Abstract
In carcinoma ex pleomorphic adenoma (CXPA), pleomorphic adenoma (PA) and diverse carcinoma components showing luminal (ductal) or non-luminal (myoepithelial) differentiation coexist. To elucidate the clinicopathological implications of cellular differentiation in CXPA and the potential role of p53, vascular endothelial growth factor (VEGF), c-erbB-2, c-kit, and glucose transporter 1 (Glut-1) in carcinogenesis, we analyzed 11 CXPAs with luminal differentiation (CXPAs-LD) and 6 CXPAs with non-luminal differentiation (CXPAs-NLD) and compared protein expressions in residual PAs and carcinomas by immunohistochemistry. Among the CXPAs-LD, 5 were invasive and 8 were histologically high-grade tumors. The 5-year survival rate was 72.7%. P53, c-erbB-2, VEGF, and Glut-1 were more immunoreactive in carcinoma components than in PAs (P = 0.008, 0.004, 0.002, and 0.024, respectively); c-erbB-2 overexpression was associated with high histological grade (P = 0.024). Carcinoma components frequently lacked c-kit expression (P = 0.009). CXPAs-NLD were all low-grade and invasive with a larger mean tumor size (5.2 cm) than CXPAs-LD (3.3 cm) (P = 0.040). The patients remained disease-free without significant immunohistochemical expression. The immunoprofiles and clinical course of CXPA differed according to cellular differentiation. Therefore, it is important to report the histological subtype and to assess potential biomarkers in diagnostic and therapeutic trials.
Keywords: Carcinoma ex Pleomorphic Adenoma, p53, c-erbB-2, Vascular Endothelial Growth Factor, c-kit, Glucose Transporter 1, Histological Types, Prognosis
INTRODUCTION
Carcinoma ex pleomorphic adenoma (CXPA) is a rare and poorly understood malignancy that develops from either a long-standing primary or a recurrent pleomorphic adenoma (PA). Among salivary gland tumors, CXPA is unique and interesting because it is histologically diverse and contains both benign pleomorphic adenoma and carcinomatous components. Most types of salivary epithelial carcinomas have been reported as malignant components of CXPA, and the morphological variability of CXPAs makes it difficult to investigate and elucidate the mechanisms of the underlying malignant transformation in PAs (1-3).
The carcinogenesis of CXPA is still unclear; however, it is probably because of the accumulation of genetic instabilities. CXPAs can be classified into 2 main subtypes according to their morphological and immunohistochemical features. The first subtype comprises carcinomas with luminal (ductal epithelial) differentiation (CXPA-LD) such as adenocarcinoma, not otherwise specified (NOS); salivary duct carcinoma (SDC); and mucoepidermoid carcinoma (MEC). The second subtype comprises carcinomas with non-luminal (myoepithelial) differentiation (CXPA-NLD) such as myoepithelial carcinoma (MC), epithelial-myoepithelial carcinoma (EMC), and adenoid cystic carcinoma (4). Since the concept of carcinoma in situ (intraductal carcinoma) has been recently proposed to be an early phase of malignant transformation in CXPA-LD, some studies have investigated ancillary biomarkers for carcinoma in situ (5-7). In CXPA-LD, the malignant transformation of ductal epithelial cells may follow a stepwise sequence and manifest as 1) carcinoma in situ, in which carcinoma cells replace the normal inner ductal epithelial layer while retaining the peripherally located intact myoepithelial layer; 2) intracapsular carcinoma, in which carcinoma cells break through the myoepithelial layer and invade the matrix of a pre-existing PA without capsular invasion; or 3) extracapsular invasive carcinoma, in which the invasion extends to the extracapsular area. In the recent World Health Organization (WHO) classification, carcinoma in situ or intraductal carcinoma and intracapsular carcinoma are included in "noninvasive" CXPA, and extracapsular carcinoma is defined as "invasive" CXPA (8).
However, this concept of carcinoma in situ cannot be applicable to CXPA-NLD. Further, there is only limited information on the differences in their pathogenesis and clinical outcome, although CXPAs-NLD exhibit histological features quite different from those of CXPAs-LD (9-11). Hence, the study of CXPAs according to cellular differentiation may give insights into the pathogenesis of CXPA and help in determining a treatment approach in the absence of definitive clinical trials, which are difficult to conduct because of the rarity of CXPA cases.
