Abstract
Objective
Important differences in gene expression have been documented in adipocytes derived from specific adipose tissue depots. We have previously documented an important role for adipocyte apoE in modulating adipocyte and adipose tissue triglyceride and lipoprotein metabolism.
Materials/Methods
We now evaluate the endogenous expression of apoE in adipocytes isolated from unique adipose tissue depots in four different species.
Results
Adipocyte apoE expression is higher in subcutaneous fat compared to visceral fat in humans, mice, rats, and baboons. In baboons, evaluation of apoE expression in five adipose tissue depots (subcutaneous abdominal, subcutaneous gluteal, visceral, pericardial, epicardial) showed that compared to subcutaneous abdominal adipocytes, the level of apoE expression is similar in subcutaneous gluteal, lower in visceral and pericardial, and higher in epicardial adipocytes. Consistent with previously demonstrated suppression of adipocyte apoE by adipose tissue inflammation, adipose tissue depots with lower apoE expression demonstrated greater infiltration of macrophages and an increased expression of TNFα mRNA. Depot-specific differences in apoE expression were maintained after in vitro differentiation. Adipocytes isolated from depots with lower apoE expression manifested lower rates of triglyceride synthesis in the absence and presence of triglyceride-rich lipoproteins. Adenoviral-mediated increase of apoE expression in omental adipocytes increased triglyceride synthesis in these cells.
Conclusions
Our results demonstrate significant heterogeneity in adipocyte apoE expression across adipose tissue depots in several species. Because of its role in modulating adipocyte triglyceride and lipoprotein metabolism, depot-specific differences in endogenous adipocyte apoE could have important implications for modulating the accumulation of lipid in these depots.
Keywords: Obesity, adipose tissue inflammation, visceral adipose tissue, apolipoproteins
Introduction
The increasing prevalence of obesity produces an increasing population risk for significant metabolic and cardiovascular complications [1–4]. Significant evidence has accrued indicating heterogeneity of adipose depots with respect to producing risk for these complications [2–6]. For example, visceral adipose tissue (as opposed to subcutaneous adipose tissue) has been more closely related to systemic inflammation, glucose dysregulation, and atherosclerotic vascular disease risk [2–6]. Results from a number of studies have also suggested that subcutaneous adipose tissue, particularly that in the lower body, can act as a sink to store and sequester excess calories, prevent its ectopic deposition, and thereby minimize cardiovascular and metabolic risk [7–9]. Altered expression of genes that could influence metabolic or cardiovascular complications has also been demonstrated in other fat depots, for example epicardial fat [10–12].
We have recently demonstrated an important role for endogenous adipocyte apoE expression in modulating adipose tissue and adipocyte substrate metabolism and storage [13–15]. ApoE, which has long been established as a surface constituent of circulating very low density lipoprotein (VLDL) and high density lipoprotein (HDL) particles in humans, was first described as an important endogenous product of adipocytes over two decades ago [16]. More recently, we have described several physiologically relevant pathways for regulating adipocyte apoE expression using in vitro and in vivo models. Physiologic regulators of adipocyte apoE expression include thiazolidinedione drugs and other peroxisome proliferator–activated receptor (PPARγ) agonists, inflammatory cytokines, and reactive oxidant species [17–21]. The onset of obesity produces substantial down-regulation of adipocyte apoE expression, and this down-regulation is mediated by inflammatory cytokines and reactive oxygen species produced by adipose tissue stromovascular macrophages [22].
We have also investigated the importance of endogenous adipocyte apoE expression for the differentiated function of these cells. Using both in vitro and in vivo models, we have produced substantial evidence that endogenous adipocyte apoE expression importantly influences adipocyte substrate metabolism and gene expression [13–15]. Moreover, these functions of endogenous adipocyte apoE cannot be substituted by the provision of exogenous apoE. This latter point is made most dramatically in studies using adipose tissue transplantation from apoE knockout (EKO) to wild-type (WT) mice. Fourteen weeks after transplantation of EKO or WT adipose tissue into WT mice, EKO adipocytes in transplanted adipose tissue are smaller and triglyceride-poor, and display a defect in triglyceride synthesis compared to WT adipocytes harvested from transplanted WT adipose tissue [14]. This result demonstrates that, in spite of being exposed to WT levels of circulating apoE in triglyceride-rich lipoproteins in vivo for 14 weeks, the lack of endogenous adipocyte apoE expression produces a defect in adipocyte substrate acquisition. With respect to a mechanism for this defect, we have also shown that absence of endogenous apoE expression in adipocytes markedly impairs the acquisition of fatty acid substrate from extracellular triglyceride-rich lipoprotein particles, and thereby impairs accumulation of triglyceride in EKO adipocytes [15].
In view of the role we have established for endogenous apoE expression in adipose tissue and adipocyte triglyceride metabolism, and the emerging role of specific adipose tissue depots for modulating systemic metabolism and cardiometabolic risk, we undertook a series of studies to evaluate the level of apoE expression in distinct adipose tissue depots. For these studies we evaluated multiple species, and investigated obese and non-obese experimental models. We further investigated the relationship between apoE expression and triglyceride metabolism in adipocytes isolated from specific depots.
Methods
Materials
All chemicals were from Sigma (St Louis, MO). Cell culture medium and fetal bovine serum were purchased from Invitrogen (Carlsbad, California). Organic solvents were from Fisher (Pittsburgh, PA). [14C]glucose was obtained from PerkinElmer Life Sciences (Waltham, MA). VLDL was isolated by sequential density gradient ultracentrifugation of human plasma as previously described in detail [13]. A goat anti-apoE antibody was purchased from International Immunology Corporation (Marietta, GA).
Human study subjects
Matched abdominal subcutaneous and visceral adipose tissue samples were obtained from 17 female adult and adolescent patients undergoing bariatric surgery, or hernia repair surgery, at the University of Illinois at Chicago (UIC) Medical Center. All subjects gave their informed consent to study, which was approved by the Institutional Review Board at UIC.
Baboon study subjects
Baboons (Papio Anubis) (n=6, female) used in this study were from the UIC primate colony containing control animals for other studies approved by the UIC Institutional Animal Care and Use Committee. Adipose tissue was harvested from euthanized animals and immediately preserved for isolation of mature adipocytes, isolation of pre-adipocytes, or isolation of RNA [13].
