Summary
The onset of chromosomal DNA replication requires highly precise and reproducible interactions between initiator proteins and replication origins to assemble a pre-replicative complex (pre-RC) that unwinds the DNA duplex. In bacteria, initiator protein DnaA, bound to specific high and low affinity recognition sites within the unique oriC locus, comprises the pre-RC, but how complex assembly is choreographed to ensure precise initiation timing during the cell cycle is not well understood. In this study, we present evidence that higher order DnaA structures are formed at oriC when DnaA monomers are closely positioned on the same face of the DNA helix by interaction with two oppositely-oriented essential arrays of closely spaced low affinity DnaA binding sites. As DnaA levels increase, peripheral high affinity anchor sites begin cooperative loading of the arrays, which is extended by sequential binding of additional DnaA monomers resulting in growth of the complexes toward the center of oriC. We suggest that this polarized assembly of unique DnaA oligomers within oriC plays an important role in mediating pre-RC activity and may be a feature found in all bacterial replication origins.
Keywords: oriC, DnaA, ORC, pre-RC
Introduction
Prior to reproducing, cells must duplicate their genomes at the correct time, and only once, during the cell cycle, reviewed in (Diffley, 2004; Sclafani and Holzen, 2007; Katayama et al., 2010). In all cell types, the initiation step of chromosome replication is tightly controlled, and requires staged binding of initiator proteins at replication origins to properly assemble pre-replicative complexes (pre-RCs) (Prasanth et al., 2004; Leonard and Grimwade, 2005). The first stage of pre-RC assembly in both bacteria and eukaryotes begins by formation of stable nucleoprotein complexes that mark replication origins and persist throughout the majority of the cell cycle (Prasanth et al., 2004; Nievera et al., 2006). In E. coli, this persistent complex (termed the bacterial ORC) forms immediately after a previous initiation event, when monomers of the initiator protein, DnaA, occupy three symmetrically arranged high affinity (Kd=5–20 nM) (Schaper and Messer, 1995) 9 mer recognition sites (R1, R2, and R4, see Fig. 1) in the unique chromosomal origin of replication, oriC (Nievera et al., 2006; Miller et al., 2009). DnaA is conserved among eubacteria (Messer et al., 1999), and, as a member of the AAA+ (ATPases associated with various cellular activities) family of proteins, also shares structural similarity with initiator proteins in eukaryotes (Orc1) and archea (Orc1/Cdc6) (Erzberger et al., 2002).
Fig. 1.
Arrays of DnaA contacts exist in each half of oriC. (A) Map of oriC with positions of DnaA, IHF, and Fis binding sites, as well as the DNA unwinding element (DUE), marked. The three high affinity sites are designated by large black squares, and the low affinity sites are marked by small black rectangles. Horizontal arrows indicate orientation of sites. C1, C2, and C3, and vertical arrows mark regions of unmapped DnaA contacts between R4 and R2. (B) In vitro DMS modification patterns of oriC, after incubating oriC plasmids with the indicated concentrations of DnaA prior to treatment with DMS. Two different primers were used to analyze modifications between R1 and R2 (left panel) and between R2 and R4 (right panel). Binding site positions are marked, with their orientations indicated by vertical arrow. Guanosine residues in position 2 or 4 are labeled, with up or down arrows indicating enhanced or diminished DMS sensitivity, respectively. (C) In vivo DMS modification patterns of oriC on purified chromosomal DNA, or DNA isolated from cells aligned at the stage of initiation just prior to helicase loading, measured by LMPCR. Guanosine residues in position 2 or 4 are labeled, with up or down arrows indicating enhanced or diminished DMS sensitivity, respectively. Filled circles indicate changes in the modification pattern in the Fis binding region. (D) Relative intensities of DMS modification at guanosine residues within DnaA binding sites.
As cells approach the time for new rounds of DNA synthesis, the bacterial ORC recruits additional DnaA to complete pre-RC assembly (Leonard and Grimwade, 2005). At least some of the additional DnaA must be in the ATP form (Grimwade et al., 2007) to couple new initiations to DnaA-ATP levels, which fluctuate during the cell cycle (Kurokawa et al., 1999). The recruited DnaA binds to multiple low affinity contact sites (Miller et al., 2009), several of which (τ sites and I sites) preferentially bind DnaA-ATP, in contrast to the R-boxes, which have equal affinities for active DnaA-ATP and inactive DnaA-ADP (McGarry et al., 2004; Ozaki et al., 2008).
Occupation of high and low affinity double stranded binding sites by DnaA leads to DNA strand separation in the AT-rich DNA unwinding element (DUE, see Fig.1A), by a process that has not yet been defined. Once the DUE is unwound, DnaA-ATP binds to single stranded DNA and stabilizes the open complex (Speck and Messer, 2001; Duderstadt et al., 2010), as well as assists the loading of replicative DNA helicase (Abe et al., 2007; Mott et al., 2008). Multiple regulatory mechanisms are focused on ensuring that each pre-RC forms at the correct cell cycle time, and only once per cycle, reviewed in (Katayama et al., 2010).
Only the three high affinity sites in oriC are capable of binding DnaA independently of other sites (Schaper and Messer, 1995; Miller et al., 2009). Thus, cooperative interactions are required to promote occupation of low affinity DnaA binding sites. DnaA-DnaA contacts are implicated in cooperative binding, with both domain I and domain III of DnaA playing a role in pre-RC formation (Weigel et al., 1999; Simmons et al., 2003; Kawakami et al., 2005). Domain I interactions may be increased or stabilized by the accessory protein DiaA, which is reported to promote DnaA oligomerization on oriC (Keyamura et al., 2007; Keyamura et al., 2009).
While strong sites are required to assist loading of weak sites, the assistance given by any single occupied strong site is limited, and facilitates loading primarily at a proximal weak site (Miller et al., 2009). Therefore, to fill all weak sites, particularly those that are flanked by other weak sites (Fig 1A), it seems logical that to propose that DnaA monomers must be positioned on oriC DNA in a way that favors cooperative interactions. In support of this idea, we recently demonstrated that while DnaA in solution is primarily monomeric, formation of DnaA oligomers is greatly enhanced when the protein is bound to oriC DNA (Miller et al., 2009). Additionally, several studies have shown that pairing of sites, as well as spacing between sites, can affect DnaA binding efficiency (Doran et al., 1999; Majka et al., 2001; Hansen et al., 2006; Hansen et al., 2007). However, based on the current oriC map (Fig 1A) it is not immediately obvious how the oriC sequence directs cooperative DnaA binding to the weak sites that are not paired with a strong site or close to any other site.
In this study, we hypothesized that after a strong site assists DnaA loading at its neighboring weak site, there may be additional DnaA contacts in oriC that direct the placement of DnaA monomers so that a stable DnaA structure can be extended. We identified an unsuspected symmetrical arrangement of specific DnaA contacts in E. coli oriC, which provide nucleotide-based instructions for ordered assembly of two DnaA oligomers that converge toward R2. In each half of oriC, four low affinity 9mer recognition sites define a helically-phased, closely-spaced array of DnaA contacts. While all the sites in each array are similarly oriented, the two arrays are in opposite orientation. Occupation of the arrays by DnaA is sequential and polarized, preferentially emanating from R4 toward R2 in oriC’s right half, and from R1 toward R2 in the left half. Alterations in the sequence, orientation, or spacing of the low affinity sites in the two arrays disrupted DnaA binding, and perturbed in vivo origin function. We propose a model in which polarized growth of helically-phased oligomers plays a key role in the function of bacterial pre-RC.
