Abstract
Extracellular matrix (ECM) and matrix receptors are intimately involved in most biological processes. The ECM plays fundamental developmental and physiological roles in health and disease, including processes underlying the development, maintenance and regeneration of the nervous system. To understand the principles of ECM-mediated functions in the nervous system, genetic model organisms like Drosophila provide simple, malleable and powerful experimental platforms. This article provides an overview of ECM proteins and receptors in Drosophila. It then focuses on their roles during three progressive phases of neural development: 1) neural progenitor proliferation, 2) axonal growth and pathfinding and 3) synapse formation and function. Each section highlights known ECM and ECM-receptor components and recent studies done in mutant conditions to reveal their in vivo functions, all illustrating the enormous opportunities provided when merging work on the nervous system with systematic research into ECM-related gene functions.
Introduction
The extracellular matrix (ECM) makes up a staggering 50–70% of the human body mass. It is composed of many families of molecules including proteoglycans, glycosaminoglycans, collagens and non-collagenous glycoproteins. These heavily glycosylated proteins provide the structural support and anchorage for cells, define tissue borders, regulate the availability of extracellular signals and directly mediate intercellular communication (Reichardt and Prokop, 2011). Transmembrane ECM receptors, including integrins, syndecans and the dystrophin-associated glycoprotein complex, are major determinants of cellular structure and intercellular signaling processes (Bökel and Brown, 2002; Häcker et al., 2005; Waite et al., 2009). One of the primary challenges for research on ECM mechanisms is the enormous number and complexity of matrix proteins and receptors, and their intimate links with many other molecular processes of cells (Rozario and DeSimone, 2010). One strategy to address such complexity is the use of genetically malleable invertebrate model organisms, which provide efficient experimental platforms for gaining mechanistic understanding in a simpler context. The use of one such model, the nematode Caenorhabditis elegans, for the study of ECM-related mechanisms in nervous system development has recently been reviewed (Escuin and Georges-Labouesse, 2007). Here, we focus on the venerable system of the fruitfly Drosophila melanogaster.
Drosophila is an excellent model to integrate research on ECM and matrix receptors with the study of nervous system development and function. First, Drosophila genes encoding ECM proteins and their receptors are well conserved, and loss-of-function analyses are facilitated by the low gene number and redundancy in the fly genome. Second, molecular and cellular understanding of nervous system development is very advanced in Drosophila, ranging from mechanisms underpinning early neural patterning through to synaptic development and plasticity in the mature brain (Broadie et al., 1993; Prokop, 1999; Tessier and Broadie, 2009). Third, the Drosophila toolkit for neurological studies is extensive (Mudher and Newman, 2007). Established cellular approaches for imaging and in vivo recording combine with sophisticated and versatile genetic research strategies, including conducting unbiased screens for molecules contributing to neurological processes of interest (St Johnston, 2002; Venken and Bellen, 2005; Venken et al., 2009; Giacomotto and Segalat, 2010; Zhang et al., 2010). For these reasons, work on Drosophila nervous system development has long been and remains instrumental in generating new ideas and novel concepts often essentially impacting on research into mammals and human disease conditions (Bellen et al., 2010). In spite of the obvious advantages of neurodevelopmental studies in Drosophila and the enormous importance of the ECM and matrix receptors for biological processes, these areas are only gradually merging into a concrete avenue of research. To encourage this development and illustrate its enormous potential, we discuss here recent examples of ECM research in the Drosophila nervous system with specific attention to neural progenitors, axonal pathfinding and synaptic function and differentiation.
ECM molecules in Drosophila
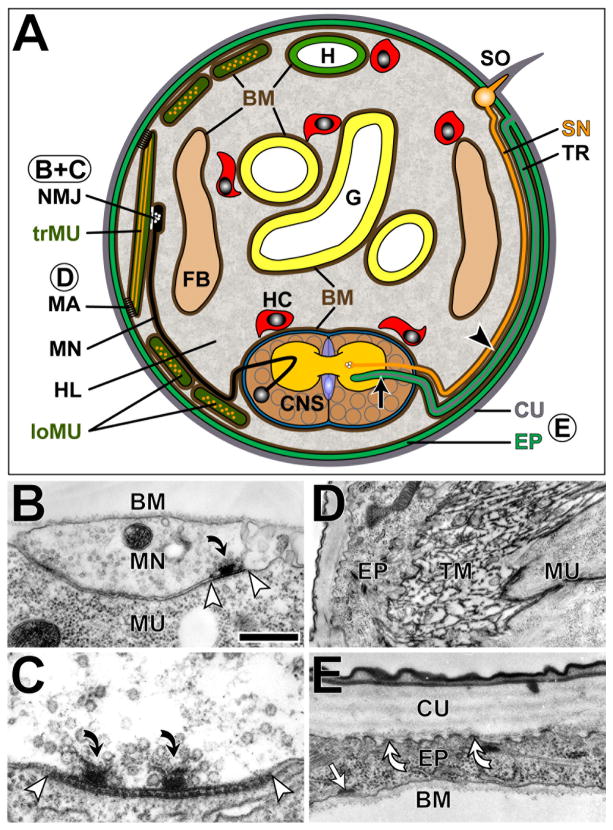
The Drosophila ECM can be divided into an external or apical fraction on the body surface, and an internal or basal fraction inside the body (Fig. 1A). Like all arthropods, Drosophila possesses an exoskeleton of cuticle secreted from the apical surfaces of the epidermis (Fig. 1E) (Anderson, 1979; Kaznowski et al., 1985). This apical ECM contains chitin polysaccharide fibrils (composed essentially of N-acetylglucosamine), and a large number of structural proteins, such as the ChLD (chitin binding, LDL receptor ligand binding, chitin deacetylase) protein family members Serpentine (Serp), Vermiform (Verm) and Chld3, and the zona pellucida (ZP) domain proteins Dumpy (Dp), Piopio (Pio) and Papillote (Pot) (Jazwinska et al., 2003; Payre, 2004; Bökel et al., 2005; Luschnig et al., 2006). Since most sensory organs of the fly comprise chitinous structures (Hartenstein, 1988), this apical ECM is likely to be of direct relevance to sensory nervous system development. This chitinous ECM is also part of the internal tracheal system, which interfaces closely with the nervous system, for example in guiding sensory neurons (Hartenstein, 1988) (Fig. 1A).
Figure 1.
ECM localization in Drosophila
Scheme of a frontal section through a late-stage Drosophila embryo (A) and micrographs of some morphological structures (B-E); letters in round fields in A refer to images B-E. A) Apical surfaces of the epidermis (EP) including certain sensory organs (SO) as well as inner walls of air-filled tracheal tubes (TR) are lined by an external cuticular ECM (CU, grey; compare E) forming the exoskeleton; tracheal branches reach into the CNS (arrow; see Fig. 2 for details on CNS morphology) and specific sensory nerves (SN) grow along these trachea (arrow head) (Hartenstein, 1988). Internal ECM is ultrastructurally visible as tendon matrix at muscle attachment sites (MA; compare D), as structured cleft material at neuromuscular junctions (NMJ; compare B, C), and as basement membranes (BM; brown lines on basal epidermal surfaces and outer surfaces of all organs including nerves and the CNS; compare B, E). Major sources for basal ECM molecules are the fat body (FB) and migratory hemocytes (HC, red). Spaces between cells and tissues are filled with liquid hemolymph (HL), kept under flow by the dorsal heart (H). Further abbreviations: G, gut; trMU, transverse muscle sectioned along its contractile filaments (orange lines); loMU, longitudinal muscles sectioned perpendicular to their contractile filaments (orange stipples). B) At NMJs, motorneuronal terminals (MN) form a close, non-conspicuous cell junction with the surfaces of muscles (MU), interspersed by prominent synapses indicated by electron dense extracellular material in the synaptic cleft (between white arrow heads) and a prominent presynaptic active zone surrounded by vesicles (curved arrow); remaining surfaces of MN and MU are covered by basement membrane (BM). C) Higher magnification of a neuromuscular synapse with two active zones showing the regular dashed arrangement of extracellular material in the synaptic cleft (between white arrow heads). D) A muscle attachment site with prominent electron dense ECM called tendon matrix (TM) connecting epidermis and muscle. E) Cuticular ECM forms a layered structure which is connected via apical hemiadherens junctions (white curved arrows) to epidermal cells, the basal surfaces of which attach to basement membrane through laminin-dependent mechanisms; only at focal hemiadherens junctions (white arrow) BM adheres via PS integrins. Scale bar in B represents 480 nm in B, 250 nm in C, 1 μm in D and 0.5 μm in E.
All internal organs and the basal surfaces of the epidermis are bathed in haemolymph from which cells are separated by a surface-associated basement membrane (BM; Fig. 1A). BMs are constituted by classical secreted ECM molecules (Yurchenco and Patton, 2009). In Drosophila, this ECM is evolutionarily well conserved, including two Laminins (Laminin A and a minor Laminin W), one Perlecan (Terribly reduced optic lobes, Trol), two genes for type IV collagen polypeptides (Cg25C and Viking, Vkg), one Nidogen (Ndg) and one BM40/SPARC (Table 1) (Hynes and Zhao, 2000). Laminins are cruciform, heterotrimeric glycoproteins. The two Drosophila Laminins both share common β and γ chains (LanB1 and LanB2, respectively), whereas there are two distinct α chains (LanA in Laminin A, Wing blister in Laminin W) with different tissue distributions (Kusche-Gullberg et al., 1992; Martin et al., 1999). The heparane sulfate proteoglycan (HSPG) Perlecan is well conserved in Drosophila and shares, with the exception of domain I, the domain structure of mammalian Perlecan (Friedrich et al., 2000; Voigt et al., 2002). In embryos, Perlecan is found localized in many BMs and co-localizes to a large extent with Laminin A (Friedrich et al., 2000). Nidogens are glycoproteins with important linker functions in BMs. Drosophila Nidogen has hardly been investigated at the functional level so far (www.flybase.org), but it is clearly a prominent constituent of BMs (Wolfstetter and Holz, 2011). Type IV collagens are triple-helical proteins forming interlaced networks contributing to the structural backbone of BMs. In Drosophila, the two collagen type IV paralogues Viking and Cg25C are thought to contribute to BMs throughout the body, whereas a third type IV-like collagen, Pericardin (Prc), is restricted in location and function to the most dorsal region of the embryo where it contributes to the formation of the Drosophila heart (Fig. 1A) (Natzle et al., 1982; Yasothornsrikul et al., 1997; Chartier et al., 2002; Myllyharju and Kivirikko, 2004). BM40/SPARC (secreted protein, acidic, rich in cysteine) is an evolutionarily conserved collagen-binding ECM glycoprotein that localizes throughout Drosophila BMs and has been suggested to be functionally required for BM formation (Martinek et al., 2002; Martinek et al., 2008).
Table 1.
Features of selected Drosophila ECM molecules and receptors
| Gene name in Drosophila | Mammalian homologues | Binding partner in Drosophila | mRNA Expression in embryo | Protein localization in embryo | Most prominent phenotypes in embryos | Neural phenotypes |
|---|---|---|---|---|---|---|
| ECM | ||||||
| laminin A (lanA) | Laminin α3/α5 | Laminin A*: αPS1βPS integrin, αPS3βPS integrin, Dystroglycan, Syndecan, Slit, | HC, FB, | BM CNS, BM brain, gut, DV, tendon, | EL, heart, somatic mesoderm, gut, | rare midline guidance errors, misrouted axons of ocellar neurons |
| wing blister (wb) | Laminin α1/α2 | Laminin W*: αPS2βPS integrin, αPS3βPS integrin, | VM, MA, DV, | BM gut, MA, | EL, tracheal tree, DV, somatic muscles, | ND |
| laminin B1 (lanB1) | Laminin β1 | see Laminin A* or Laminin W*, | HC, FB, | BM CNS, BM brain, gut, | EL, instable BM, gut, tracheae, CNS condensation, muscles, | ND |
| laminin B2 (lanB2) | Laminin γ1 | see Laminin A* or Laminin W*, | HC, FB, | BM CNS, BM brain, gut, | EL, instable BM, gut, tracheae, CNS condensation, loose pericardial cells, muscles, | ND |
| Cg25C | type IV Collagen | Dpp, Screw | HC, FB, | BM gut, brain, CNS, | EL, instable BM, | ND |
| viking (vkg) | type IV Collagen | Dpp, Screw, Sog | HC, FB, | ND | EL, instable BM, | ND |
| pericardin (prc) | type IV Collagen-like | ND | pericaridal cells, ring gland, oenocytes, | pericardial cells, basal surface DV, | ND, disorganized cardial epithelium, dorsal vessel, | ND |
| terribly reduced optic lobes (trol) | Perlecan | Dystroglycan | VM, FB, DV, | BM CNS, gut, DV, | EL, NOP, | failure to activate larval neuroblast proliferation |
| tiggrin (tig) | none | αPS2βPS integrin, Slit, | HC, FB, | MA, FB, HC, axons, | LL | wavy longitudinal tracts |
| multiplexin (mp) | Collagen XV/XVIII | ND | CNS, DV, | ND | V, developmental retardation, | motor axon guidance defect |
| faulty attraction (frac) | Fibrillin, Fibulin 1 | MMP2 | mesoderm, muscles, | muscles, MA, | V, NOP, | ISNb defasciculation, misprojection, |
| nidogen (ndg) | Nidogen | ND | muscles, MV gut, chordot. organs, founder cell, | BM VM, gut, muscles, CNS, chordot. organs, | ND | ND |
| BM40/SPARC | BM40 | ND | HC, VM, | BM brain, CNS, midgut, | EL, instable BM, CNS condensation, | ND |
| tenectin (tnc) | none | αPS2βPS integrin, | hindgut, tracheae, CNS, anal plate, | hindgut, trachea, CNS, | EL, NOP, | ND |
| thrombospondin (tsp) | Thrombospondin | αPS2βPS integrin, | tendon cells, muscles, | MA | EL, muscle detachment, | ND |
| M-spondin (mspo) | Spondin 2 | ND | CNS, muscles, | MA | V | ND |
| papilin (ppn) | Papilin | ND | HC, proventriculus, DV, | BM CNS, gut, malphigian tubules, heart, | EL | ND |
| glutactin (glt) | none | ND | HC | BM CNS, brain, gut, muscle, | ND | ND |
| peroxidasin (pxn) | Peroxidasin, VP01 | ND | HC, FB, ring gland, | HC, BM CNS, brain, gut, | ND | ND |
| Receptors | ||||||
| mew (αPS1 integrin) | α7 Integrin | Laminin A*, Slit, | as protein, | tendon cells, gut epithelium, CNS, boutons, | LL, gut, | misguided motor nerves |
| if (αPS2 integrin) | α5/8 Integrin | Laminin W*, Slit, Tenectin, Thrombospondin, | as protein, | MA, VM, CNS, boutons, | EL, muscle detachment, | misguided motor nerves, errors in CNS pathfinding |
| scab (αPS3 integrin) | α4/9 Integrin | Laminin W*, Slit, | as protein, | midgut, tracheae, salivary gland, | EL, dorsal hole, tracheae, salivary glands, | errors in CNS pathfinding, learning defect |
| αPS4 | α5/8 Integrin | ND | ND | ND | ND | ND |
| αPS5 | α5/8 Integrin | ND | ND | ND | ND | ND |
| mys (βPS integrin) | β1 Integrin | see αPS1-5, | as protein, | MA, tendon, VM, gut epithelium, CNS, boutons, | EL, muscle detachment, dorsal hole, gut, | misguided motor nerves, errors in CNS pathfinding, NMJ defects |
| βint-ν (βν integrin) | β1 Integrin | ND | as protein | midgut | V | ND |
| dystroglycan (Dg) | Dystroglycan | Laminin A*, Trol, | as protein | CNS, PNS, dorsal median cell, hindgut, midgut constriction, | V | NMJ defects (postsynaptic GluRIIA receptor number reduced, reduction of synaptic boutons) |
| division abnormally delayed (dally) | Glypican 5 | Hh, Dpp, Pent | as protein, | segmental pattern, | V | cell cycle progression of lamina precursors |
| dally-like protein (dlp) | Glypican 4 | Wg, Hh, FGF, | as protein, | segmental pattern, axons, VM, | EL, GLC: segment polarity, NMJ, | misrouted axons at CNS midline and of retinal axons |
| syndecan (sdc) | Syndecan 1 | Slit, Robo, Nrx IV Laminin A*, DLAR | as protein, | midline and axons, MA, | EL, NMJ, | pathfinding errors at CNS midline, of retinal and motoraxons |
Abbreviations (compare Fig. 1A): BM, basement membrane; VM, visceral mesoderm of the gut; MA, muscle attachments; CNS, central nervous system; DV, dorsal vessel; PNS, peripheral nervous system; FB, fat body; HC, hemocytes; EL, embryonic lethal; LL, larval lethal; V, viable; NOP, no obvious phenotype; GLC, germline clones; ND, no data.
Footnote: Laminin A and Laminin W are trimers; Laminin A is composed of Laminin subunits α3,5/LanA), β1/LanB1 and γ1/LanB2, while Laminin W is composed of Laminin subunits α1,2/Wb, β1 and γ1.
