Abstract
Caspase-8, FADD, and FLIP orchestrate apoptosis in response to death receptor ligation. Mysteriously however, these proteins are also required for normal embryonic development and immune cell proliferation, an observation that has led to their implication in several non-apoptotic processes. While many scenarios have been proposed, recent genetic and biochemical evidence points to unregulated signaling by the receptor interacting protein kinases-1 (RIPK1) and RIPK3 as the lethal defect in caspase-8, FADD and FLIP deficient animals and tissues. The RIPKs are known killers, being responsible for a non-apoptotic form of cell death with features similar to necrosis. However, the mechanism by which caspase-8, FADD, and FLIP prevent runaway RIPK activation is unknown, and the signals that trigger these events during development and immune cell activation remain at large. In this review, we will lay out the evidence as it now stands, reinterpreting earlier observations in light of new clues and considering where the investigation might lead.
Prologue: A mysterious death
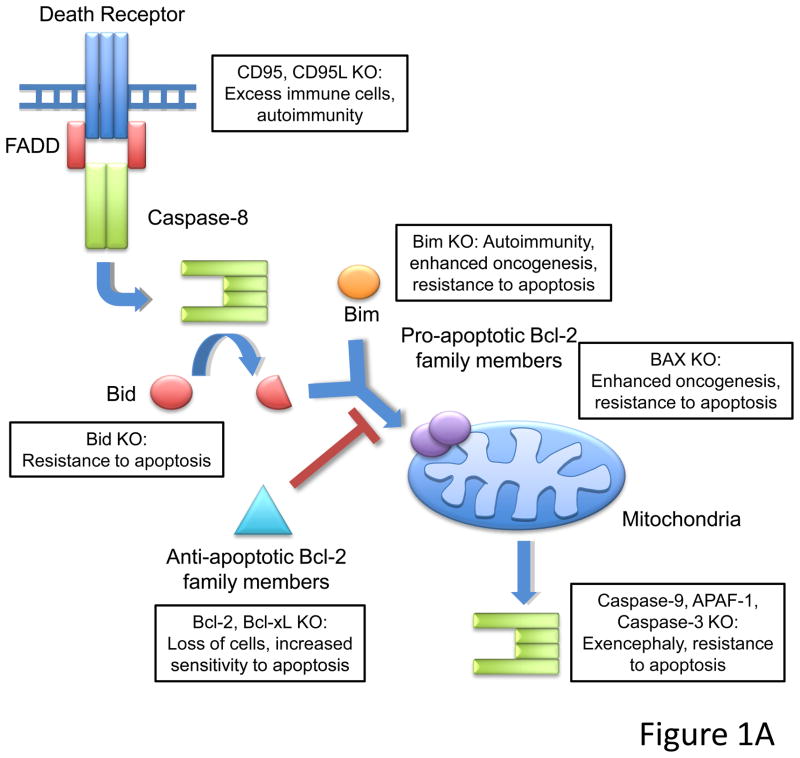
(The curtain rises on a death that has long been unexplained.) In the simple arithmetic of multicellular life, the number of cells in a tissue is the difference between cells that arise by division and/or differentiation, and those that die. Therefore, apoptosis has a fundamental role in normal development and homeostasis, and perturbations of the players in the cell death pathways generally have effects that follow from their roles. Ablation of pro-apoptotic genes tends to promote cell accumulation, autoimmunity, and/or oncogenesis, while loss of anti-apoptotic genes leads to attrition and degeneration. Some examples are provided in Figure 1A.
Figure 1.
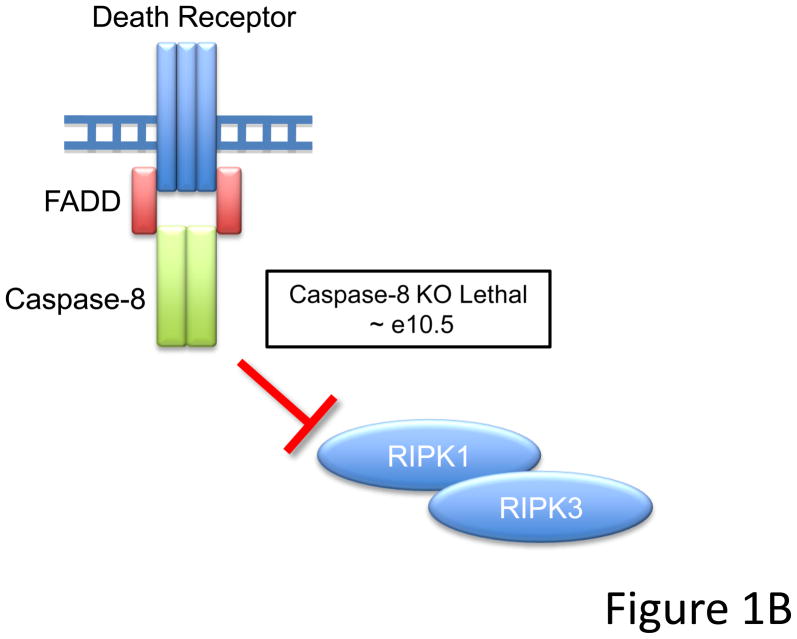
(A) Schematic of apoptotic death pathways. Extrinsic signals through death receptors like CD95 or intrinsic signals that activate pro-apoptotic Bcl-2 members like Bid and Bim lead to activation of downstream effector caspases (caspase-3). Loss of pro-apoptotic molecules lead to a range of phenotypes including embryonic lethality associated with exencephaly (caspase-9, APAF-1, or caspase-3 knockouts), and lymphoaccumulative and autoimmune disorders (CD95/CD95L or Bim knockout mice). (It should be noted, however, that while cells lacking downstream elements of the mitochondrial pathway do not undergo apoptosis via this route, cells may nevertheless die by a caspase-independent mechanism). Additionally, animals deficient in certain pro-apoptotic molecules (i.e. Bim or Bax knockouts) display enhanced oncogenesis. Cells from animals where pro-apoptotic proteins are deleted are generally resistant to apoptosis, while cells from animals missing anti-apoptotic proteins (such as Bcl-2 and Bcl-xL) have increased sensitivity to apoptotic stimuli. (B) Ablation of Caspase-8 leads to embryonic lethality circa e10.5. The simultaneous deletion of Caspase-8 and RIPK3 or FADD together with RIPK1 rescue embryonic development, suggesting that FADD and Caspase-8 act in concert to provide survival signals which may inhibit RIPK function during development.
