Highlights
► Age-related tissue stiffening has a profound effect on human morbidity and mortality. ► Scanning acoustic microscopy can quantify the localised stiffness of discrete tissue components in situ. ► Age-related aortic stiffening is both localised to collagen fibril-rich regions and associated with collagen fibrosis. ► Age-related structural remodelling events can be directly linked with in situ mechanical changes using scanning acoustic microscopy.
Abbreviations: ECM, extracellular matrix; VSMC, vascular smooth muscle cell; SAM, scanning acoustic microscopy; MLU, medial lamellar unit; STAN, soft tissue analysis
Keywords: Tissue elasticity, Arteriosclerosis, Collagen, Elastic fibers, Scanning acoustic microscopy
Abstract
Age-related loss of tissue elasticity is a common cause of human morbidity and arteriosclerosis (vascular stiffening) is associated with the development of both fatal strokes and heart failure. However, in the absence of appropriate micro-mechanical testing methodologies, multiple structural remodelling events have been proposed as the cause of arteriosclerosis. Therefore, using a model of ageing in female sheep aorta (young: <18 months, old: >8 years) we: (i) quantified age-related macro-mechanical stiffness, (ii) localised in situ micro-metre scale changes in acoustic wave speed (a measure of tissue stiffness) and (iii) characterised collagen and elastic fibre remodelling. With age, there was an increase in both macro-mechanical stiffness and mean microscopic wave speed (and hence stiffness; young wave speed: 1701 ± 1 m s−1, old wave speed: 1710 ± 1 m s−1, p < 0.001) which was localized to collagen fibril-rich regions located between large elastic lamellae. These micro-mechanical changes were associated with increases in both collagen and elastic fibre content (collagen tissue area, young: 31 ± 2%, old: 40 ± 4%, p < 0.05; elastic fibre tissue area, young: 55 ± 3%, old: 69 ± 4%, p < 0.001). Localised collagen fibrosis may therefore play a key role in mediating age-related arteriosclerosis. Furthermore, high frequency scanning acoustic microscopy is capable of co-localising micro-mechanical and micro-structural changes in ageing tissues.
1. Introduction
The mechanical functions of dynamic connective tissues are mediated primarily by highly structured extracellular matrices (ECM) in which fibrillar collagens confer tensile strength (Birk and Bruckner, 2005) and elastic fibres confer compliance and passive recoil (Kielty et al., 2002; Sherratt, 2009). However, progressive loss of both compliance and recoil, appears to be a universal pathology in ageing mammalian tissues such as skin (Nishimori et al., 2001), lungs (Janssens et al., 1999; Lai-Fook and Hyatt, 2000) and arteries (Bruel and Oxlund, 1996; Cattell et al., 1996). Whilst reduced skin elasticity is associated with the visible progression of ageing in the form of wrinkles (Nishimori et al., 2001), and loss of pulmonary compliance may increase the risk of mortality in an elderly population (Janssens et al., 1999; Meyer et al., 1998), the major clinical impact of age-related tissue stiffening arises as a consequence of functional changes within arteries (Lakatta and Levy, 2003; Wilkinson et al., 2009). The profound nature of these clinical effects led Robert and colleagues to propose that age-related loss of elasticity within the cardio-respiratory system may be responsible for the apparent 100–120 year upper-limit on human lifespan (Robert et al., 2008).
Specifically, arteriosclerosis (diffuse large artery stiffening which is pathologically distinct from localised atherosclerosis (Pickering, 1963; Wilkinson et al., 2009), appears to be a key factor in the maintenance of essential hypertension (Arribas et al., 2006) and hence in the development of adverse cardiovascular events including stroke and heart failure (Domanski et al., 2001; Pearson et al., 1994). Regrettably, whilst there is ample evidence from in vivo (O’Rourke and Hashimoto, 2007; Pearson et al., 1994; Ruitenbeek et al., 2008) and in vitro (Kielty et al., 2007; O’Connell et al., 2008) studies of a significant relationship between increasing age and the progression of arteriosclerosis, the primary cause of arterial stiffening remains contentious (Wilkinson et al., 2009). This lack of consensus is due, in part, to the structural and compositional complexity of the tissue, which limits the ability of conventional mechanical testing methods to discriminate between the functional contributions of discrete components, and also, to the complexity of the remodelling events which accompany ageing. Specifically, large arteries such as the aorta rely on the maintenance of a complex, hierarchical architecture to perform their mechanical functions (Kielty et al., 2007). However, with age the aorta undergoes significant remodelling at multiple length scales. Macroscopically, vessel dilatation and hypertrophy (Cantini et al., 2001; Lakatta, 2008; O’Rourke and Hashimoto, 2007) appear to differentially affect the intimal and medial layers (Cantini et al., 2001; Lakatta, 2008; Nagai et al., 1998; Virmani et al., 1991). At micrometre length scales age-related structural changes include fenestration and/or fragmentation of medial elastic lamellae (Bruel and Oxlund, 1996; Greenwald, 2007) and vascular smooth muscle cell (VSMC) migration, proliferation and cytoskeletal remodelling (Greenwald, 2007; Qiu et al., 2007). Finally molecular remodelling events in the ageing aorta include: (i) aberrant glycation of collagen and elastin (Bailey, 2001; Kass et al., 2001; Sims et al., 1996; Stacey et al., 2010), (ii) elastic fibre calcification (Blumenthal et al., 1944; Robert et al., 2008), (iii) proteolytic degradation and fatigue fracture (O’Rourke and Hashimoto, 2007; Rigby, 1964; Robert et al., 2008) of extracellular assemblies and (iv) gender and species dependent changes in the amount (fibrillar collagen and elastin) and subtype (type I and III collagen) of specific ECM components (Bruel and Oxlund, 1996; Cattell et al., 1996; Hosoda et al., 1984; Maurel et al., 1987; Qiu et al., 2007).
To date, as a consequence of both this multiplicity of potential pathological mechanisms and the technical challenge of mapping mechanical properties with a high spatial resolution, it has been necessary to infer a causal relationship between global mechanical stiffening and local structural remodelling events. However, the major aortic components are predominantly localised to multiple micrometre-scale medial lamellar units (MLU) (O’Connell et al., 2008) each of which is composed of an elastin-rich lamellae and a fibrillar collagen and VSMC-rich interlamellar region. We have therefore employed ultra-high frequency scanning acoustic microscopy (SAM), a technique that is well established in other disciplines (Briggs and Kolosov, 2009), to map micro-metre scale stiffness within aortic tissue excised from an established model of ageing in the sheep (Dibb et al., 2004). This technique, which quantifies tissue stiffness as a function of its longitudinal acoustic wave speed, can achieve a spatial resolution of ∼1 μm at a frequency of 1 GHz, thereby resolving individual tissue components (Akhtar et al., 2009b) such as cells (∼10 μm) and elastic fibres (2–3 μm) (Alberts et al., 2002; Sherratt et al., 2001). In this study, we have combined macro- and micro-mechanical methodologies with histological assessments of tissue structure and composition to test the hypotheses that in the ageing aorta gross mechanical stiffening (arteriosclerosis) is a consequence of localised stiffening within discrete tissue compartments.
