Abstract
Purpose
The severe reduction of salivary function (xerostomia) is a common complication following radiation therapy for head and neck cancer. Consequently, guidelines to ensure adequate function based on parotid gland tolerance dose-volume parameters have been suggested by the QUANTEC group (1) and by Ortholan et al. (2). We perform a validation test of these guidelines against a prospectively collected dataset and compared to a previously published dataset.
Method and Materials
Whole-mouth stimulated salivary flow data from 66 head and neck cancer patients treated with radiotherapy at the British Columbia Cancer Agency (BCCA) were measured, and treatment planning data were abstracted. Flow measurements were collected from 50 patients at 3 months, and 60 patients at 12 month follow-up. Previously published data from a second institution (WUSTL) were used for comparison. A logistic model was used to describe the incidence of grade 4 xerostomia as a function of the mean dose of the spared parotid gland. The rate of correctly predicting the lack of xerostomia (negative predictive value, NPV) was computed for both the QUANTEC constraints and Ortholan et al. (2) recommendation to constrain the total volume of both glands receiving more than 40 Gy to less than 33%.
Results
Both data sets showed a rate of xerostomia < 20 % when the mean dose to the least-irradiated parotid gland is kept below 20 Gy. Logistic model parameters for the incidence of xerostomia at 12 months after therapy, based on the least-irradiated gland, were D50=32.4 Gy and and γ=0.97. NPVs for QUANTEC guideline were 94% (BCCA data), 90% (WUSTL data). For Ortholan et al. (2) guideline NPVs were 85% (BCCA), and 86% (WUSTL).
Conclusion
This confirms that the QUANTEC guideline effectively avoids xerostomia, and this is somewhat more effective than constraints on the volume receiving more than 40 Gy.
Keywords: radiotherapy, xerostomia, parotid gland, head and neck cancer, dose-volume effects
Introduction
Reduction in the ability to produce saliva (xerostomia) is a common toxicity associated with radiation therapy (RT) of head and neck (HN) cancers (1). In particular, a reduction in stimulated salivary flow, which is often permanent, negatively affects patient quality of life. Modern RT techniques, in particular intensity modulated radiation therapy (IMRT), enable highly conformal dose distributions that can selectively spare critical organs at risk, such as parotid salivary glands.
Recent reviews are available which discuss the symptomatic and clinical impact of xerostomia. Additionally, the dose-volume response for measured salivary function has been investigated with various methods, including salivary gland scintigraphy (3, 4) collecting saliva from individual glands using catheters (5); or expectoration following oral stimulation (6). Clinically significant xerostomia is usually defined according to the LENT-SOMA analytic criteria: a grade 4 complication is defined as a reduction in salivary flow to below 25% of the pre-RT baseline. Measurement noise is typically non-negligible.
For glands which are irradiated to intermediate doses, some recovery is often observed, up to two years after radiotherapy. A strong dose-volume effect is apparent: mean doses to the parotid glands correlate with reduction in stimulated salivary flow (6). Despite qualitative consistency, estimates differ of the mean dose required to cause a 50% rate of complications. Partly this is due to a difference in definitions: some studies have defined a complication as relating to single gland output, whereas other studies focus on imaging surrogates for salivary flow or, whole-mouth salivary flow (similar to the LENT-SOMA definition).
A recent concerted effort by radiation oncologists, medical physicists, and statistical modelers, QUANTEC, reviewed and summarized normal tissue toxicity data, and, in some cases, suggested dose-volume treatment planning guidelines likely to be associated with low rates of complications. As such, consensus guidelines are often limited by the available data, and their validity has to be tested on independent datasets, which have not been used in generating these guidelines.
Ideally, treatment planning guidelines should be simple to understand, easy to implement, and relate closely to the clinical problem. The QUANTEC guideline to limit the probability of severe xerostomia fit these criteria: at least one parotid gland should receive less than 20 Gy mean dose, or both parotid glands should receive less than 25 Gy mean dose. As it is rare for both parotid glands to receive between 20 and 25 Gy mean dose, we simplify this to a 20 Gy spared-gland rule. This guideline would be easy to use for both treatment plan optimization (‘constrain the contra-lateral parotid gland to less than 20 Gy’) as well as treatment plan review. We believe this guideline would be easier to implement than incorporating a complex normal tissue model into the planning process. In this study, we test the effectiveness of this guideline as a correlate to a low rate of xerostomia using a prospectively collected dataset at the British Columbia Cancer Agency (BCCA; n = 66); this dataset was not used to formulate the QUANTEC recommendations. The previously published Washington University in St. Louis (WUSTL) data, used to help formulate the QUANTEC guideline, is provided for comparison.
Of interest, Ortholan et al. (2) have recently produced a treatment planning guideline (after the QUANTEC review was completed), based on sophisticated modeling of their whole mouth stimulated salivary measurements. Ortholan et al. (2) recommend that no more than 1/3 of the spared parotid gland should receive more than 40 Gy mean dose; this planning criteria will be compared with the modified QUANTEC guideline.
Methods and Materials
WUSTL cohort
The WUSTL patient cohort has previously been described in detail (6) and is therefore only summarized here: head and neck patients (HN) had stimulated whole mouth saliva collected pre-radiotherapy as well as various time intervals; for the purposes of this analysis, we used the 1 yr. follow-up time point (n=29). All measurements were captured prospectively. Treatment technique was either 3-D conformal therapy or IMRT.