Many more structural ECM molecules have been reported to form intricate relationships with BMs, and many show obvious expression in nervous tissues (Table 1). These molecules include Thrombospondin (Tsp) (Adams et al., 2003), M-spondin (Mspo; a homologue of F-spondin/VSGP/Spon1) (Umemiya et al., 1997), Papilin (Ppn; an alternatively spliced, conserved, prominent constituent of BMs with thrombospondin type-1 domains and homologies to the carboxyterminal of ADAMTS group metalloproteases) (Campbell et al., 1987; Kramerova et al., 2003; Fessler et al., 2004), Multiplexin (Mp; a homologue of collagens XV and XVIII with an endostatin domain) (Meyer and Moussian, 2009; Momota et al., 2011), Faulty attraction (Frac; a protein rich in EGF-like and calcium-binding EGF domains, related to the Fibrillin and Fibulin protein families) (Miller et al., 2011), Tiggrin (Tig; containing unique N- and C-terminal domains and a central stretch of sixteen contiguous ~75 amino acid repeats, essentially modified through mucin-type O-glycosylation) (Fogerty et al., 1994; Bunch et al., 1998; Zhang et al., 2011), Tenectin (Tnc; containing a signal peptide, a RGD tripeptide, and a c-type von Willebrand Factor domain) (Fraichard et al., 2006), Glutactin (Glt; containing a catalytically inactive acetylcholine esterase domain) (Olson et al., 1990), Peroxidasin (Pxn, containing leucin rich and immuno globulin repeats, and a functional peroxidase domain) (Nelson et al., 1994), and MDP-1 (Macrophage-derived proteoglycan-1; potentially synonymous to Papilin) (Hortsch et al., 1998). Indications of a potential Drosophila Fibronectin (Gratecos et al., 1988) have not been confirmed by subsequent studies or genome annotations. Moreover, classical fibrillar collagens have not been found in Drosophila (but exist in some other insects) (Ashhurst and Costin, 1974; Ashhurst and Bailey, 1980; Boot-Handford and Tuckwell, 2003; Aouacheria et al., 2004; Myllyharju and Kivirikko, 2004). Also tenascins are not found in Drosophila, in spite of the fact that two genes were named tenascin accessory and tenascin major for historical reasons (www.flybase.org). Additional ECM components restricted to specific localizations within tissues will be detailed below (e.g. Anachronism, Hikaru genki and Mind-the-Gap at synapses) (Ebens et al., 1993; Hoshino et al., 1996; Rushton et al., 2009).
ECM proteins are both produced by local cells and recruited from distant sources to the site of their final deposition. This has been nicely illustrated for M-spondin and Tiggrin, two constituents of the specialised BM-associated ECM at muscle attachment sites (tendon matrix; Fig. 1A) (Prokop et al., 1998). M-spondin is produced by a subset of muscles, i.e. locally near the site of its final deposition (Umemiya et al., 1997). In contrast, Tiggrin is synthesized primarily in the fat body (similar to liver) and haemocytes (a type of blood cell; Fig. 1A), transported through the haemolymph, and finally recruited to muscle attachments via integrin-independent mechanisms that are still uncharacterized (Fogerty et al., 1994; Martin-Bermudo and Brown, 2000). Similarly, wing blister laminin is primarily produced locally in specialized tissues (e.g. visceral mesoderm, muscle attachment sites and cardiac cells) (Martin et al., 1999), but reportedly not in the nervous system, whereas all other Laminins are produced primarily by fat body and migrating haemocytes and later incorporated into BMs throughout the body. The same recruitment mechanism acts for other BM components, including type IV Collagen and BM40/SPARC (Mirre et al., 1988; Montell and Goodman, 1989; Kusche-Gullberg et al., 1992; Yasothornsrikul et al., 1997; Martinek et al., 2002; Olofsson and Page, 2005; Martinek et al., 2008). Some haemocyte-derived ECM proteins, such as Laminin, Tiggrin and the type IV collagen Cg25C, acquire strong localization within the embryonic CNS (Montell and Goodman, 1989; Mirre et al., 1992; Fogerty et al., 1994). In agreement with this finding, the ablation of haemocytes leads to nervous system defects (Sears et al., 2003).
In conclusion, fat body cells and haemocytes certainly represent major ECM-producing sources, at least in the embryo. Accordingly, Drosophila haemocyte-derived Kc cell lines have been used as efficient sources for the production and purification of a number of ECM molecules (Fessler et al., 1994; Fessler et al., 1994). However, a recent report has refined this view by showing that Perlecan and Laminin are both produced by follicular and embryonic epithelia (Denef et al., 2008). This report further shows that these ECM proteins require the activity of the endocytic trafficking regulator Crag (Calmodulin-binding protein related to a Rab3 GDP/GTP exchange protein) for their correct basal deposition. Further ECM proteins discussed below are also produced by adjacent cells during developmental mechanisms. Such local mechanisms of ECM deposition agree with our knowledge about vertebrate ECM, and provide excellent means to control and refine the specification of matrices in different tissue locations over developmental time.
ECM receptors in Drosophila
Drosophila matrix proteins bind a phylogenetically-conserved repertoire of classical ECM-receptors, including integrins, dystroglycan, syndecan and glypicans. Amongst these, most attention has been given to the α/β-heterodimeric transmembrane integrin receptors. Mammalian genomes contain 18 α subunit and 8 β subunit genes forming 24 reported α/β-heterodimers that interact with a wide range of ECM proteins (Bökel and Brown, 2002; Humphries et al., 2006; Barczyk et al., 2010). In contrast, the Drosophila genome encodes only 5 α integrin subunits and two β subunits. Of these, the position specific (PS) integrins βPS (myospheroid, mys), αPS1 (inflated, if) and αPS2 (multiple edematous wings, mew) are most evolutionary conserved, whereas less well conserved subunits include βν, αPS3 (scab, Volado), αPS4 and αPS5 (Brown et al., 2000) (Table 1).
In mammals, dystroglycan is the transmembrane matrix receptor of the dystrophin-glycoprotein complex (DGC), which is closely associated with muscular dystrophies and so-called ‘dystroglycanopathies’ affecting the nervous system (Michele and Campbell, 2003; Cohn, 2005; Waite et al., 2009). Intracellularly, neuronal dystroglycan binds dystrophin and sarcoglycans. The O-linked sugar side chains of its extracellular mucin-like domain mediate binding to a number of laminin G-domain-containing ECM proteins (laminins, perlecan, agrin, neurexins). Drosophila Dystroglycan (Dg) is encoded by a single gene, but exists in differentially expressed splice versions, two of which lack the mucin-like domain (Schneider et al., 2006; Schneider and Baumgartner, 2008). Whereas the mammalian gene product is cleaved into a N-and C-terminal halves (α- and β-Dystroglycan) that stay physically attached, Drosophila Dg seems not to be cleaved (Deng et al., 2003). In the Drosophila developing nervous system, Dg is expressed together with most other members of the DGC (Dekkers et al., 2004; Schneider and Baumgartner, 2008) and has been shown to genetically interact with a broad range of signalling pathways involved in nervous system development (Kucherenko et al., 2008; Kucherenko et al., 2011).
The third class of classical ECM receptors is the heparan sulfate proteoglycan (HSPG) family of proteins. HSPGs are characterised by N-linked glycosylation, predominantly heparan sulfate glycosaminoglycan (GAG) side chains (Van Vactor et al., 2006). Drosophila contains four phylogenetically conserved HSPGs, one of which is the secreted ECM protein Trol (Perlecan; see above), whereas the other three represent membrane associated receptors: the GPI-anchored glypicans Dally (Division abnormally delayed) and Dlp (Dally-like), as well as the transmembrane protein Syndecan (Sdc) (Lin, 2004). Although their extracellular domains can be cleaved, Syndecans have been shown to serve predominantly as transmembrane ECM receptors, often acting in conjunction with other receptors in a number of signalling pathways (Lin, 2004; Häcker et al., 2005; Alexopoulou et al., 2007; Morgan et al., 2007; Couchman, 2010). In conclusion, Drosophila contains a restricted number of ECM proteins and receptors. Although their diversity is increased by differential splicing and glycosylation (Kramerova et al., 2003), and although it is probable that further ECM genes will be uncovered amongst the numerous non-investigated annotated Drosophila loci, ECM redundancy is impressively low in the fly providing a simplified experimental platform to study the principal biological functions of ECM proteins and their receptors. This notion is illustrated with concrete examples of the nervous system in the latter parts of this review.
General functions of ECM proteins and receptors in Drosophila
A number of Drosophila studies have revealed ligand-receptor relationships between ECM components and membrane receptors. Laminin A binds to Dystroglycan and αPS1/βPS integrin, but not to αPS2/βPS integrin (Gotwals et al., 1994; Schneider et al., 2006). Laminin W binds αPS2/βPS and αPS3/βPS integrin, but not αPS1-containing receptors (Graner et al., 1998; Schock and Perrimon, 2003). Drosophila Syndecan can also mediate cell adhesion to laminin substrates, whereas the GPI-anchored glypicans Dally or Dlp fail to bind (Narita et al., 2004; Yamashita et al., 2004). Therefore, like in vertebrates (Suzuki et al., 2005; Durbeej, 2010), PS-integrins, Dystroglycan and Syndecan are all receptors for laminins in Drosophila. αPS2/βPS integrin has also been shown to bind Tiggrin (Gotwals et al., 1994), and Dystroglycan serves as a receptor for Trol (Schneider et al., 2006). In spite of these demonstrated ligand-receptor relationships, we still do not know which receptors link BMs to cell surfaces. In lanA mutant embryos, the BM detaches from all cell surfaces indicating that BM association is directly or indirectly dependent on Laminin A (Prokop et al., 1998). PS Integrins are clearly not the main BM receptors on general cell surfaces, although they mediate locally restricted BM attachment at focal hemiadherens junctions in a Laminin-independent manner (Fig. 1E) (Prokop et al., 1998). Notably, BM attachment is of relevance to nervous system development, as it has been shown to contribute to neuromuscular junction adhesion (Prokop et al., 1998).
In vertebrates, loss of function of classical ECM genes frequently causes failure to progress beyond early embryonic developmental stages (Gelse et al., 2003; Durbeej, 2010). Similarly in Drosophila, loss-of-function of these same genes tends to cause embryonic lethality. However, the development of mutant embryos has never been reported to arrest prematurely, providing opportunities for analyses of the developmental functions of ECM proteins in vivo. For example, ultrastructural analyses reveal that the absence of Drosophila Laminin A and/or W (lanA or lanB1 loss of function) causes BMs to be fragile and detach from all cell surfaces (Prokop et al., 1998) (A. Koper and A.P., unpublished data). Accordingly, functional loss of the LanA α chain, LanB1 β chain or LanB2 γ chain all cause abnormal morphogenesis of major tissues such as gut, trachea, nervous system and muscles, but embryos otherwise continue to differentiate mature features, such as cuticle deposition and synapse formation (Henchcliffe et al., 1993; Yarnitzky and Volk, 1995; Prokop et al., 1998; Urbano et al., 2009; Wolfstetter and Holz, 2011). Likewise, loss of both Collagen type IV genes causes BMs to be thin and fragile and, although these embryos fail to hatch, they differentiate into mature stages (Borchiellini et al., 1996) (Andre Koper and A.P., unpublished). Loss of Vkg alone impairs processes of cell elongation during oogenesis (also requiring βPS integrin) (Haigo and Bilder, 2011). Loss of Vkg also affects bone morphogenic protein (BMP) signalling during patterning events in the Drosophila embryo (Wang et al., 2008; Bunt et al., 2010). Loss of Perlecan function causes embryonic lethality, but mutant embryos fail to show any obvious morphological aberrations (Voigt et al., 2002). Loss of BM40/SPARC also causes embryonic lethality and misregulation of integral BM proteins (Martinek et al., 2008), but no ultrastructural studies have been reported so far to examine BM structural integrity.
Amongst the classical ECM receptors of Drosophila, mutations in PS integrins cause the most profound developmental effects, as reviewed previously (Brown et al., 2000; Bökel and Brown, 2002). In general, the βPS subunit is far more abundant and functionally important than βν. Expression of βν primarily occurs in endodermal cells of the developing midgut, where it executes no essential functions, but has roles that are redundant with βPS (Yee and Hynes, 1993; Devenport and Brown, 2004). However, βν functions are not restricted to the midgut, as indicated by roles of αPS3/βν at the neuromuscular junction (Tsai et al., 2008). Nevertheless, the specificity of Drosophila integrin receptor functions is primarily conferred by the α integrin subunits, in particular the extracellular domains of α subunits (as shown for αPS1 and αPS2) (Martin-Bermudo et al., 1997). In contrast to integrins, loss-of-function of other classical ECM receptors causes surprisingly subtle phenotypes in Drosophila. Complete loss of Dystroglycan or Syndecan permits semi-viable survival (Steigemann et al., 2004; Christoforou et al., 2008). Similarly, null mutant alleles of dally and dally-like are viable (Han et al., 2004), and only complete loss of Dally-like through germline clones causes severe embryonic phenotypes primarily relating to roles in Hedgehog signalling (Han et al., 2004).
In the following sections, this review will focus on three aspects of neural development in which ECM and matrix receptors have been demonstrated to play essential roles: 1) the proliferation of neural progenitors, 2) axonal growth and pathfinding, and 3) neuromuscular synapse formation and function. Each section will highlight known ECM and ECM-receptor components, and recent studies that have been done in mutant conditions to reveal their in vivo functions.
Roles of the ECM in neural precursor cells
As detailed in another review of this special issue, the ECM plays major roles in the regulation of vertebrate neural stem cells (XXXFfrench-Constant, 2011). Similarly in Drosophila, ECM proteins and their receptors regulate progenitor cell behaviors. For example, Drosophila integrins have been proposed to maintain the stem-cell niche in the testis (Tanentzapf et al., 2007). Similarly, Perlecan and Dystroglycan have been shown to regulate or maintain the polarity of epithelial follicle cells of the gonad (Schneider et al., 2006; Mirouse et al., 2009). Most important to this review, neural progenitor polarity (a key feature of asymmetric division) is functionally linked to the polarity of the overlying epidermis in the Drosophila embryo (Wodarz, 2005; Yu et al., 2006), and this functional link might well depend on ECM-dependent mechanisms. Moreover, the Drosophila ECM has been shown to contribute to the controlled timing of neural precursor proliferation.
The proliferation of Drosophila neural progenitor cells (neuroblasts) occurs at precisely timed developmental stages (Ito and Hotta, 1992; Prokop and Technau, 1994; Sousa-Nunes et al., 2010). Towards the end of embryogenesis, virtually all neuroblasts enter a quiescence stage (Prokop and Technau, 1991). This quiescence stage is maintained by the glia cell-secreted glycoprotein Anachronism (Ana), although the mechanism is little understood (Ebens et al., 1993). Controlled re-activation of neuroblast proliferation is hormonally induced by ecdyson at early to mid larval stages, and this re-activation mechanism requires the function of Drosophila Perlecan (Trol) (Datta and Kankel, 1992; Truman et al., 1994; Datta, 1999; Voigt et al., 2002). In trol mutant larvae, Ana function is not suppressed and neuroblasts remain arrested at the late G1 phase of the mitotic cycle (Caldwell and Datta, 1998; Park et al., 2001; Park et al., 2003). However, neuroblasts can reactivate in the combined absence of both Trol and Ana, suggesting that Trol antagonises Ana but is not required as a promoting factor of proliferation (Datta, 1995). Trol does not act by downregulating Ana but by overriding its activity (Ebens et al., 1993; Datta, 1995) through well-established signalling pathways (Park et al., 2003; Lindner et al., 2007; Barrett et al., 2008). For example, trol mutant alleles genetically interact with mutations of the FGF receptors Branchless (Bnl) and Breathless (Btl). Human FGF-2 physically binds Trol, and its application to trol mutant brains partly rescues the phenotype, whereas MAPK inhibitors mimic the trol mutant phenotype (Park et al., 2003). In addition, Trol binds Hedgehog (Hh) and mammalian Sonic hedgehog (Shh). This Trol-Hh link is also relevant, since loss of Hh function decreases neuroblast divisions, gain of Hh function induces excess neuroblast divisions, and hh mutant alleles genetically interact in neuroblast activation with mutations of trol or tout-velu (ttv; required for the generation of heparan sulfate side chains) (Lin, 2004). These findings are consistent with the known regulatory roles of HSPGs in intercellular signalling (Häcker et al., 2005), and demonstrate their general importance in regulating the availability of extracellular signals.
A number of questions remain to be addressed. How does ecdysone trigger the Trol-Hh-FGF-dependent reactivation of neuroblasts? Is Trol required in the BM or otherwise within the nervous system? This latter question is essential, since neuroblasts in the larval nervous systems are separated from the BM by a double-layer of glial cells (Fig. 2) (Ito et al., 1995). Given this cellular constellation, it is also essential to resolve which cells produce and receive the Hh and FGF signals. Adressing these questions and unravelling mechanistic details of this cellular system provides an exciting opportunity to advance our knowledge of ECM-dependent mechanisms regulating stem cells, especially since Drosophila neuroblasts are amongst the best-understood neuronal precursor cell models under investigation. As a promising prospect for broadening such research avenues, additional ECM proteins and matrix receptors (Gtn, LanA, B1 and B2, Ten-m, Sdc) were discovered in a differential cDNA hybridization screen of Drosophila embryonic neuroblasts (Brody et al., 2002).
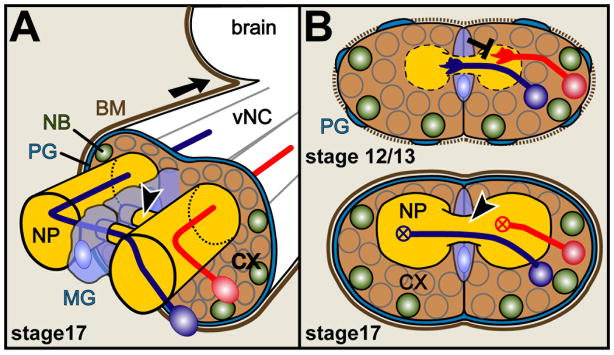
Figure 2.