For over a decade, though, one set of molecules in an apoptotic pathway have presented a deep paradox: knockout of the initiator caspase-8 (Varfolomeev et al., 1998), its adapter molecule FADD (Yeh et al., 1998), or the caspase-like regulatory molecule FLIPL (herein, FLIP) (Yeh et al., 2000) produces a fully penetrant embryonic lethality around e10.5, associated with a failure to develop vascularization of the yolk sac. This effect cannot be readily attributed to a loss of cell death, and has led to the idea (supported by in vitro experiments, conditional knockouts, and transgenic expression of dominant negative forms) that these molecules function in other processes essential to development, including cell cycle (Alappat et al., 2005; Hua et al., 2003; Kay, et al., 1998; Zhang et al., 2001), NF-kB activation (Golks et al., 2006; Kataoka and Tschopp, 2004; Lemmers et al., 2007; Su et al., 2005), cell adhesion(Rytomaa et al., 1999; Stupack et al., 2001), cell migration (Barbero et al., 2008; Barbero et al., 2009; Helfer et al., 2006; Senft et al., 2007), and suppression of inflammation (Kovalenko et al., 2009; Rajput et al., 2011).
Recently, however, another view has emerged that may force a re-evaluation of these alternative roles for FADD, caspase-8, and FLIP: mice lacking both caspase-8 and another protein, RIPK3, develop normally and are born at mendelian frequency (Kaiser et al., 2011; Oberst et al., 2011). Similarly, mice lacking both FADD and still another protein, RIPK1, develop normally as well (these mice tend to die perinatally, an effect also seen in RIPK1 single knockouts) (Zhang et al., 2011). At the very least, we can conclude that whatever caspase-8 and FADD (and possibly, FLIP, by extension) do to preserve embryonic development, the lethal effects of their ablation is inexorably tied to the functions of these kinases (see Figure 1B). Our play begins.
Act I. The players are introduced
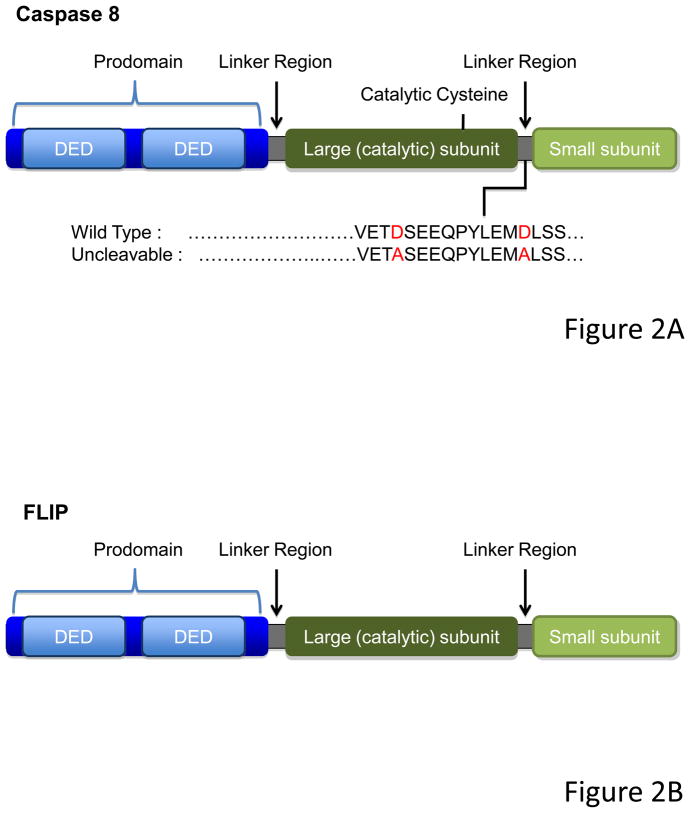
In its inactive pro-form, caspase-8 is a monomer, with a large prodomain containing two “death fold” interaction regions called Death Effector Domains (DED), followed by what will be the large and small subunits of the mature enzyme; these are separated by linker regions containing sites at which the caspase can auto-cleave (see Figure 2A). When two pro-caspase-8 molecules are dimerized at their prodomains, the enzyme is activated, and cleavage between the subunits stabilizes the active form. Non-cleavable caspase-8 (mutated at the cleavage sites) can be made to be enzymatically active by enforced dimerization in kosmotropic salts (Boatright et al., 2003) but N-terminal dimerization of such noncleavable caspase-8 does not induce enzymatic activity in normal salts, nor does it trigger apoptosis in cells (Hughes et al., 2009; Oberst et al., 2010).