Results
DnaA binds to two oppositely-oriented helically-phased arrays of low affinity sites in oriC
Recently we demonstrated that the bacterial ORC is required for further DnaA contacts with lower affinity sites in oriC (Miller et al., 2009). Studies with mutant oriC containing a single high affinity recognition site (either R1, R2, or R4) revealed that DnaA occupation was localized to low affinity sites that were immediately adjacent to the strong site, with binding to additional low affinity sites decreasing as a function of distance from the strong site (Miller et al., 2009). Since not all low affinity sites in oriC are positioned next to a high affinity R box (Fig. 1A), and, since weak sites cannot bind DnaA independently (Schaper and Messer, 1995; Miller et al., 2009) (also see Fig. S2D, E), there must be additional parameters that allow cooperative DnaA binding to extend larger distances to internal weak sites within the DNA “gap” regions between R1 and R2 (97 bp) and between R2 and R4 (65 bp).
To define these parameters, we first examined the pattern of DnaA contacts in a supercoiled oriC plasmid using high resolution in vitro dimethyl sulfate (DMS) footprinting. DnaA contacts are revealed by the distinctive and reproducible changes to the DMS modification pattern that are produced when DnaA binds to its 9mer consensus sequence 5’-TGTGNATAA-3’ (or variations of this recognition sequence) (Grimwade et al., 2000; McGarry et al., 2004; Nievera et al., 2006). Specifically, the guanosine residue in the fourth position of the 9-mer becomes hypersensitive, and modification of the guanosine in the second position is repressed (Fig. 1B, D), causing darker and lighter bands, respectively, on footprinting gels. This well-defined DnaA “footprint” not only allows resolution of individual, closely spaced DnaA recognition sites, but also predicts the nucleotide sequence of putative sites, since any repressed G signal should be in position 2, and any enhanced signal should be in position 4 (McGarry et al., 2004). At DnaA-ATP levels high enough to unwind the DUE of supercoiled oriC (80 nM, corresponding to 20–25 molecules per oriC (Bramhill and Kornberg, 1988; Grimwade et al., 2000)), a distinctive pattern of DMS modifications, indicative of four regularly-spaced low affinity DnaA contact regions, can be seen in the left gap region between R1 and R2 (Fig. 1B left panel), as well as another array of 4 contact sites in the right gap region, between R2 and R4 (Fig. 1B right panel). The contacts in the left region were mapped to the previously identified sites I2, I1, τ2, and R5M (McGarry et al., 2004; Kawakami et al., 2005), which are all in the same orientation, with each site separated by 2bp. Only minor changes in modification pattern were seen in the R3 region on this strand (Fig. S1), consistent with our previously published results (McGarry et al., 2004; Nievera et al., 2006). Binding to the τ1 site (Kawakami et al., 2005), was not detected by DMS footprinting on supercoiled templates (Fig. 1B), or in a gel shift assay (Fig. S3, where the IHF4 probe contains the predicted τ1 sequence).
In the right half of oriC, only two weak DnaA recognition sites (I3 and R3) are reported, and in the in vitro DMS footprint, only I3 can be mapped to a contact site (Fig. 1B). Of the three remaining footprints detected in the right gap region, one (C1) is positioned between R4 and I3, and the other two (C2 and C3) partially overlap the R3 sequence. Using the DMS modification pattern, we predicted specific 9mer binding sites for the three contact sites (shown in Fig. 2, with correctly positioned suppressed or enhanced G residues marked). A previous report also suggested the existence of a low affinity binding site between R4 and I3, based on weak similarity to the R box consensus (Hansen et al., 2007). Each 9mer in the right oriC array is separated by 2 bp, and oriented in the same direction as R4 and I3. Thus, the spacing and number of low affinity contact sites between R2 and R4 is identical to the left array between R2 and R1, but the two arrays are oriented in opposite directions. These data suggest that pre-RC formation in oriC proceeds by filling of two arrays of helically-phased, oppositely oriented, low affinity DnaA contacts.
Fig. 2.
Sequence of all DnaA interaction sites in oriC, based on placing hypersensitive G residues in position 4, and suppressed Gs in position 2.
We previously reported that, in vivo, the pre-RC is formed when low affinity sites become occupied just prior to the time of initiation of chromosome replication (Cassler et al., 1995; Nievera et al., 2006). To verify that all contact sites in the two arrays are filled in vivo when the pre-RC is completely assembled, we performed Ligation Mediated PCR (LMPCR) on chromosomal DNA isolated from DMS-treated cells halted at the stage of helicase loading, compared to chromosomal DNA isolated without DMS treatment (Fig. 1C). These in vivo footprints show that DnaA contacts the same arrayed sites that were detected by in vitro footprinting, and the contact pattern is verified by densitometric scans quantifying band intensities (Fig. 1D). The bands in the in vivo chromosomal footprint for the left half of oriC are separated better than those in the right half, since constraints inherent to the LMPCR procedure necessitated the use of a probe for the right half that hybridizes to a region relatively distant from oriC. We also note that there are subtle changes in the DMS modification pattern in the Fis region (marked by closed circles in Fig. 1C). We do not currently know what protein is causing these changes. We previously reported that Fis is displaced from its primary binding site in oriC at the time of initiation (Cassler et al., 1995), but it remains possible that Fis is bound to an overlapping secondary site (Hengen et al., 2003), or that some other, unidentified factor, is binding in this region.
DnaA occupation of the arrayed contact sites between R2 and R4 is sequence specific
Although the DMS footprints indicate that there are arrayed DnaA contact sites in both the right and left halves of oriC that become occupied in the pre-RC, it is possible that not all the contacts in the right array are sequence-specific, which would be required if the origin sequence is directing the precise placement of DnaA monomers. (Contact sites in the left array were previously determined to be specific binding sites (McGarry et al., 2004)). To investigate this, we constructed a simple electrophoretic mobility shift assay (EMSA) system, utilizing a series of double-stranded DNA oligonucleotide probes (see Table S1 for oligonucleotide sequence information). Previous studies (Schaper and Messer, 1995), and our control experiments (Fig. S2A) indicate that in the EMSA system, a single R4 box binds one molecule of DnaA, yielding only one shifted complex even at high DnaA concentrations. Since DnaA does not appear to “pile up” on the high affinity site, complexes with higher mobilities form only when extra DnaA molecules interact with additional sites on the DNA. Accordingly, probes containing two or three R boxes have the capacity to form 2 or 3 complexes, respectively (Fig. S2B, C).
To examine binding to the C-sites, we designed oligonucleotide probes containing R4 and R2, flanking the putative 9mer C1, C2, or C3 sites predicted by the DMS modification pattern (sequences of C-sites are shown in Fig. 2). One high affinity site is required to act as a donor site, and the use of two high affinity flanking sites provides optimal cooperative assistance to load the low affinity “test” site. The probes were end-labeled and incubated with increasing concentrations of DnaA-ATP, and the resulting DnaA-DNA complexes were resolved on polyacrylamide gels (Fig. 3). Like the probe containing three R boxes (Fig. S2C), the probes carrying R4, R2 and the putative C sites were able to form three distinct DnaA-DNA complexes at the higher DnaA concentrations (Fig. 3A–C), with relative migration rates consistent with complexes comprising 1, 2, or 3 DnaA molecules. Preliminary studies indicate that C2 and C3 preferentially bind DnaA-ATP, whereas C1 binds both nucleotide forms of DnaA equivalently (data not shown). Similar assays using probes containing known low affinity sites R5M, I2, and I3 also yield 3 complexes at the higher DnaA-ATP concentrations (data not shown). Since each probe differs in the amount of DnaA required to form complex 3, it is likely that the affinities of the C1, C2 and C3 sites vary, as would be expected from different specific binding motifs.
Fig. 3.