Cellular constellations in the embryonic ventral nerved cord of Drosophila
A) The mature ventral nerve cord (vNC; equivalent to spinal cord; arrow points anterior) at embryonic stage 17 (Campos-Ortega and Hartenstein, 1997) is ensheathed by perineurial and subperineurial glia cells (blue line; PG) (Ito et al., 1995) covered by BM (brown line). Quiescent neural precursor cells (neuroblasts, NB) and neuronal cell bodies lie in the cortex (CX) from where axons project straight into the neuropile (NP; the synaptic compartment, which is itself ensheathed by interface glia; not shown) where they constitute ascending and descending pathways, dendrites and synapses (Sánchez-Soriano et al., 2007). Contralateral neurons (blue) project across the midline through two segmental commissures (arrow head) enwrapped by midline glia (MG). B) The lower image shows a frontal cross section of the vNC in A, the upper image the same section at an earlier stage when axonal growth takes place. Slit is released from midline glia cells and repels (black T-bar) growth cones of Robo-presenting neurons (red). At this early stage, the BM (dashed brown line) and PG sheath are incomplete, and fat body/hemocyte-derived ECM proteins like Tiggrin, Cg25C or Laminin A are enriched in the NP.
Roles of the ECM in axonal growth
As detailed in another review of this special issue (Myers et al., 2011), the ECM and its receptors are of key importance for the process of axonal growth and pathfinding during nervous system development and regeneration. Axon elongation is performed by growth cones at the tips of axons, and basic properties of growth cones are well conserved in Drosophila (Sánchez-Soriano et al., 2007; Sánchez-Soriano et al., 2009; Sánchez-Soriano et al., 2010). Growth cones use ECM and cell-attached proteins as substrates and are guided by spatiotemporal patterns of extracellular cues. These extracellular cues comprise ECM components and extracellular domains of membrane-associated proteins, as well as signalling molecules anchored to ECM or membrane-associated proteins. Growth cones interpret these extracellular signals through neuron-specific cocktails of membrane-associated adhesion and signaling receptors (Araujo and Tear, 2003; Huber et al., 2003; Kiryushko et al., 2004). Intracellular signalling events elicited by these receptors trigger the morphogenetic changes that implement growth cone behaviors to advance, turn, retract and collapse (Lowery and van Vactor, 2009; Moore et al., 2010; Dent et al., 2011). Although these mechanistic principles are widely accepted, there are few examples of pathfinding events that provide a comprehensive model for the induction and mediation of morphogenetic changes during growth cone behaviors. Two of the best-known examples are derived from work on Drosophila embryos and comprise motoraxon growth cones in the peripheral muscle field (Landgraf and Thor, 2006), and decisions of axonal growth cones at the CNS midline (Sánchez-Soriano et al., 2007). These models also provide the best examples of ECM-related axonal growth mechanisms being investigated in the fly.
Roles of ECM, integrins and syndecan in motor axon guidance
Drosophila Integrins were first implicated in axonal growth almost 40 years ago when loss of βPS integrin was shown to affect the abundance of axons in primary cell cultures (Donady and Seecof, 1972). In agreement with this finding, Drosophila Laminin promotes axon outgrowth of cultured primary neurons (Takagi et al., 1996) and triggers cell spreading of neuronal BG2 cell lines and recruitment of βPS integrin into focal adhesions (Takagi et al., 1998). These focal adhesions are enriched with tyrosine-phosphorylated proteins, one of which is the actin regulator Enabled (Takagi et al., 1998; Takagi et al., 2000). In vivo analyses of motor nerve patterns in mutant embryos lacking βPS or its partner subunits αPS1 or αPS2, revealed no deficits in the principal outgrowth capacity of motoraxons but a significant increase in navigational errors. These phenotypes have been interpreted to suggest that integrins are primarily required for the “responsiveness of an axon to a large array, rather than a small specific set, of guidance cues” (Hoang and Chiba, 1998).
To gain understanding of the cross-talk mechanism of integrins with guidance signaling pathways, analysis of molecular components downstream of integrins provides a promising strategy. For example, neuronal p130CAS/CasL (Crk-associated substrate), a tyrosine-phosphorylated docking protein of integrin adhesion complexes, has been shown to phenocopy and genetically interact with mutations in PS integrin genes, providing a potential functional link from integrins to actin regulation (Huang et al., 2007). Furthermore, links of p130CAS to Mical (Molecule interacting with CasL), a known effector of Semaphorin signalling (Huber et al., 2003), suggest interesting possibilities for PS integrin crosstalk with a relevant pathfinding signalling pathway. Notably, Mical and Semaphorins both show prominent motor axon guidance phenotypes (Yu et al., 1998; Beuchle et al., 2007). Loss of PS integrins as well as p130CAS suppresses spatiotemporally regulated defasciculation of motor axons from their nerves: instead of single axons, whole nerve branches are misrouted (Hoang and Chiba, 1998; Huang et al., 2007). This finding suggests that only pioneer neurons are misrouted in the absence of integrin signaling, whereas their follower neurons simply follow suit. In agreement with this model, follower neurons in embryonic motor nerves display narrow growth cones closely adhering to the preceding neuron surface, and this cell-cell contact requires cell-cell adhesion through the N-CAM homologue Fasciclin2, rather than cell-matrix adhesion (Sánchez-Soriano and Prokop, 2005).
Apart from p130CAS, surprisingly little is known about other classical downstream factors of integrins in the context of axonal growth, many of which are well conserved in Drosophila and for which suitable genetic tools are available (Brown et al., 2000). The roles of Paxillin (Pax), Talin (Rhea), Intregrin linked kinase (Ilk), Vinculin (Vinc), PINCH (Steamer duck; Stck), α-Actinin (Actn, Actn3) or two of the Filamins (Cher and Jitterbug, Jbug) have not been studied in the nervous system (www.flybase.org). Reported nervous system functions of Cheerio, the third Filamin, relate to learning and behavior (Bolduc et al., 2010), and neural functions of Fak56D concern neuronal physiology, NMJ growth and glial differentiation (Murakami et al., 2007; Tsai et al., 2008; Ueda et al., 2008), but these molecules have likewise not been linked directly to pathfinding. GTPases are typical effectors downstream of integrins (Parsons et al., 2010) and prominent players in axonal growth in Drosophila (Hakeda-Suzuki et al., 2002; Ng et al., 2002). However, GTPases have only been loosely linked to integrin functions in the context of axon guidance (Billuart et al., 2001). Finally, although two Src kinases, Src64B and Src42A, functionally interact with signalling processes involved in embryonic CNS midline guidance, mediated by Wnt5/Derailed or the membrane bound tyrosine phosphatase PTP69D (Song et al., 2008; Wouda et al., 2008), potential links of these signalling processes to integrins have also not yet been reported.
Surprisingly, reported loss-of-function phenotypes of ECM proteins in motor axon guidance have also so far not been related to integrins, leaving open the question of what ligands the PS integrin receptors bind to in this context. For example, the secreted acetylcholine esterase domain-factor Glutactin is expressed by one particular ventral longitudinal muscle (muscle 13). On this muscle, Glutactin function is believed to prevent ectopic synapse formation of two motor axons that navigate over it to reach their appropriate target muscles (Inaki et al., 2007). Potential links of this function to integrin receptors have not been investigated. Furthermore, the collagen XV/XVIII homologue Multiplexin is crucial for the correct navigation of two particular nerve branches in developing embryos (Meyer and Moussian, 2009), and mutant phenotypes are not dissimilar to the variable aberrations observed upon loss of PS integrin functions (see above). Nevertheless, this function has also not been reported to link to integrin receptors. Recently, the muscle-derived Fibrillin/Fibulin-related protein Faulty attraction (Frac) has been shown to promote motor axon bundling during outgrowth, potentially through BMP-dependent activation of a non-canonical LIM-kinase 1 (LIMK1)-dependent pathway in motor neurons (Miller et al., 2008; Miller et al., 2011). Frac is cleaved and activated by GPI-anchored matrix metalloproteinase 2 (Mmp2) expressed by motor axon-associated exit glia and perhaps elsewhere. So far, Glutactin, Multiplexin and Faulty attraction have not been reported to represent PS integrin ligands.
Other than integrins, also the Drosophila HSPG Syndecan (Sdc) has been studied in the context of motoraxonal growth (Fox and Zinn, 2005). Sdc localizes in non-neuronal peripheral tissues along the paths of embryonic motoraxonal nerves (Spring et al., 1994) and acts as an extracellular ligand for the receptor protein tyrosin phosphatase (RPTP) Lar (Leukocyte-antigen-related-like) on motor neuron growth cones (Fox and Zinn, 2005). Lar binds Sdc in vitro with nanomolar affinity via its GAG chains, and genetic studies strongly support a model in which interaction of Lar with Sdc (but not Dlp or Dally) is functionally required for motor axon guidance (Fox and Zinn, 2005). Similarly, in vertebrates, heparin and HSPGs directly interact with avian RPTPσ, a close homolog of Drosophila Lar (Aricescu et al., 2002).
In conclusion, surprisingly few Drosophila ECM molecules and matrix receptors play known roles in motor axon guidance. The outstanding task will now be to unravel how ECM functions integrate with the numerous classical guidance factors already functionally assessed in this cellular context (Landgraf and Thor, 2006). The Sdc-RPTP interaction is an early example for such research, which will eventually enhance our general understanding of the complex functional relationships between “classical” signalling factors, ECM proteins and their respective receptors.
Roles of ECM, integrins and syndecan in CNS midline guidance
A better defined pathfinding role for ECM proteins and receptors is the regulation of CNS midline axon crossing mediated by the secreted ligand Slit and its Roundabout (Robo) receptors (Fig. 3) (Sánchez-Soriano et al., 2007). Loss-of-function slit mutations or combined removal of the three orthologous robo, robo2 and robo3 genes lead to the collapse of virtually all axons on the CNS midline. Loss-of-function mutations of integrin subunit genes (βPS/mys, αPS1/mew, αPS2/if or αPS3/scab) similarly cause moderate slit/robo-like pathfinding defects, including axon scaffold collapse and inappropriate midline crossing, and strong genetic interaction with slit mutations (Stevens and Jacobs, 2002). The αPS1/βPS ligand Laminin A and the αPS2/βPS ligand Tiggrin are both present in the Drosophila CNS (Montell and Goodman, 1989; Fogerty et al., 1994), and lanA or tig both genetically interact with slit mutations (Stevens and Jacobs, 2002). Laminin A has likewise been shown to be required for axon guidance in another in vivo context, i.e. ocellar neurons in the post-embryonic brain (Garcia-Alonso et al., 1996). In midline guidance, genetic interactions of slit with integrins and integrin ligands are as strong as with mutations in the genes encoding Robo receptors or downstream Dreadlocks (Dock), suggesting that Slit/Robo-dependent pathways in axonal midline guidance functionally converge with functions downstream of ECM to PS integrin adhesion (Fig. 2C) (Stevens and Jacobs, 2002).
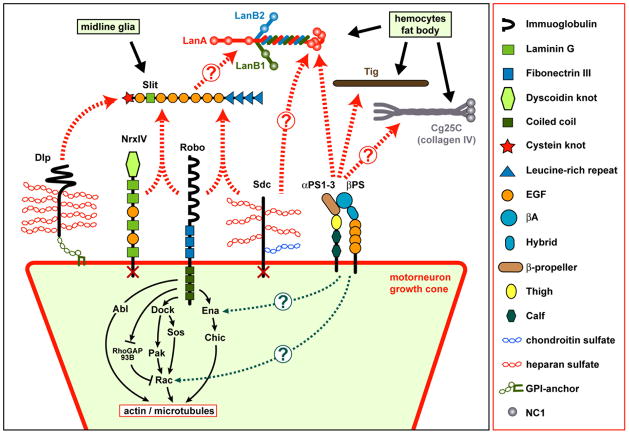
Figure 3.
Drosophila ECM proteins and matrix receptors during growth cone repulsion at the CNS midline.
Glia cell-derived Slit forms a quarternary complex with Neurexin IV (NrxIV), Syndecan (Sdc) and Roundabout (Robo) receptors presented on the surfaces of motorneuronal growth cones (red box), potentially further supported by Dlp. Only the cytoplasmic tail of Robo is required to mediate intracellular signalling function (Sánchez-Soriano et al., 2007; Chanana et al., 2009; Banerjee et al., 2010). It has not been resolved whether Slit and Sdc may interact with its other ligand Laminin A in this context. PS integrins and their ligands Tiggrin and Laminin A display strong functional interaction with Slit in midline guidance, and also the type IV collagen Cg25C as further putative integrin ligand localises in the CNS. Potential cross talk between the quarternary complexes and PS integrins could take place at the level of Ena (Takagi et al., 2000), or Rac as a common effector downstream of integrins in vertebrates (Parsons et al., 2010).
Sdc also localizes to the CNS midline and indeed throughout the embryonic synaptic neuropil (Fig. 2) (Spring et al., 1994; Johnson et al., 2004; Steigemann et al., 2004). Accordingly, loss of Sdc function causes axonal midline-misrouting phenotypes, which are reminiscent of, though much weaker than, slit or robo mutant phenotypes (Johnson et al., 2004; Steigemann et al., 2004; Chanana et al., 2009). Like integrin mutations, sdc mutations (but not dally or trol) show strong genetic interaction with robo or slit, whereas such interactions of sdc were not observed with mutations of DPTP69D (encoding another midline guidance factor) or of the robo paralogous genes robo2 and 3 (but robo2 and 3 enhance Sdc-robo interactions) (Johnson et al., 2004; Steigemann et al., 2004; Chanana et al., 2009). Similarly at larval stages during photoreceptor axon guidance into the optic lobe, loss of Sdc causes aberrations which are reminiscent of slit or robo mutant phenotypes and, accordingly, Sdc appears not to operate as a Lar ligand in this context (Tayler et al., 2004; Rawson et al., 2005; Hofmeyer and Treisman, 2009). These findings in the Drosophila CNS are in agreement with findings in vertebrates. For example, cell-surface heparan sulfates enhance slit2-robo1 binding in vitro and are required for their chemo-repulsive activity in rodent explant cultures (Hu, 2001). Moreover, mutations in heparan sulfate biosynthesis enzymes in zebrafish or mouse cause axonal guidance phenotypes reminiscent of slit or robo loss of function mutations (Inatani et al., 2003; Lee et al., 2004).
In rat and human, glypicans have been shown to bind Slit in vitro and to display overlapping localization patterns (Liang et al., 1999; Ronca et al., 2001). Interestingly, the Drosophila glypican Dlp contributes to Slit/Robo signalling: Dlp is strongly expressed in the developing CNS and dlp genetically interacts with sdc, with loss of dlp strongly enhancing sdc mutant midline axon defects and dlp over-expression partly rescuing sdc mutant phenotypes (Johnson et al., 2004; Chanana et al., 2009; Smart et al., 2011). However, the functional requirement of Sdc is clearly stronger than that of Dlp. One possible explanation for this difference is the presence of an intracellular domain in Sdc (which is lacking in the GPI-anchored Dlp), and in vertebrates this cytoplasmic tail of syndecans is known to execute signaling functions (Morgan et al., 2007). However, this possibility has been experimentally devaluated by showing that the intracellular domain of Drosophila Sdc is dispensable in axonal midline guidance, and that only association with neuronal membranes is required (Fig. 2C) (Johnson et al., 2004; Steigemann et al., 2004; Chanana et al., 2009). Detailed structure function analysis demonstrated that also the distinct core proteins of Sdc and Dlp fail to explain their functional differences at the midline (Chanana et al., 2009). Instead, deviations in their GAG chains have been pinpointed as the cause, in particular the presence of chondroitin sulfate side chains in Sdc (Fig. 2C), which are absent in Dlp (Lin, 2004; Chanana et al., 2009). This finding emphasises the regulatory importance of the enzymes catalysing GAG side chain formation, most of which have been identified in Drosophila (Lin, 2004; Häcker et al., 2005; Van Vactor et al., 2006). Accordingly, mutation of one such enzyme, tout-velu/ext1, shows strong genetic interaction with sdc in midline guidance (Chanana et al., 2009).
Dlp might play additional non-neuronal roles during Slit/Robo signalling, since the expression of Dlp (but not Sdc) in midline glia cells reportedly causes partial rescue of sdc mutant axonal midline phenotypes (Fig. 2). We note, however, that these findings are controversial, potentially due to distinct spatiotemporal expression patterns used in different experiments (Johnson et al., 2004; Chanana et al., 2009). Dlp also plays additional neuronal roles in axon guidance in the lateral CNS. Slit repulsion regulates not only midline crossing, but also the decision of whether axons project to medial, intermediate or lateral CNS positions. This function of Slit requires the Robo paralogous receptors Robo2 and 3 (Sánchez-Soriano et al., 2007). Lateral CNS fascicles are affected in dlp mutant embryos, and dlp genetically interacts with robo2 and slit in this context (Smart et al., 2011). Similarly, differential functions for Sdc and Dlp were observed for photoreceptor neurons in the larval optic lobe (Rawson et al., 2005). Finally, it needs to be pointed out that Slit not only repels growth cones, but it can also promote neuronal growth, as was similarly reported for vertebrate slit (Wang et al., 1999; Ozdinler and Erzurumlu, 2002; Whitford et al., 2002). For example, growth of motor neuron dendrites in the Drosophila embryonic CNS occurs later than axonal midline crossing (Sánchez-Soriano et al., 2005), and this phase of dendritogenesis coincides in space and time with an increase of Slit levels in the paraxial neuropile (Fig. 2). Removal of either Sdc or Robo causes reduction of Slit protein levels in the neuropile and, in parallel, the branching extent of dendrites is reduced (Johnson et al., 2004; Furrer et al., 2007). These data suggest that Sdc acts as a co-receptor for Robo in mediating growth promoting roles of Slit (Furrer et al., 2007).
A similar mechanism was suggested for the function of Sdc in repulsive midline guidance (Chanana et al., 2009). In agreement with this interpretation, Sdc, Slit and Robo were first demonstrated to form a ternary complex (Johnson et al., 2004), which was recently upgraded to a quaternary complex containing also the NeurexinIV transmembrane receptor (Fig. 3) (Banerjee et al., 2010). Therefore, Sdc could either present Slit to Robo or stabilize the Slit-Robo interaction, in agreement with reports that heparan sulfate enhances the binding affinity of rodent Slit and Robo (Hu, 2001). So far, it has not been addressed whether this Sdc co-receptor function requires interaction with ECM, such as Laminin present in the CNS (Fig. 3). Even if Sdc does not serve as an ECM receptor in this context, the Slit/Robo pathway might still couple to ECM proteins, such as Laminin A, Cg25C and Tiggrin (all known to be in the embryonic CNS; see above), through its links to PS integrins (Fig. 3). Further links to ECM-dependent mechanisms might arise from recent reports that slit and robo genetically interact with mutations of the ECM receptor gene dystroglycan in non-neuronal cells (Kucherenko et al., 2008; Kucherenko et al., 2011).