2. Materials and methods
2.1. Reagents and tissue samples
All reagents were obtained from Sigma–Aldrich Co. Ltd. (Poole, UK) or BDH Ltd. (Poole, UK) unless otherwise specified. All procedures were approved by the UK Home Office and accorded with the UK Animals (Scientific Procedures) Act 1986 (which complies with the guidelines set out in NIH Publication No. 85-23 [revised 1996]). Young (<1.5 years) and old (>8 years) female sheep (Ovis aries) were killed by intravenous injection of pentobarbitone (200 mg kg−1) (Dibb et al., 2004). Ascending aortas were immediately dissected, cut into rings of 5 mm depth and either: (i) used immediately for gross mechanical testing, (ii) snap frozen in liquid nitrogen prior to gross mechanical testing, (iii) frozen in optimal cutting temperature (OCT) compound (Sakura Finetek Europe B.V., The Netherlands) (Akhtar et al., 2009a,b) or (iv) fixed in 4% paraformaldehyde for 24 h prior to processing to paraffin wax for subsequent histological analysis of collagen and elastin content (Graham and Trafford, 2007).
2.2. Effective gross mechanical stiffness
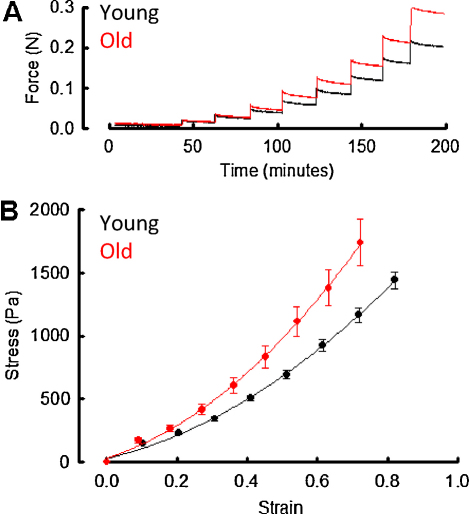
An indication of blood vessel effective stiffness [which is determined by both tissue thickness and material properties (Greenwald, 2007)] can be derived by measuring the maximum applied loads resulting from incremental extension of a tissue. Therefore, in order to quantify age-related changes in aortic wall thickness and stiffness, frozen aortic rings (n = 5 young and 6 old) were thawed, photographed with a gel documentation system (Syngene, UK) and mounted between a fixed steel hook and a force transducer in an organ bath. Gross wall thickness was measured from the captured micrographs using ImageJ (Abramoff et al., 2004). The potential effects of the freeze/thaw process on the compliance of aortic rings were characterised in additional experiments. The tissue was equilibrated in physiological saline (in mmol L−1: NaCl, 134; KCl, 5; MgSO4, 1; NaHCO3, 17; KH2PO4, 1; K2EDTA, 0.025; glucose, 5.6; CaCl2, 1.6 [pH 7.4] with 95% air and 5% CO2 at 37 °C for 30 min prior to extension at a rate of 1 mm every 20 min. The maximal force following each displacement step was recorded (Fig. 1A). The distensibility (and hence strain as derived from volume change) of large arteries is, dependent on age, species and experimental design (Faury, 2001). In this study we subjected the aortas to maximal strains of ∼0.8 encompassing both low and physiologic regions of the stress–strain curve (Sokolis et al., 2002).
Fig. 1.
Gross mechanical testing of intact aortic rings. (A) Example force vs time plot from a single young and a single old aortic ring. The tissue was elongated through 1 mm increments at 20 min intervals and the maximum force recorded following each incremental step. (B) Mean engineering stress–strain curves in young and old aorta following extension by 1 mm increments. Curves fitted to data derived from young and old animals were significantly different (ANOVA, p < 0.0001) (n = 5–6 animals/group).
2.3. Quantitative scanning acoustic microscopy
Age-related variations in acoustic wave speed and hence in the micro-mechanical stiffness of young and aged sheep aorta were mapped in unfixed and unstained tissue cryo-sections using SAM (Akhtar et al., 2009b). This instrument employs a pulse of ultra high frequency acoustic waves (100 MHz–1 GHz) which are focused to a diffraction limited spot with a concave spherical lens immersed in a coupling fluid (usually distilled water) (Briggs, 1992) By scanning in both the x and y directions a two dimensional acoustic image can be built up in the field of interest.
SAM images were analysed using a frequency scanning method which exploits the interference that is generated from signals reflected at fluid/sample and sample/substrate interfaces (Jensen et al., 2006; Jorgensen and Kundu, 2002). By varying the frequency (and hence wavelength) of the acoustic radiation, a periodic function, V(f) (voltage vs. defocus), is recorded which can be used to determine the acoustic wave speed if the sample thickness is known. In principal the technique is sensitive to small changes in elastic properties. However, the accurate determination of these properties within tissues is complicated because V(f) is a non-linear function of specimen thickness, acoustic attenuation and the relative reflection and transmission intensities at each interface. This problem can be addressed by comparing the measured V(f) signal with a computed V(f) and adjusting the variables of specimen thickness, attenuation and impedance, to optimise the fit between theoretical and experimental curves (Kundu et al., 2000). The longitudinal acoustic wave speed (νL), which we measure with the V(f) method, is related to Young's modulus (stiffness) of the tissue. In its simplest form, vL is given by Eq. (1):
| (1) |
where ρ is the mass density (kg m−3), and C11 (Pa) is the appropriate component of the stiffness tensor which can be expressed as a function of Young's modulus (E) and Poisson's ratio (ν) (Landau and Lifshitz, 1986).
Whilst ultra-high frequency ultrasound methods have previously been used to map wave speed within cardiovascular tissues (Marsh et al., 2004; Miller et al., 2003; Saijo et al., 2005; Verdonk et al., 1996), to our knowledge, all previous SAM investigations have been conducted on chemically fixed specimens. Given the profound effects of chemical fixation on the mechanical properties of tissues (Hall et al., 2000; Young, 2006), we have mapped and quantified changes in wave speed, within unfixed aortic cryo-sections excised from young and old sheep. Although technical considerations require quantitative SAM imaging to be carried out at the edge of tissue sections, by imaging the intima and adjacent inner medial layer, we were able to quantify wave speed changes in the region of the internal elastic lamina and across multiple MLUs.
Hydrated 5 μm cryosections adhered to glass cover-slips of known density were imaged by SAM (SAM 2000 [PVA Tepla Analytical Instruments GmbH, Germany]) using the V(f) method (Kundu et al., 2000; Akhtar et al., 2009b). Seven images were captured at a scan size of 200 × 200 μm and 10 MHz incremental frequencies from 960–1020 MHz (Akhtar et al., 2009b). Initially, mean longitudinal acoustic wave speed was estimated (within probability limits of 1400–2000 m s−1 (Duck, 1990) for seven young and seven old sheep using Soft Tissue Analysis (STAN) software (Aarhus University Hospital, Denmark) averaged across 20 lines (150–170 μm in length) originating from the intimal surface. Probability limits for the V(f) Simplex algorithm employed by the STAN software were set using reference data at 1400–2000 m s−1 (Duck, 1990). Subsequently, the relative contribution of both elastic lamellae and inter-lamellar regions within the MLU to the wave speed frequency distribution was determined for four young and four old animals.