Figure 2.
(A) Caspase-8 consists of a prodomain, followed by the large and the small protease subunits, separated by linker regions. The prodomain has two death “folds” named Death Effector Domains (DEDs, light blue), through which caspase-8 is recruited and dimerized. Upon dimerization, Caspase-8 become proteolytically active and processes itself in specific cleavage sites present in the linker regions. The cleavage between the large and small subunit contributes to the stabilization of the molecule and is essential to induction of cell death but is dispensable for RIPK-necrosis inhibition. (B) FLIP has a similar structure than Caspase-8, but lacks the catalytic cysteine. Although it can act as a dominant negative for caspase-8-induced death when overexpressed, FLIP acts in concert with Caspase-8 to inhibit RIPK-induced necrosis.
FLIP resembles caspase-8 and may have arisen following a duplication of the caspase-8 gene, but lacks a catalytic cysteine (Figure 2B). Although initially thought to act as a dominant negative version of caspase-8 (Irmler et al., 1997), dimerization of caspase-8 and FLIP in kosmotropic salts induces catalytic activity (Boatright et al., 2004; Micheau et al., 2002), and N-terminal dimerization in normal salts induces enzymatic function not only of native caspase-8 but also that of noncleavable mutants (Pop et al., 2011). Thus, the heterodimer of caspase-8 and FLIP is enzymatically active. However, under physiological conditions, this enzymatic complex does not induce apoptosis, either due to subtle differences with the caspase-8 homodimer in terms of substrate specificity (Pop et al., 2011) or its location in the cell (e.g. (Gonzalvez et al., 2008; Koenig et al., 2008)).
Physiologically, assembly of caspase-8 or caspase-8-FLIP is mediated by the adapter molecule, FADD, which interacts with either player via its own DED. This only occurs when FADD is itself bound and oligomerized by molecules interacting with a second region of FADD containing another death fold, the Death Domain (DD); such proteins do so via their own DDs.
Such DD-containing, FADD-binding proteins include the death receptors of the TNFR superfamily, including TNFR1, CD95, and the TRAIL receptors (Wilson et al., 2009). When bound by their ligands, these recruit and oligomerize FADD, which in turn oligomerizes caspase-8 and FLIP, inducing enzymatic activity. If FLIP is present, formation of the hetero-forms appears to be dominant, and apoptosis does not occur (e.g., (Irmler et al., 1997). If FLIP is absent, however (discussed in more detail below) then FADD engages caspase-8 and apoptosis ensues (Micheau et al., 2001).
Other proteins also appear to recruit FADD, and in turn, caspase-8 and FLIP. FADD is bound by the autophagy ATG5-12 protein (Pyo et al., 2005), polyglutamine repeat protein aggregates (Sanchez et al., 1999), and by the DD of RIPK1 (Micheau and Tschopp, 2003; Wang et al., 2008); the latter two have also been shown to lead to activation of caspase-8 (the RIPK1-FADD interaction is further discussed below).
Act II. An assembly of life and death
In the specific case of TNFR1, signaling induced by receptor ligation is more complex than we have described (this may also apply to other death receptors in some cases, although less is known about these). Ligation of TNFR1 induces recruitment of two ubiquitin ligases, TRAF2 and cIAP1, and the binding of an adapter molecule, TRADD (the latter via a DD-DD interaction). RIPK1 is recruited, and the complex is modified by a complex series of ubiquitination events carried out by both the IAPs (Bertrand et al., 2008; Varfolomeev et al., 2007; Vince et al., 2007) and by the linear ubiquitin assembly complex (LUBAC), which generates linear ubiquitin linkages (Haas et al., 2009; Ikeda et al., 2011; Tokunaga et al., 2011; Tokunaga et al., 2009). The resulting linear ubiquitin recruits the IKK complex via NEMO/IKKγ, leading to NF-κB activation (although the role of RIPK1 in NF-κB activation is controversial (Wong et al., 2010)). NF-κB then induces the expression of FLIP (Micheau et al., 2001). This NF-κB (and other signal)-inducing complex is referred to as Complex I (Micheau and Tschopp, 2003).
RIPK1 is then de-ubiquitinated by the enzymes Cezanne (Enesa et al., 2008) and CYLD (Wang et al., 2008), and the complex of TRADD and RIPK1 moves to the cytosol as Complex II. FADD is recruited to TRADD (by a DD-DD interaction), which, in turn, binds caspase-8, and FLIP. In the absence of NF-κB function, or if protein synthesis is inhibited, FLIP is not produced, and this complex triggers apoptosis (Micheau and Tschopp, 2003).