Mouse and Rat adipose tissue
C57BL/6 mice (12wo, male) were purchased from Charles River (Wilmington, MA) and maintained on either chow diet (13.4% kcal from fat, Harlan Teklad, Madison, WI) or high-fat diet (HFD) (60% kcal from fat, Harlan Teklad) for 12 weeks. Male 9–10 week old Zucker fatty rats (fa/fa) or lean littermates (fa/+) were purchased from Charles River. All animal protocols were approved by the Institutional Animal Care and Use Committees of UIC.
Adipose tissue handling and adipocyte isolation and differentiation
Adipose tissue for qRT-PCR analysis was flash frozen in liquid nitrogen. Mature floating adipocytes were isolated from freshly harvested adipose tissues for immediate use as previously described [13]. Pre-adipocytes from the adipose tissue stromovascular fraction were isolated and differentiated into adipocytes using a 3-day incubation in insulin, dexamethasone, isobutylmethylxanthine and rosiglitazone as previously described in detail [13–15]. Cells differentiated in culture were used for experiments 10–14 days later. Adenoviral transduction was used to increase endogenous cellular apoE expression as previously described [13]. Briefly, rat or human adipocytes harvested from the omental adipose tissue depot were incubated with adenovirus directing the expression of LacZ (as a control) or apoE at a multiplicity of 25 for 6h. After four additional days in complete growth medium, cells were used for experiments.
qRT-PCR analysis for mRNA levels
Quantitative reverse transcription-PCR (qRT-PCR) was performed as previously described [13–15]. For normalization, human, baboon, and mouse RNA samples were standardized to 3 housekeeping genes using the geNorm program [23]. β-actin was used for normalization of rat RNA samples. The primer sets used for this study are listed in Table 1. Data were analyzed using the comparative critical threshold method. Fold change was calculated by2−ΔΔCt.
Table 1.
PCR Primer Sequences
| Species | Gene | Forward | Reverse |
|---|---|---|---|
| Homo sapiens | apoE | CACAGGCAGGAAGATGAAGGT | AGCGCAGGTAATCCCAAAAG |
| LRP10 | AGTGGGACTGCTCCTATGTTCT | CACAATCTCAGCCTCCATCC | |
| β-actin | ACTCTTCCAGCCTTCCTTCCT | CAGTGATCTCCTTCTGCATCCT | |
| HPRTI | CTGAGGATTTGGAAAGGGTGT | AATCCAGCAGGTCAGCAAAG | |
| Papio anubis | apoE | CTCTTGGTTCACTGCCCTTCT | TTCAACTCCTTCATGGTCTCGT |
| CD68 | CGAGCATCATTCTTTCACCA | AGGAGGCCAAGAAGAACCA | |
| TNF | TCTTCACTGGAAAGGACACCA | GAAGGAGAAGAGGCTGAGGAA | |
| β-actin | ATCGTGCGTGACATTAAGGAG | CCAGGAAGGAAGGTTGGAAG | |
| Cyclophilin A | CAAGACGGAGTGGTTGGATG | TGGTGGTCTTCTTGCTGGTC | |
| GAPDH | AGCGAGATCCCTCCAAAATC | GTTCACACCCATGACGAACA | |
| Mus musculus | apoE | AGTGGCAAAGCAACCAACC | CTTCCGTCATAGTGTCCTCCA |
| β-actin | CTGGGACGACATGGAGAAGA | AGAGGCATACAGGGACAGCA | |
| 36B4 | AGCGCGTCCTGGCATTGTGTGG | GGGCAGCAGTGGTGGCAGCAGC | |
| Cyclophilin A | TGGTCTTTGGGAAGGTGAAAG | TGTCCACAGTCGGAAATGGT | |
| Rattus norvegicus | apoE | ATCAGCTCCCAGGGCAAA | TTCCGTCATAGTGTCCTCCATC |
| β-actin | CTGGGACGACATGGAGAAGA | AGAGGCATACAGGGACAGCA |
LRP10: low density lipoprotein receptor-related protein 10
HPRT1: Hypoxanthine Phosphoribosyl-Transferase 1
36B4 or RPLP0: acidic Ribosomal Phosphoprotein P0
Cyclophilin A
TG synthesis and mass
Cultured adipocytes were incubated with 0.5 µCi/ml [14C]glucose with or without 100 µg/ml VLDL in DMEM and 0.1% BSA for 6 h at 37 °C. After washing, cellular lipids were extracted and triglycerides were separated by thin layer chromatography in a solvent system of hexane:ethyl ether:acetic acid (90:30:1). The triglyceride spots were harvested, and radioactivity in spots measured in a scintillation counter. Cell protein was estimated using the Bio-Rad DC protein kit (Hercules, CA). Cellular TG mass was measured using a kit obtained from Wako as previously described [13].
Western blot
Adipocytes were lysed, and protein extracts were prepared for apoE Western blot analysis as previously described in detail [13–15]. Western blot images were quantitated using ImageQuant TL software (GE Healthcare) and corrected for actin as an internal loading control.
Statistics
Results are shown as mean ± SD as described in figure legends. Statistical differences were analyzed by Wilcoxon signed rank test for human studies; T-test or ANOVA for baboon, rodent and in vitro studies, using SPSS 18.0 (Chicago, IL). P< 0.05 was considered significant.