2.4. Histological analysis of ECM composition
Aortic fibrillar collagen and elastic fibre content were assessed by semi-quantitative histological methods. Collagen content was characterised by polarized light microscopy of picrosirius red stained sections and elastin content by brightfield microscopy of sections stained with Millers elastic stain as previously described (Graham and Trafford, 2007; Miller, 1971). In this same study, we demonstrated that both semi-quantitative histological and quantitative HPLC-based approaches are capable of identifying similar changes in collagen content in the remodelling ventricular wall. Briefly, paraffin wax-embedded aortic rings (n = 9 for both young and old sheep) were sectioned to 5 μm thickness. Montages of picrosirius red and Millers stained sections were obtained across the vessel wall from the intimal to adventitial layers at four equally spaced points around the circular tissue cross-section. Mean fibrillar collagen and elastic fibre contents are expressed as a percentage of the tissue section area.
2.5. Data analysis and statistics
Results are expressed as mean ± standard deviation error and sample numbers are noted in the methods and figure legends. In some cases non-parametric data sets were transformed using the log10 transform to better conform to normality of distribution. Statistical significance was evaluated using Student's t-test, Mann–Whitney U-test or ANOVA, with p < 0.05 considered significant.
3. Results
3.1. The effective stiffness of sheep aorta increases with age
In order to establish if ageing sheep [in common with humans and rats (Mitchell, 2008; Osborne-Pellegrin et al., 2010)], suffer from arteriosclerosis, we measured applied load after incremental extension for aortic rings excised from young and old animals (Fig. 1A). The engineering stress–strain curves plotted from these measurements show that at each given strain, stress was higher in old compared with young sheep aortas (Fig. 1B, ANOVA, p < 0.0001). Furthermore, there was no observable difference in the engineering stress–strain curves between fresh and thawed vessels (Supplemental Fig. 1) and no significant difference (Student's t-test, p = 0.548) in mean wall diameter between young (2.4 mm ± 0.3 mm) and old (2.2 mm ± 0.1 mm) vessels.
3.2. Increases in acoustic wave speed are predominantly localised to the regions between elastic lamellae
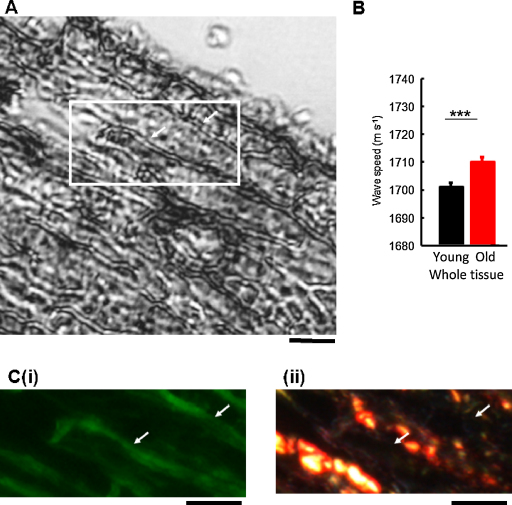
In order to determine whether these age-related gross mechanical changes were also evident at micrometre length scales we imaged sheep aorta cryosections by SAM (Fig. 2A). Mean wave speed, and hence tissue stiffness, was found to increase significantly (by approximately 9 m s−1) between young and old animals (Fig. 2B, Mann–Whitney U-test, p < 0.001). Previously, lower frequency (and hence lower resolution) SAM has been employed to identify disease-related changes in atherosclerotic (as opposed to arteriosclerotic) arteries and infarcted myocardium (Saijo et al., 1997, 2005; Verdonk et al., 1996). However, such global measurements of acoustic properties fail to exploit the resolving power of ultra-high frequency SAM and hence cannot distinguish between the mechanical contributions of discrete tissue components or the effects of localized stiffening events in ageing tissues. In contrast, at the GHz frequencies employed in this study prominent elastic lamellae-like structures were clearly discernible (Fig. 2A). By following SAM with sequential fluorescence and polarized light microscopy (of unstained and picrosirius red stained sections respectively) we were able to confirm that: (i) these discrete structures were rich in auto-fluorescent elastin and therefore were elastic lamellae and (ii) that fibrillar collagen was localised to the inter-lamellar regions (Fig. 2C).
Fig. 2.
SAM imaging of ovine aorta: tissue wave speed measurements and identification of structural features. (A) SAM image (1 GHz) of an unstained and unfixed aorta cryosection. Elastic lamellae-like tissue sub-structures were clearly evident in high-frequency SAM images (arrows). (B) Mean tissue wave speed was significantly increased in old compared with young animals across the full length of 20 line scans (n = 7 animals per group). (C) The inset region in (A) viewed by: fluorescence microscopy (i) and bright field microscopy following picrosirius red staining (ii). The lamellar structures which are evident in SAM images (A, arrows) correlate with the location of elastin autofluorescence (C(i), arrows) but not with the location of fibrillar collagen (C(ii), arrows). Scale bars = 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
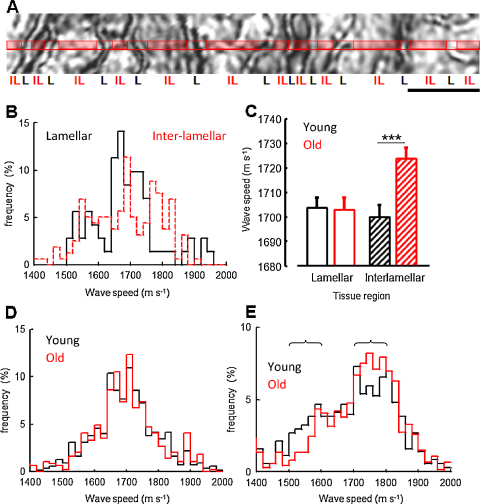
By combining ultra-high frequency SAM with conventional optical microscopy it was possible to determine the micromechanical contribution of discrete tissue regions to age-related arteriosclerosis (Fig. 3A). Using this approach, striking differences in acoustic wave speed frequency distribution were evident within the lamellar and inter-lamellar regions of sheep aorta (Fig. 3B). In contrast with elastic lamellae, where a single peak of ∼1700 m s−1 dominated the wave speed frequency distribution, the inter-lamellar region was characterised by an irregular profile, which could be interpreted as a convolution of multiple wave speed distributions centred at 1500–1600, ∼1700 and >1800 m s−1. By resolving the distinct wave speed contributions of the lamellar and inter-lamellar regions in young and old sheep, it became clear that the increase in tissue wave speed of 9 m s−1, which was evident in old animals (Fig. 2B), was due to a localized increase in wave speed (and hence stiffness) of 24 m s−1 within the collagen fibril and VSMC-rich inter-lamellar regions (Mann–Whitney U-test, p < 0.001) (Fig. 3C–E). Within these inter-lamellar regions there was an age-related reduction in the contribution of wave speeds between 1500 and 1600 m s−1 and an increased contribution of wave speeds between 1700 and 1800 m s−1 (Fig. 3E).