A third complex can also form (Complex IIb) in which RIPK1 directly oligomerizes FADD (through DD-DD interactions) to recruit caspase-8 and FLIP (Wang et al., 2008). It is possible that this RIPK1-mediated complex forms in response to other signals, such as extensive DNA damage (Biton and Ashkenazi, 2011). Under some conditions, recruitment of caspase-8 to this complex can trigger apoptosis. However, de-ubiquitinated RIPK1 can also recruit RIPK3; the two proteins interact via RHIM domains in both molecules (Cho et al., 2009). This can lead to necrotic cell death, as we will see.
In addition to these TNF-dependent RIPK1 containing complexes, two recent studies identified a RIPK1-containing complex dubbed the “ripoptosome” that forms independently of receptor ligation (Feoktistova et al., 2011; Tenev et al., 2011). This complex is proposed to be antagonized by the ubiquitin ligase activity of cIAP1 and cIAP2, and can therefore form spontaneously when the cIAPs are down-regulated, either by specific small-molecule inhibitors or by genotoxic stress. The spontaneously formed RIPK1-FADD complex may be recruited to any of several signaling platforms—the RHIM-containing toll-like receptor (TLR) adapter protein TRIF, for example—to activate either caspase-8 or RIPK3.
The complex protein-protein interactions, via death folds (the common structure that includes DD and DED) occur in multi-tiered hexagonal arrays involving multiple interfaces (Salvesen and Riedl, 2009). Thus, several different molecules assembled through such interactions in complexes I, II, and IIb are likely to involve variable (and complicated) stoichiometries that almost certainly dictate different biological outcomes.
Act III. Enter the RIPper
As we have seen, activation of caspase-8 homodimers downstream of death receptor ligation triggers apoptosis. It was therefore surprising when it was found that inhibition of caspases can promote a necrotic form of cell death upon ligation of CD95(Scheller et al., 2002) or TNFR1(Vercammen et al., 1998b), and further, that this necrotic death depends on the function of RIPK1(Vanden Berghe et al., 2004). Subsequently, an inhibitor of RIPK1 kinase activity, necrostatin-1, was identified which strikingly inhibits necrosis engaged by inhibition of caspases and ligation of death receptors(Degterev et al., 2008).
This RIPK-dependent necrosis can be observed in the presence of caspase inhibitors in response to other signaling events, including T cell activation (Kennedy et al., 1999) and Toll-like receptor (TLR) ligation(Feoktistova et al., 2011). In addition, the DNA-sensing protein, DAI, recruits RIPK1 and RIPK3(Rebsamen et al., 2009), and while its ability to promote RIPK-dependent necrosis has not been explored, it is possible that it functions in the RIPK3-dependent necrotic response to viral infection (Upton et al., 2010).
Further, it was found that in addition to RIPK1, RIPK3 activity is also required for necrosis in response to TNFR1 or viral infection(Cho et al., 2009; He et al., 2009; Zhang et al., 2009). RIPK1 and RIPK3 associate through RHIM domains in both proteins (unlike RIPK1, RIPK3 does not posses a DD). The model therefore emerges that RIPK1 activation in complex IIb leads to RIPK3 activation, which in turn causes necrotic cell death. This necrotic form of cell death is antagonized by caspase-8, which is recruited by FADD; FLIP is also required for this anti-necrotic activity, as we discuss below.
The formation of complex IIb depends on de-ubiquitination of RIPK1 by CYLD (Wang et al., 2008), and CYLD is also required for TNF-induced RIPK-dependent necrosis (O’Donnell, et al., in press). However, any requirements for the complex ubiquitination events that occur in Complex I (see Act II) are currently unexplored.
It is not clear, however, how RIPK1 and RIPK3 interact to promote necrotic cell death. RIPK3 has been suggested to activate RIPK1 by phosphorylation (Cho et al., 2009), and death induced by RIPK3 dimerization appears to depend on RIPK1. However, in response to some viral infections, RIPK3 promotes death in the absence of RIPK1 (Upton et al., 2010).
The mechanism of cell death induced by RIPK1-RIPK3 is even less clear. ROS (Lin et al., 2004; Vercammen et al., 1998a), perhaps as a result of activation of NADPH-oxidase 1 (NOX1) (Kim et al., 2007), has been implicated, but attempts to block death with ROS scavengers (He, et al., 2009) or NOX1 inhibition (Kim et al., 2007) have been unimpressive. Similarly, several metabolic enzymes were identified as RIPK3 substrates, but again, their roles in cell death have been elusive (i.e., knock down of these enzymes did not convincingly prevent death) (Zhang et al., 2009). Another candidate is the mitochondrial enzyme cyclophilin D (CYPD), implicated in ischemic injury and required for the mitochondrial permeability transition (Baines et al., 2005). However, inhibition of CYPD did not block RIPK-dependent necrosis in vitro (He et al., 2009) and while proliferation of T lymphocytes lacking caspase-8 was rescued by deletion of RIPK3, no proliferation was observed in cells lacking both caspase-8 and CYPD (Ch’en et al., 2011). Thus, while RIPK1 and RIPK3 are implicated in necrosis, the actual “executioner” remains elusive.
