulsion from hippocampal explants. Mol Cell Neurosci. 2003;23:193–209. doi: 10.1016/s1044-7431(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Neugebauer KM, Emmett CJ, Venstrom KA, Reichardt LF. Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron. 1991;6:345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- Nishimune H, Valdez G, Jarad G, Moulson CL, Muller U, Miner JH, Sanes JR. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J Cell Biol. 2008;182:1201–1215. doi: 10.1083/jcb.200805095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Noren NK, Yang NY, Silldorff M, Mutyala R, Pasquale EB. Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem J. 2009;422:433–442. doi: 10.1042/BJ20090014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri M, Buricchi F, Giannoni E, Grimaldi G, Mello T, Raugei G, Ramponi G, Chiarugi P. EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J Biol Chem. 2007;282:19619–19628. doi: 10.1074/jbc.M701319200. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139:1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus JD, Halloran MC. Zebrafish bashful/laminin-alpha 1 mutants exhibit multiple axon guidance defects. Dev Dyn. 2006;235:213–224. doi: 10.1002/dvdy.20604. [DOI] [PubMed] [Google Scholar]
- Pearlman AL, Sheppard AM. Extracellular matrix in early cortical development. Prog Brain Res. 1996;108:117–134. [PubMed] [Google Scholar]
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci. 1999;19:1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantman S, Patarroyo M, Fried K, Domogatskaya A, Tryggvason K, Hammarberg H, Cullheim S. Integrin-laminin interactions controlling neurite outgrowth from adult DRG neurons in vitro. Mol Cell Neurosci. 2008;39:50–62. doi: 10.1016/j.mcn.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- Prevost N, Woulfe DS, Jiang H, Stalker TJ, Marchese P, Ruggeri ZM, Brass LF. Eph kinases and ephrins support thrombus growth and stability by regulating integrin outside-in signaling in platelets. Proc Natl Acad Sci U S A. 2005;102:9820–9825. doi: 10.1073/pnas.0404065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racchetti G, Lorusso A, Schulte C, Gavello D, Carabelli V, D'Alessandro R, Meldolesi J. Rapid neurite outgrowth in neurosecretory cells and neurons is sustained by the exocytosis of a cytoplasmic organelle, the enlargeosome. J Cell Sci. 2010;123:165–170. doi: 10.1242/jcs.059634. [DOI] [PubMed] [Google Scholar]
- Ren XR, Ming GL, Xie Y, Hong Y, Sun DM, Zhao ZQ, Feng Z, Wang Q, Shim S, Chen ZF, Song HJ, Mei L, Xiong WC. Focal adhesion kinase in netrin-1 signaling. Nat Neurosci. 2004;7:1204–1212. doi: 10.1038/nn1330. [DOI] [PubMed] [Google Scholar]
- Renaudin A, Lehmann M, Girault J, McKerracher L. Organization of point contacts in neuronal growth cones. J Neurosci Res. 1999;55:458–471. doi: 10.1002/(SICI)1097-4547(19990215)55:4<458::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rhiner C, Gysi S, Frohli E, Hengartner MO, Hajnal A. Syndecan regulates cell migration and axon guidance in C. elegans. Development. 2005;132:4621–4633. doi: 10.1242/dev.02042. [DOI] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas RJ, Burmeister DW, Goldberg DJ. Rapid effects of laminin on the growth cone. Neuron. 1992;8:107–115. doi: 10.1016/0896-6273(92)90112-q. [DOI] [PubMed] [Google Scholar]
- Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci. 2010;30:15337–15357. doi: 10.1523/JNEUROSCI.3467-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- Robles E, Huttenlocher A, Gomez TM. Filopodial calcium transients regulate growth cone motility and guidance through local activation of calpain. Neuron. 2003;38:597–609. doi: 10.1016/s0896-6273(03)00260-5. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O'Connell S, Cowan CW, Hu L, Goldberg JL, Debant A, Corfas G, Krull CE, Greenberg ME. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Sarig-Nadir O, Seliktar D. The role of matrix metalloproteinases in regulating neuronal and nonneuronal cell invasion into PEGylated fibrinogen hydrogels. Biomaterials. 2010;31:6411–6416. doi: 10.1016/j.biomaterials.2010.04.052. [DOI] [PubMed] [Google Scholar]