In this study, we compared the clinicopathological characteristics of the CXPA-LD and CXPA-NLD groups. Moreover, to investigate the potential values of biomarkers associated with the development and progression of carcinoma in each group, we examined the expression of the immunohistochemical markers p53, endothelial growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), c-erbB-2, c-kit, Bcl-2, and glucose transporter 1 (Glut-1) in carcinomas and residual PAs. To the best of our knowledge, a comparative study for these immunoprofiles between CXPA groups according to cellular differentiation has not been reported earlier.
MATERIALS AND METHODS
Patient selection and histopathological analysis
The cases of 17 CXPA patients were studied. Their records were retrieved from the surgical pathology database of the Asan Medical Center between 1996 and 2006. All patients underwent an operation. Demographic, clinical, and follow-up data were collected from the patients' medical records in such a manner that subjects cannot be identified. Using ancillary immunohistochemical stains for p63 and smooth muscle actin (SMA), by a previously described method, we classified the CXPAs into 2 groups according to the cellular differentiation of the carcinoma component: with LD and with NLD (4). Further, the histological subtype of the malignant component was defined according to WHO classification of salivary gland tumors (12). Hematoxylin-eosin-stained slides of tumor samples from all the study patients were reviewed by 2 pathologists to confirm the diagnosis. Other histological findings, including invasiveness according to the WHO criteria (noninvasive, minimally invasive, and frankly invasive), histological subtype, tumor necrosis, presence of prominent stromal hyalinization, lymphovascular tumor invasion (LVI), perineural invasion (PNI), status of microscopic resection margin, and estimated proportion of residual PA, were also examined. The tumors were histologically examined and classified as high grade (when ≥ 2 of the following features were observed: anaplasia with nuclear pleomorphism and prominent nucleolus, frequent mitoses [≥ 5 per 10 high-power fields], atypical mitosis, and extensive coagulative tumor necrosis) or low grade.
Immunohistochemistry
The tissue blocks containing both the malignant area and benign PA were selected. We mounted 4-µm thick sections on precoated glass slides; these were deparaffinized in xylene and dehydrated in descending grades of ethanol. Immunohistochemical assays were performed using the Ventana NX automated immunohistochemistry system (Ventana Medical Systems, Tucson, AZ, USA) with monoclonal/polyclonal primary antibodies to SMA (dilution, 1:200; Clone 1A4; Neomarkers, Fremont, CA, USA), p63 (dilution, 1:50; Clone 7JUL; Novocastra, Newcastle, UK), p53 (dilution, 1:3000; Code M7001; Dako, Glostrup, Denmark), EGFR (dilution, 1:50; Clone EGFR.113; Novocastra), VEGF (dilution, 1:400; Clone G153-694; Pharmingen, San Diego, CA, USA), c-erbB-2 (dilution, 1:500; Code A0485; Dako), c-kit (dilution, 1:200; Code A4502; Dako), Bcl-2 (dilution, 1:25; Clone 124; Dako), and Glut-1 (dilution, 1:100; Clone SPM498; Neomarkers).
Interpretation of immunohistochemical staining patterns
Evaluation of immunohistochemical staining patterns in each malignant component and residual PA and reassessment of discordant results were performed by 2 pathologists. p53 staining was scored by combining both the percentage of positive tumor cells and the staining intensity (5). A factor for the intensity of staining (0, none; 1, weak; 2, moderate; and 3, strong) and a factor for the total percentage of positively stained cells (1, 1%-20%; 2, 21%-50%; 3, 51%-80%; and 4, 81%-100%) were multiplied, with the cutoff value for a positive reaction being a score of 4; this has previously yielded useful results (13). The immunostaining results of EGFR and c-erbB-2 were scored as follows: 0, no reactivity or membranous staining in ≥ 10% of the tumor cells; 1+, faint/barely perceptible membranous staining in ≥ 10% of tumor cells; 2+, weak to moderate complete membrane staining in ≥ 10% of tumor cells; and 3+, strong complete membrane staining in ≥ 10% of tumor cells. Scores of 2+ and 3+ were considered a positive reaction (14, 15). C-kit expression was defined by membranous reactivity in ≥ 10% of the cells (16). VEGF staining was scored by combining both the percentage of positive tumor cells and the staining intensity, defined as low (< 20% of tumor cells showing weak positivity) or high (≥ 20% of tumor cells showing moderate or strong positivity); high staining indicated overexpression of VEGF (17). Glut-1 expression was considered positive only if distinct membrane staining was observed. All patients were divided into the following 2 groups according to their Glut-1 expression level: low-expression group (< 15%) and high-expression group (≥ 15%) (18). Positive expression of p63 and Bcl-2 was identified by unequivocal nuclear staining, and that of SMA, by cytoplasmic staining of neoplastic cells.