In conclusion, the model of CNS midline crossing in Drosophila provides excellent possibilities to explore regulatory roles of ECM and the mechanisms by which ECM receptors cross-talk with other signalling pathways. Such studies will benefit from the well defined signalling pathways downstream of Robo (Sánchez-Soriano et al., 2007), as well as the refined knowledge at the receptor level involving at least three ECM-related receptors (Fig. 3).
Studying ECM roles at the synapse
Once growth cones have established contact with their target cells, pre- and postsynaptic partner cells jointly form synapses. The formation, function and maintenance of these highly specialized cell junctions are dependent on ECM and matrix receptors. Well-studied synaptic ECM systems include the prominent BM at the vertebrate cholinergic neuromuscular junction (NMJ) (Singhal and Martin, 2011), the conspicuous perineuronal nets in the vertebrate CNS (Kwok and Fawcett, 2011; Wlodarczyk et al., 2011), and the beautifully-structured synaptic ECM at the Drosophila glutamatergic NMJ (Prokop, 1999; Prokop, 2006; Rohrbough et al., 2007; Rushton et al., 2009). At the Drosophila NMJ, extracellular material in the parasynaptic cleft is diffuse and little conspicuous, but it exhibits an electron-dense, highly organized structure in a limited radius of 50 to 200 nm around presynaptic active zones (Fig. 1B, C), coinciding with the postsynaptic glutamate receptor (GluR) fields (Fig. 4). In cross section, this synaptic ECM displays a repeated pattern of regularly spaced densities embedded in a lighter matrix, which are located adjacent to the postsynaptic membrane. When sectioned in parallel to the membrane, this synaptic ECM appears as a “honey-comb” array (Prokop, 1999; Prokop, 2006). The ECM at the Drosophila NMJ is derived from the motor neurons, muscles and/or closely associated glia (Fuentes-Medel et al., 2009; Rushton et al., 2009), but is likely to come also from other distant sources (haemocytes and fat body; see above).
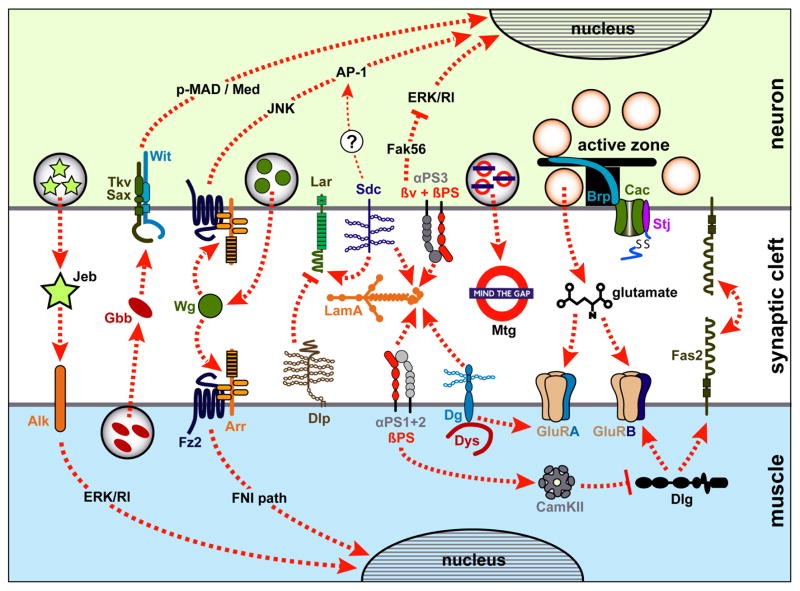
Figure 4.
Overview over mentioned molecules and pathways at the Drosophila NMJ
Aspects of presynaptic neuron, postsynaptic muscle and synaptic cleft are shown, as indicated on the right. Dashed arrows represent molecular interactions, functions or translocations that are explained in the text. Abbreviations: Arr (Arrow), Alk (Anaplastic Lymphona Kinase), AP-1 (Activator protein-1), Brp (Bruchpilot), Cac (Cacophony), CamKII (Calcium/calmodulin-dependent protein kinase II), Dg (Dystroglycan), Dlg (Discs large), Dlp (Dally-like), Dys (Dystrophin), ERK/Rl (Extracellular signal-regulated kinase/Rolled), Fak56D (Focal adhesion kinase), Fas2 (Fasciclin2), FNI (Frizzled nuclear import), Fz2 (Frizzled2), Gbb (Glass bottom boat), GluRA/B (Glutamate receptor), Jeb (Jelly belly), JNK (Jun N-terminal Kinase), LamA (Laminin A), Lar (Leukocyte-antigen-related-like), Med (Medea), Mtg (Mind the gap), pMAD (phospho-Mothers against dpp), α/βPS (PS integrin heterodimers), Sax (Saxophon), Sdc (Syndecan), Stj (Straightjacket), Tkv (Thick veins), Wg (Wingless).
Organization and remodelling of NMJ synapse ECM
Vertebrate NMJs possess a wide synaptic cleft (~70 nm), which contains a prominent BM containing three different β2 chain laminins (Singhal and Martin, 2011). Other synapse types, including the Drosophila NMJ, display far narrower clefts (<20 nm) without a BM. Although a classic BM is absent, laminins have been found at vertebrate central synapses (Egles et al., 2007), and also at the Drosophila NMJ where they are involved in regulating synaptic structure and physiology (Bogdanik et al., 2008). At embryonic stages, a BM overlays the muscle-attached motor terminal (Fig. 1B), and this BM significantly contributes to neuromuscular adhesion (Prokop et al., 1998) (A. Koper and A.P., unpublished results). Other specialized ECM components of Drosophila synapses include the neuronally secreted factor Hikaru genki (Hig; containing an RGD motif, an immunoglobulin and several complement binding domains) required for proper locomotor behaviour (Hoshino et al., 1993; Hoshino et al., 1996; Hoshino et al., 1999). At vertebrate NMJs, further prominent ECM constituents include the secreted HSPGs agrin and perlecan, which are important regulators of synapse formation, including acetylcholine receptor (AChR) clustering and acetylcholine esterase localization (Singhal and Martin, 2011). Specific probes similarly indicate localization of a heparan sulfate matrix at the Drosophila NMJ (Ren et al., 2009). Drosophila does not possess a defined agrin homologue, but the HSPGs Dlp and Sdc are both localized to the Drosophila NMJ (Fig. 4) (Johnson et al., 2006). Moreover, the use of lectins to map the carbohydrate landscape has revealed a highly organized distribution of glycosylated proteins at the Drosophila NMJ, as is similarly the case at the vertebrate NMJ (Martin, 2003). For example, Wheat Germ Agglutinin (WGA) labels dense punctate domains around synaptic boutons, which are distinct from a more diffuse Vicia Villosa Agglutinin (VVA) zone at the synaptic interface (Haines et al., 2007; Rushton et al., 2009).
An important recent advance has been the identification of Mind-the-Gap (Mtg), a secreted endogenous lectin that organizes the synaptic ECM (Fig. 4)(Rohrbough et al., 2007; Rushton et al., 2009; Rohrbough and Broadie, 2010). In mtg mutant embryos, synaptic ECM specializations are no longer detectable at the ultrastructural level, replaced by an aberrantly clear synaptic “gap”, from which the gene derives its name. Transcription of mtg is upregulated during the initial stages of NMJ synaptogenesis (12–13 hrs after egg lay, AEL), with a peak during the most active stages of synapse formation (16–18 hrs AEL) (Broadie and Bate, 1993; Broadie and Bate, 1993; Broadie and Bate, 1993; Rohrbough et al., 2007; Rushton et al., 2009). The RhoGEF gene still life involved in NMJ maturation, displays a similar expression profile (Sone et al., 2000). Secreted Mtg is proposed to act by binding N-acetylglucosamines (GlcNAc) in GAG chains of HSPGs via a conserved carbohydrate-recognition domain (CRD), a cysteine knot containing 3 disulfide bridges (Rohrbough et al., 2007; Boudko et al., 2008; Hoffmann et al., 2008; Kimura et al., 2009). This domain, referred to as the peritrophin A domain, is found in many chitin-binding proteins, such as the Drosophila cuticle proteins Serpentine, Vermiform and Chld3 mentioned in the first section of this review (Luschnig et al., 2006). Mtg is presynaptically released and immuno-EM studies show it to localize in the synaptic cleft near presynaptic active zones (Fig. 4) (Rohrbough et al., 2007). Light microscopic analyses show it within the synaptic ECM in distinctive punctae, adjacent to but not overlapping active zones labeled with Bruchpilot (Brp). Mtg is predicted to bind the same GlcNAc motif as WGA and, consistently, their synaptic localization domains overlap. Targeted deletion of Mtg from the presynaptic neuron abrogates synaptic cleft ECM development and Mtg overexpression alters both the WGA and VVA lectin domains at the NMJ synapse (Rushton et al., 2009), again suggesting that presynaptically released Mtg acts to organize the synaptic ECM landscape.
This unique phenotype makes mtg a powerful tool to assay ECM roles in synaptic development. Null mtg mutants arrest as anatomically mature, paralyzed embryos with structurally normal central/peripheral nervous systems and body wall musculature. NMJs are of normal size and display normal presynaptic architecture, but show a strong loss of functional GluR responsiveness in the muscle (Rohrbough et al., 2007). Consistently, mtg loss of function or knock-down of mtg in presynaptic neurons prevents localized accumulation of GluRs in postsynaptic domains (Fig. 4), whereas the level of GluRs in non-synaptic regions appears aberrantly high, consistent with abnormal receptor targeting or localization. This defect cannot be ameliorated by overexpressing GluR subunits in muscle, suggesting that Mtg is required to maintain functional GluRs at the surface (Rohrbough et al., 2007). The muscular expression of the Rho-type GEF Pixie (Pix), the PAK-kinase (Pak), and the scaffold proteins Dreadlocks (Dock) and Discs Large (Dlg) are all dramatically mis-regulated in the absence of neuronal Mtg function (Fig. 4), with lower levels at the synapse and, at least in some cases, higher levels in non-synaptic areas of the muscle. These components constitute the molecular pathways mediating GluR field assembly (Thomas et al., 2000; Parnas et al., 2001; Mathew et al., 2002; Albin and Davis, 2004). Therefore, these findings indicate that Mtg originating from the presynaptic neuron organizes the synaptic ECM in a mechanism required to induce proper localization and maturation of postsynaptic components in the muscle (Rohrbough et al., 2007).
Mtg expression persists into maturity, suggesting it is required to constitutively maintain the synaptic ECM. However, it is currently unknown whether the synaptic ECM may change with developmental age, or might be dynamically modulated during growth and neurotransmission. In vertebrates, a subset of the ADAMTS (A Disintegrin and Metalloprotease with Thrombospondin motifs) family of secreted metalloproteases (~20 in vertebrates), secreted matrix metalloproteinases (MMPs; ~25 in vertebrates) and Tissue Inhibitors of Metalloproteinases (TIMPs; 4 in vertebrates) actively modify the synaptic ECM through cleavage of inductive signals (e.g. agrin), ECM components (e.g. laminins) and ECM receptors (e.g. integrins, dystroglycan) (Nicholson et al., 2005; Lluri et al., 2006; Page-McCaw et al., 2007; Murphy and Nagase, 2008; Werle, 2008). Such cleavage changes receptor-binding affinities during synaptic development and modulation (Wlodarczyk et al., 2011) and regulates both synaptogenesis and the synaptic modulation underlying behavior (Ethell and Ethell, 2007; Agrawal et al., 2008), with defects linked to synaptic disorders including Fragile X syndrome (Bilousova et al., 2009). As usual, the Drosophila gene complement is simpler, comprising secreted MMP1, GPI-anchored MMP2 and a single TIMP that inhibits them both, with additional potential members of the ADAMTS family (Nicholson et al., 2005; Page-McCaw et al., 2007; Page-McCaw, 2008). MMP2 modifies dendritic and axonal development in Drosophila neurons (Miller et al., 2008; Yasunaga et al., 2010; Miller et al., 2011). Therefore, an important ongoing question will be to investigate the role of these protease mechanisms in shaping and remodelling the synaptic ECM at Drosophila NMJs.
Roles of Integrins at the NMJ synapse
At the vertebrate NMJ, three laminins containing the β2 chain and the type IV collagens α3/6 activate β1 integrin receptors, contributing to synapse architecture, and the clustering of postsynaptic AChRs and presynaptic Ca2+ channels (Singhal and Martin, 2011). At the Drosophila NMJ, βPS, βν and αPS1-3 integrin subunits are likewise found in both pre- and postsynaptic membranes (Fig. 4) (Beumer et al., 1999; Rohrbough et al., 2000; Beumer et al., 2002; Tsai et al., 2008), but Laminin is the only integrin ECM ligand established to be present (Bogdanik et al., 2008). PS integrins bind secreted developmental signals and have additional transmembrane partners in other cellular contexts (Araujo et al., 2003; Negreiros et al., 2010), and so may also have a broadened function at the NMJ. For example, the immunoglobulin family adhesion molecule Basigin (Bsg) is expressed at Drosophila NMJs (Besse et al., 2007), and βPS and basigin mutant alleles have been shown to display genetic interaction in the regulation of neuronal development in other contexts (Curtin et al., 2005). As above, integrin complex proteins (Brown et al., 2000) have hardly been investigated at the Drosophila NMJ, with the exception of Fak56D, which has been suggested to block activity of the MAP kinase ERK (Rolled, Rl) downstream of αPS3/βν function (Fig. 4) (Tsai et al., 2008). However, βPS and αPS1/2 integrin receptor subunits co-localize with the PDZ-domain scaffolding protein Dlg at the NMJ (Beumer et al., 1999), where βPS forms a physical complex with Dlg. Current data support a model in which PS integrins act upstream of postsynaptic Dlg and Calcium/calmodulin-dependent protein kinase II (CaMKII) to regulate postsynaptic localization of the immunoglobulin superfamily adhesion factor Fasciclin2 (Fas2) in synaptic assembly mechanisms (Fig. 4).
Thus, mutation of the βPS and αPS3 Integrin subunits both cause strong defects in NMJ morphological differentiation and functional maturation (Beumer et al., 1999; Rohrbough et al., 2000; Beumer et al., 2002; Suzuki et al., 2002). Similarly, acute introduction of RGD peptide to disrupt Integrin-ECM interactions changes synaptic basal function and activity-dependent modulation (Rohrbough et al., 2000). These functions are likely to relate to Dlg regulation, which helps to spatially organize postsynaptic proteins, including GluRs, ion channels and Fas2 (Fig. 4) (Budnik, 1996; Thomas et al., 2000; Ashley et al., 2005). Consistently, postsynaptic Dlg is significantly elevated at NMJs of larvae carrying the viable mysb9 gain-of-function mutant allele of the βPS gene (Beumer et al., 2002). In parallel, CaMKII levels are reduced at mysb9 mutant NMJs, and postsynaptic overexpression of CaMKII rescues mys mutant NMJ defects (Beumer et al., 2002). Vice versa, impairing CaMKII function causes altered synaptic differentiation phenotypes comparable to mys mutant NMJs (Wang et al., 1994). Similarly, synaptic integrin function in vertebrate hippocampal synapses can be blocked by a CaMKII inhibitor (Shi and Ethell, 2006). It is therefore well established that Integrins activate CaMKII (Hynes, 2002). CamKII, in turn, regulates the synaptic localization of Dlg at the Drosophila NMJ. This CamKII-DLG pathway regulates synaptic localization of postsynaptic proteins (Fig. 4). Consistently, the postsynaptic localization of Fas2 is elevated at mys mutant NMJs (Beumer et al., 2002). When a fas2 hypomorphic loss-of-function mutant allele (fasIIe86) is recombined into a mys mutant background, the integrin mutant synaptic differentiation defects are effectively rescued (Beumer et al., 2002).
It is known that surface maintenance of integrin receptors can depend on their ECM ligands (Hynes, 2002). Consistently, targeted deletion of Mtg abrogates synaptic localization of PS integrins (Rushton et al., 2009). The ECM receptors are still expressed, but fail to normally localize at the synaptic interface, both in pre- and postsynaptic membranes (Fig. 4). By mapping the synaptic ECM with lectin probes (WGA, VVA), it was shown that PS integrins also shape the extracellular carbohydrate environment during synaptogenesis (Rushton et al., 2009). Thus, there appears to be a bidirectional dependency between the synaptic ECM and these ECM receptors. Within the synaptic VVA lectin domain, Mtg and WGA lectin punctae overlap co-localized with PS Integrins (Rushton et al., 2009). Thus, Mtg and PS integrins interactively influence the composition and distribution of synaptic and perisynaptic ECM, but the molecular mechanism for this interaction is currently unknown. Part of the difficulty in testing this process is the severity of non-synaptic PS integrin mutant phenotypes, such as muscle detachment and embryonic lethality. It might be possible to circumvent this problem through the use of conditional mutants or allelic combinations that bypass early requirements via interallelic complementation. Furthermore, it would be of interest to examine possible known integrin interacting partners such as the Basigin receptor (Besse et al., 2007). Moreover, other ECM receptors reside at the Drosophila NMJ (see below), and it is unknown what happens to their expression and distribution in the absence of Mtg or PS integrin function.