As mentioned in the prologue, cells lacking caspase-8 (or FADD) display abnormalities not necessarily associated with necrosis. For example, cell migration of fibroblasts in vitro is impaired in cells lacking caspase-8 (Helfer et al., 2006; Senft et al., 2007), and this has been tied to defects in RAC1 activation. Based on the embryonic results, it is not unlikely (although currently untested) that such effects are mediated by RIPK1 and RIPK3. It is possible that the mechanisms by which these RIPKs inhibit cell movement and promote necrosis are related.
Act IV. A death averted
Restoration of catalytically active, but not inactive, caspase-8 restores resistance to TNFR-induced necrosis in cells (Oberst et al., 2011) and preserves embryogenesis in mice (Kang et al., 2008). Clearly, then, the catalytic activity of caspase-8 prevents cell death (and perhaps other effects) mediated by RIPK1 and RIPK3. But now we have another problem: How can caspase-8 do this without itself triggering apoptosis?
Several clues lead to an answer to this riddle. A noncleavable mutant of caspase-8 effectively rescues caspase-8 deficient cells (Oberst et al., 2011) and mice(Kang et al., 2008), but we know that this mutant does not form stable, active dimer and does not promote apoptosis (Oberst et al., 2010). It does, however, become stably catalytic when associated with FLIP (see Act I). Thus, it may be that it is caspase-8-FLIP heterodimers and not caspase-8 homodimers that block RIPK-necrosis. Indeed, evidence supports this view in the case of TNFR signaling: 1. Cells in which caspase-8-induced apoptosis is blocked by Bcl-xL activate caspase-8 but nevertheless undergo RIPK-dependent necrosis in the absence of FLIP (Oberst et al., 2011), and 2. The pox virus serpin CrmA prevents caspase-8-dependent apoptosis in T lymphocytes (Smith et al., 1996) and fibroblasts (Oberst et al., 2011) without promoting necrosis; CrmA preferentially inhibits caspase-8 homodimers over caspase-8-FLIP activity (Oberst et al., 2011). Therefore, the caspase-8-FLIP complex is responsible for blocking RIPK-dependent necrosis, and does so without engaging apoptosis. This satisfactorily accounts for the shared lethal phenotypes of FADD, caspase-8, and FLIP knockout mice (see Prologue).
This also establishes relative levels of caspase-8, RIPK3, FLIP and FADD as critical regulators of both the apoptotic and necrotic pathways of cell death. When FLIP, FADD or caspase-8 is absent, unchecked RIPK3 activation can trigger necrotic cell death; if RIPK3 is absent (as it is in some cell types), limited FLIP levels can be permissive for apoptotic activation of caspase-8. This cell fate equilibrium likely extends beyond death receptor signaling (Feoktistova et al., 2011; Tenev et al., 2011). The RIPK1-containing complex IIb may represent a signaling “module” that can be recruited to other signaling platforms, such as TRIF or the DNA sensor DAI, both of which interact with RIPK1.
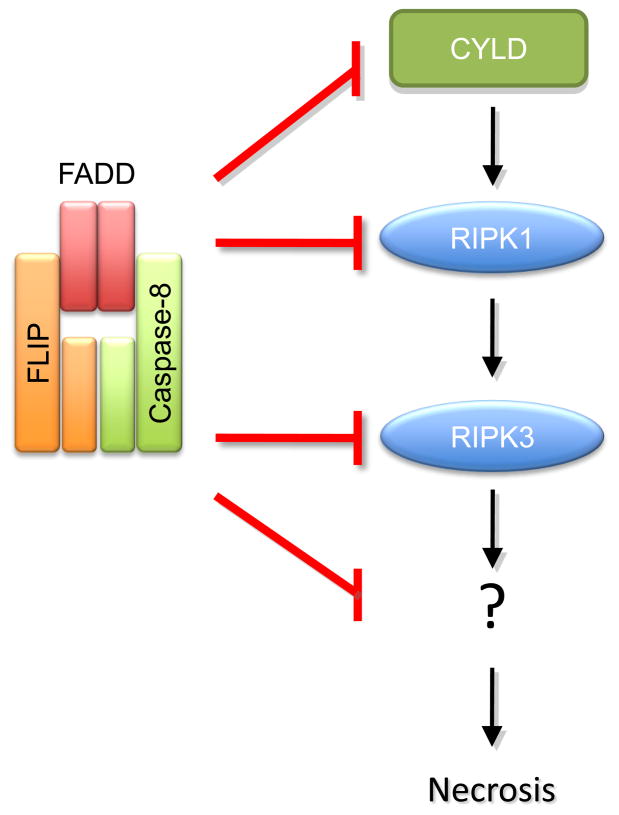
When caspase-8-FLIP is active, the RIPK1-RIPK3 signaling is suppressed, but exactly how this occurs is not definitively known. Both RIPK1 and RIPK3 can by cleaved by caspase-8 (Feng et al., 2007; Rebe et al., 2007), and cleavage of RIPK1 by the caspase-8-FLIP complex has been observed both in vitro and in cultured cells (Feoktistova et al., 2011; Pop et al., 2011). This may explain the effect, but there are other possibilities. The deubiquitases Cezanne and/or CYLD are required for RIPK1 activity leading to necrosis, and CYLD contains a caspase-8 cleavage site. Expression of CYLD, mutated at this site, yields cells that undergo necrosis despite caspase-8 and FLIP (O’Donnell, et al., 2011), and thus this may be a key substrate of the inhibitory caspase-8-FLIP activity. The proposed functions of caspase-8-FLIP in blocking RIPK-dependent necrosis are outlined in Figure 3.