British Columbia Cancer Agency cohort
66 HN cancer patients who received RT at the Vancouver Cancer Centre, BCCA, between February 2005 and April 2008 were used in this study. The prospective collection of salivary flow measurements is standard practice for HN patients at the BCCA; patients consenting to RT also consent to dental follow-up, including salivary flow measurements. A research ethics board approval was obtained to analyze and publish the data. Patients diagnosed with a HN malignancy who received high dose radiotherapy to the pharynx and neck were eligible for this study. Unilateral and bilateral volumes were included; all patients underwent CT-planning with a conformal field or IMRT delivery technique (Eclipse, Varian, Palo Alto, CA). Table 1 summarizes this patient cohort.
Table 1.
Characteristics of the BCCA cohort.
| Patients | 66 |
| Male/Female | 44 (67%)/22 (33%) |
| Mean age at RT (range), years | 59.2 (19–84.9) |
| Chemotherapy Yes/No | 26 (39%)/40 (61%) |
| Tumor subsite | 5 (7.6%) Hypopharynx |
| 3 (4.5%) Larynx | |
| 17 (25.8) Nasopharynx | |
| 9 (13.6%) Oral cavity | |
| 12 (18.2%) Oropharynx – tonsil | |
| 12 (18.2%) Oropharynx – base of tongue | |
| 8 (12.1%) – Other | |
| Treatment technique | 23 (35 %) Bilateral parallel-opposed pair |
| 22 (33%) IMRT | |
| 21 (32%) Unilateral | |
| Dose, Gy /# fractions | 2 (3%) 40/15 |
| 1 (1.5%) 47.5/25 | |
| 3 (5%) 50/25 | |
| 1 (1.5%) 52.5/20 | |
| 2 (3%) 55/20 | |
| 1 (1.5%) 55/25 | |
| 15 (23%) 60/25 | |
| 5 (8%) 60/30 | |
| 1 (1.5%) 60/35 | |
| 7 (11%) 66/33 | |
| 28 (42%) 70/35 | |
| Measurements at 3 months | 50 |
| 27 (54%) Salivary flow ≤ 25% baseline (grade 4 xerostomia) | |
| 23 (46%) Salivary flow > 25% baseline | |
| Measurements at 1 year | 60 |
| 20 (33%) Salivary flow ≤ 25% baseline (grade 4 xerostomia) | |
| 40 (67%) Salivary flow > 25% baseline | |
Exclusion criteria included previous radiotherapy to the neck, the use of direct electron fields to the primary site, surgery involving a parotid gland, the use of multiple planning CT datasets during the course of RT, missed follow-up appointments or failure to comply with the saliva collection protocol. One patient underwent pre-RT bilateral neck dissection, but no salivary glands were removed. Three patients received an ipsilateral neck dissection post-RT, which included submandibulectomy. These patients were not censored. Patients receiving dose to parotid glands from scattered radiation only were excluded from the analysis. The study cohort of 66 patients is a subset of 88 patients initially enrolled to this study.
Radiation therapy
All patients scheduled for HN radiotherapy were seen in the Department of Oral Oncology to review the oral complications of therapy and any needed dental extractions. Patients then had 3.2 mm thick commercial thermoplastic immobilization shells constructed whilst in the supine position. All CT datasets were obtained in the treatment position with a slice thickness 2.5 or 3 mm
The choice of treatment laterality and technique depended on the patient's disease configuration. Well lateralized primary tumors with small bulk disease in the ipsilateral neck at low risk for contralateral disease were treated using a unilateral, gland sparing, technique. Despite this, at least some portion of the contralateral parotid gland was in the beam’s eye view of at least one field for all patients treated with this technique. Midline tumours, or patients with bulky unilateral or contralateral neck disease were treated with a bilateral parallel-opposed pair (BLPOP) technique. Shielding on lateral ports was conformed to planning target volume delineated on CT. Additional anterior neck fields contributed a minimal amount of dose to the parotid glands (typically < 2% of the prescription dose), and so were not explicitly included in dose summations. Cord blocks combined with skin-matched electron fields were used for 16 of 23 patients treated with BLPOP, limiting the spinal cord dose below 46 Gy; 9 or 12 MeV electrons were used (SSD=115 cm). In beam’s eye view (BEV) electron fields either abutted or slightly overlapped (~0.5 cm) the posterior-inferior edge of a parotid gland. Based on electron beam scanning data in a water phantom the mean dose to the parotids from electrons was calculated as less than 1.5 Gy. Mean parotid gland doses in BLPOP plans were always above 20 Gy from photons alone (average 47.1 Gy); therefore, dose from electrons was omitted.
IMRT was first used in this clinic in 2002. For patients requiring bilateral radiotherapy, there has been a gradual transition from parallel-opposed fields to IMRT, initially for nasopharyngeal tumours followed by base of tongue and thyroid cancers. IMRT is now considered standard therapy for midline tumours. Patients were evenly distributed between the three techniques (see Table 1.) Irrespective of the treatment technique, RT was delivered one fraction per weekday.
Measurements of saliva flow
The protocol to measure salivary flow closely follows the WUSTL protocol (6, 7). In brief, patients refrained from consuming food for at least one hour prior to measurement. Both un-stimulated and stimulated whole saliva flow measurements were performed in a span of 5 min, the latter by chewing on a paraffin block. Saliva was collected in a pre-weighed cup and the flow was calculated as a proportion relative to pre-RT measurement. Only stimulated flow data, which have generally demonstrated higher correlations to dose-volume parameters vs. un-stimulated measurements, were used in this study. Baseline measurements were performed prior to commencement of RT, one to two weeks before the first fraction. Two post-RT measurements were scheduled, the first at three months and the second at one year after completing RT. Overall, 50 measurements were available for the 3 months follow-up; the median time between completion of RT and the measurement was 95 days. For the one year follow-up, 60 patients produced samples; the median follow-up was 14.8 months. Both measurements were available for 44 patients. The LENT-SOMA criteria was applied to define clinical grade xerostomia: salivary flow of 25% or less compared to pre-RT was scored as complication. The raw incidence of complications at 3 months and one year after completion of RT is shown in Table 1. As is typical for reports on this endpoint, actuarial methods are not used; any potential sampling bias due to lack of complete follow-up is thought to be minimal for this toxicity endpoint, although it cannot be ruled out.
