Abstract
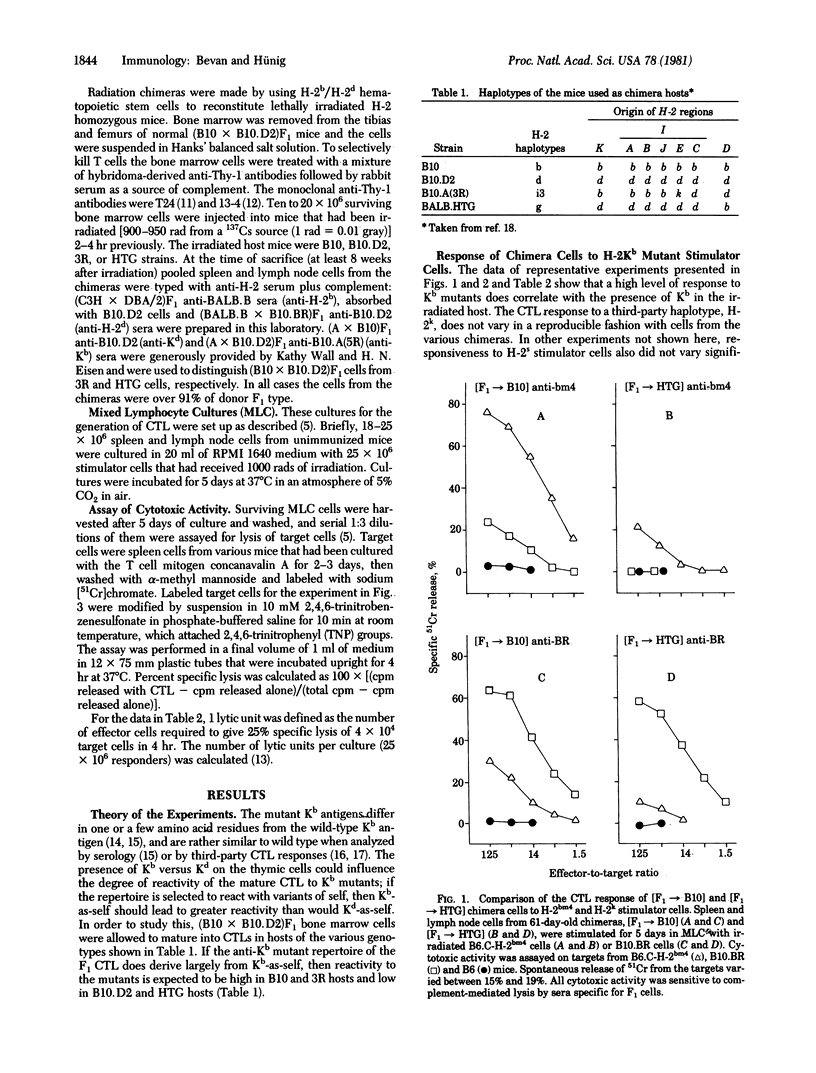
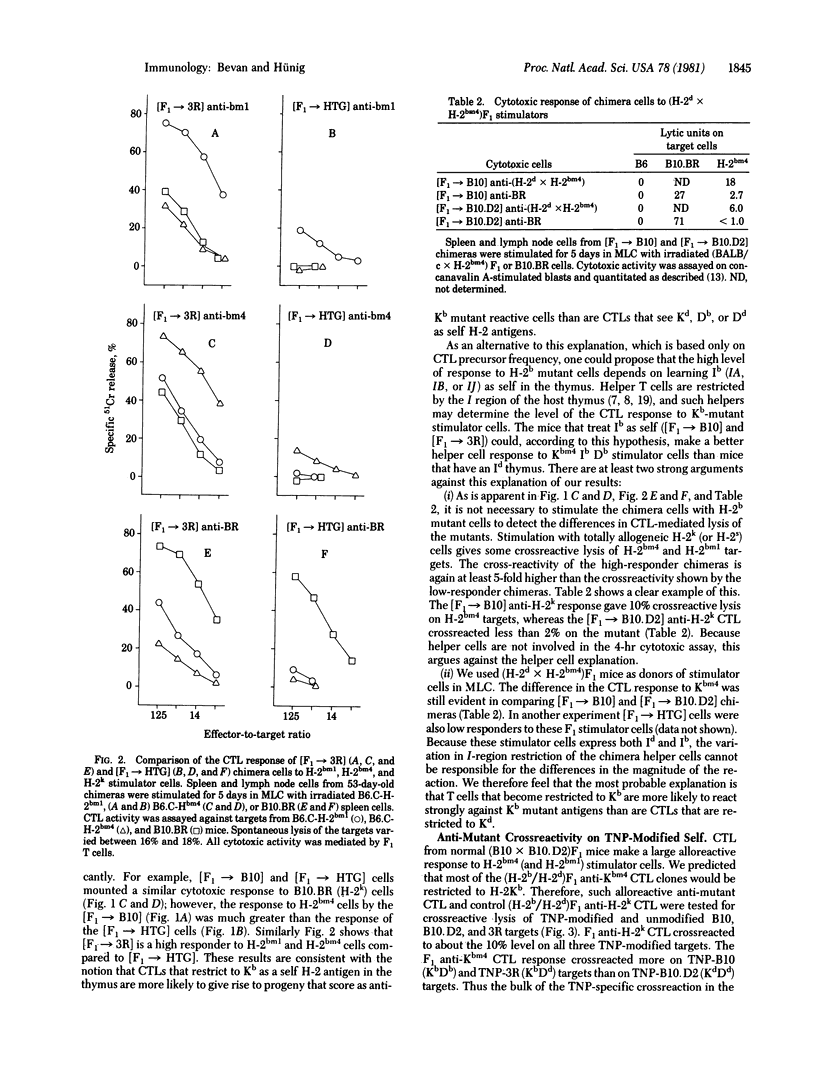
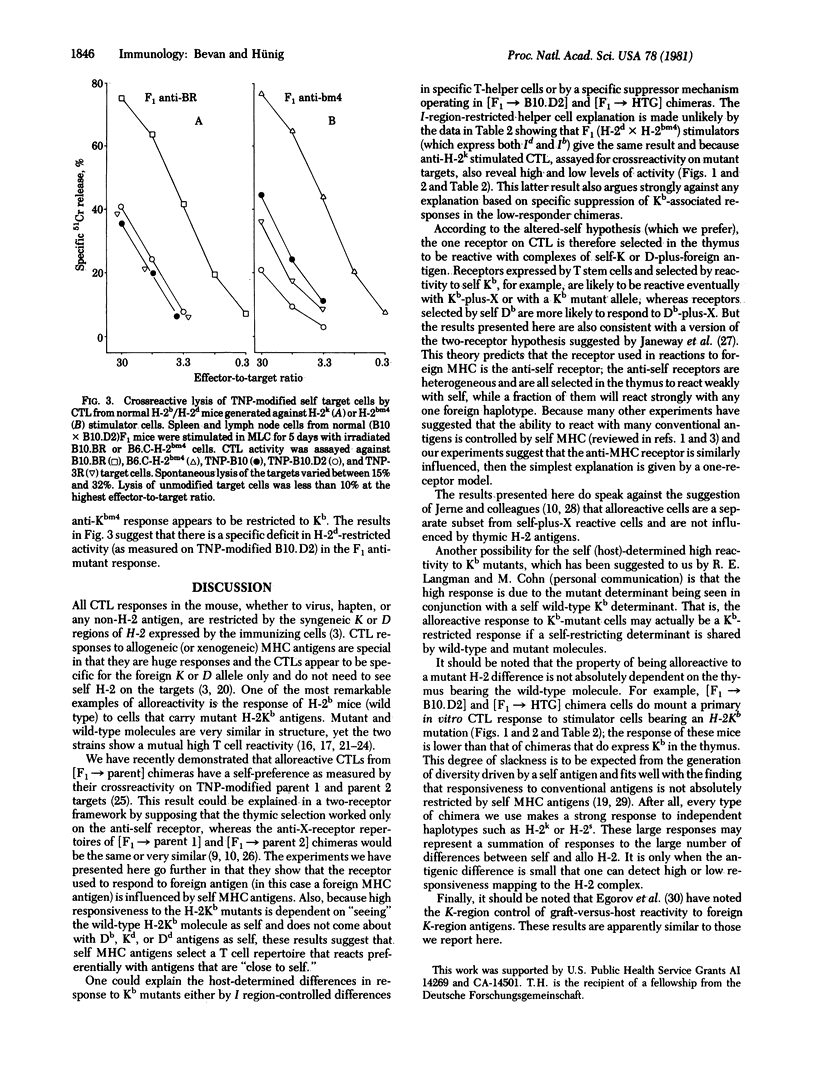
We have constructed bone marrow irradiation chimeras to investigate the influence of self antigens on the specificity of the T lymphocyte receptor repertoire. Bone marrow cells from (A X B)F1 mice heterozygous for the major histocompatibility genes were allowed to mature into T cells in irradiated parent A or parent B strains. More than 8 weeks after irradiation, when the lymphoid system had regenerated from the F1 stem cells, the degree of T cell reactivity to mutant major histocompatibility antigens, A', was assessed. It was found that T cells that had matured in the irradiated A mice, [F1 leads to A] chimeras, responded better to A' antigen than did T cells from the [F1 leads to B] chimeras. Because the mutant histocompatibility antigen A' is very similar in structure to A, differing only by one or a few residues, this suggests that the T cell repertoire in [F1 leads to parent] chimeras reacts preferentially with foreign antigens that are slight variants of the self antigens expressed on radiation-resistant cells--probably cells in the thymus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J., Fink P. J. The influence of thymus H-2 antigens on the specificity of maturing killer and helper cells. Immunol Rev. 1978;42:3–19. doi: 10.1111/j.1600-065x.1978.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977 Sep 29;269(5627):417–418. doi: 10.1038/269417a0. [DOI] [PubMed] [Google Scholar]