Statistical analysis
The clinicopathological variables and the incidence of overexpression detected by immunohistochemical staining were compared by t test and Pearson's chi-square test. The Cox proportional hazard model was used for multivariate analysis, and the 5-yr survival rate was calculated by the Kaplan-Meier method. All statistical analyses were performed using the Statistical Package for Social Sciences software program, version 17.0 (SPSS Inc., Chicago, IL, USA), and the results were considered statistically significant when the P value was ≤ 0.05.
Ethics statement
Institutional review board exempted review of this study protocol on the basis of being a study of existing pathological specimens.
RESULTS
Histological findings and clinical course
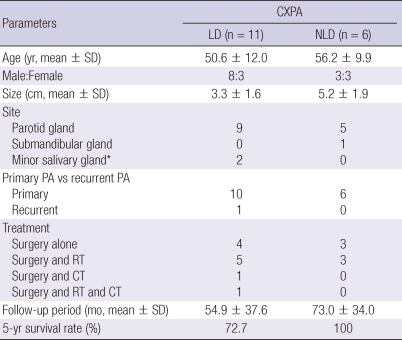
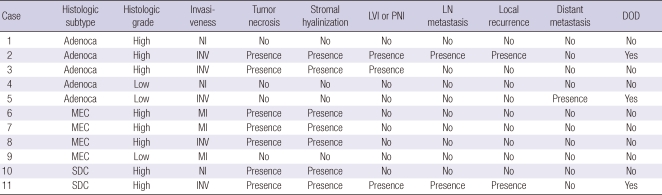
CXPA-LD was predominant and was observed in 11 (64.7%) patients (Table 1). It was found to be more prevalent in men than in women (8 men and 3 women). The mean age of the patients was 50.6 yr, and the mean size of the tumors was 3.3 cm. The histological subtype of the malignant component was classified as adenocarcinoma, NOS in 5 tumors, MEC in 4, and SDC in 2 tumors (Table 2). There were 8 tumors of a histologically high grade and 3 tumors of low grade. All high-grade tumors, except for 1, had a prominent hyalinized stroma and coagulative tumor necrosis. Low-grade tumors however did not show stromal hyalinization or necrosis. The percentage of the malignant component ranged from 45% to 95% (mean, 75.5%). There were 5 invasive, 3 minimally invasive, and 3 noninvasive carcinomas. Of the 5 patients with invasive CXPAs-LD, 3 (27.3%, 2 of high grade and 1 of low grade) died from the disease. One with high-grade adenocarcinoma, NOS, a positive surgical margin, and regional lymph node metastasis died 2 months after the operation and radiation therapy. Another patient with high-grade SDC and lymph node metastasis showed disease recurrence and underwent radiotherapy but died 15 months after the operation, and 1 patient with low-grade adenocarcinoma, NOS developing from recurrent PA was treated with chemotherapy for distant metastasis to the lung but died 21 months after the operation. The 5-yr survival rate was 72.7%, and the disease mortality rate was higher in CXPA-LD than in CXPA-NLD; however, the difference was not statistically significant (P = 0.178).
Table 1.
Clinical parameters of study population and 5-yr survival rate
*Located in parapharynx (n = 2). CXPA, carcinoma ex pleomorphic adenoma; PA, pleomorphic adenoma; LD, luminal differentiation; NLD, non-luminal differentiation; n, number of cases; yr, years; SD, standard deviation; RT, radiation treatment; CT, chemotherapy; mo, months.