Results
ApoE expression in visceral compared to subcutaneous adipocytes in obese humans
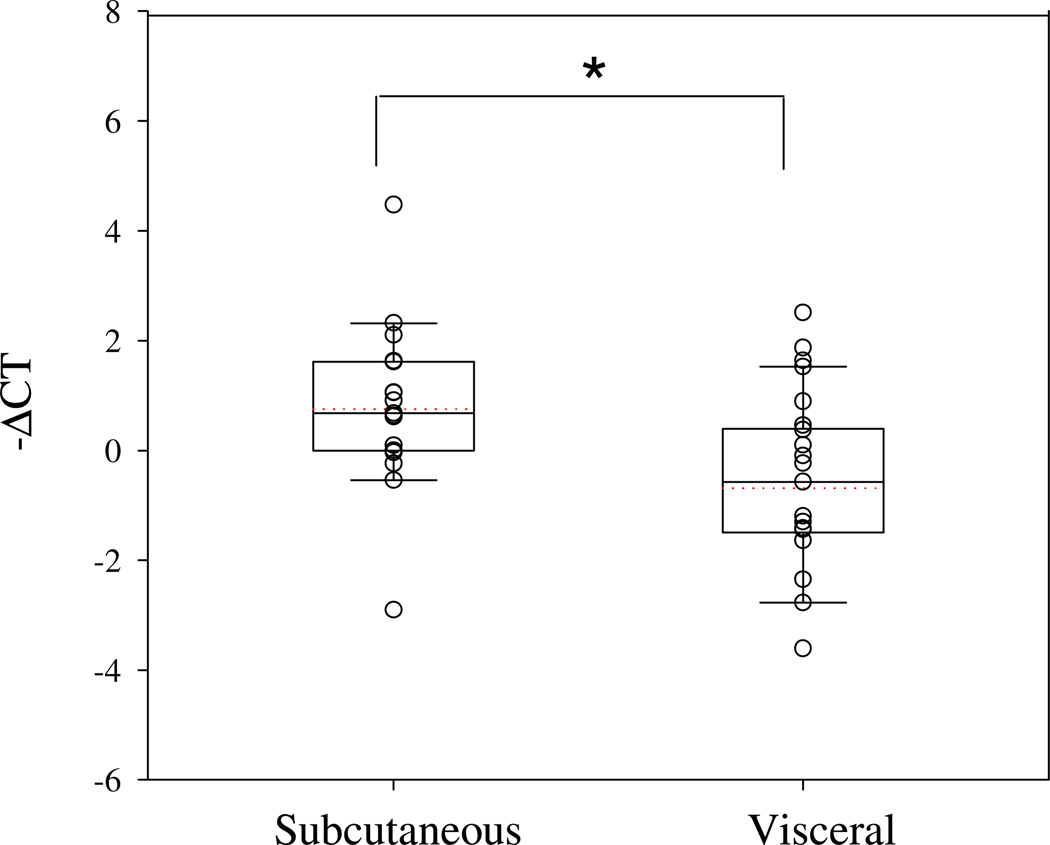
ApoE mRNA level was measured in adipocytes isolated from freshly harvested adipose tissue from 17 adolescent and adult female subjects undergoing bariatric surgery, or hernia repair surgery at UIC. The characteristics of the subjects are shown in Table 2. Subjects were female with a median age of 30 years (range 14–55 years). Median body weight was 130.9kg, and median BMI was 48.0. Co-morbidities included diabetes and hyperlipidemia. One subject was a current smoker. Figure 1 shows apoE mRNA level in adipocytes harvested from subcutaneous or visceral adipose tissue presented as a box plot of the cycle threshold number for apoE compared to the cycle threshold number for the housekeeping genes (see Figure Legend and Methods). Based on the difference in cycle number, adipocytes from visceral adipose tissue expressed 0.71 ± 0.26 of the apoE mRNA level found in adipocytes isolated from subcutaneous adipose tissue (p<0.05).
Table 2.
Characteristics of Human Subjects
| Median (range) | |
|---|---|
| N | 17 |
| Age (years) | 30.0 (14.0–55.0) |
| Gender | Female |
| Ethnicity | White (9/17) |
| Hispanic (3/17) | |
| African-American (5/17) | |
| Body Weight (Kg) | 130.9 (102.3–204.5) |
| BMI | 48.0 (34.4–62.8) |
| Diabetes | 4 |
| Hyperlipidemia | 6 |
| Smoking | 1 |
Figure 1. ApoE mRNA levels in human subcutaneous and visceral mature adipocytes.
Mature adipocytes were isolated from matched sets of freshly harvested subcutaneous and visceral adipose tissue depots (n=17) as described in Methods. ApoE mRNA levels were measured by qRT-PCR. Results presented in the box plot graph are ΔCT number of subcutaneous and visceral samples as described in Methods. Each sample was run in duplicate. *P<0.05 for the difference in apoE mRNA level between subcutaneous and visceral adipocytes.
ApoE expression in subcutaneous and intra-abdominal fat harvested from lean and obese rodents
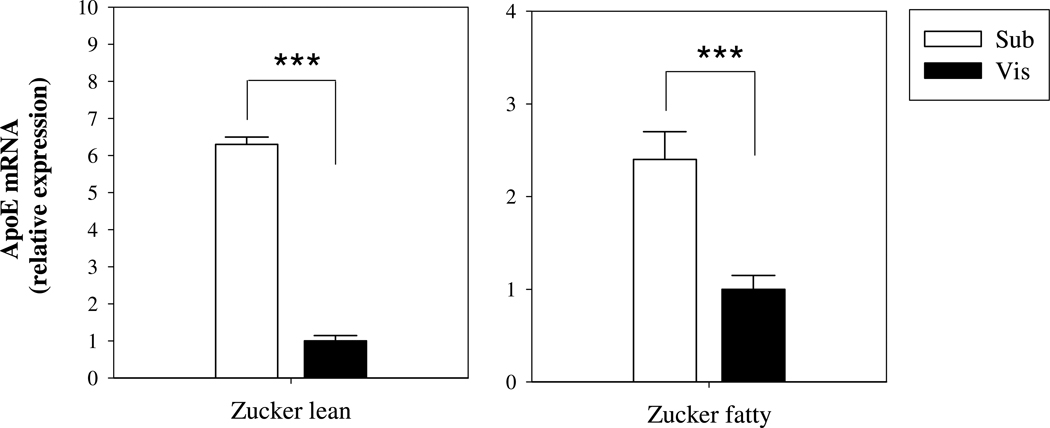
Adipocytes were harvested from adipose tissue obtained from the subcutaneous or omental depots of Zucker fatty rats or lean littermates (Fig. 2). Lean and obese rats weighed 225–250g and 350–375g respectively. ApoE mRNA levels were approximately 6-fold higher in subcutaneous compared to visceral adipocytes harvested from lean rats. With obesity, the difference between visceral and subcutaneous fat was reduced. However, subcutaneous adipocytes demonstrated 2–3 fold higher levels of apoE mRNA compared to omental adipocytes.