Dose-volume data extraction
All planning data sets, i.e., CT, RT structures, and associated RT plans and dose matrices, were anonymized prior to export for analysis. Both parotid glands were contoured by a qualified radiation oncologist specializing in HN RT and further validated by a principal investigator from radiation oncology (JW). For patients who showed gross artifacts on their CT scans from tooth fillings, regions with artifacts were assigned unit density. Dose was calculated on a 2.5 mm grid and mean doses generated by Eclipse treatment planning system (Varian, Palo Alto, CA) was recorded. Plans were later converted to the Computational Environment for Radiotherapy Research (CERR) format (8). Mean doses were recalculated and checked against original plan data for consistency, and agreement was on average within 3% except for mean doses from 2 to 3 Gy in which case agreement was within 0.15 Gy.
Dose-response modeling based on the spared parotid gland
For patients with a pronounced laterality of the primary tumor, the contralateral parotid gland was always spared to a lower mean dose. For patients treated with BLPOP, designating a spared parotid in treatment planning was not always obvious, and depended on the proximity/overlap of high dose PTV and a parotid gland. In IMRT planning, if both parotid glands could not be spared, the objective of keeping the mean dose to one gland below 26 Gy was invoked, and subsequently this gland is considered the ‘spared’ gland for our analysis. For patients receiving comparable doses to both parotid glands the gland receiving the smaller mean dose was nominally deemed the spared gland for this analysis.
A logistic model was used to fit dose-response for the incidence of severe xerostomia (post-treatment saliva flow < 25% of pre-treatment flow) as a function of mean dose to the spared parotid gland, as reported by the planning system:
| (1) |
where P is the rate of complications; D is mean dose to the spared parotid gland; D50 is the dose predicting a 50% risk of complications; and γ50 is normalized slope, i.e., change in P in units of percent per 1% change in dose (9). The best-fitting values for D50 and γ50 were found using maximum likelihood analysis, and the 95% confidence intervals were found using the profile likelihood method (10). Although fitting used individual data points, figures group patient mean doses in bins of 5 Gy width.
We also report negative predictive power (NPP), which gives the rate of avoidance of xerostomia when the guideline is fulfilled based on dose-volume data converted to CERR. A high NPP would support the validity of a guideline, though plans near the guideline threshold are certainly less risky than those far from the threshold. This analysis was performed for both QUANTEC (1) and Ortholan et al. (2) guidelines.
Results
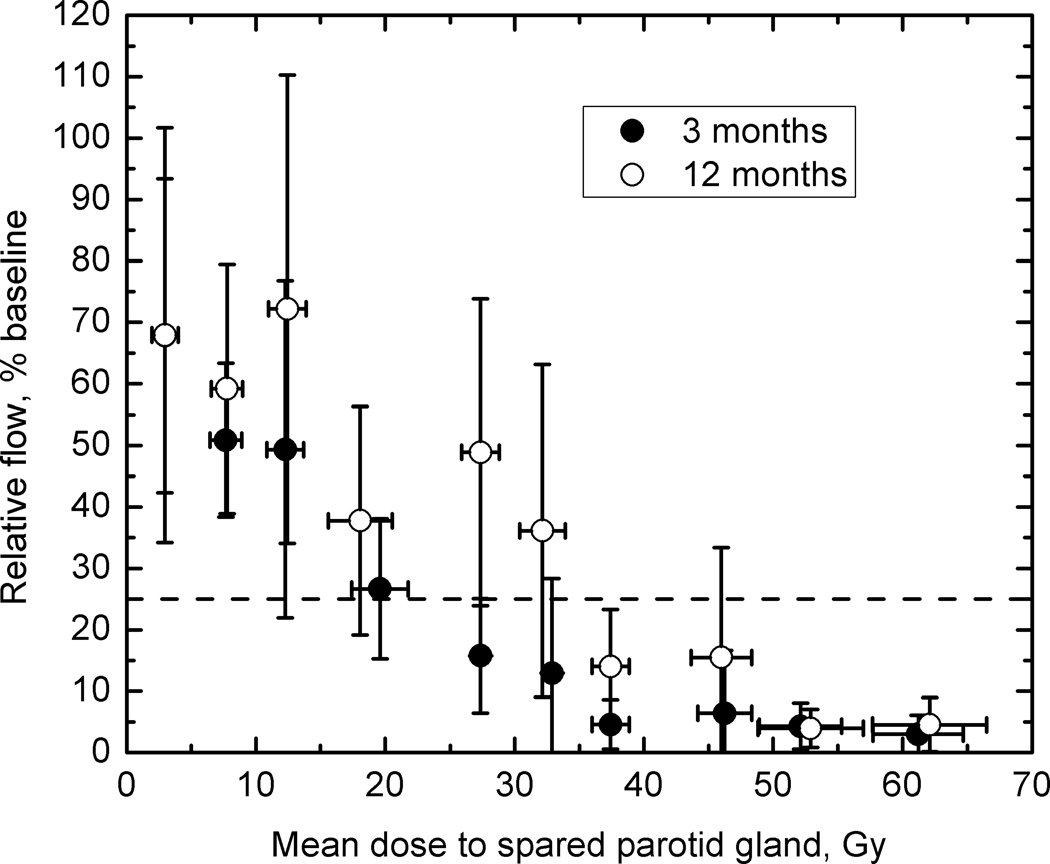
Figure 1 shows mean salivary flow as a function of dose to the spared parotid gland. At 3 months follow-up, the flow decreases to below 25%, which is a definition of grade 4 xerostomia, at doses to the spared parotid of 20 Gy. The figure shows that there is a recovery of salivary function between 3 months and 1 year except for doses in excess of 50 Gy. At 1 year follow-up a dose to the spared parotid gland of approximately 35 Gy causes the relative flow to decrease to 25%.