Table 2.
Histopathologic features and clinical outcome of carcinoma ex pleomorphic adenoma with luminal differentiation
Adenoca, adenocarcinoma, not otherwise specified; MEC, mucoepidermoid carcinoma; SDC, salivary duct carcinoma; NI, noninvasive; INV, invasive; MI, minimally invasive; LVI, lymphovascular tumor invasion; PNI, perineural invasion; LN, lymph node, DOD, died of disease.
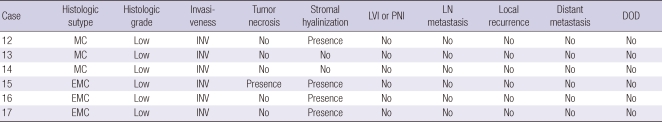
CXPA-NLD, which showed positive reactions for p63 and SMA and demonstrated myoepithelial differentiation, was observed in 6 (35.3%) patients, with equal numbers of men and women being affected. The mean age of the patients was 56.2 yr and the mean size of the tumors was 5.2 cm, which was larger than that of CXPA-LD (P = 0.040). The tumors developed from a primary PA. The histological subtype of the malignant component was MC in 3 tumors and EMC in 3 tumors (Table 3). All tumors were low-grade and invasive. All the 3 MCs and 1 EMC showed hyalinized stroma. One MC displayed coagulative necrosis. The percentage of the malignant component ranged from 38% to 90% (mean, 85.0%). No tumor showed lymph node metastasis. All patients remained disease-free after surgical excision irrespective of whether they received postoperative radiation treatment.
Table 3.
Histopathologic features and clinical outcome of carcinoma ex pleomorphic adenoma with non-luminal differentiation
MC, myoepithelial carcinoma; EMC, epithelial-myoepithelial carcinoma; NI, noninvasive; INV, invasive; MI, minimally invasive; LVI, lymphovascular tumor invasion; PNI, perineural invasion; LN, lymph node, DOD, died of disease.
Immunohistochemical analysis
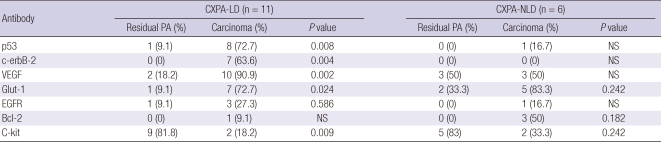
In CXPA-LD, p53, VEGF and Glut-1 was more often overexpressed in the carcinoma components than in the residual PA components (P = 0.008, 0.002, and 0.024, respectively) (Table 4). Positive staining for c-erbB-2 (score, 3+) was observed in the carcinoma components of 7 tumors but not in the PA components (P = 0.004) (Fig. 1). Moreover, c-erbB-2 overexpression was associated with a high histological grade (P = 0.024). Typical examples of different patterns of immunoreactivity for c-kit are shown in Fig. 2. We observed that c-kit was expressed in the luminal cells of the PA but not in the myoepithelial cells. Immunopositivity of c-kit was found in the PA component of 9 tumors (81.8%), whereas the carcinoma components of 9 tumors lacked c-kit expression (P = 0.009). Overexpression of EGFR (score, 2+) was noted in the malignant components of 3 tumors (27.3%) and in the residual PA of 1 tumor (9.1%). Bcl-2 was weakly stained in the carcinoma component of only 1 sample. No biomarker was found to be associated with invasiveness, and no significant prognostic factor was identified in the Cox proportional hazard model.
Table 4.
Immunohistochemical expression status of carcinoma and residual pleomorphic adenoma in carcinoma ex pleomorphic adenomas
n, number of cases with overexpression; CXPA-LD, carcinoma ex pleomorphic adenoma with luminal differentiation; CXPA-NLD, carcinoma ex pleomorphic adenoma with non-luminal differentiation; NS, not significant.