Fig. 3.
Comparison of lamellar and inter-lamellar wave speeds in young and old sheep. (A) Extracted region from a SAM image of an aorta cryo-section. Tissue wave speed was determined at pixel intervals across lines subdivided into lamellar (L) and inter-lamellar (IL) regions. (B) Differential wave speed frequency distribution within the lamellar and inter-lamellar regions of a single young sheep. (C) Whilst mean inter-lamellar wave speed was significantly increased in old compared with young sheep (Mann–Whitney U-test, ***p < 0.001) there was no significant age-related difference in wave speed within the elastic lamellae. (D and E) Wave speed frequency distributions of lamellar (D) and inter-lamellar (E) regions from young (black line) and old (red line) sheep aorta (n = 4 animals per group). Age related differences in wave speed frequency distribution are indicated by brackets. Scale bar = 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Age-related extracellular matrix remodelling is localised within the aortic wall
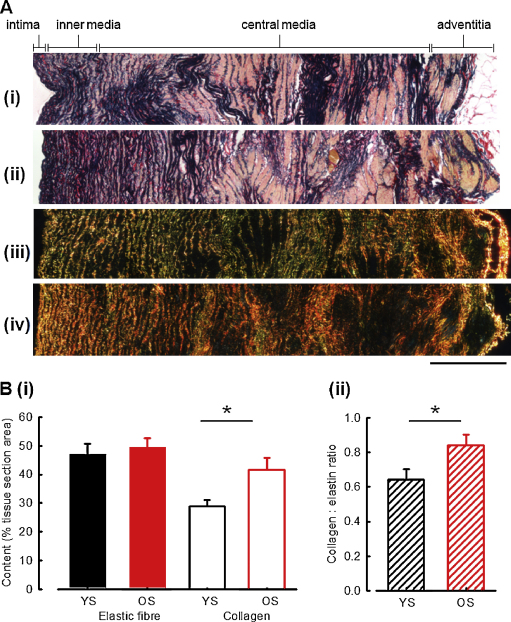
Aberrant remodelling of the ECM is likely to play a key role in mediating age-related arteriosclerosis. Therefore, we used histological approaches to quantify aortic elastic fibre and collagen fibril content in young and old sheep aorta. Regardless of age, Millers staining revealed numerous elastic lamellae whose packing density varied with distance from the intimal surface (Fig. 4A(i and ii)). Elastic fibre content (per tissue section area) was also invariant across the entire wall thickness in young and old animals (Fig. 4B(i), old: 50 ± 3%, young: 47 ± 4%). In contrast, fibrillar collagen content increased with age (4A(iii and iv)), young: 29 ± 2%, old: 42 ± 4.0%, Student's t-test, p < 0.05). Consequently, there was an age-dependent increase in the collagen to elastic fibre ratio (Fig. 4B(ii), 0.64 ± 0.06 vs 0.84 ± 0.06, Student's t-test, p < 0.05) across the whole vessel wall.
Fig. 4.
Collagen and elastic fibre distribution across the whole aortic wall. (A) Optical microscopy images of full thickness aortic wall. (i and ii) Young (i) and old (ii) sheep aorta stained with Millers elastic stain (which pigments elastic fibres [and hence elastic lamellae] blue/black and smooth muscle cells yellow). The density of lamellar packing decreases with distance from the intimal surface. (iii and iv) Young (iii) and old (iv) sheep aorta stained with picrosirius red and imaged under polarized light microscopy. Under these conditions fibrillar collagen is exclusively visualized as red, green or yellow regions. (B) Mean elastin and collagen contents (i) and collagen:elastic fibre ratios (ii) in young sheep (YS) and old sheep (OS) aortas, determined from four optical microscopy montages per animal as illustrated in panel (A), n = 9 animals/group, *p < 0.05. Scale bar = 400 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The structure and composition of the arterial wall however, varies not only with age (Feldman and Glagov, 1971), but also, between species (Stergiopulos et al., 2001) and with tissue location. As a consequence of this heterogeneity, assessments of global ECM composition (Bruel and Oxlund, 1996; Cattell et al., 1996; Osborne-Pellegrin et al., 2010) are unlikely to identify localized changes in collagen or elastic fibre abundance (Osborne-Pellegrin et al., 2010; Selvin et al., 2010; Timmins et al., 2010). Therefore, we additionally characterised ECM remodelling within the intimal and inner medial layer of the vessel wall (Fig. 5A(i–iv)); within this region, ageing was associated with an increase in both collagen (young: 31% ± 2%, old: 39% ± 3%, Student's t-test, p < 0.01) and elastic fibre composition (young: 55% ± 3%, old: 69% ± 4%, Student's t-test p < 0.001) (Fig. 5B). As a result, the collagen to elastic fibre ratio, which was significantly increased across the whole vessel wall, remained unchanged in the inner region of the medial layer in ageing sheep aorta.
Fig. 5.
Age-related ECM remodelling in the inner vessel wall. (A) Millers (i and ii) and picrosirius red (iii and iv) stained aortic wall sections (200 μm from the intimal surface) excised from young (YS) and old (OS) sheep. (B) Mean elastin and collagen contents (i) and collagen:elastic fibre ratios (ii) within the intimal/inner medial region of young sheep (YS) and old sheep (OS) aortas, n = 9 animals/group, **p < 0.01 and ***p < 0.001.
4. Discussion
Whilst generalized stiffening of the vasculature is a key contributor to cardiovascular disease in older individuals (Shirwany and Zou, 2010) and hence plays a central role in determining lifespan (Robert et al., 2008), the causative mechanisms remain poorly defined (Wilkinson et al., 2009). Here, we use ultra-high frequency SAM to demonstrate that gross mechanical stiffening of the aorta in ageing sheep is localized to the collagen fibril-rich interlamellar regions of the medial layer.
Ageing arteries are characterised by arteriosclerosis and ECM remodelling in both humans (Nejjar et al., 1990; Selvin et al., 2010) and rats (Bruel and Oxlund, 1996; Osborne-Pellegrin et al., 2010) and whilst human and ovine cardiovascular functions are physiologically similar (Dibb et al., 2004), to our knowledge, there are no data on the prevalence of arteriosclerosis in ageing sheep. From our engineering stress vs engineering (Cauchy) strain curves (Fig. 1) it is evident that a greater stress was required to produce a given strain in old compared with young sheep aorta. At a physiological strain of 30%, we report stresses of 0.34 kPa and 0.48 kPa in the young and old sheep respectively. Although beyond the scope of our study, more accurate stress–strain curves could be determined with a sophisticated uniaxial testing rig (Sokolis et al., 2002) or a biaxial testing setup that is more representative of physiological stresses (Choudhury et al., 2009).