Figure 3.
Caspase-8-FLIP complex blocks RIPK-dependent necrosis, although the precise mechanism behind this inhibition is still unknown. RIPK1 and RIPK3, as well as upstream deubiquitinase CYLD, are caspase-8 cleavage targets, suggesting that cleavage of any of these proteins might be critical for necrosis inhibition. However, as little is known about what is downstream RIPK1/RIPK3 activation, other targets for caspase-8 may yet be implicated.
Alternatively, it is possible that the proteolytic activity of caspase-8-FLIP results in rapid degradation of the complex and the associated RIPK molecules. Indeed, cleavage of FLIP has been shown to result in degradation of the complex (Feokistova, et al., 2011). The role of FLIP cleavage in control of RIPK-dependent necrosis has not been explored, however.
Lurking in the wings is a protease known as MALT1/paracaspase whose job is to regulate NFkB signaling downstream of B and T cell receptors. Targets for MALT1 include the ubiquitin editors A20 and CYLD (Coornaert et al., 2008; Staal et al., 2011) and one wonders what we can learn by comparing the biological functions of MALT1 (which is, interestingly, an “Argase” not an “Aspase”) with caspase-8. There is even a claim that MALT1 can heterodimerize with caspase-8, raising the intriguing notion that this complex regulates necrosis in lymphocyte activation (Kawadler et al., 2008). The role of specific proteolysis in survival signaling may go beyond caspase-8-FLIP.
In humans, a kindred with an inactivating mutation in the caspase-8 gene has been described (Chun et al., 2002), raising the possibility that human embryogenesis proceeds independently of caspase-8. It is possible, however, that the closely related caspase-10 (expressed in humans and other vertebrates but not in rodents) is also recruited by FADD (Sprick et al., 2002), and effectively blocks the lethal effects of RIPK1 and RIPK3 in humans (in association with FLIP?) This idea is complicated by the observation that differentiation of human villous trophoblast, in vitro, appears to require caspase-8 (Black et al., 2004), although the roles of RIPK1 and RIPK3 in this setting have not yet been explored.
Interlude: Lymphoaccumulative disease in caspase-8-RIPK3 double knockout mice
While mice lacking caspase-8 and RIPK3 develop normally, they display a profound lymphoaccumulative disease as adults, and this is ultimately lethal (Kaiser et al., 2011; Oberst et al., 2011). This disease closely resembles that of mice and humans lacking CD95 or its ligand (Rieux-Laucat et al., 1995; Takahashi et al., 1994; Watanabe-Fukunaga et al., 1992) and involves accumulation of a B220+, CD3+, CD4−, CD8− T cell population. In both C57Bl/6 and 129 backgrounds, however, CD95 deficiency results in a milder disease than that observed in the caspase-8-RIPK3 double knockout mouse (although the latter was on a mixed C57Bl/6-129 background). It is possible that the effect in the double knockout may be a consequence not only of defective CD95-induced apoptosis (to which these mice are resistant (Oberst et al., 2011)) but also that of other death receptors. Indeed, deficiency in both CD95L and TRAIL results in exacerbated disease(Sedger et al., 2010). More extensive genetic analyses would be required to test this possibility.
Further, while deletion of caspase-8 only in T cells of RIPK3-null mice similarly results in this lymphoaccumulative disease (Ch’en et al., 2011), conditional deletion of CD95 only in wild type T cells on the same background does not (Hao et al., 2004). This, then, represents a mini-mystery that remains unresolved (and as we will see, it is one of many).
Act V. Identifying the killer
Here we return to our main mystery, the one that drives the story: Why do mice lacking caspase-8, FADD, or FLIP fail to develop beyond e10.5?
In the initial characterization of the FADD knockout, compelling data in blastocyst chimeras mapped the defect to the heart (Yeh et al., 1998). Subsequent studies with conditional knockouts, however, found that deletion of caspase-8 in the heart produced no lethality, while deletion in endothelium (using CRE driven by the TIE1 promoter) phenocopied the embryonic lethality (Kang et al., 2004).
These apparently contrasting results can be reconciled. During embryogenesis, a stem cell precursor of blood and endothelium arises in the embryonic aorta (around e9) and migrates to the yolk sac (Boisset et al., 2010). Deletion of the angiopoietin receptor TIE2 (Sato et al., 1995), or angiopoietin-1 (Suri et al., 1996) causes defective yolk sac vascularization and e10.5 lethality. Therefore, while not proven, it seems likely that it is this precursor population that is compromised by RIPK1–RIPK3 in caspase-8, FADD, or FLIP knockouts.
While we may therefore have a victim, we do not have a killer, the signal responsible for engaging the players (FADD, caspase-8, FLIP, RIPK1, and RIPK3, at least). There are suspects, however.