- Schimmelpfeng K, Gogel S, Klambt C. The function of leak and kuzbanian during growth cone and cell migration. Mech Dev. 2001;106:25–36. doi: 10.1016/s0925-4773(01)00402-6. [DOI] [PubMed] [Google Scholar]
- Schlomann U, Schwamborn JC, Muller M, Fassler R, Puschel AW. The stimulation of dendrite growth by Sema3A requires integrin engagement and focal adhesion kinase. J Cell Sci. 2009;122:2034–2042. doi: 10.1242/jcs.038232. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Anton ES. Role of integrins in the development of the cerebral cortex. Cereb Cortex. 2003;13:219–224. doi: 10.1093/cercor/13.3.219. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Granato M. The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration. Neuron. 2006;50:683–695. doi: 10.1016/j.neuron.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9:1046–1056. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Sharfe N, Nikolic M, Cimpeon L, Van De Kratts A, Freywald A, Roifman CM. EphA and ephrin-A proteins regulate integrin-mediated T lymphocyte interactions. Mol Immunol. 2008;45:1208–1220. doi: 10.1016/j.molimm.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Moore SW, Tritsch NX, Morris SJ, Bouchard JF, Kennedy TE. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J Neurosci. 2005;25:3132–3141. doi: 10.1523/JNEUROSCI.1920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM. Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci. 2009;29:8129–8142. doi: 10.1523/JNEUROSCI.4681-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Simonson WT, Franco SJ, Huttenlocher A. Talin1 regulates TCR-mediated LFA-1 function. J Immunol. 2006;177:7707–7714. doi: 10.4049/jimmunol.177.11.7707. [DOI] [PubMed] [Google Scholar]
- Singhal N, Martin P. Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev Neurobiol. 2011 doi: 10.1002/dneu.20953. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YJ, Patton BL, Sanes JR. Induction of presynaptic differentiation in cultured neurons by extracellular matrix components. Eur J Neurosci. 1999;11:3457–3467. doi: 10.1046/j.1460-9568.1999.00766.x. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci U S A. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniszewska I, Sariyer IK, Lecht S, Brown MC, Walsh EM, Tuszynski GP, Safak M, Lazarovici P, Marcinkiewicz C. Integrin alpha9 beta1 is a receptor for nerve growth factor and other neurotrophins. J Cell Sci. 2008;121:504–513. doi: 10.1242/jcs.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigemann P, Molitor A, Fellert S, Jackle H, Vorbruggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Stettler EM, Galileo DS. Radial glia produce and align the ligand fibronectin during neuronal migration in the developing chick brain. J Comp Neurol. 2004;468:441–451. doi: 10.1002/cne.10987. [DOI] [PubMed] [Google Scholar]
- Stewart GR, Pearlman AL. Fibronectin-like immunoreactivity in the developing cerebral cortex. J Neurosci. 1987;7:3325–3333. doi: 10.1523/JNEUROSCI.07-10-03325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh LH, Oster SF, Soehrman SS, Grenningloh G, Sretavan DW. L1/Laminin modulation of growth cone response to EphB triggers growth pauses and regulates the microtubule destabilizing protein SCG10. J Neurosci. 2004;24:1976–1986. doi: 10.1523/JNEUROSCI.1670-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi H, Laub F, Yoshioka H, Ramirez F. Embryonic expression of type XIX collagen is transient and confined to muscle cells. Dev Dyn. 2001;220:155–162. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1099>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Sund M, Vaisanen T, Kaukinen S, Ilves M, Tu H, Autio-Harmainen H, Rauvala H, Pihlajaniemi T. Distinct expression of type XIII collagen in neuronal structures and other tissues during mouse development. Matrix Biol. 2001;20:215–231. doi: 10.1016/s0945-053x(01)00134-2. [DOI] [PubMed] [Google Scholar]