Figure 1.
Whole stimulated salivary flow measured at 3 and 12 months, relative to the pre-RT baseline (%), as a function of mean dose to the (most) spared parotid gland. Error bars show standard deviations in binned patient cohorts. The horizontal dashed line indicates the 25% flow (the threshold for grade 4 xerostomia).
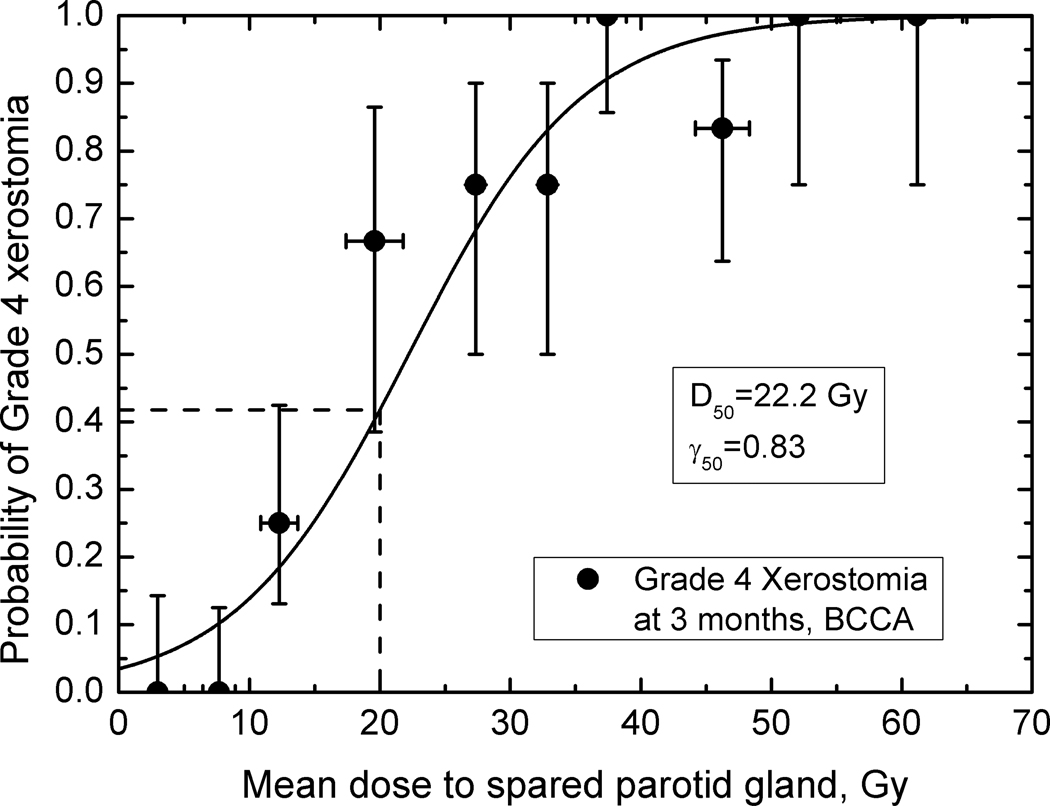
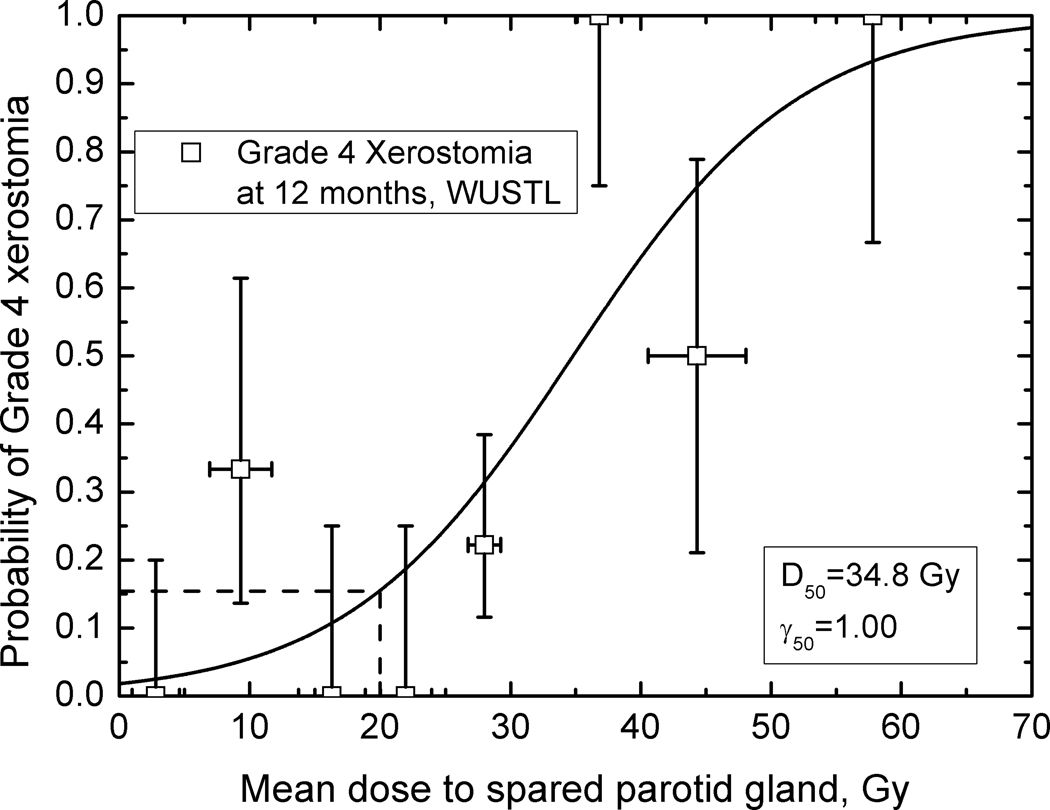
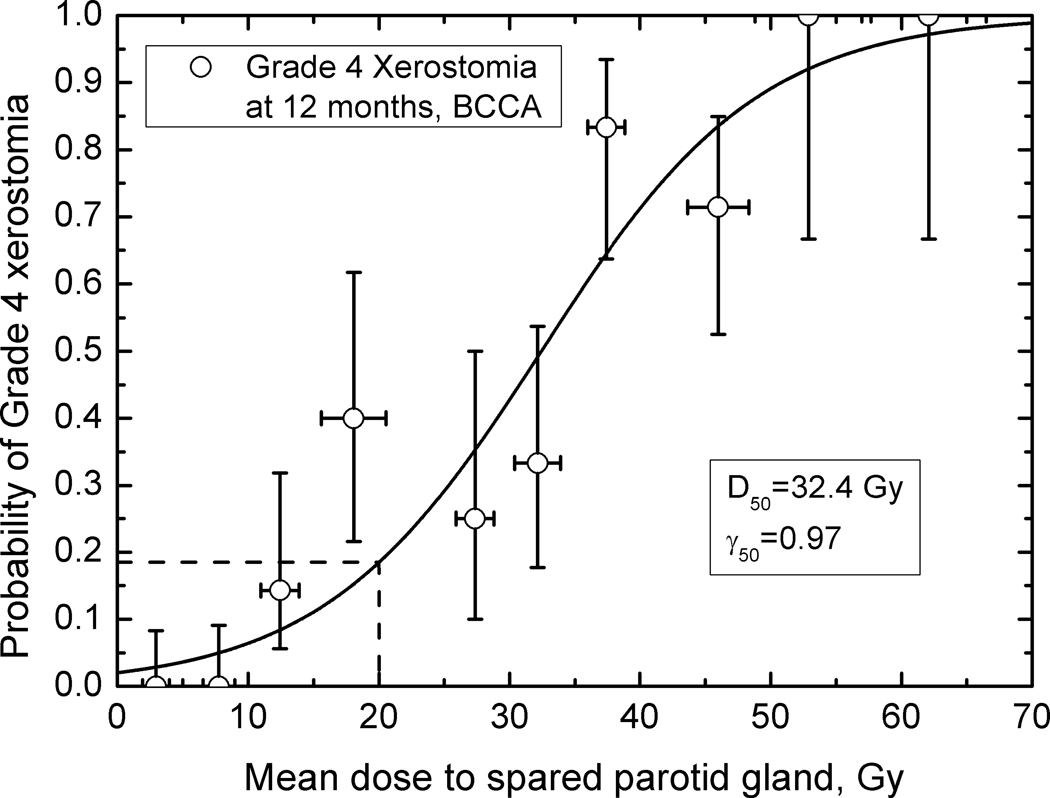
Figures 2 – 4 show observed data and fitted dose-response curves for incidence of xerostomia at 3 and 12 months after completion of RT. Fitted parameters are given in Table 2. There is a 10 Gy difference in D50 values for the fitted BCCA data: 32.4 Gy at 12 and 22.2 Gy at 3 months, indicating recovery. For plans consistent with the modified-QUANTEC recommendation, the incidence of grade 4 xerostomia is 42 % at 3 months but decreases to 18 % at 12 months. In comparison, at 25 Gy to the spared gland, the incidence is 60% and 29% at 3 and 12 months, respectively. Agreement between model parameters obtained for the BCCA and WUSTL data at 12 months is excellent, despite only using the spared parotid gland mean dose. The incidence of grade 4 xerostomia predicted based on the WUSTL data, at 1 yr. follow-up, is 15% at 20 Gy and 24% at 25 Gy mean dose to the spared parotid. Both data sets therefore show a rate of xerostomia < 20 % when the mean dose to the spared parotid is kept below 20 Gy.