One approach to measuring the relative mechanical contribution of individual tissue components to age-related aortic stiffening is to selectively extract all other constituents before characterising the mechanical properties of the remaining material (Marsh et al., 2004). Alternatively, individual components, such as VSMC, can be isolated from ageing tissues (Qiu et al., 2010) prior to mechanical testing in vitro. However, the use of highly reactive chemical extraction regimes and in vitro culture conditions has the potential to profoundly disrupt complex macromolecular structures (Shon, 1997) and to influence cellular phenotype (Eguchi et al., 1994), thereby irreversibly altering the mechanical properties of interest. Hence, it is preferable to measure the mechanical properties of native tissue components in situ. Previous studies have employed SAM operated at frequencies of 100–200 MHz to relate acoustic attenuation to pathological changes in formalin fixed infarcted myocardium (Saijo et al., 1997). Here, we have used tissue cryo-sections and whilst there is no consensus as to the effects of freezing on the mechanical properties of blood vessels, Stemper et al. (2007) recently demonstrated that compared with refrigeration, freezing does not significantly affect the mechanical properties of porcine aorta. Therefore it appears that, in general, the mechanical properties of ECM-rich tissues such as tendon (Goh et al., 2008) and aorta (Stemper et al., 2007 and this study) are relatively insensitive to freezing. By using SAM at frequencies around 1 GHz, we demonstrate that acoustic wave speeds within chemically untreated tissues, are positively correlated with age-related increases in gross mechanical stiffness. Furthermore, at these frequencies we can resolve the fundamental structural element of the aorta, the MLU.
Each MLU is composed of elastin-rich lamellae and an interlamellar region which predominantly contains VSMCs and collagen fibrils (O’Connell et al., 2008) (Fig. 1). In both young and old sheep the wave speed frequency distribution measured in the elastic lamellae was dominated by a central peak at ∼1650 m s−1 which, in the absence of an established literature value, we suggest corresponds to the wave speed of unfixed elastin. Crucially, there was no difference in mean wave speed between young and old animals within this tissue compartment. Therefore, whilst the elastic fibre remodelling that occurs with age (Osborne-Pellegrin et al., 2010) may play other roles in the pathogenesis of arteriosclerosis, our data suggest that the stiffness of elastic lamellae remains unperturbed by the ageing process. In contrast, not only was the acoustic wave speed distribution of the inter-lamellar region more complex (reflecting the disparate composition of this tissue compartment) but we also observed a significant age-related increase in wave speed (and hence tissue stiffness) in this region. Typically, the acoustic wave speed profile in young animals was characterised by three peaks corresponding to: VSMCs (<1600 m s−1 (Kinoshita et al., 1998)), fibrillar collagen (literature values range from 1700 m s−1 (Goss and O’Brien, 1979) to 2100 m s−1 (Lees et al., 1983)) and, as already discussed, elastin (1650 m s−1). With age however, there was a marked loss of lower wave speeds <1600 m s−1 and a concomitant gain of wave speeds >1700 m s−1, which may result from: (i) VSMC migration or cytoskeletal stiffening (Greenwald, 2007; Qiu et al., 2010) or (ii) collagen fibrosis ((Greenwald, 2007; Selvin et al., 2010; Vogel, 1991; Zieman and Kass, 2004) and Figs. 4 and 5, this study), cross-linking (Norton et al., 1997) or subtype modulation (Qiu et al., 2007).
The histological observations reported in Figs. 4 and 5, support the hypothesis that collagen fibrosis, which occurs throughout the vessel wall, plays a key role in mediating local and hence global tissue-wide arteriosclerosis in ageing mammals (Greenwald, 2007; Selvin et al., 2010; Vogel, 1991; Zieman et al., 2005). The potential consequences of these age-related compositional changes are profound. Fibrillar collagen in mammalian tendon, for example, is three orders of magnitude stiffer (Gosline et al., 2002) than highly compliant elastin which has an estimated Young's modulus of ∼1 MPa (Aaron and Gosline, 1981). As a result, even small changes in collagen concentration may have a major influence on tissue stiffness. Within the cardiovascular system, there is mounting evidence for the involvement of aberrant TGF-β signalling as a potent mediator of collagen fibrosis (Goumans et al., 2009; Rosenkranz et al., 2002) and for the role of elastic fibre remodelling (Arribas et al., 2006; Cattell et al., 1996; Osborne-Pellegrin et al., 2010) (Fig. 5, this study) in disrupting TGF-β sequestration (Neptune et al., 2003). Therefore, a causative pathway may link: elastic fibre remodelling, the release of fibrillin microfibril-bound TGF-β and the induction of VSMC-mediated interlamellar collagen fibrosis which in turn stiffens the vessel at micro- to macro-scopic length scales. As a consequence, treatment with TGF-β antagonists (Habashi et al., 2006), may hold the key to preventing not only age-related arteriosclerosis but also stiffening within the heart (Ho et al., 2010)
In conclusion, the present investigation, by combining structural characterisation of the ageing sheep aorta with quantitation of macro- and micro-mechanical stiffness, demonstrates that: (i) both tissue stiffness and acoustic wave speed increase in old compared with young tissue, (ii) this increase in acoustic wave speed (and hence stiffness) is localised to the collagen fibril- and VSMC-rich regions of the medial layer and not to the elastic lamellae and (iii) whilst collagen fibrosis may be a driver of age-related arteriosclerosis in sheep, ECM remodelling (of collagen and elastin) is spatially regulated in the ageing vessel wall. Micro-mechanical methodologies such as SAM therefore, may be employed to localise age-related changes in stiffness and hence to directly link structural and functional changes in situ. In the case of the ageing aorta, the application of these methodologies suggests that age-related arteriosclerosis (as distinct from atherosclerosis) may be due to fibrillar collagen remodelling rather than elastic fibre calcification, glycation or fragmentation.
Acknowledgements
This work was supported by the British Heart Foundation (PG 07/099/23758 to H.K.G., A.W.T. and M.J.S., and FS/08/036/25364 to R.A. and B.D.); Research into Ageing (266 to M.J.S) and the Wellcome Trust (WT085981AIA to B.D. and M.J.S.). The authors would like to thank Dr. Sebastian Brand (Fraunhofer Institute of Material Mechanics, Germany) and Professor Kay Raum (Julius Wolff Institut & Berlin-Brandenburg School for Regenerative Therapies, Germany) who developed the MATSAM software used to collect the acoustic images and Dr. Carsten Riis (Aarhus University Hospital, Denmark) who provided access to STAN software. The authors are also extremely grateful to the Strategic Promotion of Ageing Research Capacity (SPARC) and to Dr. Xuegen Zhao (who contributed to the development of the SAM methodology).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.mad.2011.07.003.
Appendix A. Supplementary data
Comparison of gross mechanical properties of fresh and frozen intact aortic rings. In this study, aortic tissue from the same group of animals was used in the assessment of gross tissue mechanics, tissue wave speed (micro-mechanical tissue properties) and collagen and elastin content. This experimental approach necessitated the freezing of aortic rings for storage prior to testing. Therefore, to determine whether the freezing process had an effect on tissue stiffness, we measured applied load at incremental tissue extensions. Mean engineering stress–strain curves derived for freshly prepared (grey) and frozen/thawed aortic rings following extensions of 1 mm increments were not significantly different (ANOVA) (n = 4–5 animals/group).