DnaA contacts at specific low affinity 9mer sites are detected by EMSA. Double-stranded DNA oligonucleotides containing R4 and R2 boxes flanking the putative low affinity binding site C1 (A); C2 (B); or C3 (C) were incubated with DnaA-ATP at DnaA/DNA molar ratios of 0:1, 0.5:1, 1:1, 2:1, 5:1, 10:1, 20:1, and 50:1, and the resulting complexes were resolved on polyacrylamide gels. The position of the unbound probe, and complexes resulting from 1, 2 or 3 molecules of DnaA bound to the probe are marked. The last lane of each panel shows complexes formed by incubating DnaA-ATP (50:1 DnaA/DNA) with an equivalent probe, in which the putative site is scrambled (see text).
To provide further evidence that DnaA interaction with C1, C2, and C3 is sequence-specific, we designed probes in which these sites were scrambled by converting purines to non-complementary pyrimidines, and vice versa (see Table S1 for sequences). In our EMSA system, we found that scrambling the putative binding sites prevented the formation of complex 3 on all of the probes, even at high DnaA levels (Fig. 3A–C, last lanes). These data indicate that specific DnaA recognition sites can include sequence motifs that diverge significantly (4 or more positions) from consensus (Fig. 2).
Although the DMS footprints revealed only four DnaA contact sites in each of the DNA gap regions bounded by high affinity R boxes, we used our EMSA system to scan for additional DnaA binding sites in the sequences 5’ of each array, where binding sites are mapped for the DNA bending proteins Fis (right half) (Gille et al., 1991) and IHF (left half; this region also contains the τ1 site) (Roth et al., 1994). However, at the DnaA levels used to identify C1, C2, and C3, very little, if any, binding was detected in these regions (Fig. S3), indicating a lack of specific contact.
Cooperative binding between low affinity sites is dependent on site orientation and spacing
Low affinity sites in the arrays are in the same orientation and separated by 2 bp. If this arrangement is required for cooperative DnaA binding, then inverting arrayed sites, or placing sites further apart should prevent the arrays from becoming filled, and thus prevent pre-RC assembly. We first tested whether site orientation played a role in extension of DnaA oligomers within an array (Fig. 4). Oligonucleotide probes, containing a peripheral donor strong site and two weak sites, in varying orientations, were incubated with increasing concentrations of DnaA, and the complexes that formed were examined using EMSA. When the two weak sites were in the same orientation, three complexes were formed, by binding to the donor site (complex 1), as well as to the donor site plus the proximal (middle) site (complex 2) or both weak sites (complex 3) (Fig. 4A, D). When the two weak sites were in opposite orientations, only two complexes could form, indicating that only one weak site could be occupied in this configuration (Fig. 4B, C). Given that a low affinity site must be paired with a strong site to bind DnaA (Fig. S2D, E), it is likely that it is the middle weak site that is bound in complex 2. Extension of DnaA from the high affinity anchor site was less dependent on site orientation, since the strong site assisted loading of the middle weak site on all probes, regardless of the orientation of the anchor (Fig. 4C, D), although DnaA binding was most efficient when all three sites were in the same orientation.
Fig. 4.
Cooperative binding between low affinity sites is dependent on site orientation, while strong sites in either orientation can donate DnaA. The EMSA system described in the text was used to examine DnaA-ATP binding to double-stranded DNA oligonucleotide probes containing R4 flanking two low affinity sites (C1 and R5M) in varying orientations, shown by the drawing below each panel (A–D). End-labeled probes were incubated with DnaA-ATP at DnaA/DNA molar ratios of 0:1, 20:1, and 50:1, and the resulting complexes were resolved on polyacrylamide gels.
To determine if the 2 bp spacing between arrayed sites is also critical for cooperative binding between low affinity sites, we designed probes for EMSA that had a donor strong site and two low affinity sites, with varying number of bases (1–4) between the weak sites (Fig. 5). When DnaA was incubated with these probes, a clear dependency on site spacing was observed. Extension of DnaA from the middle to the distal weak site was optimal when sites were separated by 2 bp, shown by the formation of complex 3 in gel shift assay shown in Fig 5B. Spacing of 1bp and 4 bp abolished binding (Fig. 5A, D), and increasing the spacing to 3bp decreased binding to the distal weak site by approximately 2-fold (Fig. 5C). Together, our studies on site sequence-specificity, orientation, and spacing are consistent with pre-RC assembly being directed by arrayed low affinity sites, which precisely position DnaA protomers in both halves of oriC.
Fig. 5.
Extension of DnaA requires precise spacing between low affinity sites. Double-stranded oligonucleotide probes containing R4, C1, and R5M were designed so that C1 was separated from R5M by 1bp (A), 2bp (B), 3 bp (C), or 4 bp (D). All sites were in the same orientation. The configuration of the probes is indicated below each panel. End-labeled probes were incubated with DnaA-ATP at DnaA/DNA molar ratios of 0:1, 20:1, and 50:1, and the resulting complexes (1, 2, and 3) were resolved on polyacrylamide gels.
Strong sites can assist loading of a proximal weak site over several bases
The experiments described above show that cooperative binding of DnaA between a high and low affinity site does not require that the sites be in the same orientation, while the rules governing extension of DnaA between low affinity sites are more stringent. To determine if there is also more flexibility in the spacing allowed between strong and weak sites, we designed probes for EMSA in which the donor high affinity site was separated from the neighboring weak site by varying numbers of base pairs (Fig. 6). After incubation of these probes with increasing concentrations of DnaA, we observed that there was a range in the number of base pairs tolerated between a strong and weak site; donation of DnaA to the weak site could be observed when the two sites were separated by 2–5 bp (Fig. 6).
Fig. 6.
Cooperative binding between high and low affinity sites can extend over a range of bases. Double-stranded oligonucleotide probes containing R4 and R5M were designed so that the sites were separated by 1bp, 2bp, 3 bp, or 5 bp, or 7 bp. All sites were in the same orientation. The configuration of the probes is indicated below each panel. End-labeled probes were incubated with DnaA-ATP at DnaA/DNA molar ratios of 0:1, 20:1, and 50:1, and the resulting complexes (1, 2, and 3) were resolved on polyacrylamide gels.
DnaA extends in the array in a preferred direction
DnaA binding at a high affinity site is required as a nucleation point for extension of additional DnaA molecules into proximal arrayed weak sites (Miller et al., 2009). The data shown in Fig. 6 indicate that both the orientation and spacing requirements for this donation are more flexible than for DnaA binding within an array. If the direction of complex assembly is determined by the nucleating site, extension of DnaA into the arrays could be either from a single strong site (e.g. unidirectional) or from two strong sites flanking a gap region (bidirectional), since each array is located in a gap region between two strong sites. To determine if DnaA is extended in a preferred direction, we used site-directed mutagenesis to scramble the low affinity sites at the outer positions of each array on an oriC plasmid, and measured DnaA binding to the remaining sites by DMS footprinting. In the left half of oriC, mutations that diminished binding to R5M also reduced binding to I1, τ2, and I2, while mutations of I2 had a minimal affect on DnaA binding to the rest of the sites in the left array (Fig. 7A, right panel and graph). These results indicate that in the left half of oriC, a DnaA oligomer is predominantly extended from R1 (Fig. 7A, middle panel and graph), with much less extension emanating from R2. We are currently investigating how DnaA is donated from R1 to begin the extension, with particular focus on the possible role for DNA bending as a mechanism to decrease the distance between R1 and R5M. In the right array, the footprints show that DnaA extends preferentially from R4, since oriC plasmids with a scrambled C1 site were unable to bind DnaA at I3, and also lack the subtle footprints seen at C2 and C3 (Fig. 7B, middle panel, and graph). This conclusion was verified by examining DnaA binding to oriC plasmid with a C3 mutation; in these plasmids, binding to I3 was observed, as expected from directional assembly of DnaA oligomers starting at R4 (Fig. 7B, right panel and graph). Binding to C2 was less robust than observed using wt oriC, possibly due to the lack of a stabilizing cooperative interaction from C3. Mutations that inverted I3 also resulted in loss of binding to C2 and C3 (Fig. S4), as would be expected if stable extension from R4 is possible only when all arrayed sites are in the same orientation. Inversion of I3 also resulted in less binding observed at C1, consistent with the idea that in oriC, flanking cooperative interactions stabilize low affinity binding. Combined, these data indicate that progressive and directional growth of DnaA structures in each oriC half is guided by the positioning and orientation of low affinity arrayed sites.