Figure 2.
The fitted incidence of grade 4 xerostomia (salivary flow 25% or less relative to pre-RT) at three months as a function of mean dose to spared parotid gland; BCCA data. Error bars on dose values are standard deviations; error bars on incidence values are 68% confidence limits. The dashed line indicates the estimated rate of xerostomia at 20 Gy, 42%.
Figure 4.
The fitted incidence of grade 4 xerostomia (salivary flow 25% or less relative to pre-RT) at twelve months as a function of mean dose to spared parotid gland; WUSTL data. Error bars on dose values are standard deviations, error bars on incidence values are 68% confidence limits. The dashed line shows the estimated incidence of xerostomia at 20 Gy, 15%.
Table 2.
Dose-response parameters for incidence of grade 4 xerostomia as a function of mean dose to the spared parotid gland.
| Follow-up, months, data | D50, Gy (95% CI) | γ50 (95% CI) |
|---|---|---|
| 3, BCCA | 22.2 (16.4, 28.4) | 0.83 (0.44, 1.36) |
| 12, BCCA | 32.4 (26.3, 39.9) | 0.97 (0.58, 1.53) |
| 12, WUSTL | 34.8 (26.7,52.8) | 1.00 (0.41, 1.93) |
An overall summary of the ability of the 20 Gy spared-parotid-mean-dose rule to predict a low rate of xerostomia is given in Figure 5. BCCA and WUSTL data are both shown. Both datasets show a very low incidence of xerostomia when the spared-parotid-mean-dose is below 20 Gy. The negative predictive values are: 94% (BCCA), 90% (WUSTL) and 93% for combined data.