References
- Aaron B.B., Gosline J.M. Elastin as a random-network elastomer: a mechanical and optical analysis of single elastin fibers. Biopolymers. 1981;20:1247–1260. [Google Scholar]
- Abramoff M.D., Magelhaes P.J., Ram S.J. Image processing with Image. J. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Akhtar R., Schwarzer N., Sherratt M.J., Watson R.E.B., Graham H.K., Trafford A.W., Mummery P.M., Derby B. Nanoindentation of histological specimens: mapping the elastic properties of soft tissues. J. Mater. Res. 2009;24:638–646. doi: 10.1557/JMR.2009.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar R., Sherratt M.J., Watson R.E.B., Kundu T., Derby B. Mapping the micromechanical properties of cryo-sectioned aortic tissue with scanning acoustic microscopy. Mater. Res. Soc. Symp. Proc. 2009;1132E(1132-Z03-07) doi: 10.1557/PROC-1132-Z03-07. pp. ukpmcpa27262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K. Garland Science; 2002. Molecular Biology of the Cell. [Google Scholar]
- Arribas S.M., Hinek A., Gonzalez M.C. Elastic fibres and vascular structure in hypertension. Pharmacol. Ther. 2006;111:771–791. doi: 10.1016/j.pharmthera.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Bailey A.J. Molecular mechanisms of ageing in connective tissues. Mech. Ageing Dev. 2001;122:735–755. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Birk D.E., Bruckner P. Collagen suprastructures. Collagen. Top Curr. Chem. 2005;245:185–205. [Google Scholar]
- Blumenthal H.T., Lansing A.I., Wheeler P.A. Calcification of the media of the human aorta and its relation to intimal arteriosclerosis, ageing and disease. Am. J. Pathol. 1944;20:665–687. [PMC free article] [PubMed] [Google Scholar]
- Briggs A. Clarendon Press; Oxford: 1992. Acoustic Microscopy. [Google Scholar]
- Briggs G.A.D., Kolosov O.V. 2nd ed. Oxford University Press; 2009. Acoustic Microscopy. [Google Scholar]
- Bruel A., Oxlund H. Changes in biomechanical properties, composition of collagen and elastin, and advanced glycation endproducts of the rat aorta in relation to age. Atherosclerosis. 1996;127:155–165. doi: 10.1016/s0021-9150(96)05947-3. [DOI] [PubMed] [Google Scholar]
- Cantini C., Kieffer P., Corman B., Liminana P., Atkinson J., Lartaud-Idjouadiene I. Aminoguanidine and aortic wall mechanics, structure, and composition in aged rats. Hypertension. 2001;38:943–948. doi: 10.1161/hy1001.096211. [DOI] [PubMed] [Google Scholar]
- Cattell M.A., Anderson J.C., Hasleton P.S. Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin. Chim. Acta. 1996;245:73–84. doi: 10.1016/0009-8981(95)06174-6. [DOI] [PubMed] [Google Scholar]
- Choudhury N., Bouchot O., Rouleau L., Tremblay D., Cartier R., Butany J., Mongrain R., Leask R.L. Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovasc. Pathol. 2009;18:83–91. doi: 10.1016/j.carpath.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Dibb K.M., Rueckschloss U., Eisner D.A., Isenberg G., Trafford A.W. Mechanisms underlying enhanced cardiac excitation contraction coupling observed in the senescent sheep myocardium. J. Mol. Cell. Cardiol. 2004;37:1171–1181. doi: 10.1016/j.yjmcc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Domanski M., Norman J., Wolz M., Mitchell G., Pfeffer M. Cardiovascular risk assessment using pulse pressure in the First National Health and Nutrition Examination Survey (NHANES I) Hypertension. 2001;38:793–797. doi: 10.1161/hy1001.092966. [DOI] [PubMed] [Google Scholar]
- Duck F.A. Academic Press; London: 1990. Physical Properties of Tissue. [Google Scholar]
- Eguchi S., Hirata Y., Imai T., Kanno K., Marumo F. Phenotypic change of endothelin receptor subtype in cultured rat vascular smooth-muscle cells. Endocrinology. 1994;134:222–228. doi: 10.1210/endo.134.1.8275937. [DOI] [PubMed] [Google Scholar]
- Faury G. Function–structure relationship of elastic arteries in evolution: from microfibrils to elastin and elastic fibres. Pathol. Biol. (Paris) 2001;49:310–325. doi: 10.1016/s0369-8114(01)00147-x. [DOI] [PubMed] [Google Scholar]
- Feldman S.A., Glagov S. Transmedial collagen and elastin gradients in human aortas: reversal with age. Atherosclerosis. 1971;13:385–394. doi: 10.1016/0021-9150(71)90081-5. [DOI] [PubMed] [Google Scholar]
- Goh K.L., Holmes D.F., Lu H.Y., Richardson S., Kadler K.E., Purslow P.P., Wess T.J. Ageing changes in the tensile properties of tendons: influence of collagen fibril volume fraction. J. Biomech. Eng. 2008;130(April (2)) doi: 10.1115/1.2898732. pp. 021011. [DOI] [PubMed] [Google Scholar]
- Gosline J., Lillie M., Carrington E., Guerette P., Ortlepp C., Savage K. Elastic proteins: biological roles and mechanical properties. Phil. Trans. R. Soc. Lond. B. 2002;357:121–132. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss S.A., O’Brien W.D. Direct ultrasonic velocity measurement of mammalian collagen threads. J. Acoust. Soc. Am. 1979;85:507–511. [Google Scholar]
- Goumans M.J., Liu Z., ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- Graham H.K., Trafford A.W. Spatial disruption and enhanced degradation of collagen with the transition from compensated ventricular hypertrophy to symptomatic congestive heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1364–H1372. doi: 10.1152/ajpheart.00355.2006. [DOI] [PubMed] [Google Scholar]
- Greenwald S.E. Ageing of the conduit arteries. J. Pathol. 2007;211:157–172. doi: 10.1002/path.2101. [DOI] [PubMed] [Google Scholar]
- Habashi J.P., Judge D.P., Holm T.M., Cohn R.D., Loeys B.L., Cooper T.K., Myers L., Klein E.C., Liu G.S., Calvi C., Podowski M., Neptune E.R., Halushka M.K., Bedja D., Gabrielson K., Rifkin D.B., Carta L., Ramirez F., Huso D.L., Dietz H.C. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.S., Dent C.L., Scott M.J., Wickline S.A. High-frequency ultrasound detection of the temporal evolution of protein cross linking in myocardial tissue. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2000;47:1051–1058. doi: 10.1109/58.852089. [DOI] [PubMed] [Google Scholar]
- Ho C.Y., López B.A., Coelho-Filho O.R., Lakdawala N.K., Cirino A.L., Jarolim P., Kwong R., González A., Colan S.D., Seidman J.G., Díez J., Seidman C.E. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N. Engl. J. Med. 2010;363:552–563. doi: 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda Y., Kawano K., Yamasawa F., Ishii T., Shibata T., Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary-artery. Angiology. 1984;35:615–621. doi: 10.1177/000331978403501001. [DOI] [PubMed] [Google Scholar]