Fig. 7.
DnaA extends from high affinity anchor sites in a preferred direction. Wild-type oriC plasmids, or oriC plasmids that are mutated in one of the DnaA contact sites at the end of an array were incubated with the indicated concentrations of DnaA-ATP and treated with DMS. Binding sites are marked. (A) DMS modified nucleotide positions in the array between R1 and R2 are shown for wt oriC (left panel); scramble (scr) mutation in R5M (middle panel); scr mutation in I2 (right panel). (B) DMS modified nucleotide positions in the array between R2 and R4 are shown for wt oriC (left panel); scr C1 mutation (middle panel); scr C3 mutation (right panel). Graphs of relative intensities (arbitrary units) of the DMS modified G4 in each arrayed site (marked by arrows in gel scans) are shown on the right of each set of panels.
Mutations that disrupt filling of arrays also reduce oriC function on plasmids in vivo
The data shown above suggests that pre-RC assembly is directed by precise placement of DnaA protomers . One consequence of disrupting the sequence, orientation, or spacing of the arrayed sites that are guiding this placement should be a perturbation of pre-RC formation, and a decrease of oriC function in vivo. oriC function can be measured using a plasmid-based assay, in which a chimeric plasmid, harboring both the pBR322 origin and the mutant oriC, is used to transform a polA strain whose chromosome has a wt oriC sequence (Langer et al., 1996; Grimwade et al., 2007). Since the pBR322 origin will not function without Pol I activity, transformants are obtained only if the mutant oriC can assemble a functional pre-RC that unwinds oriC and recruits helicase, and do this before the triggering of chromosomal oriC causes a rapid decrease in cellular DnaA-ATP levels (Katayama and Sekimizu, 1999). While under normal conditions, there is no incompatibility between minichromsomes and chromosomal oriC (Lobner-Olesen, 1999), any mutation that prevents pre-RC assembly, or makes pre-RC assembly less efficient than the wt oriC on the chromosome, will result in decreased plasmid oriC function. Incompatibility has also been observed when plasmid and chromosomal oriC copies do not initiate at the same time in the cell cycle (Skarstad and Lobner-Olesen, 2003). This plasmid-based assay was used to evaluate three types of mutations that might affect pre-RC assembly: 1) mutations that diminish DnaA binding to an arrayed site; 2) mutations that invert an arrayed site so that it was not in the same orientation as the other sites in the array; and 3) mutations that insert bases between sites in the array, so that that the arrayed sites were no longer in helical phase (Table 1). As predicted by the gap-filling model, all mutations in oriC of a type that disrupted complete extension of DnaA through the arrayed sites also resulted in more than 99% loss of activity of the plasmid-borne oriC in vivo. It should be noted that the correlation of DnaA binding to C1, C2 and C3 with oriC function further supports our assignment of these 9mer sequences as low affinity DnaA boxes. These data are consistent with previous findings that inversions of R5M (Langer et al., 1996), as well as binding mutations in R5M (Langer et al., 1996), I2, and I3 (McGarry et al., 2004) also decreased oriC function on plasmids. It is possible that, like other oriC mutations (Weigel et al., 2001; Riber et al., 2009), some of our changes that knock down function in the plasmid assay would be tolerated if placed on the chromosome, where there would be strong selective pressure for viability, and no other origin firing before the mutated copy. This scenario seems especially likely for those mutations that could be overcome by higher cellular DnaA-ATP levels, and we are currently investigating this possibility. It should be noted that the majority of oriC mutations that are inactive on plasmids, but viable when harbored on the chromosome, display mild to severe defects in initiation timing (Weigel et al., 2001; Riber et al., 2009), indicating that pre-RC assembly is not normal in the mutant cells. Studies on DnaA binding to the mutated chromosomal oriCs will be required for a clear understanding of how these origins are functioning.
Table 1.
In vivo replication activity of oriC plasmids harboring mutated low affinity Dna1 A binding sites.
| Site | mutation type | activity in polA (relative to wt)* |
|---|---|---|
| --- | none | 1 |
| C1 | scramblea | <0.01 |
| C2 | scramble | <0.01 |
| C3 | scramble | <0.01 |
| I3 | inversion | <0.01 |
| I2 | inversion | <0.02 |
| I3-C2 | spacingb | <0.04 |
| C2-C3 | spacingc | <0.01 |
| τ2-I1 | spacingd | <0.01 |
| I1-I2 | spacingd | <0.01 |
Transformants of P3478 polA/W3110 by test plasmid, normalized for transformation by wt oriC plasmid
Sites were scrambled by changing purines to the non-complementary pyrimidines, and vice versa.
CC was inserted between the two sites.
AT was inserted between the two sites.
GC was inserted between the two sites.
R3 is not in the appropriate position or orientation in oriC to participate in pre-RC formation
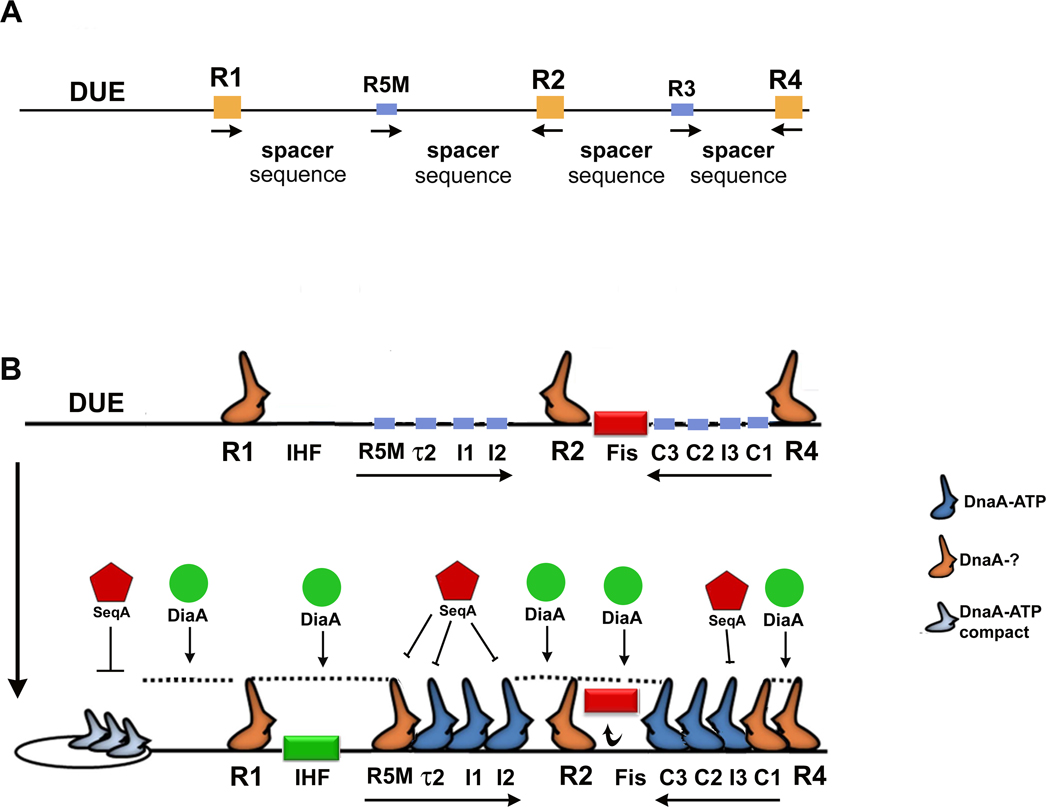
The map of arrayed DnaA contact sites in the right half of oriC, presented here, raises new questions regarding the role of R3 in pre-RC assembly. Although diverging from the canonical R box sequence by only one base, R3’s unusually low affinity for DnaA (Schaper and Messer, 1995) was previously difficult to explain. We suggest that both the position and orientation of R3 reduces its ability to bind DnaA and participate in joining DnaA into oligomeric complexes. R3 is unlikely to accept DnaA directly from R4, due to the intervening I3 and C1 sites. The lack of DnaA contact sites in the Fis binding region also reduces the likelihood that R3 accepts DnaA directly from R2. Additionally, R3 is not in the correct orientation or position to accept DnaA from I3. The apparent binding detected at R3, both by our lab (Cassler et al., 1995) and by other investigators (Fuller et al., 1984; Margulies and Kaguni, 1996), is more likely due to DnaA contacting the flanking C2 and C3 sequences, rather than R3 itself. To provide more evidence for our revision of the oriC map, we tested DnaA binding to the R3 sequence, using our EMSA system. Oligonucleotide probes containing R4 and R2 flanking R3 in its oriC configuration were incubated with DnaA (Fig. S5). While this probe was clearly able to bind 2 molecules of DnaA, forming complexes 1 and 2, complex 3 was essentially absent, indicating that R3 was not bound at these DnaA concentrations. Combined with our other results, these data suggest that the R3 sequence is a “red herring” that has diverted attention away from the symmetrical arrangement of DnaA recognition sites in oriC. We have revised the map to reflect the array of DnaA contacts in both halves of oriC (Fig. 8).