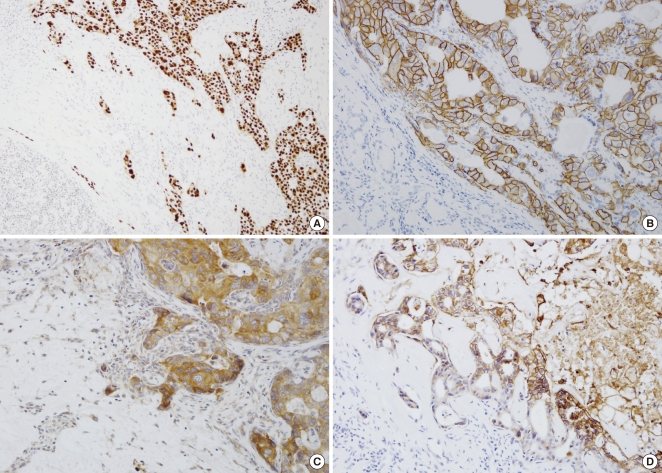
Fig. 1.
In CXPA-LD, strong immunohistochemical staining for p53 (A), c-erbB-2 (B), VEGF (C), and Glut-1 (D) was observed in the malignant components (top right), whereas no immunohistochemical reaction to the biomarkers was observed in the residual PA components (bottom left).
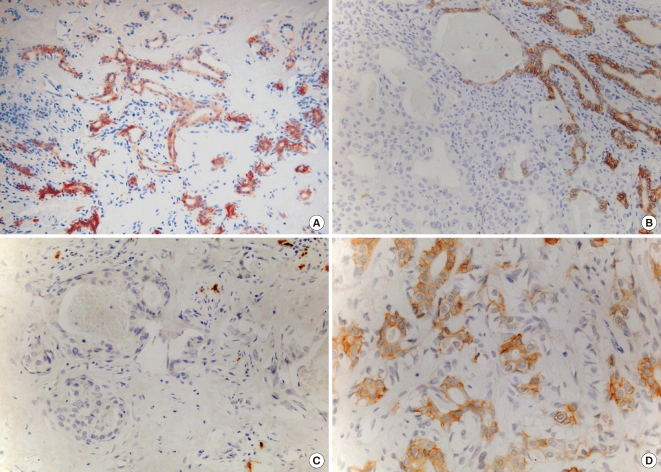
Fig. 2.
C-kit expression in residual PA and malignant components. In PA, strong membranous positivity was noted in the luminal cells of duct-like structures (A, B-top right), but in noninvasive and invasive components of CXPA-LD, the result of c-kit immunohistochemical staining was negative (B-bottom left and C). In CXPA-NLD with EMC subtype, c-kit was expressed only in the inner luminal cells of ductal structures (D).
In CXPA-NLD, Bcl-2 and Glut-1 were more frequently expressed in the malignant component than in the benign component; however, the difference was statistically non-significant. Two carcinoma components were faintly stained for c-erbB-2 (score, 1+) (Fig. 3). Immunohistochemical staining for p53, VEGF, and EGFR showed no significant difference in expression between the benign and malignant components. C-kit immunostain was expressed in the residual PA of 5 tumors (83%). In the carcinoma components, c-kit was not expressed in the MC subtype but was focally expressed only in the inner duct-like structure of 2 EMCs (33.3%, P = 0.242) (Fig. 2).
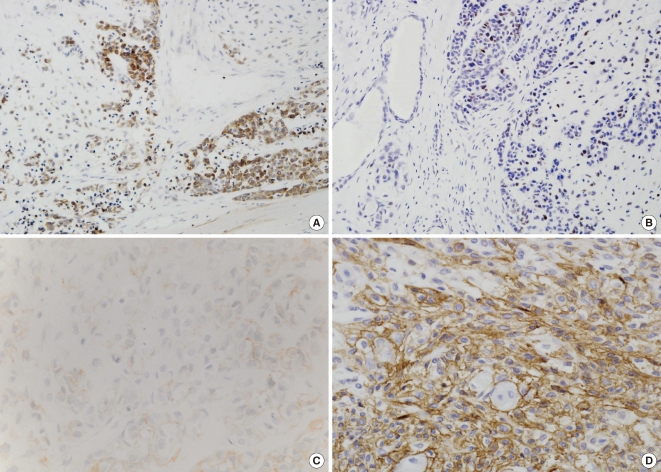
Fig. 3.