- Janssens J.P., Pache J.C., Nicod L.P. Physiological changes in respiratory function associated with ageing. Eur. Respir. J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- Jensen A.S., Baandrup U., Hasenkam J.M., Kundu T., Jorgensen C.S. Distribution of the microelastic properties within the human anterior mitral leaflet. Ultrasound Med. Biol. 2006;32:1943–1948. doi: 10.1016/j.ultrasmedbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Jorgensen C.S., Kundu T. Measurement of material elastic constants and density of cancellous bone: a micromechanical analytic study using a 1 GHz acoustic microscope. J. Orthop. Res. 2002;20:151–158. doi: 10.1016/S0736-0266(01)00061-4. [DOI] [PubMed] [Google Scholar]
- Kass D.A., Shapiro E.P., Kawaguchi M., Capriotti A.R., Scuteri A., deGroof R.C., Lakatta E.G. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- Kielty C.M., Sherratt M.J., Shuttleworth C.A. Elastic fibres. J Cell Sci. 2002;115(July(14)):2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Kielty C.M., Stephan S., Sherratt M.J., Williamson M., Shuttleworth C.A. Applying elastic fibre biology in vascular tissue engineering. Phil. Trans. R. Soc. Lond. B. 2007;362:1293–1312. doi: 10.1098/rstb.2007.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A., Senda S., Mizushige K., Masugata H., Sakamoto S., Kiyomoto H., Matsuo H. Evaluation of acoustic properties of the live human smooth-muscle cell using scanning acoustic microscopy. Ultrasound Med. Biol. 1998;24:1397–1405. doi: 10.1016/s0301-5629(98)00121-5. [DOI] [PubMed] [Google Scholar]
- Kundu T., Bereiter-Hahn J., Karl I. Cell property determination from the acoustic microscope generated voltage versus frequency curves. Biophys. J. 2000;78:2270–2279. doi: 10.1016/S0006-3495(00)76773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Fook S.J., Hyatt R.E. Effects of age on elastic moduli of human lungs. J. Appl. Physiol. 2000;89:163–168. doi: 10.1152/jappl.2000.89.1.163. [DOI] [PubMed] [Google Scholar]
- Lakatta E.G. Arterial aging is risky. J. Appl. Physiol. 2008;105:1321–1322. doi: 10.1152/japplphysiol.91145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I. Aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Landau L.D., Lifshitz E.M. 3rd ed. Butterworth Heinemann; Oxford, UK: 1986. Theory of Elasticity. [Google Scholar]
- Lees S., Heeley J.D., Ahern J.M., Oravecz M.G. Axial phase-velocity in rat tail tendon fibers at 100 MHz by ultrasonic microscopy. IEEE Trans. Sonics Ultrason. 1983;30:85–90. [Google Scholar]
- Marsh J.N., Takiuchi S., Lin S.J., Lanza G.M., Wickline S.A. Ultrasonic delineation of aortic microstructure: the relative contribution of elastin and collagen to aortic elasticity. J. Acoust. Soc. Am. 2004;115:2032–2040. doi: 10.1121/1.1698887. [DOI] [PubMed] [Google Scholar]
- Maurel E., Shuttleworth C.A., Bouissou H. Interstitial collagens and aging in human aorta. Virchows Arch. A. Pathol. Pathol. Anat. 1987;410:383–390. doi: 10.1007/BF00712757. [DOI] [PubMed] [Google Scholar]
- Meyer K.C., Rosenthal N.S., Soergel P., Peterson K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech. Ageing Dev. 1998;104:169–181. doi: 10.1016/s0047-6374(98)00065-7. [DOI] [PubMed] [Google Scholar]
- Miller C.E., Wong C.L., Sedmera D. Pressure overload alters stress–strain properties of the developing chick heart. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1849–H1856. doi: 10.1152/ajpheart.00384.2002. [DOI] [PubMed] [Google Scholar]
- Miller P.J. An elastin stain. Med. Lab. Technol. 1971;28:148–149. [PubMed] [Google Scholar]
- Mitchell G.F. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J. Appl. Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Matter E.J., Earley C.J., Kemper M.K., Becker L.C., Lakatta E.G., Fleg J.L. Increased carotid artery intimal–medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98:1504–1509. doi: 10.1161/01.cir.98.15.1504. [DOI] [PubMed] [Google Scholar]
- Nejjar I., Pieraggi M.T., Thiers J.C., Bouissou H. Age-related changes in the elastic tissue of the human thoracic aorta. Atherosclerosis. 1990;80:199–208. doi: 10.1016/0021-9150(90)90027-g. [DOI] [PubMed] [Google Scholar]
- Neptune E.R., Frischmeyer P.A., Arking D.E., Myers L., Bunton T.E., Gayraud B., Ramirez F., Sakai L.Y., Dietz H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Nishimori Y., Edwards C., Pearse A., Matsumoto K., Kawai M., Marks R. Degenerative alterations of dermal collagen fiber bundles in photodamaged human skin and UV-irradiated hairless mouse skin: possible effect on decreasing skin mechanical properties and appearance of wrinkles. J. Invest. Dermatol. 2001;117:1458–1463. doi: 10.1038/jid.2001.2. [DOI] [PubMed] [Google Scholar]
- Norton G.R., Tsotetsi J., Trifunovic B., Hartford C., Candy G.P., Woodiwiss A.J. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation. 1997;96:1991–1998. doi: 10.1161/01.cir.96.6.1991. [DOI] [PubMed] [Google Scholar]
- O’Connell M.K., Murthy S., Phan S., Xu C., Buchanan J., Spilker R., Dalman R.L., Zarins C.K., Denk W., Taylor C.A. The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal and electron microscopy imaging. Matrix Biol. 2008;27:171–181. doi: 10.1016/j.matbio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke M.F., Hashimoto J. Mechanical factors in arterial aging—a clinical perspective. J. Am. Coll. Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- Osborne-Pellegrin M., Labat C., Mercier N., Challande P., Lacolley P. Changes in aortic stiffness related to elastic fiber network anomalies in the Brown Norway rat during maturation and aging. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H144–H152. doi: 10.1152/ajpheart.00040.2010. [DOI] [PubMed] [Google Scholar]
- Pearson A.C., Guo R.Q., Orsinelli D.A., Binkley P.F., Pasierski T.J. Transesophageal echocardiographic assessment of the effects of age, gender, and hypertension on thoracic aortic-wall size, thickness, and stiffness. Am. Heart J. 1994;128:344–351. doi: 10.1016/0002-8703(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Pickering G.W. Arteriosclerosis and atherosclerosis—the need for clear thinking. Am. J. Med. 1963;34:7–18. doi: 10.1016/0002-9343(63)90035-4. [DOI] [PubMed] [Google Scholar]