Fig. 8.
Model of pre-RC assembly based on a revised oriC map. In the original (1980s era) map of oriC (A), five R boxes were identified, with the remaining regions as “spacer sequences” without defined function. High affinity R boxes are shown in yellow, and low affinity boxes are blue. In panel B, a revised map of oriC is presented, with a model of pre-RC assembly. In the first stage of pre-RC assembly (top, showing the bacterial ORC), DnaA occupies the three high affinity sites, and Fis is bound. It is not known if these DnaA molecules must be in a particular nucleotide form. In the second stage, R1 and R4 act as nucleation sites for a DnaA oligomer which is extended by sequential binding of protomers to arrayed sites, toward R2 (shown by arrows). Some sites must bind DnaA-ATP (marked in blue). Oligomer extension displaces Fis (red rectangle), and IHF (green rectangle) binds in this stage, modulating the distance between strong and weak sites. The negative regulator SeqA (red pentagon) prevents oligomer extension and unwinding by blocking sites containing a GATC, and the positive regulator DiaA (green circle) is proposed to stabilize the DnaA interactions that are required to extend DnaA from the nucleation sites, and to connect the two converging oligomers at R2. After arrays are filled and joined, a compact helical filament of DnaA-ATP extends from R1 into the DUE, and DiaA may also stabilize this extension.
Discussion
Historically, bacterial replication origins have been described as containing two types of nucleotide sequence information necessary for biological function: canonical DnaA recognition sites (R-boxes) to recruit initiator protein, and “spacer” regions of low sequence specificity, that were proposed to properly separate the R-box bound DnaA molecules (Oka et al., 1984; Zyskind and Smith, 1986; Woelker and Messer, 1993). The resulting origin maps, exemplified by E. coli oriC (Fig. 8A), provided few obvious clues regarding how the R-boxes and spacer regions work together to assemble pre-RC during the cell cycle. This work, combined with previously published studies (McGarry et al., 2004; Kawakami et al., 2005), demonstrates that regions with “spacer” function contain nucleotide sequence motifs that consist of four closely-spaced specific low affinity initiator recognition sites, arranged into oppositely-oriented symmetrical arrays in each half of the origin (Fig. 8B). The newly identified C sites, combined with the previously identified I sites (Grimwade et al., 2000), τ2 site (Kawakami et al., 2005), and R5M site (Matsui et al., 1985), yield 11 mapped DnaA contacts in E. coli oriC, with additional DnaA occupying the A-T rich DUE (see below). Thus, the revised map moves closer to accommodating the 15–20 DnaA monomers previously estimated by EM (Fuller et al., 1984) and in vitro unwinding assays to be required for complete pre-RC assembly (Ryan et al., 2004), and promotes development of a testable model for properly timed pre-RC assembly during the E. coli cell cycle. Our studies do, however, raise questions about the role of R3 and τ1 as specific DnaA contact sites in oriC. Although R3 was one of the earliest identified R boxes, with close similarity to the consensus sequence, its unusually low affinity for DnaA (Schaper and Messer, 1995) has always been difficult to explain. Additionally, mutations in R3 that eliminate DnaA binding have not been observed to have any discernible effect on oriC function (Langer et al., 1996; Weigel et al., 2001). Based on our studies, it is clear that the low affinity of R3 is, in part, due to the difference in orientation and helical phase relative to nearby sites. Although our model predicts that R3 is a "red herring" in oriC function, additional analysis will be required to completely rule out any role for this site. We also are currently unable to assign a role to τ1 in pre-RC formation, since, although at first glance this site appears as if it could be part of the left array, τ1 has never been detectable in our lab when supercoiled templates are used, or in our gel shift assays. The reasons for this are not clear, but it is possible that binding to this site is both very low affinity and affected by DNA topology.
Precise spacing and orientation of arrayed sites was required for proper pre-RC assembly, and disrupting a contact site at one end of the array perturbed further downstream contacts, indicating polarity in the sequential placement of DnaA. This polarity was determined by the arrangement of low affinity sites, rather than the orientation of the nucleating site. These data are consistent with a model of pre-RC assembly in which the arrayed sites, by precisely and closely positioning DnaA monomers, direct sequential growth of two DnaA oligomers anchored by R1 and R4, both converging on R2. In this model, regulators of oriC activity would function by targeting oligomer extension. For example, the negative regulator SeqA, previously shown to prevent DnaA from re-binding to low affinity sites after initiation (Nievera et al., 2006) would prevent oligomer growth in both halves of oriC. Fis, which also inhibits DnaA binding to low affinity sites (Ryan et al., 2004), might block extension of the right filament (see Fig. 8B). The polarized formation of two oppositely oriented DnaA oligomers also suggests a mechanism for origin unwinding. Although the two oligomers appear to assemble independently, it is possible that they ultimately become joined into a contiguous filament. Since the two arrays in each oriC half are not in helical phase, connecting the two DnaA oligomers as they converge at R2 may require twisting of the DNA strand, which would impart torsional stress on the duplex DNA and aid strand separation in the DUE. Alternatively, preventing free rotation of the DNA helix by having it locked by multiple contacts within the DNA oligomer may be sufficient to focus topological stress within the A-T rich region.