In CXPA-NLD, focally positive staining for Bcl-2 (A) and p53 (B) was observed in the malignant components. Also, positive immunohistochemical staining for c-erbB-2 (score 1+, [C]) and Glut-1 (high expression, [D]) was noted in some carcinoma cells.
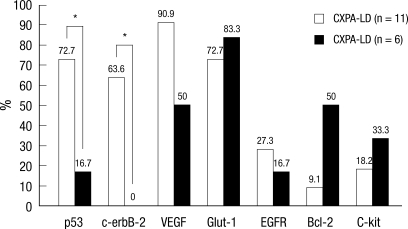
In addition, we compared the expression rate of biomarkers in malignant components between CXPA-LD and CXPA-NLD and found that p53 and c-erbB-2 overexpression was significantly frequent in the malignant component of CXPA-LD (P = 0.050 and 0.035, respectively) (Fig. 4).
Fig. 4.
Comparison of the expression rates of biomarkers between carcinoma ex pleomorphic adenomas with luminal and non-luminal differentiations in malignant components. p53 and c-erbB-2 overexpression rates in CXPA-LD are significantly higher than those in CXPA-NLD. *Statistically significant (P ≤ 0.05)
DISCUSSION
Carcinoma ex pleomorphic adenoma (CXPA) is the third common malignancy of the salivary gland and comprises approximately 3.6% of all salivary tumors and 12% of all salivary malignancies (19). Recently, Altemani et al. reported the following 2 types of carcinomas arising in a PA: 1) CXPAs composed of malignant epithelial cells, which have an immunoprofile comparable to that of ductal luminal cells in a PA and are probably derived from cells already committed to differentiation into ductal luminal cells and 2) CXPAs composed of myoepithelial cells, which are likely derived from a common precursor of myoepithelial and ductal cells (bipotent cell) (4, 20).
Here, we compared the CXPA-LD and NLD groups with respect to biomarker expression and clinical outcome. Our results showed substantial differences between the 2 groups with respect to the expression of ancillary biomarkers as well as in clinical features such as patient survival, tumor size, and gender.
With regard to the clinical outcome of CXPAs, many studies have demonstrated that the degree of tumor invasion is correlated with the prognosis of CXPA, and only a few studies have characterized CXPA according to histological subtype. Recently, Katabi et al. (10) published a study with 43 CXPAs showing that the MC subtype tends to increase the risk of recurrence and that a higher frequency of MC was identified in frankly invasive tumors. In our study, frankly invasive tumors were more frequent in CXPAs-NLD (100%) than in CXPS-LD (45.5%) like the previous study. However, the disease mortality rate was higher in CXPA-LD (27.3%) than in CXPA-NLD. And 66.7% of the dead had histologically high grade tumors. Therefore, we propose that not only invasiveness but also the histological features, such as subtype and grade, may be important prognostic factors. Katabi et al. did not describe the prognostic value of the histological grade in each carcinoma subtype but showed that histological parameters such as frequent mitoses (> 5 per 10 high-power fields), atypical mitosis, and necrosis correlated with disease-free survival. Also with regard to the evaluation of disease prognosis, consideration of the follow-up period is necessary. As a rule, low-grade carcinomas of the salivary gland have a tendency to progress slowly and require a long-term follow-up of more than 5 yr (21). And there was a report indicating that longer follow-up led to greater mortality in CXPAs-NLD than in CXPAs-LD (10). We therefore, consider that more long-term follow-up is mandatory for the exact evaluation of CXPA prognosis.