- Qiu H., Depre C., Ghosh K., Resuello R.G., Natividad F.F., Rossi F., Peppas A., Shen Y.T., Vatner D.E., Vatner S.F. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation. 2007;116:669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- Qiu H.Y., Zhu Y., Sun Z., Trzeciakowski J.P., Gansner M., Depre C., Resuello R.R.G., Natividad F.F., Hunter W.C., Genin G.M., Elson E.L., Vatner D.E., Meininger G.A., Vatner S.F. Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ. Res. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby R.J. Effect of cyclic extension on physical properties of tendon collagen and its possible relation to biological ageing of collagen. Nature. 1964;202:1072–1074. doi: 10.1038/2021072a0. [DOI] [PubMed] [Google Scholar]
- Robert L., Robert A.M., Fulop T. Rapid increase in human life expectancy: will it soon be limited by the aging of elastin? Biogerontology. 2008;9:119–133. doi: 10.1007/s10522-007-9122-6. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S., Flesch M., Amann K., Haeuseler C., Kilter H., Seeland U., Schluter K.D., Bohm M. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1) Am J Physiol Heart Circ Physiol. 2002;283:H1253–H1262. doi: 10.1152/ajpheart.00578.2001. [DOI] [PubMed] [Google Scholar]
- Ruitenbeek A.G., van der Cammen T.J.M., van den Meiracker A.H., Mattace-Raso F.U.S. Age and blood pressure levels modify the functional properties of central but not peripheral arteries. Angiology. 2008;59:290–295. doi: 10.1177/0003319707305692. [DOI] [PubMed] [Google Scholar]
- Saijo Y., Sasaki H., Yambe T., Tanaka M. Ieee, 2005. Speed of sound microscopy for biomedical applications, 2005 IEEE Ultrasonics Symposium; vols. 1–4; New York: IEEE; 2005. pp. 423–426. [Google Scholar]
- Saijo Y., Tanaka M., Okawai H., Sasaki H., Nitta S.I., Dunn F. Ultrasonic tissue characterization of infarcted myocardium by scanning acoustic microscopy. Ultrasound Med. Biol. 1997;23:77–85. doi: 10.1016/s0301-5629(96)00174-3. [DOI] [PubMed] [Google Scholar]
- Selvin E., Najjar S.S., Cornish T.C., Halushka M.K. A comprehensive histopathological evaluation of vascular medial fibrosis: insights into the pathophysiology of arterial stiffening. Atherosclerosis. 2010;208:69–74. doi: 10.1016/j.atherosclerosis.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt M.J., Wess T.J., Baldock C., Ashworth J., Purslow P.P., Shuttleworth C.A., Kielty C.M. Fibrillin-rich microfibrils of the extracellular matrix: ultrastructure and assembly. Micron. 2001;32:185–200. doi: 10.1016/s0968-4328(99)00082-7. [DOI] [PubMed] [Google Scholar]
- Sherratt M.J. Tissue elasticity and the ageing elastic fibre. Age. 2009;31(December(4)):305–325. doi: 10.1007/s11357-009-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirwany N.A., Zou M.H. Arterial stiffness: a brief review. Acta Pharmacol. Sin. 2010;31:1267–1276. doi: 10.1038/aps.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon Y.H. Extracellular matrix of fresh and cryopreserved porcine aortic tissues. J. Biochem. Mol. Biol. 1997;30:106–112. [Google Scholar]
- Sims T.J., Rasmussen L.M., Oxlund H., Bailey A.J. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia. 1996;39:946–951. doi: 10.1007/BF00403914. [DOI] [PubMed] [Google Scholar]
- Sokolis D.P., Boudoulas H., Karayannacos P.E. Assessment of the aortic stress–strain relation in uniaxial tension. J. Biomech. 2002;35:1213–1223. doi: 10.1016/s0021-9290(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Stacey R.B., Bertoni A.G., Eng J., Bluemke D.A., Hundley W.G., Herrington D. Modification of the effect of glycemic status on aortic distensibility by age in the multi-ethnic study of atherosclerosis. Hypertension. 2010;55:26–32. doi: 10.1161/HYPERTENSIONAHA.109.134031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemper B.D., Yoganandan N., Stineman M.R., Gennarelli T.A., Baisden J.L., Pintar F.A. Mechanics of fresh, refrigerated, and frozen arterial tissue. J. Surg. Res. 2007;139:236–242. doi: 10.1016/j.jss.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Stergiopulos N., Vulliemoz S., Rachev A., Meister J.J., Greenwald S.E. Assessing the homogeneity of the elastic properties and composition of the pig aortic media. J. Vasc. Res. 2001;38:237–246. doi: 10.1159/000051052. [DOI] [PubMed] [Google Scholar]
- Timmins L.H., Wu Q.F., Yeh A.T., Moore J.E., Greenwald S.E. Structural inhomogeneity and fiber orientation in the inner arterial media. Am. J. Physiol. Heart. Circ. Physiol. 2010;298:H1537–H1545. doi: 10.1152/ajpheart.00891.2009. [DOI] [PubMed] [Google Scholar]
- Verdonk E.D., Hoffmeister B.K., Wickline S.A., Miller J.G. Anisotropy of the slope of ultrasonic attenuation in formalin fixed human myocardium. J. Acoust. Soc. Am. 1996;99:3837–3843. doi: 10.1121/1.415001. [DOI] [PubMed] [Google Scholar]
- Virmani R., Avolio A.P., Mergner W.J., Robinowitz M., Herderick E.E., Cornhill J.F., Guo S.Y., Liu T.H., Ou D.Y., Orourke M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis—comparison between Occidental and Chinese communities. Am. J. Pathol. 1991;139:1119–1129. [PMC free article] [PubMed] [Google Scholar]
- Vogel H.G. Species-differences of elastic and collagenous tissue—influence of maturation and age. Mech. Ageing Dev. 1991;57:15–24. doi: 10.1016/0047-6374(91)90021-q. [DOI] [PubMed] [Google Scholar]
- Wilkinson I.B., McEniery C.M., Cockcroft J.R. Arteriosclerosis and atherosclerosis guilty by association. Hypertension. 2009;54:1213–1215. doi: 10.1161/HYPERTENSIONAHA.109.142612. [DOI] [PubMed] [Google Scholar]
- Young A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006;92:80–85. doi: 10.1016/j.pbiomolbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Zieman S.J., Kass D.A. Advanced glycation endproduct crosslinking in the cardiovascular system—potential therapeutic target for cardiovascular disease. Drugs. 2004;64:459–470. doi: 10.2165/00003495-200464050-00001. [DOI] [PubMed] [Google Scholar]
- Zieman S.J., Melenovsky V., Kass D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of gross mechanical properties of fresh and frozen intact aortic rings. In this study, aortic tissue from the same group of animals was used in the assessment of gross tissue mechanics, tissue wave speed (micro-mechanical tissue properties) and collagen and elastin content. This experimental approach necessitated the freezing of aortic rings for storage prior to testing. Therefore, to determine whether the freezing process had an effect on tissue stiffness, we measured applied load at incremental tissue extensions. Mean engineering stress–strain curves derived for freshly prepared (grey) and frozen/thawed aortic rings following extensions of 1 mm increments were not significantly different (ANOVA) (n = 4–5 animals/group).