Roles of Dystroglycan and Syndecan at the Drosophila NMJ
In mammals and humans, the dystrophin-glycoprotein complex plays major roles in muscle disease and also in a wide range of neurological disorders (Michele and Campbell, 2003; Cohn, 2005; Waite et al., 2009). In total, mutations in 10 DGC genes cause muscular dystrophies, and related ECM defects cause congenital myasthenic syndrome and Ehlers-Danlos syndrome (Dalkilic and Kunkel, 2003; Engel et al., 2008). At the vertebrate NMJ, one mutant copy of Dystrophin combined with one mutant copy of α7 integrin causes disruption of β2-laminin expression and NMJ development (Rooney et al., 2006). At the Drosophila NMJ, a postsynaptically localized Dystrophin scaffold regulates Drosophila NMJ maturation (van der Plas et al., 2006). Dystroglycan (Dg) regulates synaptic levels of both Dystrophin and Laminin (Fig. 4), as well as NMJ morphology, postsynaptic composition and presynaptic transmitter release probability (Fig. 4) (Haines et al., 2007; Bogdanik et al., 2008; Wairkar et al., 2008). Specifically, dg null mutants exhibit reduced postsynaptic GluRIIA receptor abundance and a reduction in synaptic bouton number. Interestingly, dg overexpression elevates VVA lectin levels at the NMJ, dependent on the Dg extracellular domain (Haines et al., 2007). Like observed for loss of Mtg or PS integrin function, Dg mutant NMJs exhibit altered synaptic versus extrasynaptic protein distribution, suggesting that the two ECM receptors PS integrin and Dg jointly enable the directed localization and/or anchoring of synaptic components at the Drosophila NMJ.
The mammalian ECM receptor syndecan-2 is implicated in the formation and morphological modulation of hippocampal synapses (Ethell and Ethell, 2007). Similarly at the Drosophila NMJ, both Sdc and Dlp accumulate in or near the synaptic cleft (Fig. 4), and Sdc facilitates the positive growth of the motor neuron terminal (Johnson et al., 2006). Double mutant studies with the receptor tyrosine phosphatase Lar demonstrate that Sdc and Dlp function upstream of this receptor, acting as Lar’s synaptic ligands (Kaufmann et al., 2002; Johnson et al., 2006), as during motor axon pathfinding (Fox and Zinn, 2005) (see above). Regulation of Lar appears to be antagonistically controlled by Sdc and Dlp, with Sdc activating Lar to promote growth and enhance presynaptic function. Notably, lar loss-of-function mutant phenotypes at NMJs resemble mys mutant defects. Accordingly, Lar has been shown to functionally interact with PS integrins in ECM-mediated actin filament regulation in non-neuronal tissues (Bateman et al., 2001). Sdc was also identified in a genetic screen to interact with Activator Protein-1 (AP-1; (Fig. 4) (Franciscovich et al., 2008). The transcription factor AP-1 (a Fos/Jun heterodimer) is one regulator of NMJ morphology and function(Sanyal et al., 2002). Links to this regulatory pathway provide exciting new directions to explore Sdc-dependent mechanisms at the synapse.
Glycosylation of the synaptic ECM
Carbohydrate chains (glycans) can be found on many lipids (glycolipids) and on virtually all secreted or membrane-associated proteins (glycoproteins and proteoglycans) (Freeze, 2006; Taylor and Drickamer, 2011). At the narrow synaptic cleft, they form a diverse and complex glycocalyx (Dityatev and Schachner, 2006) in which glycans mediate much of the molecular contacts (often through lectins like Mtg) essential for mechanisms of intercellular signaling and development (Kleene and Schachner, 2004; Dani and Broadie, 2011). Glycans can be classified as O- or N-linked (O- or N-glycans), the former comprising the GAG chains of proteoglycans. Glycan synthesis and their linkage to carrier proteins occurs mostly in ER and Golgi through glycosyltransferases, often intercalated by modification steps performed by specialised enzymes, such as epimerases (converting certain sugar residues), N-deacetylases (removing acetyl groups), or N- or O-sulfotransferases (sulfation of GAG chains; see next section). Genetic defects of N-glycan biosynthesis (congenital disorders of glycosylation) are associated with neuroanatomical and psychomotor defects, suggesting the possible importance of glycosylation at neuronal synapses (Freeze, 2006). Similarly, O-glycan defects have been associated with a number of disease conditions, such as congenital muscular dystrophy due to glycosylation defects of dystroglycan, or synaptic transmission impairment, neuronal connectivity defects, or debilitating neurological diseases upon pharmacological or genetic manipulations of HSPGs (Freeze, 2006; Van Vactor et al., 2006; Kul’chitskii et al., 2009).
Enzymes of the common glycan synthesis pathways are well conserved in Drosophila (Fabini and Wilson, 2001). For example, the Drosophila genome harbours two GlcNAc transferases, Mgat1 and Mgat2. Loss of Mgat1 function causes a lack of paucimannose N-glycans (including the pan-neuronal epitope detected by the widely used anti-horseradish peroxidase antibody) (Jan and Jan, 1982; Desai et al., 1994) and leads to defects in locomotor activity and brain development (Sarkar et al., 2006). Mgat2 is predicted to generate Mtg ligands (Sarkar et al., 2006). The two O-mannosyl transferases POMT1 (Rotated abdomen, Rt) and POMT2 (Twisted, Tw) glycosylate Dg (Ichimiya et al., 2004). Consistent with an O-linked mannose requirement on Dg, POMT loss-of-function mutant phenotypes resemble those of Dg mutants (Haines et al., 2007). Finally, heparan sulphate is enriched at Drosophila NMJs. The HSPGs Dlp and Sdc presumably contribute prominently to this local synaptic heparan sulphate enrichment (in agreement with their functional requirement at the NMJ; see previous section), and it can be abolished when deleting the heparan sulfate synthesizing enzymes tout-velu/Ext1 (ttv) and brother of ttv/Extl3 (botv) (Lin, 2004; Johnson et al., 2006; Ren et al., 2009). Loss of heparan sulphate in ttv/botv mutants, or of specific sulfate modifications in N-deacetylase/N-sulfotransferane mutants (encoded by sfl), causes differential structural and functionional defects at NMJs, including elevated synaptic vesicle endocytosis (Ren et al., 2009).
In addition to sulfation modifications, the addition of sialic acid or polysialic acid at the termini of extracellular oligosaccharides may play critical roles in synaptic development. For example, the synaptic neuronal cell adhesion molecule N-CAM is essentially regulated through sialylation (Kleene and Schachner, 2004). Sialic and polysialic acids are synthesised and typically linked to GlcNAc at terminal glycan positions through the activity of sialyltransferases in the Golgi, and they are also found in Drosophila including its nervous system (Roth et al., 1992). Accordingly, mutation of the Drosophila sialyltransferase ST6Gal/DSiaT causes NMJ undergrowth and reduction in neurotransmission current amplitudes (Repnikova et al., 2010). Dysfunction of these enzymes is one way to cause hyposialylation, but other possibilities have been suggested (Morin et al., 2004). For example, Sialin is an 8-pass transmembrane transporter protein believed to recycle sialic acid from degraded sialoglycoconjugates in lysosomes. Accordingly, sialin mutations are linked to the lysosomal storage diseases Salla Disease and Infantile Sialic Acid Storage Disease, both characterized by cognitive impairments suggestive of synaptic defects (Morin et al., 2004; Wreden et al., 2005; Yarovaya et al., 2005; Ruivo et al., 2008). Drosophila mutants of the sialin homologue Fuseless (Fusl) are viable, but display >75% reduction in NMJ current amplitudes and severely impaired synaptic vesicle cycling (Long et al., 2008). In ultrastructural studies, vesicles arrest in clustered and docked pools at fusl mutant presynaptic active zones. These defects are causally linked with severe loss or mislocalization of the voltage-gated Ca2+ channel Cacophony (Cac) from presynaptic active zones (Fig. 4) (Kawasaki et al., 2004; Xing et al., 2005). Whether other recently identified Ca2+ channel components (e.g. Straightjacket, Stj/α2δ3; Fig. 4) (Ly et al., 2008; Kurshan et al., 2009) are similarly regulated by sialylation is currently not known. It has been proposed that Bruchpilot (Brp) stabilizes active zone formation by integration of Cacophony Ca2+ channels with internal active zone components (Fig. 4) (Kittel et al., 2006; Wagh et al., 2006; Fouquet et al., 2009). Extracellularly, Fusl-dependent sialylation may likewise link the synaptic cleft ECM to Ca2+ channels during active zone assembly, providing a means to correctly anchor Ca2+ channels at the synaptic cleft interface during synaptogenesis.
ECM Dependence of Trans-Synaptic Signaling
Trans-synaptic signals including WNTs and TGFβ/BMPs play major roles in the regulation of synapse formation and function in vertebrates and invertebrates (Fig. 4) (Packard et al., 2003; Salinas and Zou, 2008; Wu et al., 2010; Bayat et al., 2011). The ECM in the synaptic cleft likely acts as a sink and/or barrier properly retaining, presenting and controlling the activity of such trans-synaptic signals and their receptors. Much pioneering work on trans-synaptic signaling has been done at the Drosophila NMJ. Two pathways are well-established: The two WNTs Wingless (Wg) and Wnt5 act as predominantly anterograde signals through their receptors Frizzled2 (Fz2) and Arrow (Arr/LRP) or Derailed (Drl), respectively, whereas the BMP Glass Bottom Boat (Gbb) acts mainly retrogradely through receptor complexes of the type-I receptors Saxophone (Sax) and Thick veins (Tkv) with the type-II receptor Wishful thinking (Wit) (Fig. 4). FGFs have also recently been identified as trans-synaptic signals at the Drosophila NMJ (Sen et al., 2011). In non-synaptic ECMs of vertebrates and invertebrates alike, HSPGs have co-receptor functions that regulate ligand-receptor interactions by enhancing the stability and concentration of ligand binding or facilitating oligomerization of the receptor or even ligand, thus impacting the extracellular distribution of WNT, BMP and FGF signals (Christian, 2000; Kuo et al., 2010). For example, reducing the sulfation state of HSPGs through mutations in Drosophila Sulf1 positively influences Wg association with its receptor Frizzled (Fz) and increases Wg expression/signalling in the developing wing disk (Kleinschmit, 2010), and this mechanism appears evolutionarily conserved (Ai et al., 2003). Similarly, cell surface HSPGs of mammalian myoblasts sequester BMP2 extracellularly via their heparan sulfate chains (Jiao et al., 2007). Vertebrate Sulf1 regulates BMP-receptor interactions, and the binding to heparan sulfate can both activate and inhibit BMP signaling (Christian, 2000; Viviano et al., 2004). Therefore, it is likely that the synaptic ECM environment at the Drosophila NMJ may modify the trans-synaptic signaling required for synaptic development and function, possibly through similar HSPG-dependent mechanisms to modulate trans-synaptic WNT and BMP signalling.
Another recently defined anterograde signaling pathway involves presynaptic secretion of Jelly Belly (Jeb) as a ligand, to activate the postsynaptic receptor tyrosine kinase Anaplastic Lymphona Kinase (Alk) (Fig. 4) (Rohrbough and Broadie, 2010). In the developing Drosophila visual system, Jeb/Alk signalling drives ERK activation to regulate transcription of downstream genes including the transmembrane adhesion molecules Kin of irre/Dumbfounded (Kirre), Roughest/IrreC (Rst) and Starry night/Flamingo (Stan) (Bazigou et al., 2007; Palmer et al., 2009). At the Drosophila NMJ, this trans-synaptic signaling pathway is strongly shaped by the Mtg-dependent ECM (Rohrbough and Broadie, 2010). Loss of Mtg causes dramatic accumulation of Jeb in the extracellular space surrounding synaptic boutons, and concomitant reduction of postsynaptic Alk levels (Rohrbough and Broadie, 2010). The change of extracellular Jeb at mtg mutant NMJs (increased amount, number and size of ECM punctae) indicates that the Mtg-dependent ECM is acting directly to limit the Jeb trans-synaptic signal. Interestingly, Alk is N-glycosylated by Mgat1 (Palmer et al., 2009) and is also independently maintained by the Mtg-dependent ECM (Rohrbough and Broadie, 2010). This signaling mechanism may also work in CNS synapses, where Jeb, Alk and Mtg are likewise synaptically localized. In addition, other ECM components and the matrix receptors Dystroglycan and PS integrins localize to CNS synapses, either globally or in subsets of synapses (Rohrbough et al., 2000; Baines, 2004; Bazigou et al., 2007; Rohrbough et al., 2007; Fradkin et al., 2008; Rushton et al., 2009; Rohrbough and Broadie, 2010). This suggests that ECM-dependent modulation of trans-synaptic signaling is important also for central synaptogenesis (Featherstone et al., 2005; Daniels et al., 2008; Okada et al., 2009). Notably, a range of central neural circuits, including the giant fiber, circadian clock and mushroom body learning/memory circuits, are available to test emerging ECM mechanisms with the power of Drosophila genetics (Huang et al., 2006; Prokop and Meinertzhagen, 2006; Tessier and Broadie, 2008; Gatto and Broadie, 2009; Tessier and Broadie, 2011).
Conclusions and perspectives
The three neurodevelopmental themes covered by this article clearly demonstrate the importance of ECM and matrix receptors, and the opportunities for merging work on the Drosophila nervous system in vivo with systematic investigations of ECM-related genes. Many examples given here demonstrate strong evolutionary conservation with mammalian ECM mechanisms, and illustrate the power of using Drosophila to unravel the conserved molecular pathways by which the ECM impacts nervous system development and functional differentiation. Certainly, there are limitations to the use of Drosophila and cases of less conservation, such as the relatively mild fak56D mutant phenotypes (Grabbe et al., 2004; Tsai et al., 2008; Ueda et al., 2008) which deviate from mammalian reports (Dansie and Ethell, 2011; Myers et al., 2011). Nevertheless, Drosophila studies benefit enormously from the cellular detail of in vivo experiments in the developing nervous system, the enormous genetic malleability of the fly, and the advantages provided by low ECM gene number in the genome. Notably, low genetic redundancy does not only concern the ECM genes and their receptors, but also the other molecular pathways they functionally interlink with. Advancing our core understanding of such crosstalk at the systems level clearly is an essential strength of Drosophila, with the required techniques, genetic tools and cellular knowledge already mostly available. Clearly, the full potential of this research avenue has not yet been realized, not least due to the fact that work on ECM in Drosophila has historically never gained the broad appreciation it deserves. Certainly, a lot more could be understood in the future through the use of this valuable little model organism.
Acknowledgments
We would like to thank Neil Dani and Emma Rushton from the Broadie Laboratory and Mark Bass and Dave Thornton from the Wellcome Trust Centre for Cell-Matrix Research in Manchester for fruitful discussions during the preparation of this manuscript. This work was supported by NIH R01 grants GM54544, MH07504 and MH084989 to K.B., by funds from the Swedish Research Council to S.B., and by the BBSRC grant BB/I002448/1 to A.P. We would like to thank the FlyBase team for their invaluable services without which the preparation of this review would have been a far more challenging task.