Figure 5.
Overall summary of the QUANTEC (1) guideline (shown as solid lines) applied to the BCCA and WUSTL data. As seen, the rate of xerostomia for plans meeting the QUANTEC guideline is low, resulting in NPV’s of 94% for the BCCA data and 90% for the WUSTL data.
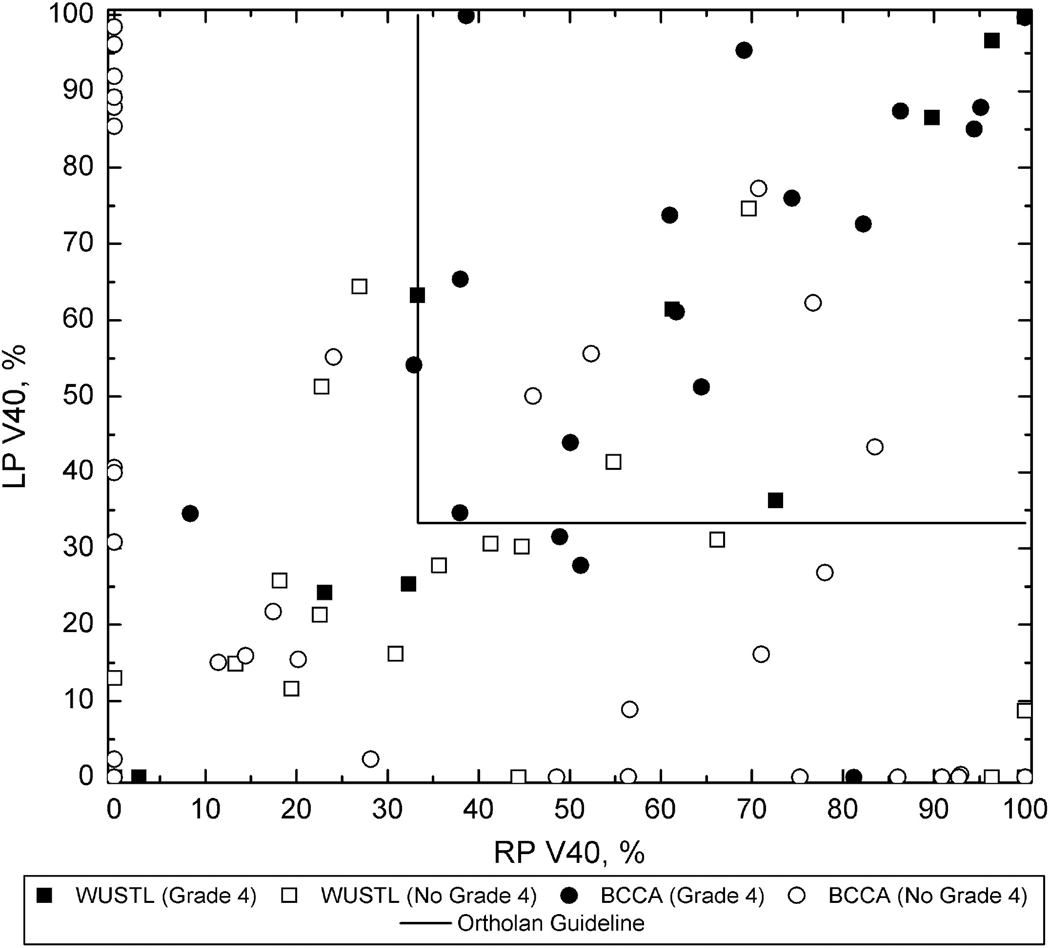
The Ortholan guideline (V40 less than 33%) is similarly summarized in Figure 6. It can be seen that the negative predictive power, 85% (BCCA), 86% (WUSTL) and 85% (combined data) is lower than the QUANTEC rule NPV, though still substantial.
Figure 6.
Overall summary of the Ortholan et al. (2) guideline (shown as solid lines) applied to the BCCA and WUSTL data. The rate of xerostomia for plans meeting the guideline is low, but not as low as for the QUANTEC guideline (Figure 5). The NPV is 85% for the BCCA data and 86% for the WUSTL data.
Discussion
Thresholds vs. treatment planning guidance
The 20 Gy spared-gland-mean-dose rule as published by Deasy et al. (1) was meant to encapsulate a guideline which, if met, would imply a low rate of xerostomia. Hence, it was not meant to be the ‘best estimate’ of the 50% rate of xerostomia, which clearly resides at higher dose values. Accordingly, Figure 3 gives an estimated rate of xerostomia of 18% for those plans consistent with the 20 Gy lesser-mean-dose rule. This rate is consistent with the WUSTL data shown in Figure 4.
Figure 3.
The fitted incidence of grade 4 xerostomia (salivary flow 25% or less relative to pre-RT) at twelve months as a function of mean dose to spared parotid gland; BCCA data. Error bars on dose values are standard deviations; error bars on incidence values are 68% confidence limits. The dashed line shows the estimated incidence of xerostomia at 20 Gy, 18%.
The usefulness of focusing on the spared parotid gland is emphasized in Figures 1–4, which show an orderly and predictable dependence of overall whole-mouth salivary function on the mean dose to the (mostly highly) spared gland. Dose-response parameters for incidence of grade 4 threshold at 12 months are consistent between two datasets, Figures 3 and 4. This leads to a consistent prediction of grade 4 xerostomia at 20 Gy to the most spared gland, 18% for the BCCA and 15% for the WUSTL patient cohorts.