Normal pre-RC assembly must also be coordinated with the growth rate of the cell, to ensure proper initiation timing during the cell cycle (Leonard and Grimwade, 2010). Based on spacing, one of the earliest DnaA-DnaA interactions in pre-RC assembly should be between DnaA monomers occupying R4 and the proximal C1 site, and therefore the right half of oriC might play a key role in proper timing of pre-RC assembly. Consistent with this idea, cells harboring R4 binding mutations or deletions on chromosomal oriC exhibit dramatic perturbations in initiation timing (Bates et al., 1995; Riber et al., 2009), and chromosomal oriC mutants lacking the entire R2–R4 region, while viable, show profound replication initiation and growth defects, and are particularly sensitive to rich media (Stepankiw et al., 2009). The DnaA binding pattern in the pre-RCs formed from these mutant origins is not currently known, but since initiation timing is not normal, it seems logical to suggest that pre-RC assembly is altered, and somehow uncoupled from cellular mass accumulation. Since the left half of oriC is reported to be required for viability (Stepankiw et al., 2009), it is possible that the oligomer assembled in this half plays a greater role in DNA unwinding and helicase loading.
The structure of the higher order DnaA complexes formed on the arrays remains to be determined. Each array contains sites that preferentially bind DnaA-ATP suggesting that at least some of the positioned DnaA monomers must be in the ATP-form. Domain III-specific interactions between adjacent DnaA-ATP molecules have been previously proposed to form a right-handed helical filament (Erzberger et al., 2006), but, as currently modeled, this structure is not capable of double-stranded DNA binding (Duderstadt et al., 2010). While the modeled DnaA-ATP filament is able to interact with single-stranded DNA in an unwound DUE, the closely spaced DnaA-DNA interactions within the arrays are likely to promote assembly of a novel, alternative DnaA filament, in which the protomers are less compact and capable of stable interaction with double-stranded oriC DNA. Additionally, all the DnaA in the alternative filament structure may not be in the ATP form, since both in vitro and in vivo studies have shown that some DnaA-ADP is permitted in the pre-RC (Yung et al., 1990; Grimwade et al., 2007). We are currently evaluating the requirement for DnaA-ATP and domain III interactions in array filling.
It is noteworthy that the criteria governing cooperative interaction between high and low affinity DnaA recognition sites are less stringent than those for low affinity site interactions, which suggests that a different type of DnaA-DnaA contact may be used to extend DnaA from a high affinity binding site. We previously demonstrated that DnaA defective in domain I oligomerization was capable of binding strong sites, but could not occupy low affinity sites (Miller et al., 2009). Since domain I is attached to the flexible domain II linker region (Nozaki and Ogawa, 2008), by using domain I interactions, it should be possible to extend a DnaA monomer from a nucleating site to a weak site over variable distances, and we are investigating this possibility. Accessory factors, such as DiaA (Ishida et al., 2004), which associate with Domain I and increase initiation efficiency, may also promote or stabilize these longer-range DnaA-DnaA interactions (Fig. 8B). Additionally, the effective proximity between strong and weak sites could be modulated by the DNA bending proteins Fis and IHF, which bind in the right and left half of oriC, respectively (Fig. 8), and are known to regulate pre-RC assembly (Ryan et al., 2004). IHF would increase interaction of R1 with R5M, which are separated by 46 bp, and Fis might affect the ability of R2 to interact with C3 (separated by 20 bp). Further studies will be necessary to determine how removal of the Fis and IHF sites, as well as altering the distance between high and low affinity sites, affects pre-RC assembly and initiation timing.
Can this model for pre-RC assembly be relevant for other bacterial replication origins? DnaA is the initiator protein for all bacteria, but DnaA binding sites in bacterial replication origins have been identified primarily by sequence similarity to the consensus DnaA R box. Further, the arrangement of mapped DnaA recognition sites in other bacteria is very different from those in E. coli, for example see (Zawilak-Pawlik et al., 2005). Our studies reveal that extreme deviation from the consensus recognition site is tolerated for DnaA recognition, although deviations greatly reduce binding affinity. Since it appears that clustered low affinity contacts adjacent to high affinity DnaA recognition sites play a key role in E. coli pre-RC assembly, it will be necessary to re-evaluate DnaA binding to other origins to determine if these features are shared by all bacteria.
Experimental procedures
Strains and Plasmids
pOC170 (3,852 bp) was used as the starting plasmid for all mutant oriC constructions. It carries both the pBR322 replication origin as well as oriC. Supercoiled plasmids were isolated using the QIAPrep Spin plasmid preparation kit (Qiagen). Site directed mutagenesis was performed as described (McGarry et al., 2004), with all mutations being verified by sequence analysis. For evaluation of in vivo replication of oriC plasmids, pOC170 or mutant plasmids were transformed into either P3478 polA1, thyA36 deoC2 IN(rrnD-rrnE)1, λ-, or its isogenic parent, W3110, grown in LB agar containing 100 µg ml−1 ampicillin. For in vivo footprinting experiments, PC2(dnaC(ts), leu-6 thyA47 deoC3 rpsL) was grown in minimal salts media (Bogan and Helmstetter, 1997) supplemented with 0.1% glucose, 0.2% casamino acids, and 10 mg ml−1 thymine. To align cells at initiation, exponentially growing cultures at 27°C were shifted to 39°C for 1 hr.
Chemicals, Proteins, and Enzymes
Reagent grade chemicals were purchased from Amresco, Fisher Scientific, or Sigma. Media components were from Difco. All enzymes were from Sigma, New England Biolabs or Bioline. Poly (dI-dC) was purchased from Roche. Sequencing gel reagents were purchased from National Diagnostics. Amino-terminal His10-tagged DnaA was isolated as described by Li and Crooke (Li and Crooke, 1999).
Electrophoretic Mobility Shift Assays
Complementary single-stranded oligonucleotides were purchased from Integrated DNA Technologies. Sequences of the oligonucleotides are shown in Table S1. Oligonucleotides were resuspended at a concentration of 10µg ml−1 in deionized water. DNA strands were annealed by incubating at 90°C for 10 minutes followed by a 50°C incubation for 10 minutes and subsequent cooling to room temperature. 100µg of annealed oligonucleotide was applied to a 10% polyacrylamide gel, and double-stranded DNA was separated from remaining single stranded oligonucleotide by electrophoresis in Tris-Borate-EDTA buffer. The double-stranded fragment was localized in the gel by UV shadowing, excised, and eluted by incubation in 0.3M sodium acetate pH 4.5 overnight at 37°C. Eluted fragments were extracted with 24:1:1 phenol:chloroform:isoamyl alcohol, and precipitated with ethanol. The double-stranded fragments were radio-labeled at the 5’ end using polynucleotide kinase.
Binding reactions were set up in a mixture containing 20mM HEPES•KOH pH 8, 2.5mM magnesium acetate, 1mM EDTA, 4mM DTT, 100µM ATP, 5mg ml−1 BSA, 0.2% Triton X-100, 5% glycerol, 100ng poly(dI-dC), 4nM of end-labeled fragment, and 2 nM, 4 nM, 8nM, 20nM, 40 nM, 80nM, or 200nM of DnaA-ATP. Reactions were incubated at 37°C for 8 minutes, and then loaded onto a 7% polyacrylamide gel. Complexes were separated by electrophoresis, using 45mM Tris-Borate, 1mM EDTA as the running buffer. Dried gels were scanned on a Bio-Rad Molecular Image FX PhosphorImager.
DMS Modification and Primer Extension
DMS modifications of chromosomal DNA in vivo, and LMPCR were performed as described previously (Nievera et al., 2006). DMS modification of DNA (0.75µg) in vitro was performed as described (McGarry et al., 2004). DnaA was preincubated in reaction buffer with 5 mM ATP for 5 minutes before addition to reactions at the concentrations indicated in the Figures. No other proteins were added to the in vitro reactions. DMS- treated samples were extended with radio-labeled primer as described (McGarry et al., 2004). Two primers were used, a left primer hybridizing at bases 272–290 to analyze top strand modifications of plasmid template, and a right primer hybridizing at bases 124–142 to analyze bottom-strand modifications. Extension products were resolved on 6% polyacrylamide sequencing gels, and dried gels were scanned on a Bio-Rad Molecular Imager FX PhosphorImager. Representative scans are shown in the Figures. Images were analyzed by using Bio-Rad QUANTITY ONE software. Intensities of bands in binding sites (relative to the total intensity of all selected bands) were calculated. Deviations in band intensities among experiments were <10%.