First, there is TNF. Without FADD, caspase-8, or FLIP, TNF kills cells expressing RIPK3 (and RIPK3 is widely expressed in hematopoietic cells, and thus, perhaps, in our victim (Newton et al., 2004)). But we may have a problem with convicting this suspect: mice lacking elements of the NF-κB pathway die during development as a consequence of TNF-induced liver destruction (Li et al., 1999; Tanaka et al., 1999) (probably as a consequence of failed FLIP expression), but this does not occur until e12.5–e15 (dependent on the knockout). But if TNF were the killer in our mystery (perhaps a serial killer?) then the NF-kB pathway knockout mice should phenocopy the e10.5 lethality, and they clearly do not. We may have to let TNF go (but we will be watching—it remains possible that TNF acts in concert with other ligands of death receptors).
As an aside, the late lethality in the NF-κB pathway knockouts poses another problem: What induces the expression of FLIP, clearly required for survival beyond e10.5? We have only hints. The transcription factor, FOXO1, can induce FLIP expression (Park et al., 2009), and deletion of FOXO1 causes lethality at e10.5 with features of our mystery (Hosaka et al., 2004). Perhaps whatever induces FOXO1 also triggers RIPK-dependent lethality? In any case, the evidence is circumstantial, and we have no culprit.
Another suspect is autophagy, perhaps via ATG5-12 and its association with FADD? While not proof of innocence, proliferation of T cells lacking caspase-8 is not rescued by deletion of ATG7, required for ATG5-12 generation (Ch’en et al., 2011). It may be that autophagy is off the hook as the killer.
Other suspects lurk in the shadows: TRIF (and TLR signaling), and the DNA sensor DAI, but we have insufficient reason to implicate them, and no evidence to hold them. Further complicating the case, the apparently spontaneous formation of a lethal RIPK1-FADD complex independent of receptor platforms (Feoktistova et al., 2011; Tenev et al., 2011) leaves our killer very much at large.
And thus we are left in the final, post-modern finale. No killer in tow, and the suspects celebrating with winks and nods. And one final note, delivered at the denouement: why is the system built this way? What does it do? Why are FADD and caspase-8-FLIP vitally linked to a lethal system in embryogenesis? What are they protecting us from? We have reached, not the end, but the conclusion. Curtain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alappat EC, Feig C, Boyerinas B, Volkland J, Samuels M, Murmann AE, Thorburn A, Kidd VJ, Slaughter CA, Osborn SL, et al. Phosphorylation of FADD at serine 194 by CKIalpha regulates its nonapoptotic activities. Mol Cell. 2005;19:321–332. doi: 10.1016/j.molcel.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Barbero S, Barila D, Mielgo A, Stagni V, Clair K, Stupack D. Identification of a critical tyrosine residue in caspase 8 that promotes cell migration. J Biol Chem. 2008;283:13031–13034. doi: 10.1074/jbc.M800549200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero S, Mielgo A, Torres V, Teitz T, Shields DJ, Mikolon D, Bogyo M, Barila D, Lahti JM, Schlaepfer D, et al. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 2009;69:3755–3763. doi: 10.1158/0008-5472.CAN-08-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-alpha feedforward signaling. Cell. 2011;145:92–103. doi: 10.1016/j.cell.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Black S, Kadyrov M, Kaufmann P, Ugele B, Emans N, Huppertz B. Syncytial fusion of human trophoblast depends on caspase 8. Cell Death Differ. 2004;11:90–98. doi: 10.1038/sj.cdd.4401307. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382:651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J Exp Med. 2011;208:633–641. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- Coornaert B, Baens M, Heyninck K, Bekaert T, Haegman M, Staal J, Sun L, Chen ZJ, Marynen P, Beyaert R. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat Immunol. 2008;9:263–271. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enesa K, Zakkar M, Chaudhury H, Luong le A, Rawlinson L, Mason JC, Haskard DO, Dean JL, Evans PC. NF-kappaB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. J Biol Chem. 2008;283:7036–7045. doi: 10.1074/jbc.M708690200. [DOI] [PubMed] [Google Scholar]
- Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, Macfarlane M, Hacker G, Leverkus M. cIAPs Block Ripoptosome Formation, a RIP1/Caspase-8 Containing Intracellular Cell Death Complex Differentially Regulated by cFLIP Isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golks A, Brenner D, Krammer PH, Lavrik IN. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med. 2006;203:1295–1305. doi: 10.1084/jem.20051556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, Petit PX, Vaz FM, Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, Feltham R, Vince J, Warnken U, Wenger T, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Hao Z, Hampel B, Yagita H, Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J Exp Med. 2004;199:1355–1365. doi: 10.1084/jem.20032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Helfer B, Boswell BC, Finlay D, Cipres A, Vuori K, Bong Kang T, Wallach D, Dorfleutner A, Lahti JM, Flynn DC, et al. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006;66:4273–4278. doi: 10.1158/0008-5472.CAN-05-4183. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua ZC, Sohn SJ, Kang C, Cado D, Winoto A. A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its C-terminal region. Immunity. 2003;18:513–521. doi: 10.1016/s1074-7613(03)00083-9. [DOI] [PubMed] [Google Scholar]