Figure 2. ApoE mRNA levels in rat subcutaneous and omental mature adipocytes.
Mature adipocytes were isolated from freshly harvested subcutaneous or omental fat depots from Zucker fatty rats or lean littermates. ApoE mRNA levels were measured by qRT-PCR. Results shown are mean ± SD of 3 rats per group. ***P<0.001 for the difference in apoE mRNA level between subcutaneous and omental adipocytes.
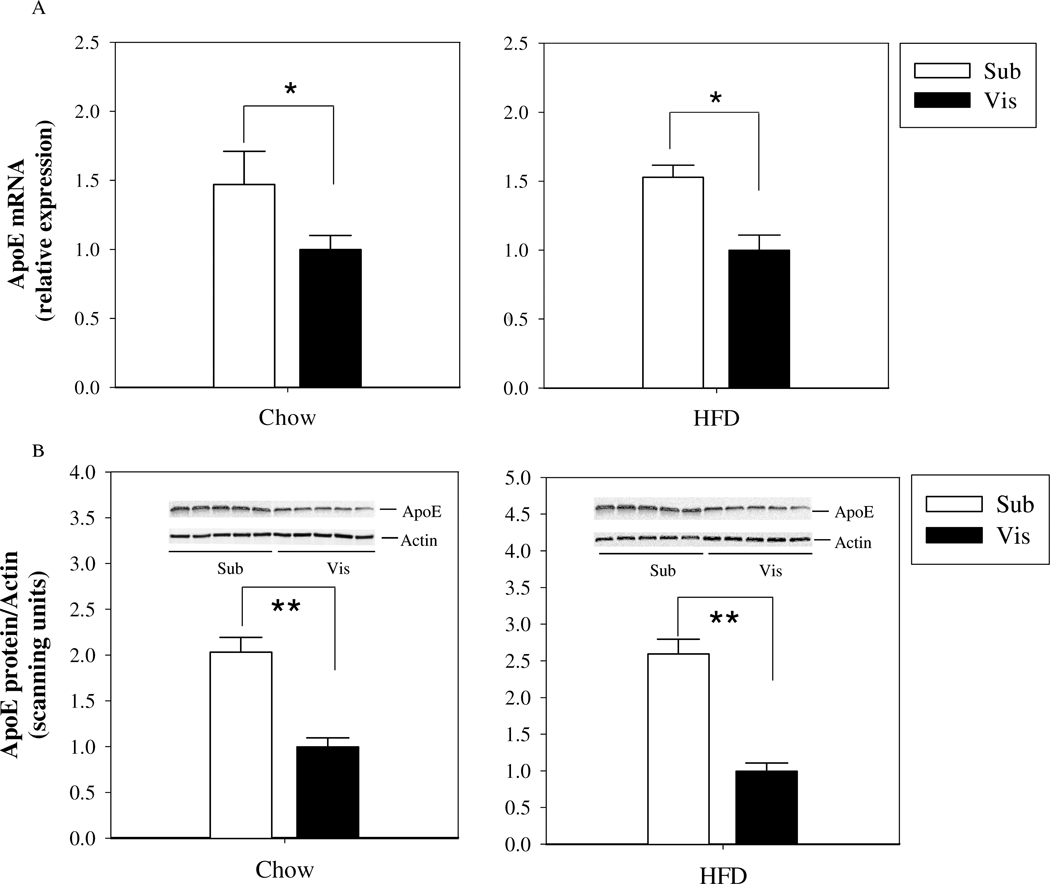
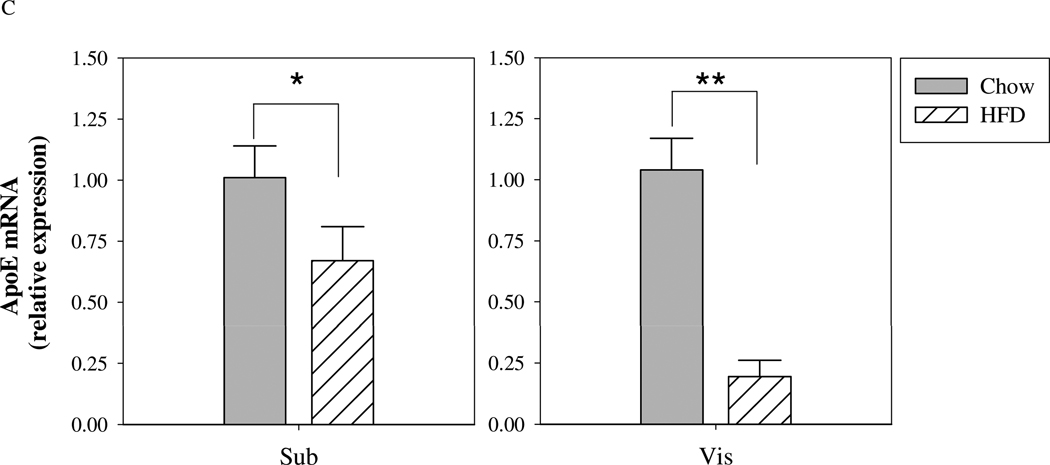
Figure 3 presents results for apoE mRNA and protein levels in subcutaneous compared to intra-abdominal (epididymal fat pad) adipocytes isolated from chow-fed mice or mice maintained on a HFD. At time of harvest, body weight for chow-fed mice was 33 ± 4g, and for those maintained on HFD 50 ± 2g. For both chow and high-fat fed mice, apoE mRNA (3A) and protein (3B) were significantly higher in adipocytes isolated from the subcutaneous fat depot. Figure 3C demonstrates suppression of apoE gene expression in each depot in response to the HFD. Interestingly, suppression in visceral adipocytes tended to be greater.
Figure 3. ApoE mRNA and protein expression in mouse subcutaneous and intra-abdominal mature adipocytes.
Mature adipocytes were isolated from freshly harvested subcutaneous and visceral fat depots from chow fed or high-fat fed (12 weeks) mice (n=6–8 mice per group). (A) ApoE mRNA levels were measured by qRT-PCR, and (B) ApoE protein expression was estimated by Western blot. ApoE and β-actin signals are shown from the Western blot of samples from 5 different mice. (C) Changes in apoE expression in visceral and subcutaneous adipocytes with HFD-induced obesity. *P<0.05, **P<0.01 for the indicated comparison.