Note that it is undoubtedly the case that some differences between the institutional datasets remain. In particular, treatment setup and technique, including beam directions and the resulting impact on spatial dose distribution characteristics, should not be assumed to be strictly equivalent between the two datasets. Although the contouring approach appears similar on visual review, BCCA parotid gland volumes are on average 15% larger than WUSTL gland volumes, indicating that some differences in contouring exist. Despite presumptive differences, the overall consistency is remarkable between the datasets, as shown in Figures 3 and 4, and the resulting model parameters are in excellent agreement (Table 2).
Although whole mouth salivary function has been shown to relate to quality of life measurement questionnaires (6), we note that the 25% threshold has not itself been validated as particularly crucial. Perhaps the weakest point of this entire approach is to focus on such a threshold, which is somewhat artificial although clearly related to patient outcomes. Measurement noise, reported as 30% by Blanco et al., (6) should also be kept in mind, and clearly affects the results. If a better threshold, or if a ‘transition zone’ of poor ratio values is identified in relation to QOL results, then treatment planning guidelines will need to be updated. More research in this area is needed.
Radiotherapy planning technique likely has some influence on parotid sparing, particularly when bilateral treatment is required. Ipsilateral radiotherapy provides the optimal opportunity to spare the contralateral gland, whereas IMRT allows for intermediate sparing. In contrast, none of 23 patients treated with BLPOP had any parotid gland spared to mean dose < 20 Gy (versus 8 of 22 IMRT). While these data are indicative of benefits of IMRT it should be noted that no attempt to spare parotid glands in BLPOP planning was made. Treatment planning for IMRT patients was done prior to QUANTEC publication, therefore this QUANTEC compliance in the IMRT group is a result of putting planning constraints on a parotid gland, but not with a 20 Gy mean dose objective in mind.
The reference follow-up time of the prediction is important
As noted in review articles (1, 11), salivary function tends to recover unless both glands receive high doses, and recovery, when present, is not complete for up to 2 years. Recovery of damaged glandular function likely involves stem cell rescue, as shown by the Groningen group using transplanted stem cells in rat parotid glands (12). We therefore emphasize that we are testing a prediction rule for 12 month follow-up, and that higher doses could be tolerated if referencing 2 year or greater follow-up times. The recovery in fractional stimulated salivary function between 6 months and 12 months is sizable (e.g., from an average of 0.30 to 0.41 for stimulated whole mouth salivary function in the series reported recently by Kam et al. (13)).
Models vs. threshold rules
The focus of this paper is the test of the QUANTEC guideline that is simple enough to be used clinically. Models that predict post-treatment salivary function level, while crucial in the search for a detailed understanding of xerostomia, may not be necessary for treatment planning and, moreover, are usually not available in treatment planning systems. The QUANTEC 25/20 rule is here simplified to a 20/20 rule because we observe very few plans which have both parotid mean doses falling between 20 and 25 Gy. The rule focuses on the spared parotid gland, similar to the recent report by Ortholan et al. (2), which found that the best prediction rule to avoid xerostomia was to keep the volume of the spared parotid receiving more than 40 Gy to less than 1/3 of the total volume.
The precise threshold at which xerostomia becomes more likely is not a precise value, as shown by Figure 5 and the variation around the logistic fit shown in Figures 2–4. 20 Gy should therefore not be taken as an inviolable rule, mean doses in the range of 20–25 Gy will also produce low rates of xerostomia below 30%.
The relationship to single-gland NTCP modeling
The NTCP modeling by Houweling et al. (14), which combined the Utrecht and Michigan data, applies to single parotid glands alone. Strictly speaking, this is not NTCP modeling per se, as complications arise only from the combination of all gland contributions. To relate those calculations to whole mouth salivary function measurements, the potential contribution of submandibular glands must also be considered. Submandibular glands contribute to stimulated saliva as well as salivary function at rest. Although the precise contribution of submandibular glands to stimulated saliva is uncertain, several references put this at 15–30% (15, 16). The surgical transfer of submandibular glands outside the radiation field, and their surgical return after therapy, has been shown to significantly reduce grade four xerostomia for stimulated function, confirming their important role (15, 16). In these two cohorts, all patients received significant doses to their submandibular glands, usually 50 Gy or more due to nearby subdigastric nodes, targeted as potential paths of disease spread. Assuming submandibular glands contribute 30–40% of stimulated salivary function, but this is lost due to high local doses, then the parotid gland function needs only be reduced by factors of 60–65%, rather than 75%, to reach the whole mouth xerostomia threshold. Instead of a threshold of 39 Gy (reported by Houweling et al.(14) for a 75% reduction), the threshold for 60–65% reduction is more relevant to our results, but is not readable directly from their single gland modeling results. This may explain why our D50 parameters are smaller (32.4 and 34.8 Gy), but a direct comparison is not available.
The submandibular glands are adjacent to the Level 1B/2 lymph nodes; if this lymph node region is an intended target, then it is very difficult to spare the adjacent gland(s). Furthermore, the minor salivary glands are distributed throughout the oral cavity and oropharyngeal mucosa; we cannot isolate or spare them dosimetrically, nor can we separate the objective contribution of the minor salivary glands with respect to total salivary output. It should be noted that the data used to formulate the QUANTEC rule primarily involved plans which delivered a mean dose >40–50 Gy to the submandibular glands (6). The submandibular and minor salivary glands were not analyzed in this paper.