- Sydor AM, Su AL, Wang FS, Xu A, Jay DG. Talin and vinculin play distinct roles in filopodial motility in the neuronal growth cone. J Cell Biol. 1996;134:1197–1207. doi: 10.1083/jcb.134.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Tate MC, Garcia AJ, Keselowsky BG, Schumm MA, Archer DR, LaPlaca MC. Specific beta1 integrins mediate adhesion, migration, and differentiation of neural progenitors derived from the embryonic striatum. Mol Cell Neurosci. 2004;27:22–31. doi: 10.1016/j.mcn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Tojima T, Hines JH, Henley JR, Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat Rev Neurosci. 2011;12:191–203. doi: 10.1038/nrn2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol. 2009;21:676–683. doi: 10.1016/j.ceb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Doherty P, Emmett CJ, Damsky CH, Walsh FS, Reichardt LF. Expression of beta 1 integrins in sensory neurons of the dorsal root ganglion and their functions in neurite outgrowth on two laminin isoforms. J Neurosci. 1993;13:4880–4888. doi: 10.1523/JNEUROSCI.13-11-04880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Hall DE, Flier LA, Gehlsen KR, Turner DC, Carbonetto S, Reichardt LF. A neuronal cell line (PC12) expresses two beta 1-class integrins-alpha 1 beta 1 and alpha 3 beta 1-that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990;5:651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- Treloar HB, Ray A, Dinglasan LA, Schachner M, Greer CA. Tenascin-C is an inhibitory boundary molecule in the developing olfactory bulb. J Neurosci. 2009;29:9405–9416. doi: 10.1523/JNEUROSCI.2356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Mearow KM. Peripheral sensory axon growth: from receptor binding to cellular signaling. Can J Neurol Sci. 2008;35:551–566. doi: 10.1017/s0317167100009331. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Rahimtula M, Mearow KM. Integrin activation and neurotrophin signaling cooperate to enhance neurite outgrowth in sensory neurons. J Comp Neurol. 2005;486:267–280. doi: 10.1002/cne.20518. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Rahimtula M, Mearow KM. Laminin and growth factor receptor activation stimulates differential growth responses in subpopulations of adult DRG neurons. Eur J Neurosci. 2006;24:676–690. doi: 10.1111/j.1460-9568.2006.04963.x. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Rahimtula M, Mearow KM. Src and FAK are key early signalling intermediates required for neurite growth in NGF-responsive adult DRG neurons. Cell Signal. 2008;20:241–257. doi: 10.1016/j.cellsig.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Hagios C, Chiquet-Ehrismann R. Tenascin-Y in the developing and adult avian nervous system. Dev Neurosci. 1999;21:126–133. doi: 10.1159/000017374. [DOI] [PubMed] [Google Scholar]
- Turney SG, Bridgman PC. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- Van Vactor D, Wall DP, Johnson KG. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr Opin Neurobiol. 2006;16:40–51. doi: 10.1016/j.conb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Venstrom K, Muller U, Kypta R, Backus C, Chiquet M, Reichardt LF. The integrin receptor alpha 8 beta 1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron. 1995;14:1213–1222. doi: 10.1016/0896-6273(95)90268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venstrom K, Reichardt L. Beta 8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin. Mol Biol Cell. 1995;6:419–431. doi: 10.1091/mbc.6.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venstrom KA, Reichardt LF. Extracellular matrix. 2: Role of extracellular matrix molecules and their receptors in the nervous system. FASEB J. 1993;7:996–1003. doi: 10.1096/fasebj.7.11.8370483. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Nakamura M, Okano H, Toyama Y. Establishment of three-dimensional culture of neural stem/progenitor cells in collagen Type-1 Gel. Restor Neurol Neurosci. 2007;25:109–117. [PubMed] [Google Scholar]