- Hughes MA, Harper N, Butterworth M, Cain K, Cohen GM, MacFarlane M. Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell. 2009;35:265–279. doi: 10.1016/j.molcel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- Kang TB, Oh GS, Scandella E, Bolinger B, Ludewig B, Kovalenko A, Wallach D. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181:2522–2532. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol. 2004;24:2627–2636. doi: 10.1128/MCB.24.7.2627-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawadler H, Gantz MA, Riley JL, Yang X. The paracaspase MALT1 controls caspase-8 activation during lymphocyte proliferation. Mol Cell. 2008;31:415–421. doi: 10.1016/j.molcel.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Harris AW, Bath ML, Smith KG, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Koenig A, Russell JQ, Rodgers WA, Budd RC. Spatial differences in active caspase-8 defines its role in T-cell activation versus cell death. Cell Death Differ. 2008;15:1701–1711. doi: 10.1038/cdd.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, Ohashi PS, Jurisicova A, Lenardo M, Hakem R, et al. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem. 2007;282:7416–7423. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grutter MG. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Pop C, Tremblay AG, Blais V, Denault JB, Salvesen GS, Green DR. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem. 2010;285:16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT. Caspase 8 inhibits programmed necrosis by processing CYLD. Nature Cell Biol. 2011 doi: 10.1038/ncb2362. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Sohn HY, Yoon J, Park SI. Down-regulation of FoxO-dependent c-FLIP expression mediates TRAIL-induced apoptosis in activated hepatic stellate cells. Cell Signal. 2009;21:1495–1503. doi: 10.1016/j.cellsig.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Pop C, Oberst A, Drag M, Van Raam BJ, Riedl SJ, Green DR, Salvesen GS. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem Journal. 2011;433:447–457. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, Cho DH, Choi B, Lee H, Kim JH, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, Du J, Wallach D. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Rebe C, Cathelin S, Launay S, Filomenko R, Prevotat L, L’Ollivier C, Gyan E, Micheau O, Grant S, Dubart-Kupperschmitt A, et al. Caspase-8 prevents sustained activation of NF-kappaB in monocytes undergoing macrophagic differentiation. Blood. 2007;109:1442–1450. doi: 10.1182/blood-2006-03-011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, Hofmann K, Vazquez J, Benedict CA, Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, de Fischer A, Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- Rytomaa M, Martins LM, Downward J. Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Curr Biol. 1999;9:1043–1046. doi: 10.1016/s0960-9822(99)80454-0. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Riedl SJ. Structure of the Fas/FADD complex: a conditional death domain complex mediating signaling by receptor clustering. Cell Cycle. 2009;8:2723–2727. doi: 10.4161/cc.8.17.9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez I, Xu CJ, Juo P, Kakizaka A, Blenis J, Yuan J. Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron. 1999;22:623–633. doi: 10.1016/s0896-6273(00)80716-3. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Scheller C, Sopper S, Ehrhardt C, Flory E, Chen P, Koutsilieri E, Ludwig S, ter Meulen V, Jassoy C. Caspase inhibitors induce a switch from apoptotic to proinflammatory signaling in CD95-stimulated T lymphocytes. Eur J Immunol. 2002;32:2471–2480. doi: 10.1002/1521-4141(200209)32:9<2471::AID-IMMU2471>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sedger LM, Katewa A, Pettersen AK, Osvath SR, Farrell GC, Stewart GJ, Bendall LJ, Alexander SI. Extreme lymphoproliferative disease and fatal autoimmune thrombocytopenia in FasL and TRAIL double-deficient mice. Blood. 2010;115:3258–3268. doi: 10.1182/blood-2009-11-255497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft J, Helfer B, Frisch SM. Caspase-8 interacts with the p85 subunit of phosphatidylinositol 3-kinase to regulate cell adhesion and motility. Cancer Res. 2007;67:11505–11509. doi: 10.1158/0008-5472.CAN-07-5755. [DOI] [PubMed] [Google Scholar]
- Smith KG, Strasser A, Vaux DL. CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. EMBO J. 1996;15:5167–5176. [PMC free article] [PubMed] [Google Scholar]
- Sprick MR, Rieser E, Stahl H, Grosse-Wilde A, Weigand MA, Walczak H. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. EMBO J. 2002;21:4520–4530. doi: 10.1093/emboj/cdf441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal J, Driege Y, Bekaert T, Demeyer A, Muyllaert D, Van Damme P, Gevaert K, Beyaert R. T-cell receptor-induced JNK activation requires proteolytic inactivation of CYLD by MALT1. EMBO J. 2011;30:1742–1752. doi: 10.1038/emboj.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:459–470. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, Macfarlane M, Cain K, et al. The Ripoptosome, a Signaling Platform that Assembles in Response to Genotoxic Stress and Loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, Tanaka K, Nakano H, Iwai K. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11:123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Berghe T, van Loo G, Saelens X, Van Gurp M, Brouckaert G, Kalai M, Declercq W, Vandenabeele P. Differential signaling to apoptotic and necrotic cell death by Fas-associated death domain protein FADD. J Biol Chem. 2004;279:7925–7933. doi: 10.1074/jbc.M307807200. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998a;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998b;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- Wong WW, Gentle IE, Nachbur U, Anderton H, Vaux DL, Silke J. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ. 2010;17:482–487. doi: 10.1038/cdd.2009.178. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kabra NH, Cado D, Kang C, Winoto A. FADD-deficient T cells exhibit a disaccord in regulation of the cell cycle machinery. J Biol Chem. 2001;276:29815–29818. doi: 10.1074/jbc.M103838200. [DOI] [PubMed] [Google Scholar]