ApoE expression in adipose tissue and adipocytes harvested from non-human primates
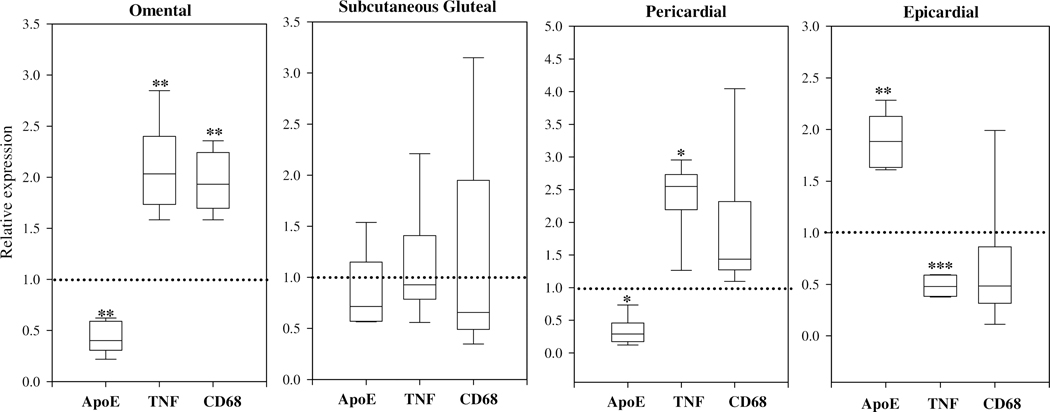
We next evaluated apoE expression in adipose tissue and adipocytes isolated from a nonhuman primate model. This model afforded the opportunity to evaluate apoE expression in multiple adipose tissue depots, and provided sufficient adipose tissue to allow comparison of apoE expression between freshly isolated adipocytes and cultured adipocytes. The characteristics of the baboons used in this study are shown in Table 3. Figure 4 shows levels of apoE, TNFα, and CD68 mRNA in adipose tissue. The level of expression in omental, subcutaneous gluteal, pericardial, and epicardial adipose tissue is presented relative to that found in subcutaneous abdominal adipose tissue. For each panel, therefore, the level of expression of apoE, TNFα, and CD68 in subcutaneous abdominal tissue is assigned a value of 1 (represented by the hatched line). In adipose tissue isolated from the omental depot, apoE expression is reduced by approximately 50%, whereas the expression of both CD68 (a marker of macrophage infiltration) and TNFα (an inflammatory cytokine we have previously shown down-regulates adipocyte apoE expression) are elevated by about 2-fold. There are no substantial differences in apoE, TNFα, and CD68 expression between subcutaneous gluteal and subcutaneous abdominal tissue. The pattern of expression for pericardial tissue is similar to that observed in omental adipose tissue with reduced apoE expression, increased TNFα, and increased CD68 expression compared to subcutaneous abdominal tissue. On the other hand, epicardial adipose tissue has increased levels of apoE expression compared to subcutaneous abdominal tissue with reduced expression of TNFα and a trend toward reduction in CD68 expression.
Table 3.
Characteristics of Study Baboons (Papio Anubis)
| Median (range) | |
|---|---|
| N | 6 |
| Gender | Female |
| Body weight (Kg) | 20.2 (17.5–32.1) |
| Diabetes | No |
| Medications | No |
| Hyperlipidemia | No |
| Smoking | No |
Figure 4. Evaluation of apoE, CD68, and TNFα levels in five adipose tissue depots from the baboon.
Subcutaneous abdominal, omental, subcutaneous gluteal, pericardial, and epicardial adipose tissue was harvested from 6 baboons. ApoE, CD68 and TNF mRNA levels were measured by qRT-PCR. Expression levels in omental, subcutaneous gluteal, pericardial, and epicardial adipose tissue depots are expressed in box plot format relative to expression in subcutaneous abdominal, which is represented by the dashed line at “1” in each panel. Results are presented as fold change. *P<0.05, **P<0.01, ***P<0.001 for the difference compared to the subcutaneous abdominal fat depot.
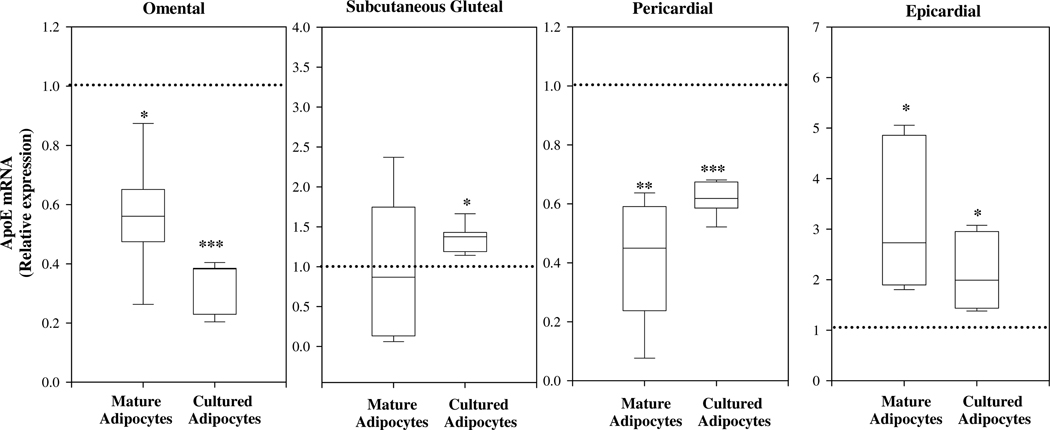
Figure 5 shows level of apoE expression in mature adipocytes studied immediately after isolation from freshly harvested adipose tissue, and that in adipocytes differentiated from pre-adipocytes in vitro and maintained in culture for 10–14 days. The level of apoE expression in freshly isolated mature omental, subcutaneous gluteal, pericardial, and epicardial adipocytes is compared to freshly isolated mature subcutaneous abdominal adipocytes. The level of apoE expression in cultured omental, subcutaneous gluteal, pericardial and epicardial adipocytes is compared to cultured subcutaneous abdominal adipocytes. Consistent with our observations in whole adipose tissue, apoE expression is substantially decreased in isolated mature adipocytes freshly harvested from omental compared to the subcutaneous adipose tissue depot. This reduction in apoE expression in omental adipocytes is maintained even when cells have been differentiated from pre-adipocytes in culture, and maintained in culture for 10–14 days. Likewise, the relationship of apoE expression in subcutaneous gluteal, pericardial, and epicardial mature adipocytes compared to subcutaneous mature adipocytes is maintained in the cultured adipocytes.