Supplementary Material
Acknowledgements
We thank Payal Pradham and Mohammed Abdulwahhab for their assistance in making the mutant oriC plasmids. We are grateful to all of our colleagues for sharing information with us, and for their many helpful discussions. This work was supported by NIH-GM54042. T.R. was supported by a Graduate Teaching Fellowship from NSF grants DGE 0440529 and 0638702 (Florida Tech InSTEP Program).
References
- Abe Y, Jo T, Matsuda Y, Matsunaga C, Katayama T, Ueda T. Structure and function of DnaA N-terminal domains: specific sites and mechanisms in inter-DnaA interaction and in DnaB helicase loading on oriC. J Biol Chem. 2007;282:17816–17827. doi: 10.1074/jbc.M701841200. [DOI] [PubMed] [Google Scholar]
- Bates DB, Asai T, Cao Y, Chambers MW, Cadwell GW, Boye E, Kogoma T. The DnaA box R4 in the minimal oriC is dispensable for initiation of Escherichia coli chromosome replication. Nucleic Acids Res. 1995;23:3119–3125. doi: 10.1093/nar/23.16.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan JA, Helmstetter CE. DNA sequestration and transcription in the oriC region of Escherichia coli. Mol Microbiol. 1997;26:889–896. doi: 10.1046/j.1365-2958.1997.6221989.x. [DOI] [PubMed] [Google Scholar]
- Bramhill D, Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988;52:743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Cassler MR, Grimwade JE, Leonard AC. Cell cycle-specific changes in nucleoprotein complexes at a chromosomal replication origin. EMBO J. 1995;14:5833–5841. doi: 10.1002/j.1460-2075.1995.tb00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley JF. Regulation of early events in chromosome replication. Curr Biol. 2004;14:R778–R786. doi: 10.1016/j.cub.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Doran KS, Helinski DR, Konieczny I. A critical DnaA box directs the cooperative binding of the Escherichia coli DnaA protein to the plasmid RK2 replication origin. J Biol Chem. 1999;274:17918–17923. doi: 10.1074/jbc.274.25.17918. [DOI] [PubMed] [Google Scholar]
- Duderstadt KE, Mott ML, Crisona NJ, Chuang K, Yang H, Berger JM. Origin remodeling and opening in bacteria rely on distinct assembly states of the DnaA initiator. J Biol Chem. 2010;285:28229–28239. doi: 10.1074/jbc.M110.147975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzberger JP, Mott ML, Berger JM. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat Struct Mol Biol. 2006;13:676–683. doi: 10.1038/nsmb1115. [DOI] [PubMed] [Google Scholar]
- Erzberger JP, Pirruccello MM, Berger JM. The structure of bacterial DnaA: implications for general mechanisms underlying DNA replication initiation. EMBO J. 2002;21:4763–4773. doi: 10.1093/emboj/cdf496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RS, Funnell BE, Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984;38:889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Gille H, Egan JB, Roth A, Messer W. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli. Nucleic Acids Res. 1991;19:4167–4172. doi: 10.1093/nar/19.15.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwade JE, Ryan VT, Leonard AC. IHF redistributes bound initiator protein, DnaA, on supercoiled oriC of Escherichia coli. Mol Microbiol. 2000;35:835–844. doi: 10.1046/j.1365-2958.2000.01755.x. [DOI] [PubMed] [Google Scholar]
- Grimwade JE, Torgue JJ, McGarry KC, Rozgaja T, Enloe ST, Leonard AC. Mutational analysis reveals Escherichia coli oriC interacts with both DnaA-ATP and DnaA-ADP during pre-RC assembly. Mol Microbiol. 2007;66:428–439. doi: 10.1111/j.1365-2958.2007.05930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen FG, Christensen BB, Atlung T. Sequence characteristics required for cooperative binding and efficient in vivo titration of the replication initiator protein DnaA in E. coli. J Mol Biol. 2007;367:942–952. doi: 10.1016/j.jmb.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Hansen FG, Christensen BB, Nielsen CB, Atlung T. Insights into the quality of DnaA boxes and their cooperativity. J Mol Biol. 2006;355:85–95. doi: 10.1016/j.jmb.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Hengen PN, Lyakhov IG, Stewart LE, Schneider TD. Molecular flip-flops formed by overlapping Fis sites. Nucleic Acids Res. 2003;31:6663–6673. doi: 10.1093/nar/gkg877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Akimitsu N, Kashioka T, Hatano M, Kubota T, Ogata Y, Sekimizu K, Katayama T. DiaA, a novel DnaA-binding protein, ensures the timely initiation of Escherichia coli chromosome replication. J Biol Chem. 2004;279:45546–45555. doi: 10.1074/jbc.M402762200. [DOI] [PubMed] [Google Scholar]
- Katayama T, Ozaki S, Keyamura K, Fujimitsu K. Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC. Nat Rev Microbiol. 2010;8:163–170. doi: 10.1038/nrmicro2314. [DOI] [PubMed] [Google Scholar]
- Katayama T, Sekimizu K. Inactivation of Escherichia coli DnaA protein by DNA polymerase III and negative regulations for initiation of chromosomal replication. Biochimie. 1999;81:835–840. doi: 10.1016/s0300-9084(99)00213-8. [DOI] [PubMed] [Google Scholar]
- Kawakami H, Keyamura K, Katayama T. Formation of an ATP-DnaA-specific initiation complex requires DnaA Arginine 285, a conserved motif in the AAA+ protein family. J Biol Chem. 2005;280:27420–27430. doi: 10.1074/jbc.M502764200. [DOI] [PubMed] [Google Scholar]
- Keyamura K, Abe Y, Higashi M, Ueda T, Katayama T. DiaA dynamics are coupled with changes in initial origin complexes leading to helicase loading. J Biol Chem. 2009;284:25038–25050. doi: 10.1074/jbc.M109.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyamura K, Fujikawa N, Ishida T, Ozaki S, Su'etsugu M, Fujimitsu K, Kagawa W, Yokoyama S, Kurumizaka H, Katayama T. The interaction of DiaA and DnaA regulates the replication cycle in E. coli by directly promoting ATP DnaA-specific initiation complexes. Genes Dev. 2007;21:2083–2099. doi: 10.1101/gad.1561207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Nishida S, Emoto A, Sekimizu K, Katayama T. Replication cycle-coordinated change of the adenine nucleotide-bound forms of DnaA protein in Escherichia coli. EMBO J. 1999;18:6642–6652. doi: 10.1093/emboj/18.23.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer U, Richter S, Roth A, Weigel C, Messer W. A comprehensive set of DnaA-box mutations in the replication origin, oriC, of Escherichia coli. Mol Microbiol. 1996;21:301–311. doi: 10.1046/j.1365-2958.1996.6481362.x. [DOI] [PubMed] [Google Scholar]
- Leonard AC, Grimwade JE. Building a bacterial orisome: emergence of new regulatory features for replication origin unwinding. Mol Microbiol. 2005;55:978–985. doi: 10.1111/j.1365-2958.2004.04467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AC, Grimwade JE. Chapter 4.4.1 Initiation of DNA Replication. In: Bock A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Nystrom T, Slauch JM, Squires CL, Ussery D, editors. EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. Washington, DC: ASM Press; 2010. [Google Scholar]