Effect of chemotherapy
The QUANTEC report states that chemotherapy typically is not associated with risk of xerostomia (1). In the BCCA cohort xerostomia at 3 months was seen in 13 of 22 patients receiving concurrent chemotherapy and 14 of 28 patients treated with RT alone (Fisher exact test p=0.57). Mean doses to spared parotid glands were not significantly different, 29.3±17.5 Gy in concurrent chemotherapy and 23.8±18.9 Gy in RT alone groups (p=0.30). Logistic function parameters were D50=24.2 Gy (95% confidence interval 13.7 – 33.1 Gy) and γ50=0.89 (0.26 – 1.94) for the chemotherapy group. For RT only patients corresponding values were 20.4 Gy (13.4 – 29.4 Gy) and 0.84 (0.36 – 1.58). At 12 months, the incidence of xerostomia was 7 in 24 patients receiving chemotherapy and 13 in 36 patients treated with RT alone (p=0.78). Due to the small number of events at 12 months, it was not possible to analyze dose-response at this time point. Overall, no association between chemotherapy and risk of xerostomia was observed.
Caveats
As stated in the QUANTEC paper, despite having a goal of 20 Gy mean dose or less to the spared gland, lower mean dose usually preserves more gland function, and should be pursued as a planning goal when consistent with adequate target dose coverage.
The discrimination accuracies quoted here are for data generated without use of the 20 Gy spared-parotid-mean-dose rule, though 26 Gy was used as a planning target in the BCCA cohort. Because many of those analyzed plans have spared-parotid-gland mean doses much lower than 20 Gy, the discrimination accuracies (NPV) for plans close to the threshold will be smaller than the cohort figures quoted here (94% for BCCA and 90% for WUSTL).
Conclusion
We independently tested two proposed treatment planning guidelines for the avoidance of sever xerostomia. Both guidelines are simple to implement clinically, and, based on their discrimination power in these two datasets, are expected to result in a relatively low risk of xerostomia at 1 year follow-up time. The QUANTEC guideline to spare at least one parotid gland to less than 20 Gy has a high negative predictive value (93%) on the new (BCCA) dataset, consistent with performance on the WUSTL dataset. The Ortholan et al. guideline was not as predictive but still performed well. Both data sets showed a rate of xerostomia < 20 % when the mean dose to spared parotid is kept below 20 Gy. Based on these results, we believe the clinical use of the (slightly modified) QUANTEC 20 Gy spared-gland-mean-dose guideline is justified.
Acknowledgements
This research was partially supported by NIH grant R01 CA85181.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of itnetrest: none
References
- 1.Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58–S63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortholan C, Chamorey E, Benezery K, et al. Modeling of salivary production recovery after radiotherapy using mixed models: Determination of optimal dose constraint for IMRT planning and construction of convenient tools to predict salivary function. Int J Radiat Oncol Biol Phys. 2009;73:178–186. doi: 10.1016/j.ijrobp.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 3.Roesink JM, Schipper M, Busschers W, et al. A comparison of mean parotid gland dose with measures of parotid gland function after radiotherapy for head-and-neck cancer: Implications for future trials. Int J Radiat Oncol Biol Phys. 2005;63:1006–1009. doi: 10.1016/j.ijrobp.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Munter MW, Hoffner S, Hof H, et al. Changes in salivary gland function after radiotherapy of head and neck tumors measured by quantitative pertechnetate scintigraphy: Comparison of intensity-modulated radiotherapy and conventional radiation therapy with and without amifostine. Int J Radiat Oncol Biol Phys. 2007;67:651–659. doi: 10.1016/j.ijrobp.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 5.Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 6.Blanco AI, Chao KS, El Naqa I, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055–1069. doi: 10.1016/j.ijrobp.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 7.Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: Initial results. Int J Radiat Oncol Biol Phys. 2001;49:907–916. doi: 10.1016/s0360-3016(00)01441-3. [DOI] [PubMed] [Google Scholar]
- 8.Deasy JO, Blanco AI, Clark VH. CERR: A computational environment for radiotherapy research. Med Phys. 2003;30:979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 9.Bentzen SM, Tucker SL. Quantifying the position and steepness of radiation dose-response curves. Int J Radiat Biol. 1997;71:531–542. doi: 10.1080/095530097143860. [DOI] [PubMed] [Google Scholar]
- 10.Roberts SA, Hendry JH. The delay before onset of accelerated tumour cell repopulation during radiotherapy: a direct maximum-likelihood analysis of a collection of worldwide tumour-control data. Radiother Oncol. 1993;29:69–74. doi: 10.1016/0167-8140(93)90175-8. [DOI] [PubMed] [Google Scholar]
- 11.Bhide SA, Miah AB, Harrington KJ, et al. Radiation-induced xerostomia: Pathophysiology, prevention and treatment. Clin Oncol (R Coll Radiol) 2009;21:737–744. doi: 10.1016/j.clon.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Lombaert IM, Brunsting JF, Wierenga PK, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 14.Houweling AC, Philippens ME, Dijkema T, et al. A comparison of dose-response models for the parotid gland in a large group of head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2010;76:1259–1265. doi: 10.1016/j.ijrobp.2009.07.1685. [DOI] [PubMed] [Google Scholar]
- 15.Jha N, Seikaly H, McGaw T, et al. Submandibular salivary gland transfer prevents radiation-induced xerostomia. Int J Radiat Oncol Biol Phys. 2000;46:7–11. doi: 10.1016/s0360-3016(99)00460-5. [DOI] [PubMed] [Google Scholar]
- 16.Seikaly H, Jha N, McGaw T, et al. Submandibular gland transfer: A new method of preventing radiation-induced xerostomia. Laryngoscope. 2001;111:347–352. doi: 10.1097/00005537-200102000-00028. [DOI] [PubMed] [Google Scholar]