Figure 5. ApoE expression in freshly isolated mature adipocytes compared to cultured adipocytes.
RNA was isolated from mature adipocytes isolated from the 5 adipose tissue depots noted in Figure 4. RNA was also isolated from adipocytes differentiated from preadipocytes isolated from the same 5 depots, and maintained in culture for 10–14 days. ApoE mRNA level was measured by qRT-PCR, and its level of expression in freshly isolated mature adipocytes in each depot was compared to that measured in freshly isolated mature adipocytes from the subcutaneous abdominal fat depot. The level of expression in cultured adipocytes from each depot was compared to that in cultured adipocytes from the subcutaneous abdominal fat depot. Results are presented in box plot format as fold changes with the dashed line at “1” representing level of expression in the mature adipocytes or cultured adipocytes from the subcutaneous abdominal depot. *P<0.05, **P<0.01, ***P<0.001 for the difference in expression level compared to mature adipocytes or cultured adipocytes isolated from the subcutaneous abdominal fat depot.
We have previously reported changes in gene expression in cultured EKO compared to WT adipocytes. The expression of acetyl-CoA dehydrogenase, medium chain (ACADM), PPARγ coactivator (PGC-1α), carnitine palmitoyltransferase-1 (CPT-1), and acyl-CoA oxidase (ACO) was higher, and expression of adiponectin, CCAAT/enhancer binding protein (CEBPα), PPARγ, and caveolin was lower comparing EKO to WT adipocytes. Similar, but smaller, changes were observed in gene expression comparing visceral (with lower apoE gene expression) to subcutaneous (with higher apoE gene expression) cultured adipocytes. The level of expression of ACADM (1.5 ± 0.4), PGC-1α (2.0 ± 0.3), and CPT-1 (1.9 ± 0.7) was significantly higher, and the expression of CEBPα (0.4 ± 0.3), PPARγ (0.3 ± 0.2), and caveolin (0.4 ± 0.2) was significantly lower comparing visceral to subcutaneous cultured adipocytes.
Implications of depot-specific differences in apoE expression for adipocyte triglyceride homeostasis
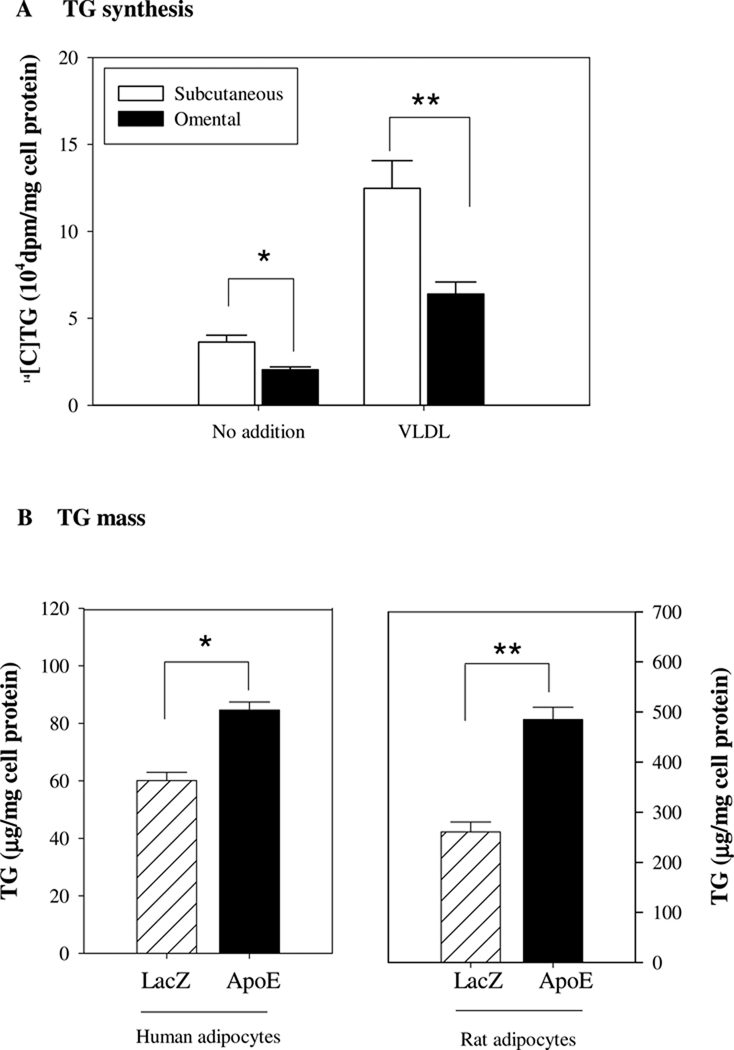
The above information clearly establishes significant differences in apoE expression across adipose tissue depots in four different species. Results previously published from our laboratory suggest that these differences should impact adipocyte triglyceride metabolism [13–15]. We addressed this question using adipocytes isolated from the baboon model. Pre-adipocytes were isolated from the subcutaneous abdominal or omental depots of two different baboons, and the rate of triglyceride synthesis was measured (as described in Methods) in the absence or presence of VLDL. The latter serves as a source of substrate driving adipocyte triglyceride synthesis, and we have previously shown that adipocyte triglyceride synthesis measured in both the presence and absence of VLDL is lower in adipocytes with reduced apoE expression [13–15]. Consistent with the reduced apoE expression in omental adipocytes, triglyceride synthesis is significantly lower in omental compared to subcutaneous adipocytes in both the presence and absence of VLDL (Fig. 6A). In order to confirm that decreased TG synthesis in omental adipose tissue was related to decreased endogenous adipocyte apoE expression level, we used an apoE expressing adenovirus to increase apoE expression in adipocytes cultured from omental adipose tissue in humans and rats (Fig. 6B). A LacZ adenovirus was used as control. In multiple experiments, transduction with the apoE adenovirus led to a 4– 10 fold increase in apoE expression compared to the LacZ adenovirus. Increased expression of apoE in omental adipocytes from both species was associated with a significant increase in adipocyte TG content after incubation in lipoprotein-containing growth medium.