- Bevan M. J., Langman R. E., Cohn M. H-2 antigen-specific cytotoxic T cells induced by concanavalin A: estimation of their relative frequency. Eur J Immunol. 1976 Mar;6(3):150–156. doi: 10.1002/eji.1830060303. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Andrew M. E. Primary anti-viral cytotoxic T-cell responses in semiallogeneic chimeras are not absolutely restricted to host H-2 type. J Exp Med. 1979 Feb 1;149(2):535–538. doi: 10.1084/jem.149.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M., Epstein R. T-cell inhibition of humoral responsiveness. II. Theory on the role of restrictive recognition in immune regulation. Cell Immunol. 1978 Aug;39(1):125–153. doi: 10.1016/0008-8749(78)90089-8. [DOI] [PubMed] [Google Scholar]
- Dennert G., Hyman R., Lesley J., Trowbridge I. S. Effects of cytotoxic monoclonal antibody specific for T200 glycoprotein on functional lymphoid cell populations. Cell Immunol. 1980 Aug 1;53(2):350–364. doi: 10.1016/0008-8749(80)90335-4. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Bevan M. J. H-2 antigens of the thymus determine lymphocyte specificity. J Exp Med. 1978 Sep 1;148(3):766–775. doi: 10.1084/jem.148.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hünig T., Bevan M. J. Self H-2 antigens influence the specificity of alloreactive cells. J Exp Med. 1980 May 1;151(5):1288–1298. doi: 10.1084/jem.151.5.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Wigzell H., Binz H. Two different VH gene products make up the T-cell receptors. Scand J Immunol. 1976;5(9):993–1001. doi: 10.1111/j.1365-3083.1976.tb03051.x. [DOI] [PubMed] [Google Scholar]
- Langman R. E. Cell-mediated immunity and the major histocompatibility complex. Rev Physiol Biochem Pharmacol. 1978;81:1–37. doi: 10.1007/BFb0034090. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F., Bach F. H. Human lymphocytes recognise mouse alloantigens. Nature. 1975 Apr 17;254(5501):607–609. doi: 10.1038/254607a0. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Paul W. E., Benacerraf B. Functional specificity of thymus- dependent lymphocytes. Science. 1977 Mar 25;195(4284):1293–1300. doi: 10.1126/science.320663. [DOI] [PubMed] [Google Scholar]