References
- Adams JC, Monk R, Taylor AL, Ozbek S, Fascetti N, Baumgartner S, Engel J. Characterisation of Drosophila thrombospondin defines an early origin of pentameric thrombospondins. J Mol Biol. 2003;328:479–494. doi: 10.1016/s0022-2836(03)00248-1. [DOI] [PubMed] [Google Scholar]
- Agrawal SM, Lau L, Yong VW. MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol. 2008;19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Ai XB, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol. 2003;162:341–351. doi: 10.1083/jcb.200212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin SD, Davis GW. Coordinating structural and functional synapse development: postsynaptic p21-activated kinase independently specifies glutamate receptor abundance and postsynaptic morphology. J Neurosci. 2004;24:6871–6879. doi: 10.1523/JNEUROSCI.1538-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Anderson SO. Biochemistry of insect cuticle. A Rev Ent. 1979;24:29–61. [Google Scholar]
- Aouacheria A, Cluzel C, Lethias C, Gouy M, Garrone R, Exposito JY. Invertebrate data predict an early emergence of vertebrate fibrillar collagen clades and an anti-incest model. J Biol Chem. 2004;279:47711–47719. doi: 10.1074/jbc.M408950200. [DOI] [PubMed] [Google Scholar]
- Araujo H, Negreiros E, Bier E. Integrins modulate Sog activity in the Drosophila wing. Development. 2003;130:3851–3864. doi: 10.1242/dev.00613. [DOI] [PubMed] [Google Scholar]
- Araujo SJ, Tear G. Axon guidance mechanisms and molecules: lessons from invertebrates. Nat Rev Neurosci. 2003;4:910–922. doi: 10.1038/nrn1243. [DOI] [PubMed] [Google Scholar]
- Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol Cell Biol. 2002;22:1881–1892. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashhurst DE, Bailey AJ. Locust collagen: morphological and biochemical characterization. Eur J Biochem. 1980;103:75–83. doi: 10.1111/j.1432-1033.1980.tb04290.x. [DOI] [PubMed] [Google Scholar]
- Ashhurst DE, Costin NM. The development of a collagenous connective tissue in the locust, Locusta migratoria. Tissue Cell. 1974;6:279–300. [PubMed] [Google Scholar]
- Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005;25:5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA. Synaptic strengthening mediated by bone morphogenetic protein-dependent retrograde signaling in the Drosophila CNS. J Neurosci. 2004;24:6904–6911. doi: 10.1523/JNEUROSCI.1978-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Blauth K, Peters K, Rogers SL, Fanning AS, Bhat MA. Drosophila neurexin IV interacts with Roundabout and is required for repulsive midline axon guidance. J Neurosci. 2010;30:5653–5667. doi: 10.1523/JNEUROSCI.6187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AL, Krueger S, Datta S. Branchless and Hedgehog operate in a positive feedback loop to regulate the initiation of neuroblast division in the Drosophila larval brain. Dev Biol. 2008;317:234–245. doi: 10.1016/j.ydbio.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J, Reddy RS, Saito H, Van Vactor D. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr Biol. 2001;11:1317–1327. doi: 10.1016/s0960-9822(01)00420-1. [DOI] [PubMed] [Google Scholar]
- Bayat V, Jaiswal M, Bellen HJ. The BMP signaling pathway at the Drosophila neuromuscular junction and its links to neurodegenerative diseases. Curr Opin Neurobiol. 2011;21:182–188. doi: 10.1016/j.conb.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Apitz H, Johansson J, Loren CE, Hirst EM, Chen PL, Palmer RH, Salecker I. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128:961–975. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 2010;11:514–522. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse F, Mertel S, Kittel RJ, Wichmann C, Rasse TM, Sigrist SJ, Ephrussi A. The Ig cell adhesion molecule Basigin controls compartmentalization and vesicle release at Drosophila melanogaster synapses. J Cell Biol. 2007;177:843–855. doi: 10.1083/jcb.200701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuchle D, Schwarz H, Langegger M, Koch I, Aberle H. Drosophila MICAL regulates myofilament organization and synaptic structure. Mech Dev. 2007;124:390–406. doi: 10.1016/j.mod.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Beumer K, Matthies HJ, Bradshaw A, Broadie K. Integrins regulate DLG/FAS2 via a CaM kinase II-dependent pathway to mediate synapse elaboration and stabilization during postembryonic development. Development. 2002;129:3381–3391. doi: 10.1242/dev.129.14.3381. [DOI] [PubMed] [Google Scholar]
- Beumer KJ, Rohrbough J, Prokop A, Broadie K. A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development. 1999;126:5833–5846. doi: 10.1242/dev.126.24.5833. [DOI] [PubMed] [Google Scholar]
- Billuart P, Winter CG, Maresh A, Zhao X, Luo L. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell. 2001;107:195–207. doi: 10.1016/s0092-8674(01)00522-0. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- Bogdanik L, Framery B, Frölich A, Franco B, Mornet D, Bockaert J, Sigrist SJ, Grau Y, Parmentier ML. Muscle dystroglycan organizes the postsynapse and regulates presynaptic neurotransmitter release at the Drosophila neuromuscular junction. PLoS ONE. 2008;3:e2084. doi: 10.1371/journal.pone.0002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bökel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 2002;3:311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- Bökel C, Prokop A, Brown NH. Papillote and Piopio: Drosophila ZP-domain proteins required for cell adhesion to the apical extracellular matrix and microtubule organization. J Cell Sci. 2005;118:633–642. doi: 10.1242/jcs.01619. [DOI] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Rosenfelt C, Cox H, Tully T. Fragile x mental retardation 1 and filamin a interact genetically in Drosophila long-term memory. Front Neural Circuits. 2010;3:22. doi: 10.3389/neuro.04.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot-Handford RP, Tuckwell DS. Fibrillar collagen: the key to vertebrate evolution? A tale of molecular incest. Bioessays. 2003;25:142–151. doi: 10.1002/bies.10230. [DOI] [PubMed] [Google Scholar]
- Borchiellini C, Coulon J, Le Parco Y. The function of type IV collagen during Drosophila muscle development. Mech Dev. 1996;58:179–191. doi: 10.1016/s0925-4773(96)00574-6. [DOI] [PubMed] [Google Scholar]
- Boudko SP, Engel J, Okuyama K, Mizuno K, Bachinger HP, Schumacher MA. Crystal structure of human type III collagen Gly991–Gly1032 cystine knot-containing peptide shows both 7/2 and 10/3 triple helical symmetries. J Biol Chem. 2008;283:32580–32589. doi: 10.1074/jbc.M805394200. [DOI] [PubMed] [Google Scholar]
- Broadie K, Bate M. Activity-dependent development of the neuromuscular synapse during Drosophila embryogenesis. Neuron. 1993;11:607–619. doi: 10.1016/0896-6273(93)90073-z. [DOI] [PubMed] [Google Scholar]
- Broadie K, Bate M. Innervation directs receptor synthesis and localization in Drosophila embryo synaptogenesis. Nature. 1993;361:350–353. doi: 10.1038/361350a0. [DOI] [PubMed] [Google Scholar]
- Broadie K, Sink H, Van Vactor D, Fambrough D, Whitington PM, Bate M, Goodman CS. From growth cone to synapse: the life history of the RP3 motor neuron. Dev Suppl. 1993:227–238. [PubMed] [Google Scholar]
- Broadie KS, Bate M. Development of the embryonic neuromuscular synapse of Drosophila melanogaster. J Neurosci. 1993;13:144–166. doi: 10.1523/JNEUROSCI.13-01-00144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody T, Stivers C, Nagle J, Odenwald WF. Identification of novel Drosophila neural precursor genes using a differential embryonic head cDNA screen. Mech Dev. 2002;113:41–59. doi: 10.1016/s0925-4773(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Brown NH, Gregory SL, Martin-Bermudo MD. Integrins as mediators of morphogenesis in Drosophila. Dev Biol. 2000;223:1–16. doi: 10.1006/dbio.2000.9711. [DOI] [PubMed] [Google Scholar]
- Budnik V. Synapse maturation and structural plasticity at Drosophila neuromuscular junctions. Curr Opin Neurobiol. 1996;6:858–867. doi: 10.1016/s0959-4388(96)80038-9. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Graner MW, Fessler LI, Fessler JH, Schneider KD, Kerschen A, Choy LP, Burgess BW, Brower DL. The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila. Development. 1998;125:1679–1689. doi: 10.1242/dev.125.9.1679. [DOI] [PubMed] [Google Scholar]
- Bunt S, Hooley C, Hu N, Scahill C, Weavers H, Skaer H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell MC, Datta S. Expression of cyclin E or DP/E2F rescues the G1 arrest of trol mutant neuroblasts in the Drosophila larval central nervous system. Mech Dev. 1998;79:121–130. doi: 10.1016/s0925-4773(98)00178-6. [DOI] [PubMed] [Google Scholar]
- Campbell AG, Fessler LI, Salo T, Fessler JH. Papilin: a Drosophila proteoglycan-like sulfated glycoprotein from basement membranes. J Biol Chem. 1987;262:17605–17612. [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin: Springer Verlag; 1997. p. 227. [Google Scholar]
- Chanana B, Steigemann P, Jäckle H, Vorbrüggen G. Reception of Slit requires only the chondroitin-sulphate-modified extracellular domain of Syndecan at the target cell surface. Proc Natl Acad Sci U S A. 2009;106:11984–11988. doi: 10.1073/pnas.0901148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier A, Zaffran S, Astier M, Semeriva M, Gratecos D. Pericardin, a Drosophila type IV collagen-like protein is involved in the morphogenesis and maintenance of the heart epithelium during dorsal ectoderm closure. Development. 2002;129:3241–3253. doi: 10.1242/dev.129.13.3241. [DOI] [PubMed] [Google Scholar]
- Christian JL. BMP, Wnt and Hedgehog signals: how far can they go? Current Opinion in Cell Biology. 2000;12:244–249. doi: 10.1016/s0955-0674(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Christoforou CP, Greer CE, Challoner BR, Charizanos D, Ray RP. The detached locus encodes Drosophila Dystrophin, which acts with other components of the Dystrophin Associated Protein Complex to influence intercellular signalling in developing wing veins. Dev Biol. 2008;313:519–532. doi: 10.1016/j.ydbio.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Cohn RD. Dystroglycan: important player in skeletal muscle and beyond. Neuromuscul Disord. 2005;15:207–217. doi: 10.1016/j.nmd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- Curtin KD, Meinertzhagen IA, Wyman RJ. Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture. J Cell Sci. 2005;118:2649–2660. doi: 10.1242/jcs.02408. [DOI] [PubMed] [Google Scholar]
- Dalkilic I, Kunkel LM. Muscular dystrophies: genes to pathogenesis. Curr Opin Genet Dev. 2003;13:231–238. doi: 10.1016/s0959-437x(03)00048-0. [DOI] [PubMed] [Google Scholar]
- Dani N, Broadie K. Glycosylated synaptomatrix regulation of trans-synaptic signaling. Dev Neurobiol. 2011 doi: 10.1002/dneu.20891. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Gelfand MV, Collins CA, DiAntonio A. Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J Comp Neurol. 2008;508:131–152. doi: 10.1002/cne.21670. [DOI] [PubMed] [Google Scholar]
- Dansie LE, Ethell IM. Casting a net on dendritic spines: the extracellular matrix and its receptors. Dev Neurobiol. 2011 doi: 10.1002/dneu.20963. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system. Development. 1995;121:1173–1182. doi: 10.1242/dev.121.4.1173. [DOI] [PubMed] [Google Scholar]
- Datta S. Activation of neuroblast proliferation in explant culture of the Drosophila larval CNS. Brain Res. 1999;818:77–83. doi: 10.1016/s0006-8993(98)01292-x. [DOI] [PubMed] [Google Scholar]
- Datta S, Kankel DR. l(1)trol and l(1)devl, loci affecting the development of the adult central nervous system in Drosophila melanogaster. Genetics. 1992;130:523–537. doi: 10.1093/genetics/130.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers LC, van der Plas MC, van Loenen PB, den Dunnen JT, van Ommen GJ, Fradkin LG, Noordermeer JN. Embryonic expression patterns of the Drosophila dystrophin-associated glycoprotein complex orthologs. Gene Expr Patterns. 2004;4:153–159. doi: 10.1016/j.modgep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Denef N, Chen Y, Weeks SD, Barcelo G, Schupbach T. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 2008;14:354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgärtner S, Ruohola-Baker H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–184. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai CJ, Popova E, Zinn K. A Drosophila receptor tyrosine phosphatase expressed in the embryonic CNS and larval optic lobes is a member of the set of proteins bearing the “HRP” carbohydrate epitope. J Neurosci. 1994;14:7272–7283. doi: 10.1523/JNEUROSCI.14-12-07272.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Brown NH. Morphogenesis in the absence of integrins: mutation of both Drosophila {beta} subunits prevents midgut migration. Development. 2004;131:5405–5415. doi: 10.1242/dev.01427. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. The extracellular matrix and synapses. Cell Tissue Res. 2006;326:647–654. doi: 10.1007/s00441-006-0217-1. [DOI] [PubMed] [Google Scholar]
- Donady JJ, Seecof RL. Effect of the gene lethal (1) myospheroid on Drosophila embryonic cells in vitro. In Vitro. 1972;8:7–12. doi: 10.1007/BF02617937. [DOI] [PubMed] [Google Scholar]
- Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- Ebens AJ, Garren H, Cheyette BN, Zipursky SL. The Drosophila anachronism locus: a glycoprotein secreted by glia inhibits neuroblast proliferation. Cell. 1993;74:15–27. doi: 10.1016/0092-8674(93)90291-w. [DOI] [PubMed] [Google Scholar]
- Egles C, Claudepierre T, Manglapus MK, Champliaud MF, Brunken WJ, Hunter DD. Laminins containing the beta2 chain modulate the precise organization of CNS synapses. Mol Cell Neurosci. 2007;34:288–298. doi: 10.1016/j.mcn.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Engel AG, Shen XM, Selcen D, Sine SM. Further observations in congenital myasthenic syndromes. Ann N Y Acad Sci. 2008;1132:104–113. doi: 10.1196/annals.1405.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escuin S, Georges-Labouesse E. Adhesion-induced intracellular mechanisms of neurite elongation. In: de Curtis I, editor. Intracellular mechanisms for neuritogenesis. Springer; US: 2007. pp. 1–24. [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Fabini G, Wilson IBH. Glycosyltransferases in Drosophila melanogaster. Dros Inf Serv. 2001;84:122–129. [Google Scholar]
- Featherstone DE, Rushton E, Rohrbough J, Liebl F, Karr J, Sheng Q, Rodesch CK, Broadie K. An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J Neurosci. 2005;25:3199–3208. doi: 10.1523/JNEUROSCI.4201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler JH, Kramerova I, Kramerov A, Chen Y, Fessler LI. Papilin, a novel component of basement membranes, in relation to ADAMTS metalloproteases and ECM development. Int J Biochem Cell Biol. 2004;36:1079–1084. doi: 10.1016/j.biocel.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Fessler JH, Nelson RE, Fessler LI. Preparation of extracellular matrix. Methods Cell Biol. 1994;44:303–328. doi: 10.1016/s0091-679x(08)60921-8. [DOI] [PubMed] [Google Scholar]
- Fessler LI, Nelson RE, Fessler JH. Drosophila extracellular matrix. Methods Enzymol. 1994;245:271–294. doi: 10.1016/0076-6879(94)45016-1. [DOI] [PubMed] [Google Scholar]
- Fogerty FJ, Fessler LI, Bunch TA, Yaron Y, Parker CG, Nelson RE, Brower DL, Gullberg D, Fessler JH. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development. 1994;120:1747–1758. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. J Cell Biol. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AN, Zinn K. The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol. 2005;15:1701–1711. doi: 10.1016/j.cub.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Fradkin LG, Baines RA, van der Plas MC, Noordermeer JN. The dystrophin Dp186 isoform regulates neurotransmitter release at a central synapse in Drosophila. J Neurosci. 2008;28:5105–5114. doi: 10.1523/JNEUROSCI.4950-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraichard S, Bouge AL, Chauvel I, Bouhin H. Tenectin, a novel extracellular matrix protein expressed during Drosophila melanogaster embryonic development. Gene Expr Patterns. 2006;6:772–776. doi: 10.1016/j.modgep.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Franciscovich AL, Mortimer AD, Freeman AA, Gu J, Sanyal S. Overexpression screen in Drosophila identifies neuronal roles of GSK-3 beta/shaggy as a regulator of AP-1-dependent developmental plasticity. Genetics. 2008;180:2057–2071. doi: 10.1534/genetics.107.085555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- Friedrich MV, Schneider M, Timpl R, Baumgartner S. Perlecan domain V of Drosophila melanogaster. Sequence, recombinant analysis and tissue expression. Eur J Biochem. 2000;267:3149–3159. doi: 10.1046/j.1432-1327.2000.01337.x. [DOI] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Logan MA, Ashley J, Ataman B, Budnik V, Freeman MR. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009;7:e1000184. doi: 10.1371/journal.pbio.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer MP, Vasenkova I, Kamiyama D, Rosado Y, Chiba A. Slit and Robo control the development of dendrites in Drosophila CNS. Development. 2007;134:3795–3804. doi: 10.1242/dev.02882. [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso L, Fetter RD, Goodman CS. Genetic analysis of Laminin A in Drosophila: extracellular matrix containing laminin A is required for ocellar axon pathfinding. Development. 1996;122:2611–2621. doi: 10.1242/dev.122.9.2611. [DOI] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. Temporal requirements of the fragile x mental retardation protein in modulating circadian clock circuit synaptic architecture. Front Neural Circuits. 2009;3:8. doi: 10.3389/neuro.04.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Giacomotto J, Segalat L. High-throughput screening and small animal models, where are we? Br J Pharmacol. 2010;160:204–216. doi: 10.1111/j.1476-5381.2010.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotwals PJ, Fessler LI, Wehrli M, Hynes RO. Drosophila PS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc Natl Acad Sci U S A. 1994;91:11447–11451. doi: 10.1073/pnas.91.24.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe C, Zervas CG, Hunter T, Brown NH, Palmer RH. Focal adhesion kinase is not required for integrin function or viability in Drosophila. Development. 2004 doi: 10.1242/dev.01462. [DOI] [PubMed] [Google Scholar]
- Graner MW, Bunch TA, Baumgartner S, Kerschen A, Brower DL. Splice variants of the Drosophila PS2 integrins differentially interact with RGD-containing fragments of the extracellular proteins tiggrin, ten-m, and D-laminin 2. J Biol Chem. 1998;273:18235–18241. doi: 10.1074/jbc.273.29.18235. [DOI] [PubMed] [Google Scholar]