Figure 6. ApoE expression and TG synthesis/mass.
A. Baboon adipocytes were prepared from preadipocytes isolated from omental and subcutaneous adipose tissues and maintained in culture for 14 days as described in Methods. To measure TG synthesis, adipocytes were incubated with no addition or with 100µg/ml VLDL in DMEM medium containing [14C]glucose and 0.1%BSA for 6h. TG synthesis over 6h was measured as described in Methods. Results presented are mean of experiments from 2 separate baboons, each run in triplicate. *P<0.05, **P<0.01 for the difference between subcutaneous and omental adipocytes. B. Rat or human cultured adipocytes from the omental adipose tissue depot were incubated with an apoE or LacZ adenovirus as described in Methods. After an additional four days incubation in lipoprotein-containing growth medium, cells were harvested for measurement of TG mass. Results shown are mean of 2 separate experiments, each ran in triplicate. *P<0.05, **P<0.01 for the indicated comparison.
Discussion
The results in this report establish that adipocytes from the visceral fat depot express lower levels of apoE compared to those from the subcutaneous abdominal fat depot in humans, mice, rats, and baboons. Depot differences are significant in obese humans but are somewhat smaller than in the animal experimental models, perhaps related to less marked genetic heterogeneity present in the animal models. In rats and mice, we demonstrate that depot-specific differences are maintained in obesity. In baboons, we demonstrate that compared to subcutaneous abdominal fat, there is no difference in apoE expression in subcutaneous gluteal fat, but that apoE expression in pericardial fat is lower (similar to visceral fat) but higher in epicardial fat. Provocatively, results in freshly isolated mature adipocytes compared to those in adipocytes differentiated from pre-adipocytes in vitro, indicate that precursor cells are already committed to depot-specific differences in apoE expression.
We have previously shown that reactive oxygen species or TNFα from macrophages in the adipose tissue stromovascular fraction can significantly reduce adipocyte apoE expression [22]. It seems reasonable, therefore, to assume that the lower levels of adipocyte apoE measured in visceral and pericardial adipose tissue depots could result from the increased macrophage infiltration (as marked by increased CD68 expression) and an increased inflammatory milieu (as marked by increased TNFα expression) we detect in these depots. However, our results also demonstrate that depot-specific differences in adipocyte apoE expression are maintained in precursor cells that have divided, undergone differentiation, and been maintained in culture for 14 days. This observation suggests that adipocyte precursor cells in unique adipose tissue depots are already committed to a specific level of apoE expression. This observation is consistent with those of others showing that depot-specific differences in adipocyte gene expression are also maintained in longterm culture for other genes including adiponectin and genes involved in insulin signaling [24]. In fact, pre-adipocyte strains derived from single pre-adipocytes have been shown to maintain depot-specific cell characteristics after 40 divisions in culture [25, 26]. In the case of apoE expression, the long-term exposure to differing adipose tissue inflammatory milieu (Fig. 4) could play a role in imprinting preadipocyte precursors.
Our previous work has established that endogenous adipocyte apoE expression has important implications for the acquisition of substrate from triglyceride-rich lipoprotein particles like VLDL [14]. ApoE knockout adipocytes are lipid-poor and triglyceride synthesis is lower compared to WT adipocytes in the absence and presence of extracellular VLDL. We have also previously shown that the difference in triglyceride synthesis between EKO and WT adipocytes can be corrected by adenoviral expression of apoE in EKO adipocytes [13]. Based on the results of these prior studies, adipocytes derived from depots with lower expression of apoE would be predicted to manifest lower levels of triglyceride synthesis in the absence and presence of VLDL (Fig. 6A); and increasing the expression of apoE in adipocytes harvested from depots with low apoE expression should increase acquisition of substrate and TG mass (Fig. 6B). Although our study does not address depot-specific differences in apoE expression as they relate to gender, it is interesting to speculate that a gender effect could contribute to the well-established differences in adipose tissue distribution between human males and females.
Many examples of differential gene expression in cells derived from subcutaneous versus visceral fat have been documented, along with important systemic implications deriving from this differential expression. Depot-specific effects for gene expression in pericardial and epicardial depots have more recently come to attention [10–12]. Cross-sectional associations between altered gene expression in pericardial and epicardial fat, and the presence of human disease (i.e. coronary atherosclerosis or obesity) have also recently been reported [10–12]. Our results establish a depot-specific influence on apoE expression level in pericardial and epicardial adipose tissue depots. ApoE expression in the pericardial depot is lower than that in subcutaneous fat, similar to that observed in visceral fat. However, apoE expression in epicardial adipose tissue is higher than that observed in any depot examined. An interesting potential implication of apoE gene expression in epicardial fat relates to the absence of a fascia between the epicardial fat pad and the epicardial coronary arteries; the site of atherothrombotic disease that gives rise to clinically important myocardial ischemia. There are several well-established mechanisms by which apoE secreted from the epicardial fat could favorably impact the evolution of atherothrombotic disease in the coronary arteries [27–29].
In summary, our results demonstrate significant heterogeneity in the expression of adipocyte apoE across adipose tissue depots in several species. These differences in adipocyte apoE expression have implications for adipocyte triglyceride and VLDL metabolism, as predicted by the results of prior in vitro and in vivo mechanistic studies. Some differences in adipocyte apoE expression across adipose tissue depots could be related to depot-specific differences in macrophage infiltration and inflammatory microenvironment as we have already demonstrated that TNFα and reactive oxygen species produced by adipose tissue macrophages can significantly suppress adipocyte apoE expression. Because of the important role that has been established for endogenous adipocyte apoE expression in overall adipose tissue substrate flux, the depot-specific changes we currently demonstrate in adipocyte apoE expression could have important implications for modulating the accumulation of triglyceride in these depots.