- Pimsler M., Forman J. Estimates of the precursor frequency of cytotoxic T lymhocytes against antigens controlled by defined regions of the H-2 gene complex: comparison of the effect of H-2 differences due to intra-H-2 recombination vs mutation. J Immunol. 1978 Oct;121(4):1302–1305. [PubMed] [Google Scholar]
- Pimsler M., Forman J. Use of H-2 mutations to quantitate alloreactivity: Alloreactivity is strongest against H-2 antigens which are closet to self. Immunogenetics. 1980;11(2):111–121. doi: 10.1007/BF01567777. [DOI] [PubMed] [Google Scholar]
- Sprent J. Role of H-2 gene products in the function of T helper cells from normal and chimeric mice in vivo. Immunol Rev. 1978;42:108–137. doi: 10.1111/j.1600-065x.1978.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Waldmann H. The influence of the major histocompatibility complex on the function of T-helper cells in antibody formation. Immunol Rev. 1978;42:202–223. doi: 10.1111/j.1600-065x.1978.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Widmer M. B., MacDonald H. R. Cytolytic T lymphocyte precursors reactive against mutant Kb alloantigens are as frequent as those reactive against a whole foreign haplotype. J Immunol. 1980 Jan;124(1):48–51. [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Haas W., Jerne N. K. Major histocompatibility complex-linked immune-responsiveness is acquired by lymphocytes of low-responder mice differentiating in thymus of high-responder mice. Proc Natl Acad Sci U S A. 1978 May;75(5):2439–2442. doi: 10.1073/pnas.75.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]