- Gratecos D, Naidet C, Astier M, Thiery JP, Semeriva M. Drosophila fibronectin: a protein that shares properties similar to those of its mammalian homologue. EMBO J. 1988;7:215–223. doi: 10.1002/j.1460-2075.1988.tb02802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines N, Seabrooke S, Stewart BA. Dystroglycan and protein O-mannosyltransferases 1 and 2 are required to maintain integrity of Drosophila larval muscles. Mol Biol Cell. 2007;18:4721–4730. doi: 10.1091/mbc.E07-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Wang B, Lin X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development. 2004;131:601–611. doi: 10.1242/dev.00958. [DOI] [PubMed] [Google Scholar]
- Hartenstein V. Development of Drosophila larval sensory organs: spatiotemporal pattern of sensory neurones, peripheral axonal pathways and sensilla differentiation. Development. 1988;102:869–886. [Google Scholar]
- Henchcliffe C, Garcia-Alonso L, Tang J, Goodman CS. Genetic analysis of laminin A reveals diverse functions during morphogenesis in Drosophila. Development. 1993;118:325–337. doi: 10.1242/dev.118.2.325. [DOI] [PubMed] [Google Scholar]
- Hoang B, Chiba A. Genetic analysis on the role of integrin during axon guidance in Drosophila. J Neurosci. 1998;18:7847–7855. doi: 10.1523/JNEUROSCI.18-19-07847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Funkner A, Neumann P, Juhnke S, Walther M, Schierhorn A, Weininger U, Balbach J, Reuter G, Stubbs MT. Biophysical characterization of refolded Drosophila Spätzle, a cystine knot protein, reveals distinct properties of three isoforms. J Biol Chem. 2008;283:32598–32609. doi: 10.1074/jbc.M801815200. [DOI] [PubMed] [Google Scholar]
- Hofmeyer K, Treisman JE. The receptor protein tyrosine phosphatase LAR promotes R7 photoreceptor axon targeting by a phosphatase-independent signaling mechanism. Proc Natl Acad Sci U S A. 2009;106:19399–19404. doi: 10.1073/pnas.0903961106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M, Olson A, Fishman S, Soneral SN, Marikar Y, Dong R, Jacobs JR. The expression of MDP-1, a component of Drosophila embryonic basement membranes, is modulated by apoptotic cell death. Int J Dev Biol. 1998;42:33–42. [PubMed] [Google Scholar]
- Hoshino M, Matsuzaki F, Nabeshima Y, Hama C. hikaru genki, a CNS-specific gene identified by abnormal locomotion in Drosophila, encodes a novel type of protein. Neuron. 1993;10:395–407. doi: 10.1016/0896-6273(93)90329-p. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Suzuki E, Miyake T, Sone M, Komatsu A, Nabeshima Y, Hama C. Neural expression of hikaru genki protein during embryonic and larval development of Drosophila melanogaster. Dev Genes Evol. 1999;209:1–9. doi: 10.1007/s004270050221. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Suzuki E, Nabeshima Y-i, Hama C. Hikaru genki protein is secreted into synaptic clefts from an early stage of synapse formation in Drosophila. Development. 1996;122:589–597. doi: 10.1242/dev.122.2.589. [DOI] [PubMed] [Google Scholar]
- Hu H. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci. 2001;4:695–701. doi: 10.1038/89482. [DOI] [PubMed] [Google Scholar]
- Huang FD, Woodruff E, Mohrmann R, Broadie K. Rolling blackout is required for synaptic vesicle exocytosis. J Neurosci. 2006;26:2369–2379. doi: 10.1523/JNEUROSCI.3770-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Yazdani U, Thompson-Peer KL, Kolodkin AL, Terman JR. Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development. 2007;134:2337–2347. doi: 10.1242/dev.004242. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Zhao Q. The evolution of cell adhesion. J Cell Biol. 2000;150:F89–F96. doi: 10.1083/jcb.150.2.f89. [DOI] [PubMed] [Google Scholar]
- Ichimiya T, Manya H, Ohmae Y, Yoshida H, Takahashi K, Ueda R, Endo T, Nishihara S. The twisted abdomen phenotype of Drosophila POMT1 and POMT2 mutants coincides with their heterophilic protein O-mannosyltransferase activity. J Biol Chem. 2004;279:42638–42647. doi: 10.1074/jbc.M404900200. [DOI] [PubMed] [Google Scholar]
- Inaki M, Yoshikawa S, Thomas JB, Aburatani H, Nose A. Wnt4 is a local repulsive cue that determines synaptic target specificity. Curr Biol. 2007;17:1574–1579. doi: 10.1016/j.cub.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Inatani M, Irie F, Plump AS, Tessier-Lavigne M, Yamaguchi Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302:1044–1046. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol. 1992;149:134–148. doi: 10.1016/0012-1606(92)90270-q. [DOI] [PubMed] [Google Scholar]
- Ito K, Urban J, Technau GM. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux’s Arch Dev Biol. 1995;204:284–307. doi: 10.1007/BF02179499. [DOI] [PubMed] [Google Scholar]
- Jan Y-N, Jan L-L. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and grasshopper embryos. Proc Natl Acad Sci USA. 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinska A, Ribeiro C, Affolter M. Epithelial tube morphogenesis during Drosophila tracheal development requires Piopio, a luminal ZP protein. Nat Cell Biol. 2003;5:895–901. doi: 10.1038/ncb1049. [DOI] [PubMed] [Google Scholar]
- Jiao XY, Billings PC, O’Connell MP, Kaplan FS, Shore EM, Glaser DL. Heparan sulfate proteoglycans (HSPGs) modulate BMP2 osteogenic bioactivity in C2C12 cells. Journal of Biological Chemistry. 2007;282:1080–1086. doi: 10.1074/jbc.M513414200. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, Flanagan JG, Van Vactor D. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Zou B, Xu X, Ordway RW. Active zone localization of presynaptic calcium channels encoded by the cacophony locus of Drosophila. J Neurosci. 2004;24:282–285. doi: 10.1523/JNEUROSCI.3553-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaznowski CE, Schneiderman HA, Bryant PJ. Cuticle secretion during larval growth in Drosophila melanogaster. J Insect Physiol. 1985;31:801–813. [Google Scholar]
- Kimura RH, Levin AM, Cochran FV, Cochran JR. Engineered cystine knot peptides that bind alphavbeta3, alphavbeta5, and alpha5beta1 integrins with low-nanomolar affinity. Proteins. 2009;77:359–369. doi: 10.1002/prot.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryushko D, Berezin V, Bock E. Regulators of neurite outgrowth: role of cell adhesion molecules. Ann N Y Acad Sci. 2004;1014:140–154. doi: 10.1196/annals.1294.015. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, Hell SW, Buchner E, Heckmann M, Sigrist SJ. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci. 2004;5:195–208. doi: 10.1038/nrn1349. [DOI] [PubMed] [Google Scholar]
- Kleinschmit A, et al. Drosophila heparan sulfate 6-O endosulfatase regualtes Wingless morphogen gradient formation. Dev Biol. 2010:345. doi: 10.1016/j.ydbio.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerova IA, Kramerov AA, Fessler JH. Alternative splicing of papilin and the diversity of Drosophila extracellular matrix during embryonic morphogenesis. Dev Dyn. 2003;226:634–642. doi: 10.1002/dvdy.10265. [DOI] [PubMed] [Google Scholar]
- Kucherenko MM, Marrone AK, Rishko VM, Magliarelli Hde F, Shcherbata HR. Stress and muscular dystrophy: A genetic screen for Dystroglycan and Dystrophin interactors in Drosophila identifies cellular stress response components. Dev Biol. 2011;352:228–242. doi: 10.1016/j.ydbio.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Kucherenko MM, Pantoja M, Yatsenko AS, Shcherbata HR, Fischer KA, Maksymiv DV, Chernyk YI, Ruohola-Baker H. Genetic modifier screens reveal new components that interact with the Drosophila dystroglycan-dystrophin complex. PLoS One. 2008;3:e2418. doi: 10.1371/journal.pone.0002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kul’chitskii SV, Yakubovich NV, Emel’yanova AA, Garkun YS, Pashkevich SG, Kul’chitskii VA. Changes in neuropil ultrastructure in hippocampal field CA1 in rat pups after application of hyaluronidase. Neurosci Behav Physiol. 2009;39:517–521. doi: 10.1007/s11055-009-9162-2. [DOI] [PubMed] [Google Scholar]
- Kuo W, Digman M, Lander A. Heparan Sulfate acts as a BMP co-receptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell. 2010;21:4028–4041. doi: 10.1091/mbc.E10-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci. 2009;12:1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusche-Gullberg M, Garrison K, MacKrell AJ, Fessler LI, Fessler JH. Laminin A chain: expression during Drosophila development and genomic sequence. Embo J. 1992;11:4519–4527. doi: 10.1002/j.1460-2075.1992.tb05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok J, Fawcett J. XXXSpecial-Issue: regeneration. Dev Neurobiol. 2011 in press. [Google Scholar]
- Landgraf M, Thor S. Development of Drosophila motoneurons: specification and morphology. Semin Cell Dev Biol. 2006;17:3–11. doi: 10.1016/j.semcdb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Lee JS, von der Hardt S, Rusch MA, Stringer SE, Stickney HL, Talbot WS, Geisler R, Nusslein-Volhard C, Selleck SB, Chien CB, Roehl H. Axon sorting in the optic tract requires HSPG synthesis by ext2 (dackel) and extl3 (boxer) Neuron. 2004;44:947–960. doi: 10.1016/j.neuron.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Liang Y, Annan RS, Carr SA, Popp S, Mevissen M, Margolis RK, Margolis RU. Mammalian homologues of the Drosophila slit protein are ligands of the heparan sulfate proteoglycan glypican-1 in brain. J Biol Chem. 1999;274:17885–17892. doi: 10.1074/jbc.274.25.17885. [DOI] [PubMed] [Google Scholar]
- Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Hillman PR, Barrett AL, Jackson MC, Perry TL, Park Y, Datta S. The Drosophila Perlecan gene trol regulates multiple signaling pathways in different developmental contexts. BMC Dev Biol. 2007;7:121. doi: 10.1186/1471-213X-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluri G, Langlois GD, McClellan B, Soloway PD, Jaworski DM. Tissue inhibitor of metalloproteinase-2 (TIMP-2) regulates neuromuscular junction development via a beta1 integrin-mediated mechanism. J Neurobiol. 2006;66:1365–1377. doi: 10.1002/neu.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AA, Kim E, Leung HT, Woodruff E, 3rd, An L, Doerge RW, Pak WL, Broadie K. Presynaptic calcium channel localization and calcium-dependent synaptic vesicle exocytosis regulated by the Fuseless protein. J Neurosci. 2008;28:3668–3682. doi: 10.1523/JNEUROSCI.5553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig S, Batz T, Armbruster K, Krasnow MA. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr Biol. 2006;16:186–194. doi: 10.1016/j.cub.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Ly CV, Yao CK, Verstreken P, Ohyama T, Bellen HJ. straightjacket is required for the synaptic stabilization of cacophony, a voltage-gated calcium channel alpha1 subunit. J Cell Biol. 2008;181:157–170. doi: 10.1083/jcb.200712152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo MD, Brown NH. The localized assembly of extracellular matrix integrin ligands requires cell-cell contact. J Cell Sci. 2000;113(Pt 21):3715–3723. doi: 10.1242/jcs.113.21.3715. [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo MD, Dunin-Borkowski OM, Brown NH. Specificity of PS integrin function during embryogenesis resides in the alpha subunit extracellular domain. Embo J. 1997;16:4184–4193. doi: 10.1093/emboj/16.14.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Zusman S, Li X, Williams EL, Khare N, DaRocha S, Chiquet-Ehrismann R, Baumgartner S. wing blister, a new Drosophila laminin alpha chain required for cell adhesion and migration during embryonic and imaginal development. J Cell Biol. 1999;145:191–201. doi: 10.1083/jcb.145.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PT. Glycobiology of the neuromuscular junction. J Neurocytol. 2003;32:915–929. doi: 10.1023/B:NEUR.0000020632.41508.83. [DOI] [PubMed] [Google Scholar]
- Martinek N, Shahab J, Saathoff M, Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J Cell Sci. 2008;121:1671–1680. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- Martinek N, Zou R, Berg M, Sodek J, Ringuette M. Evolutionary conservation and association of SPARC with the basal lamina in Drosophila. Dev Genes Evol. 2002;212:124–133. doi: 10.1007/s00427-002-0220-9. [DOI] [PubMed] [Google Scholar]
- Mathew D, Gramates LS, Packard M, Thomas U, Bilder D, Perrimon N, Gorczyca M, Budnik V. Recruitment of scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr Biol. 2002;12:531–539. doi: 10.1016/s0960-9822(02)00758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Moussian B. Drosophila multiplexin (Dmp) modulates motor axon pathfinding accuracy. Dev Growth Differ. 2009;51:483–498. doi: 10.1111/j.1440-169X.2009.01111.x. [DOI] [PubMed] [Google Scholar]
- Michele DE, Campbell KP. Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem. 2003;278:15457–15460. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- Miller CM, Liu N, Page-McCaw A, Broihier HT. Drosophila mmp2 regulates the matrix molecule faulty attraction (frac) to promote motor axon targeting in Drosophila. J Neurosci. 2011;31:5335–5347. doi: 10.1523/JNEUROSCI.4811-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CM, Page-McCaw A, Broihier HT. Matrix metalloproteinases promote motor axon fasciculation in the Drosophila embryo. Development. 2008;135:95–109. doi: 10.1242/dev.011072. [DOI] [PubMed] [Google Scholar]
- Mirouse V, Christoforou CP, Fritsch C, St Johnston D, Ray RP. Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Dev Cell. 2009;16:83–92. doi: 10.1016/j.devcel.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mirre C, Cecchini JP, Le Parco Y, Knibiehler B. De novo expression of a type IV collagen gene in Drosophila embryos is restricted to mesodermal derivatives and occurs at germ band shortening. Development. 1988;102:369–376. doi: 10.1242/dev.102.2.369. [DOI] [PubMed] [Google Scholar]
- Mirre C, Le Parco Y, Knibiehler B. Collagen IV is present in the developing CNS during Drosophila neurogenesis. J Neurosci Res. 1992;31:146–155. doi: 10.1002/jnr.490310120. [DOI] [PubMed] [Google Scholar]
- Momota R, Naito I, Ninomiya Y, Ohtsuka A. Drosophila type XV/XVIII collagen, Mp, is involved in Wingless distribution. Matrix Biol. 2011 doi: 10.1016/j.matbio.2011.03.008. in press. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Goodman CS. Drosophila laminin: sequence of B2 subunit and expression of all three subunits during embryogenesis. J Cell Biol. 1989;109:2441–2453. doi: 10.1083/jcb.109.5.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin P, Sagne C, Gasnier B. Functional characterization of wild-type and mutant human sialin. EMBO J. 2004;23:4560–4570. doi: 10.1038/sj.emboj.7600464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudher A, Newman TA, Mudher A, Newman TAs. Drosophila: a toolbox for the study of neurodegenerative disease. New York: Taylor & Francis; 2007. [Google Scholar]
- Murakami S, Umetsu D, Maeyama Y, Sato M, Yoshida S, Tabata T. Focal adhesion kinase controls morphogenesis of the Drosophila optic stalk. Development. 2007;134:1539–1548. doi: 10.1242/dev.001529. [DOI] [PubMed] [Google Scholar]
- Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J, Santiago-Medina M, Gomez TM. ECM and matrix receptors in axonal growth. Dev Neurobiol. 2011 doi: 10.1002/dneu.20931. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Narita R, Yamashita H, Goto A, Imai H, Ichihara S, Mori H, Kitagawa Y. Syndecan-dependent binding of Drosophila hemocytes to laminin alpha3/5 chain LG4-5 modules: potential role in sessile hemocyte islets formation. FEBS Lett. 2004;576:127–132. doi: 10.1016/j.febslet.2004.08.073. [DOI] [PubMed] [Google Scholar]
- Natzle JE, Monson JM, McCarthy BJ. Cytogenetic location and expression of collagen-like genes in Drosophila. Nature. 1982;296:368–371. doi: 10.1038/296368a0. [DOI] [PubMed] [Google Scholar]
- Negreiros E, Fontenele M, Camara AR, Araujo H. alphaPS1betaPS integrin receptors regulate the differential distribution of Sog fragments in polarized epithelia. Genesis. 2010;48:31–43. doi: 10.1002/dvg.20579. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Nicholson AC, Malik SB, Logsdon JM, Jr, Van Meir EG. Functional evolution of ADAMTS genes: evidence from analyses of phylogeny and gene organization. BMC Evol Biol. 2005;5:11. doi: 10.1186/1471-2148-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada R, Awasaki T, Ito K. Gamma-aminobutyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J Comp Neurol. 2009;514:74–91. doi: 10.1002/cne.21971. [DOI] [PubMed] [Google Scholar]
- Olofsson B, Page DT. Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol. 2005;279:233–243. doi: 10.1016/j.ydbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Olson PF, Fessler LI, Nelson RE, Sterne RE, Campbell AG, Fessler JH. Glutactin, a novel Drosophila basement membrane-related glycoprotein with sequence similarity to serine esterases. Embo J. 1990;9:1219–1227. doi: 10.1002/j.1460-2075.1990.tb08229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdinler PH, Erzurumlu RS. Slit2, a branching-arborization factor for sensory axons in the mammalian CNS. J Neurosci. 2002;22:4540–4549. doi: 10.1523/JNEUROSCI.22-11-04540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Mathew D, Budnik V. Wnts and TGF beta in synaptogenesis: old friends signalling at new places. Nat Rev Neurosci. 2003;4:113–120. doi: 10.1038/nrn1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A. Remodeling the model organism: matrix metalloproteinase functions in invertebrates. Semin Cell Dev Biol. 2008;19:14–23. doi: 10.1016/j.semcdb.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420:345–361. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Fujioka M, Kobayashi M, Jaynes JB, Datta S. even skipped is required to produce a trans-acting signal for larval neuroblast proliferation that can be mimicked by ecdysone. Development. 2001;128:1899–1909. doi: 10.1242/dev.128.10.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Ng C, Datta S. Induction of string rescues the neuroblast proliferation defect in trol mutant animals. Genesis. 2003;36:187–195. doi: 10.1002/gene.10216. [DOI] [PubMed] [Google Scholar]
- Park Y, Rangel C, Reynolds MM, Caldwell MC, Johns M, Nayak M, Welsh CJ, McDermott S, Datta S. Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev Biol. 2003;253:247–257. doi: 10.1016/s0012-1606(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Parnas D, Haghighi AP, Fetter RD, Kim SW, Goodman CS. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron. 2001;32:415–424. doi: 10.1016/s0896-6273(01)00485-8. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payre F. Genetic control of epidermis differentiation in Drosophila. Int J Dev Biol. 2004;48:207–215. [PubMed] [Google Scholar]
- Prokop A. Integrating bits and pieces - synapse formation in Drosophila embryos. Cell Tissue Res. 1999;297:169–186. doi: 10.1007/s004410051345. [DOI] [PubMed] [Google Scholar]