- Li Z, Crooke E. Functional analysis of affinity-purified polyhistidine-tagged DnaA protein. Protein Expr Purif. 1999;17:41–48. doi: 10.1006/prep.1999.1094. [DOI] [PubMed] [Google Scholar]
- Lobner-Olesen A. Distribution of minichromosomes in individual Escherichia coli cells: implications for replication control. EMBO J. 1999;18:1712–1721. doi: 10.1093/emboj/18.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka J, Zakrzewska-Czerwinska J, Messer W. Sequence recognition, cooperative interaction, and dimerization of the initiator protein DnaA of Streptomyces. J Biol Chem. 2001;276:6243–6252. doi: 10.1074/jbc.M007876200. [DOI] [PubMed] [Google Scholar]
- Margulies C, Kaguni JM. Ordered and sequential binding of DnaA protein to oriC, the chromosomal origin of Escherichia coli. J Biol Chem. 1996;271:17035–17040. doi: 10.1074/jbc.271.29.17035. [DOI] [PubMed] [Google Scholar]
- Matsui M, Oka A, Takanami M, Yasuda S, Hirota Y. Sites of dnaA protein-binding in the replication origin of the Escherichia coli K-12 chromosome. J Mol Biol. 1985;184:529–533. doi: 10.1016/0022-2836(85)90299-2. [DOI] [PubMed] [Google Scholar]
- McGarry KC, Ryan VT, Grimwade JE, Leonard AC. Two discriminatory binding sites in the Escherichia coli replication origin are required for DNA strand opening by initiator DnaA-ATP. Proc Natl Acad Sci USA. 2004;101:2811–2816. doi: 10.1073/pnas.0400340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W, Blaesing F, Majka J, Nardmann J, Schaper S, Schmidt A, Seitz H, Speck C, Tüngler D, Wegrzyn G, Weigel C, Welzeck M, Zakrzewska-Czerwinska J. Functional domains of DnaA proteins. Biochimie. 1999;81:819–825. doi: 10.1016/s0300-9084(99)00215-1. [DOI] [PubMed] [Google Scholar]
- Miller DT, Grimwade JE, Betteridge T, Rozgaja T, Torgue JJ, Leonard AC. Bacterial origin recognition complexes direct assembly of higher-order DnaA oligomeric structures. Proc Natl Acad Sci USA. 2009;106:18479–18484. doi: 10.1073/pnas.0909472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott ML, Erzberger JP, Coons MM, Berger JM. Structural synergy and molecular crosstalk between bacterial helicase loaders and replication initiators. Cell. 2008;135:623–634. doi: 10.1016/j.cell.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievera C, Torgue JJ, Grimwade JE, Leonard AC. SeqA blocking of DnaA-oriC interactions ensures staged assembly of the E. coli pre-RC. Mol Cell. 2006;24:581–592. doi: 10.1016/j.molcel.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki S, Ogawa T. Determination of the minimum domain II size of Escherichia coli DnaA protein essential for cell viability. Microbiology. 2008;154:3379–3384. doi: 10.1099/mic.0.2008/019745-0. [DOI] [PubMed] [Google Scholar]
- Oka A, Sasaki H, Sugimoto K, Takanami M. Sequence organization of replication origin of the Escherichia coli K-12 chromosome. J Mol Biol. 1984;176:443–458. doi: 10.1016/0022-2836(84)90171-2. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Kawakami H, Nakamura K, Fujikawa N, Kagawa W, Park SY, Yokoyama S, Kurumizaka H, Katayama T. A common mechanism for the ATP-DnaA-dependent formation of open complexes at the replication origin. J Biol Chem. 2008;283:8351–8362. doi: 10.1074/jbc.M708684200. [DOI] [PubMed] [Google Scholar]
- Prasanth SG, Mendez J, Prasanth KV, Stillman B. Dynamics of pre-replication complex proteins during the cell division cycle. Philos Trans R Soc Lond B Biol Sci. 2004;359:7–16. doi: 10.1098/rstb.2003.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riber, Fujimitsu, Katayama T, Løbner-Olesen Loss of Hda activity stimulates replication initiation from I-box, but not R4 mutant origins in Escherichia coli. Mol Microbiol. 2009;71:107–122. doi: 10.1111/j.1365-2958.2008.06516.x. [DOI] [PubMed] [Google Scholar]
- Roth A, Urmoneit B, Messer W. Functions of histone-like proteins in the initiation of DNA replication at oriC of Escherichia coli. Biochimie. 1994;76:917–923. doi: 10.1016/0300-9084(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Ryan VT, Grimwade JE, Camara JE, Crooke E, Leonard AC. Escherichia coli prereplication complex assembly is regulated by dynamic interplay among Fis, IHF and DnaA. Mol Microbiol. 2004;51:1347–1359. doi: 10.1046/j.1365-2958.2003.03906.x. [DOI] [PubMed] [Google Scholar]
- Schaper S, Messer W. Interaction of the initiator protein DnaA of Escherichia coli with its DNA target. J Biol Chem. 1995;270:17622–17626. doi: 10.1074/jbc.270.29.17622. [DOI] [PubMed] [Google Scholar]
- Sclafani RA, Holzen TM. Cell cycle regulation of DNA replication. Annu Rev Genet. 2007;41:237–280. doi: 10.1146/annurev.genet.41.110306.130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons LA, Felczak M, Kaguni JM. DnaA Protein of Escherichia coli: oligomerization at the E. coli chromosomal origin is required for initiation and involves specific N-terminal amino acids. Mol Microbiol. 2003;49:849–858. doi: 10.1046/j.1365-2958.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- Skarstad K, Lobner-Olesen A. Stable co-existence of separate replicons in Escherichia coli is dependent on once-per-cell-cycle initiation. EMBO J. 2003;22:140–150. doi: 10.1093/emboj/cdg003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck C, Messer W. Mechanism of origin unwinding: sequential binding of DnaA to double- and single-stranded DNA. EMBO J. 2001;20:1469–1476. doi: 10.1093/emboj/20.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepankiw N, Kaidow A, Boye E, Bates D. The right half of the Escherichia coli replication origin is not essential for viability, but facilitates multi-forked replication. Mol Microbiol. 2009;74:467–479. doi: 10.1111/j.1365-2958.2009.06877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel C, Messer W, Preiss S, Welzeck M, Morigen, Boye E. The sequence requirements for a functional Escherichia coli replication origin are different for the chromosome and a minichromosome. Mol Microbiol. 2001;40:498–507. doi: 10.1046/j.1365-2958.2001.02409.x. [DOI] [PubMed] [Google Scholar]
- Weigel C, Schmidt A, Seitz H, Tüngler D, Welzeck M, Messer W. The N-terminus promotes oligomerization of the Escherichia coli initiator protein DnaA. Mol Microbiol. 1999;34:53–66. doi: 10.1046/j.1365-2958.1999.01568.x. [DOI] [PubMed] [Google Scholar]
- Woelker B, Messer W. The structure of the initiation complex at the replication origin, oriC, of Escherichia coli. Nucleic Acids Res. 1993;21:5025–5033. doi: 10.1093/nar/21.22.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung BY, Crooke E, Kornberg A. Fate of the DnaA initiator protein in replication at the origin of the Escherichia coli chromosome in vitro. J Biol Chem. 1990;265:1282–1285. [PubMed] [Google Scholar]
- Zawilak-Pawlik A, Kois A, Majka J, Jakimowicz D, Smulczyk-Krawczyszyn A, Messer W, Zakrzewska-Czerwińska J. Architecture of bacterial replication initiation complexes: orisomes from four unrelated bacteria. Biochem J. 2005;389:471–481. doi: 10.1042/BJ20050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind JW, Smith DW. The bacterial origin of replication, oriC. Cell. 1986;46:489–490. doi: 10.1016/0092-8674(86)90873-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.