Acknowledgements
The authors thank Stephanie Thompson for assistance with manuscript preparation.
Funding
This work was supported by grants DK71711 and UL1RR029879 from the National Institutes of Health (to TM), and a grant from the Association of Women Surgeons Foundation (to AH).
Abbreviations
- VLDL
very low density lipoprotein
- HDL
high density lipoprotein
- EKO
apoE knockout
- WT
wild-type
- qRT-PCR
quantitative reverse transcription-PCR
- HFD
high-fat diet
- ACADM
acetyl-CoA dehydrogenase, medium chain
- PPARγ
peroxisome proliferator–activated receptor
- PGC-1α
PPARγ coactivator
- CPT-1
carnitine palmitoyltransferase-1
- ACO
acyl-CoA oxidase
- CEBPα
CCAAT/enhancer binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors have nothing to disclose.
Author Contributions
ZHH performed experiments, analyzed data, critically evaluated and approved the final manuscript; DJE performed experiments, analyzed data, critically evaluated and approved the final manuscript, AU recruited human subjects, obtained human adipose tissue, critically evaluated and approved the final manuscript; AH assisted with collection of human visceral and subcutaneous adipose tissue, critically reviewed and approved the final manuscript; JV assisted with collection of human visceral and subcutaneous adipose tissue, critically reviewed and approved the final manuscript; TM planned experiments, analyzed data, wrote final manuscript.
Reference List
- 1.Ford ES, Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab. 2008;93 Suppl 1:S1–S8. doi: 10.1210/jc.2008-1356. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 3.Canoy D, Boekholdt SM, Wareham N, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116:2933–2943. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 4.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 5.Sam S, Haffner S, Davidson MH, et al. Relationship of abdominal visceral and subcutaneous adipose tissue to lipoprotein particle number and size in Type 2 diabetes. Diabetes. 2008;57:2022–2027. doi: 10.2337/db08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sam S, Haffner S, Davidson MH, et al. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes Care. 2009;32:1916–1920. doi: 10.2337/dc09-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Pelt RE, Jankowski CM, Gozansky WS, et al. Lower-body adiposity and metabolic rotection in postmenopausal women. J Clin Endocrinol Metab. 2005;90:4573–4578. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terry RB, Stefanick ML, Haskell WL, et al. Contributions of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism. 1991;40:733–740. doi: 10.1016/0026-0495(91)90093-c. [DOI] [PubMed] [Google Scholar]
- 9.Porter SA, Massaro JM, Hoffmann U, et al. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouwens DM, Sell H, Greulich S, et al. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konishi M, Sugiyama S, Sato Y, et al. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis. 2010;213:649–655. doi: 10.1016/j.atherosclerosis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Karastergiou K, Evans I, Ogston N, et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1340–1346. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- 13.Huang ZH, Reardon CA, Mazzone T. Endogenous apoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 2006;55:3394–3402. doi: 10.2337/db06-0354. [DOI] [PubMed] [Google Scholar]
- 14.Huang ZH, Gu D, Mazzone T. Role of adipocyte-derived apoE for modulating adipocyte size, lipid metabolism, and gene expression in vivo. Am J Physiol Endocrinol Metab. 2009;296:E1110–E1119. doi: 10.1152/ajpendo.90964.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang ZH, Minshall RD, Mazzone T. Mechanism for endogenously expressed APOE modulation of adipocyte VLDL metabolism: role in endocytic and lipase-mediated metabolic pathways. J Biol Chem. 2009;284:31512–31522. doi: 10.1074/jbc.M109.004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zechner R, Moser R, Newman TC, et al. Apolipoprotein E gene expression in mouse 3T3-L1 adipocytes and human adipose tissue and its regulation by differentiation and lipid content. J Biol Chem. 1991;266:10583–10588. [PubMed] [Google Scholar]
- 17.Yue L, Rassouli N, Ranganathan G, et al. Divergent effects of PPAR agonists and TNF on adipocyte apoE expression. J Biol Chem. 2004;279:47626–47632. doi: 10.1074/jbc.M408461200. [DOI] [PubMed] [Google Scholar]
- 18.Rao P, Huang ZH, Mazzone T. Angiotensin II regulates adipocyte apolipoprotein E expression. Journal of Clinical Endocrinology and Metabolism. 2007;92:4366–4372. doi: 10.1210/jc.2007-1592. [DOI] [PubMed] [Google Scholar]
- 19.Yue L, Christman JW, Mazzone T. Tumor necrosis factor-alpha-mediated suppression of adipocyte apolipoprotein E gene transcription: primary role for the nuclear factor (NF)-kappaB pathway and NFkappaB p50. Endocrinology. 2008;149:4051–4058. doi: 10.1210/en.2008-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue L, Mazzone T. Peroxisome proliferator-activated receptor {gamma} stimulation of adipocyte ApoE gene transcription mediated by the liver receptor X pathway. J Biol Chem. 2009;284:10453–10461. doi: 10.1074/jbc.M808482200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang ZH, Luque RM, Kineman RD, et al. Nutritional regulation of adipose tissue apolipoprotein E expression. Am J Physiol Endocrinol Metab. 2007;293:E203–E209. doi: 10.1152/ajpendo.00118.2007. [DOI] [PubMed] [Google Scholar]
- 22.Espiritu DJ, Mazzone T. Oxidative stress regulates adipocyte apolipoprotein E and suppresses its expression in obesity. Diabetes. 2008;57:2992–2998. doi: 10.2337/db08-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandesompele J, De PK, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.1-research0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrini S, Laviola L, Cignarelli A, et al. Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia. 2008;51:155–164. doi: 10.1007/s00125-007-0841-7. [DOI] [PubMed] [Google Scholar]
- 25.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 26.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 27.Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 28.Moore ZW, Zhu B, Kuhel DG, et al. Vascular apolipoprotein E expression and recruitment from circulation to modulate smooth muscle cell response to endothelial denudation. Am J Pathol. 2004;164:2109–2116. doi: 10.1016/S0002-9440(10)63769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Eckardstein A. Cholesterol efflux from macrophages and other cells. Curr Opin Lipidol. 1996;7:308–319. doi: 10.1097/00041433-199610000-00009. [DOI] [PubMed] [Google Scholar]