- Prokop A. Organisation of the efferent system and structure of neuromuscular junctions in Drosophila The fly neuromuscular junction: structure and function. In: Budnik V, Ruiz-Cañada C, Budnik V, Ruiz-Cañada Cs, editors. Int Rev Neurobiol. San Diego: Elsevier Academic Press; 2006. pp. 71–91. [Google Scholar]
- Prokop A, Martín-Bermudo MD, Bate M, Brown N. Absence of PS integrins or laminin A affects extracellular adhesion, but not intracellular assembly, of hemiadherens and neuromuscular junctions in Drosophila embryos. Dev Biol. 1998;196:58–76. doi: 10.1006/dbio.1997.8830. [DOI] [PubMed] [Google Scholar]
- Prokop A, Meinertzhagen IA. Development and structure of synaptic contacts in Drosophila. Semin Cell Dev Biol. 2006;17:20–30. doi: 10.1016/j.semcdb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Prokop A, Technau GM. The origin of postembryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Development. 1991;111:79–88. doi: 10.1242/dev.111.1.79. [DOI] [PubMed] [Google Scholar]
- Prokop A, Technau GM. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev Biol. 1994;161:321–337. doi: 10.1006/dbio.1994.1034. [DOI] [PubMed] [Google Scholar]
- Rawson JM, Dimitroff B, Johnson KG, Ge X, Van Vactor D, Selleck SB. The heparan sulfate proteoglycans dally-like and syndecan have distinct functions in axon guidance and visual-system assembly in Drosophila. Curr Biol. 2005;15:833–838. doi: 10.1016/j.cub.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Reichardt L, Prokop A. The role of extracellular matrix in nervous system development and maintenance. Dev Neurobiol. 2011 doi: 10.1002/dneu.20975. in press. [DOI] [PubMed] [Google Scholar]
- Ren Y, Kirkpatrick CA, Rawson JM, Sun M, Selleck SB. Cell type-specific requirements for heparan sulfate biosynthesis at the Drosophila neuromuscular junction: effects on synapse function, membrane trafficking, and mitochondrial localization. J Neurosci. 2009;29:8539–8550. doi: 10.1523/JNEUROSCI.5587-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repnikova E, Koles K, Nakamura M, Pitts J, Li H, Ambavane A, Zoran MJ, Panin VM. Sialyltransferase regulates nervous system function in Drosophila. J Neurosci. 2010;30:6466–6476. doi: 10.1523/JNEUROSCI.5253-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K. Anterograde jelly belly ligand to Alk receptor signaling at developing synapses is regulated by mind the gap. Development. 2010;137:3523–3533. doi: 10.1242/dev.047878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000;20:6868–6878. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Rushton E, Woodruff E, 3rd, Fergestad T, Vigneswaran K, Broadie K. Presynaptic establishment of the synaptic cleft extracellular matrix is required for post-synaptic differentiation. Genes Dev. 2007;21:2607–2628. doi: 10.1101/gad.1574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronca F, Andersen JS, Paech V, Margolis RU. Characterization of Slit protein interactions with glypican-1. J Biol Chem. 2001;276:29141–29147. doi: 10.1074/jbc.M100240200. [DOI] [PubMed] [Google Scholar]
- Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci. 2006;119:2185–2195. doi: 10.1242/jcs.02952. [DOI] [PubMed] [Google Scholar]
- Roth J, Kempf A, Reuter G, Schauer R, Gehring WJ. Occurrence of sialic acids in Drosophila melanogaster. Science. 1992;256:673–675. doi: 10.1126/science.1585182. [DOI] [PubMed] [Google Scholar]
- Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruivo R, Sharifi A, Boubekeur S, Morin P, Anne C, Debacker C, Graziano JC, Sagne C, Gasnier B. Molecular pathogenesis of sialic acid storage diseases: insight gained from four missense mutations and a putative polymorphism of human sialin. Biol Cell. 2008;100:551–559. doi: 10.1042/BC20070166. [DOI] [PubMed] [Google Scholar]
- Rushton E, Rohrbough J, Broadie K. Presynaptic secretion of mind-the-gap organizes the synaptic extracellular matrix-integrin interface and postsynaptic environments. Dev Dyn. 2009;238:554–571. doi: 10.1002/dvdy.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- Sánchez-Soriano N, Gonçalves-Pimentel C, Beaven R, Haessler U, Ofner L, Ballestrem C, Prokop A. Drosophila growth cones: a genetically tractable platform for the analysis of axonal growth dynamics. Dev Neurobiol. 2010;70:58–71. doi: 10.1002/dneu.20762. [DOI] [PubMed] [Google Scholar]
- Sánchez-Soriano N, Löhr R, Bottenberg W, Haessler U, Kerassoviti A, Knust E, Fiala A, Prokop A. Are dendrites in Drosophila homologous to vertebrate dendrites? Dev Biol. 2005;288:126–138. doi: 10.1016/j.ydbio.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Sánchez-Soriano N, Prokop A. The influence of pioneer neurons on a growing motor nerve in Drosophila requires the neural cell adhesion molecule homolog FasciclinII. J Neurosci. 2005;25:78–87. doi: 10.1523/JNEUROSCI.2377-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Soriano N, Tear G, Whitington P, Prokop A. Drosophila as a genetic and cellular model for studies on axonal growth. Neural Develop. 2007;2:9. doi: 10.1186/1749-8104-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Soriano N, Travis M, Dajas-Bailador F, Goncalves-Pimentel C, Whitmarsh AJ, Prokop A. Mouse ACF7 and Drosophila Short stop modulate filopodia formation and microtubule organisation during neuronal growth. J Cell Sci. 2009;122:2534–2542. doi: 10.1242/jcs.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Sandstrom DJ, Hoeffer CA, Ramaswami M. AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature. 2002;416:870–874. doi: 10.1038/416870a. [DOI] [PubMed] [Google Scholar]
- Sarkar M, Leventis PA, Silvescu CI, Reinhold VN, Schachter H, Boulianne GL. Null mutations in Drosophila N-acetylglucosaminyltransferase I produce defects in locomotion and a reduced life span. J Biol Chem. 2006;281:12776–12785. doi: 10.1074/jbc.M512769200. [DOI] [PubMed] [Google Scholar]
- Schneider M, Baumgartner S. Differential expression of Dystroglycan-spliceforms with and without the mucin-like domain during Drosophila embryogenesis. Fly (Austin) 2008:2. doi: 10.4161/fly.5726. [DOI] [PubMed] [Google Scholar]
- Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock F, Perrimon N. Retraction of the Drosophila germ band requires cell-matrix interaction. Genes Dev. 2003;17:597–602. doi: 10.1101/gad.1068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears HC, Kennedy CJ, Garrity PA. Macrophage-mediated corpse engulfment is required for normal Drosophila CNS morphogenesis. Development. 2003;130:3557–3565. doi: 10.1242/dev.00586. [DOI] [PubMed] [Google Scholar]
- Sen A, Yokokura T, Kankel MW, Dimlich DN, Manent J, Sanyal S, Artavanis-Tsakonas S. Modeling spinal muscular atrophy in Drosophila links Smn to FGF signaling. J Cell Biol. 2011;192:481–495. doi: 10.1083/jcb.201004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26:1813–1822. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal N, Martin P. Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev Neurobiol. 2011 doi: 10.1002/dneu.20953. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart AD, Course MM, Rawson J, Selleck S, Van Vactor D, Johnson KG. Heparan sulfate proteoglycan specificity during axon pathway formation in the Drosophila embryo. Dev Neurobiol. 2011 doi: 10.1002/dneu.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M, Suzuki E, Hoshino M, Hou D, Kuromi H, Fukata M, Kuroda S, Kaibuchi K, Nabeshima Y, Hama C. Synaptic development is controlled in the periactive zones of Drosophila synapses. Development. 2000;127:4157–4168. doi: 10.1242/dev.127.19.4157. [DOI] [PubMed] [Google Scholar]
- Song JK, Giniger E, Desai CJ. The receptor protein tyrosine phosphatase PTP69D antagonizes Abl tyrosine kinase to guide axons in Drosophila. Mech Dev. 2008;125:247–256. doi: 10.1016/j.mod.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Nunes R, Cheng LY, Gould AP. Regulating neural proliferation in the Drosophila CNS. Curr Opin Neurobiol. 2010;20:50–57. doi: 10.1016/j.conb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Spring J, Paine-Saunders SE, Hynes RO, Bernfield M. Drosophila syndecan: conservation of a cell-surface heparan sulfate proteoglycan. Proc Natl Acad Sci U S A. 1994;91:3334–3338. doi: 10.1073/pnas.91.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D. The art and design of genetic screens: Drosophila melanogaster. Nat Rev Genet. 2002;3:176–188. doi: 10.1038/nrg751. [DOI] [PubMed] [Google Scholar]
- Steigemann P, Molitor A, Fellert S, Jäckle H, Vorbrüggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Stevens A, Jacobs JR. Integrins regulate responsiveness to Slit repellent signals. J Neurosci. 2002;22:4448–4455. doi: 10.1523/JNEUROSCI.22-11-04448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Grinnell AD, Kidokoro Y. Hypertonicity-induced transmitter release at Drosophila neuromuscular junctions is partly mediated by integrins and cAMP/protein kinase A. J Physiol. 2002;538:103–119. doi: 10.1113/jphysiol.2001.012901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Yokoyama F, Nomizu M. Functional sites in the laminin alpha chains. Connect Tissue Res. 2005;46:142–152. doi: 10.1080/03008200591008527. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nomizu M, Gullberg D, MacKrell AJ, Keene DR, Yamada Y, Fessler JH. Conserved neuron promoting activity in Drosophila and vertebrate laminin alpha1. J Biol Chem. 1996;271:18074–18081. doi: 10.1074/jbc.271.30.18074. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Ui-Tei K, Hirohashi S. Adhesion-dependent tyrosine phosphorylation of Enabled in Drosophila neuronal cell line. Biochem Biophys Res Commun. 2000;270:482–487. doi: 10.1006/bbrc.2000.2458. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Ui-Teib K, Miyakec T, Hirohashia S. Laminin-dependent integrin clustering with tyrosinephosphorylated molecules in a Drosophila neuronal cell line. Neurosci Letters. 1998;244:149–152. doi: 10.1016/s0304-3940(98)00145-1. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler TD, Robichaux MB, Garrity PA. Compartmentalization of visual centers in the Drosophila brain requires Slit and Robo proteins. Development. 2004 doi: 10.1242/dev.01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ME, Drickamer K. Introduction to glycobiology. Oxford University Press; 2011. p. 283. [Google Scholar]
- Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–1557. doi: 10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. Activity-dependent modulation of neural circuit synaptic connectivity. Front Mol Neurosci. 2009;2:8. doi: 10.3389/neuro.02.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. The fragile X mental retardation protein developmentally regulates the strength and fidelity of calcium signaling in Drosophila mushroom body neurons. Neurobiol Dis. 2011;41:147–159. doi: 10.1016/j.nbd.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U, Ebitsch S, Gorczyca M, Koh YH, Hough CD, Woods D, Gundelfinger ED, Budnik V. Synaptic targeting and localization of discs-large is a stepwise process controlled by different domains of the protein. Curr Biol. 2000;10:1108–1117. doi: 10.1016/s0960-9822(00)00696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman JW, Talbot WS, Fahrbach SE, Hogness DS. Ecdysone receptor expression in the CNS correlates with stage-specific responses to ecdysteroids during Drosophila and Manduca development. Development. 1994;120:219–234. doi: 10.1242/dev.120.1.219. [DOI] [PubMed] [Google Scholar]
- Tsai PI, Kao HH, Grabbe C, Lee YT, Ghose A, Lai TT, Peng KP, Van Vactor D, Palmer RH, Chen RH, Yeh SR, Chien CT. Fak56 functions downstream of integrin alphaPS3betanu and suppresses MAPK activation in neuromuscular junction growth. Neural Dev. 2008;3:26. doi: 10.1186/1749-8104-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Grabbe C, Lee J, Lee J, Palmer RH, Wu CF. Mutation of Drosophila focal adhesion kinase induces bang-sensitive behavior and disrupts glial function, axonal conduction and synaptic transmission. Europ J Neurosci. 2008;27:2860–2870. doi: 10.1111/j.1460-9568.2008.06252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya T, Takeichi M, Nose A. M-spondin, a novel ECM protein highly homologous to vertebrate F-spondin, is localized at the muscle attachment sites in the Drosophila embryo. Dev Biol. 1997;186:165–176. doi: 10.1006/dbio.1997.8591. [DOI] [PubMed] [Google Scholar]
- Urbano JM, Torgler CN, Molnar C, Tepass U, Lopez-Varea A, Brown NH, de Celis JF, Martin-Bermudo MD. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development. 2009;136:4165–4176. doi: 10.1242/dev.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas MC, Pilgram GS, Plomp JJ, de Jong A, Fradkin LG, Noordermeer JN. Dystrophin is required for appropriate retrograde control of neurotransmitter release at the Drosophila neuromuscular junction. J Neurosci. 2006;26:333–344. doi: 10.1523/JNEUROSCI.4069-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vactor D, Wall DP, Johnson KG. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr Opin Neurobiol. 2006;16:40–51. doi: 10.1016/j.conb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Venken KJ, Bellen HJ. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet. 2005;6:167–178. doi: 10.1038/nrg1553. [DOI] [PubMed] [Google Scholar]
- Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, Spokony R, Wan KH, Koriabine M, de Jong PJ, White KP, Bellen HJ, Hoskins RA. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat Methods. 2009;6:431–434. doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist noggin. Journal of Biological Chemistry. 2004;279:5604–5611. doi: 10.1074/jbc.M310691200. [DOI] [PubMed] [Google Scholar]
- Voigt A, Pflanz R, Schafer U, Jäckle H. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev Dyn. 2002;224:403–412. doi: 10.1002/dvdy.10120. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wairkar YP, Fradkin LG, Noordermeer JN, DiAntonio A. Synaptic defects in a Drosophila model of congenital muscular dystrophy. J Neurosci. 2008;28:3781–3789. doi: 10.1523/JNEUROSCI.0478-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite A, Tinsley CL, Locke M, Blake DJ. The neurobiology of the dystrophin-associated glycoprotein complex. Ann Med. 2009;41:344–359. doi: 10.1080/07853890802668522. [DOI] [PubMed] [Google Scholar]
- Wang J, Renger JJ, Griffith LC, Greenspan RJ, Wu CF. Concomitant alterations of physiological and developmental plasticity in Drosophila CaM kinase II-inhibited synapses. Neuron. 1994;13:1373–1384. doi: 10.1016/0896-6273(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- Werle MJ. Cell-to-cell signaling at the neuromuscular junction: the dynamic role of the extracellular matrix. Ann N Y Acad Sci. 2008;1132:13–18. doi: 10.1196/annals.1405.035. [DOI] [PubMed] [Google Scholar]
- Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M, Chedotal A, Ghosh A. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33:47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk J, Mukhina I, Kaczmarek L, Dityatev A. Extracellular matrix molecules, their receptors and secreted proteases in synaptic plasticity. Dev Neurobiol. 2011 doi: 10.1002/dneu.20958. in press. [DOI] [PubMed] [Google Scholar]
- Wodarz A. Molecular control of cell polarity and asymmetric cell division in Drosophila neuroblasts. Curr Opin Cell Biol. 2005;17:475–481. doi: 10.1016/j.ceb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Wolfstetter G, Holz A. The role of LamininB2 (LanB2) during mesoderm differentiation in Drosophila. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-00011-00652-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda RR, Bansraj MR, de Jong AW, Noordermeer JN, Fradkin LG. Src family kinases are required for WNT5 signaling through the Derailed/RYK receptor in the Drosophila embryonic central nervous system. Development. 2008;135:2277–2287. doi: 10.1242/dev.017319. [DOI] [PubMed] [Google Scholar]
- Wreden CC, Wlizla M, Reimer RJ. Varied mechanisms underlie the free sialic acid storage disorders. J Biol Chem. 2005;280:1408–1416. doi: 10.1074/jbc.M411295200. [DOI] [PubMed] [Google Scholar]
- Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017–1033. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Ashleigh Long A, Harrison DA, Cooper RL. Developmental consequences of neuromuscular junctions with reduced presynaptic calcium channel function. Synapse. 2005;57:132–147. doi: 10.1002/syn.20165. [DOI] [PubMed] [Google Scholar]
- XXXFfrench-Constant C. XXXSpecial-Issue: stem cell niche. Dev Neurobiol. 2011 in press. [Google Scholar]
- Yamashita H, Goto A, Kadowaki T, Kitagawa Y. Mammalian and Drosophila cells adhere to the laminin alpha4 LG4 domain through syndecans, but not glypicans. Biochem J. 2004;382:933–943. doi: 10.1042/BJ20040558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitzky T, Volk T. Laminin is required for heart, somatic muscles, and gut development in the Drosophila embryo. Dev Biol. 1995;169:609–618. doi: 10.1006/dbio.1995.1173. [DOI] [PubMed] [Google Scholar]
- Yarovaya N, Schot R, Fodero L, McMahon M, Mahoney A, Williams R, Verbeek E, de Bondt A, Hampson M, van der Spek P, Stubbs A, Masters CL, Verheijen FW, Mancini GM, Venter DJ. Sialin, an anion transporter defective in sialic acid storage diseases, shows highly variable expression in adult mouse brain, and is developmentally regulated. Neurobiol Dis. 2005;19:351–365. doi: 10.1016/j.nbd.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Yasothornsrikul S, Davis WJ, Cramer G, Kimbrell DA, Dearolf CR. viking: identification and characterization of a second type IV collagen in Drosophila. Gene. 1997;198:17–25. doi: 10.1016/s0378-1119(97)00274-6. [DOI] [PubMed] [Google Scholar]
- Yasunaga K, Kanamori T, Morikawa R, Suzuki E, Emoto K. Dendrite reshaping of adult Drosophila sensory neurons requires matrix metalloproteinase-mediated modification of the basement membranes. Dev Cell. 2010;18:621–632. doi: 10.1016/j.devcel.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Yee GH, Hynes RO. A novel, tissue-specific integrin subunit, beta nu, expressed in the midgut of Drosophila melanogaster. Development. 1993;118:845–858. doi: 10.1242/dev.118.3.845. [DOI] [PubMed] [Google Scholar]
- Yu F, Kuo CT, Jan YN. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron. 2006;51:13–20. doi: 10.1016/j.neuron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Yu H-H, Araj HH, Ralls SA, Kolodkin AL. The transmembrane Semaphorin Sema I is required in Drosophila for embryonic motor and CNS axon guidance. Neuron. 1998;20:207–220. doi: 10.1016/s0896-6273(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Freeman MR, Waddell S, Zhang B, Freeman MR, Waddell Ss. Drosophila Neurobiology: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2010. [Google Scholar]
- Zhang L, Tran DT, Ten Hagen KG. An O-glycosyltransferase promotes cell adhesion during development by influencing secretion of an extracellular matrix integrin ligand. J Biol Chem. 2011;285:19491–19501. doi: 10.1074/jbc.M109.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]