- Weaver CD, Yoshida CK, de Curtis I, Reichardt LF. Expression and in vitro function of beta 1-integrin laminin receptors in the developing avian ciliary ganglion. J Neurosci. 1995;15:5275–5285. doi: 10.1523/JNEUROSCI.15-07-05275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber CA, Hocking JC, Yong VW, Stange CL, McFarlane S. Metalloproteases and guidance of retinal axons in the developing visual system. J Neurosci. 2002;22:8091–8100. doi: 10.1523/JNEUROSCI.22-18-08091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P, Bartsch U, Rasband MN, Czaniera R, Lang Y, Bluethmann H, Margolis RU, Levinson SR, Shrager P, Montag D, Schachner M. Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci. 1999;19:4245–4262. doi: 10.1523/JNEUROSCI.19-11-04245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P, Montag D, Schachner M, Bernhardt RR. Zebrafish tenascin-W, a new member of the tenascin family. J Neurobiol. 1998;35:1–16. [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Weinl C, Drescher U, Lang S, Bonhoeffer F, Loschinger J. On the turning of Xenopus retinal axons induced by ephrin-A5. Development. 2003;130:1635–1643. doi: 10.1242/dev.00386. [DOI] [PubMed] [Google Scholar]
- Willett M, Pollard HJ, Vlasak M, Morley SJ. Localisation of translation initiation factors to talin/beta3-integrin-enriched adhesion complexes in spreading and migrating mammalian cells. Biol Cell. 2009 doi: 10.1042/BC20090141. [DOI] [PubMed] [Google Scholar]
- Williamson T, Gordon-Weeks PR, Schachner M, Taylor J. Microtubule reorganization is obligatory for growth cone turning. Proc Natl Acad Sci U S A. 1996;93:15221–15226. doi: 10.1073/pnas.93.26.15221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman MA, Sittaramane VK, Essner JJ, Yost HJ, Chandrasekhar A, Halloran MC. Transient axonal glycoprotein-1 (TAG-1) and laminin-alpha1 regulate dynamic growth cone behaviors and initial axon direction in vivo. Neural Dev. 2008;3:6. doi: 10.1186/1749-8104-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S, Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J Neurosci. 2006;26:1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S, Rowan DJ, Gomez TM. Retinotopic mapping requires focal adhesion kinase-mediated regulation of growth cone adhesion. J Neurosci. 2009;29:13981–13991. doi: 10.1523/JNEUROSCI.4028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Baier H. Lamina-specific axonal projections in the zebrafish tectum require the type IV collagen Dragnet. Nat Neurosci. 2007;10:1529–1537. doi: 10.1038/nn2002. [DOI] [PubMed] [Google Scholar]
- Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2955–2967. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- Xiao ZC, Taylor J, Montag D, Rougon G, Schachner M. Distinct effects of recombinant tenascin-R domains in neuronal cell functions and identification of the domain interacting with the neuronal recognition molecule F3/11. Eur J Neurosci. 1996;8:766–782. doi: 10.1111/j.1460-9568.1996.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Yang P, Baker KA, Hagg T. The ADAMs family: coordinators of nervous system development, plasticity and repair. Prog Neurobiol. 2006;79:73–94. doi: 10.1016/j.pneurobio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Yebra M, Montgomery AM, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Hurford R, Florkiewicz E, Tessier-Lavigne M, Cirulli V. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Hishiyama S, Yamaguchi M, Hashiguchi M, Miyamoto Y, Kaminogawa S, Hisatsune T. Decrease in expression of alpha 5 beta 1 integrin during neuronal differentiation of cortical progenitor cells. Exp Cell Res. 2003;287:262–271. doi: 10.1016/s0014-4827(03)00158-7. [DOI] [PubMed] [Google Scholar]
- Yu YM, Cristofanilli M, Valiveti A, Ma L, Yoo M, Morellini F, Schachner M. The extracellular matrix glycoprotein tenascin-C promotes locomotor recovery after spinal cord injury in adult zebrafish. Neuroscience. 2011;183:238–250. doi: 10.1016/j.neuroscience.2011.03.043. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller J, Granato M. The zebrafish diwanka gene controls an early step of motor growth cone migration. Development. 1999;126:3461–3472. doi: 10.1242/dev.126.15.3461. [DOI] [PubMed] [Google Scholar]
- Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci. 1998;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]