In previous reports, the positive rates for the p53 protein were 0%-41% for PA and 45.2%-75% for CXPA, and some authors postulated that the p53 mutation was an early event in the malignant transformation of PA (5, 7, 22, 23). The expression of the 2 members of the epidermal growth factor receptor family, i.e., EGFR and c-erbB-2, has also been investigated in several studies as prognostic factors, and it was found that the amplification of the c-erbB-2 gene or overexpression of the c-erbB-2 protein was associated with poor prognosis in salivary gland malignancies, including CXPA (24-26). Recently, DeRoche et al. suggested that markers such as c-erbB-2 and p53 may also be expressed in benign PA, and that these markers are not reliable for predicting early carcinomatous transformation in PA (14). However, in our study, p53 was expressed in only 1 PA (score 4) among the 17 tumor samples. Also, c-erbB-2 overexpression (score 3+) was significantly frequent in the carcinoma of the CXPA-LD group but was not present in the benign component of the CXPA-LD group or any component of CXPA-NLD. Therefore, we reasonably conclude that p53 and c-erB-B-2 biomarkers are associated with the carcinogenesis of CXPA-LD. In the absence of a chemotherapeutic standard, we propose that c-erbB-2 expression should be assessed in CXPA, and that c-erbB-2-targeted therapy may provide significant clinical benefit in an appropriately selected patient group. Thus far, only a few patients who showed strong c-erbB-2 immunoreactivity and were treated with trastuzumab have been reported to show a sustained response (27).
VEGF induces endothelial differentiation, which is regulated by the hypoxia-responsive elements of the promoter region of the VEGF gene. Swelam et al. (28) proposed that tumor cells of PA produce VEGF in several functional forms, and that VEGF expression is controlled by the hypoxic condition of poorly vascularized PA. Because stroma with scarring, hyalinization, and poor vascularization were observed in many CXPAs, we examined VEGF expression. Unexpectedly, the carcinoma cells were strongly stained in the LD group, whereas the VEGF expression pattern was nonspecific in the NLD group. There was no association between VEGF expression and stromal hyalinization.
The interaction of c-kit transmembrane tyrosine kinase receptor with its ligand, stem cell factor promotes phosphorylation and the activation of intracytoplasmic signal cascades, which are essential in embryogenesis, hematopoiesis, development, proliferation, and migration of germ cells. In salivary gland tumors, there is only limited information on the immunoreaction of c-kit in a PA, and a study on c-kit expression in benign and malignant components of CXPA has not been reported (16, 29). We observed the loss of c-kit expression in the invasive and noninvasive carcinoma components but not in the benign component of CXPA-LD; this suggests that the loss of c-kit is associated with the malignant transformation of CXPA-LD and c-kit may serve as a useful ancillary diagnostic marker for CXPA-LD in combination with p53, c-erbB2, and VEGF. We think that further study with larger cohorts is needed to demonstrate the diagnostic utility of c-kit for the differential diagnosis between early stage CXPA-LD and atypical PA, which it is sometimes challenging to distinguish them based on the morphologic features alone. In CXPA-NLD cases, c-kit was expressed in the most residual PAs (83.3%) and only in the inner luminal cells of an EMC; this result was similar to that observed in previous reports (29). These expression patterns also suggest that c-kit may play a role in the differentiation of common precursor cells of myoepithelial and ductal cells.
In the evaluation of salivary gland tumors, the use of 18-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET) remains a matter of debate, and there are only few reports describing the clinical significance of Glut-1 expression (18, 30). In this study, 18F-FDG PET was used for the detection of primary tumor or metastasis in 6 tumor samples, the results of which showed an increase in the maximum standardized uptake value (SUV), with a mean value of 4.3 (range, 2.3-5.8). Four high-grade CXPAs-LD (1 adenocarcinoma, NOS, 2 MEC, and 1 SDC) and 1 low-grade CXPA-NLD (EMC) had a maximum SUV of at least 3.7 and showed high Glut-1 expression. In 1 case of low-grade CXPA-LD (adenocarcinoma, NOS subtype), the maximum SUV was 2.3, and it showed low expression of Glut-1. The results illustrate an association between high FDG uptake and Glut-1 overexpression, and offer a basis for the clinical application of 18F-FDG PET and Glut-1 for differential diagnosis between CXPA and PA.
In order to investigate the possible relation between apoptosis and malignant transformation, we examined the frequency of Bcl-2 expression in CXPA (25). We observed a higher rate of positive staining for Bcl-2 in the malignant component of CXPA-NLD, but the difference was not statistically significant.
Although the number of cases analyzed in this study is limited, we show that the immunoprofiles and clinical course of CXPA differed according to its cellular differentiation. The distinct expression of biomarkers in CXPA-LD and CXPA-NLD indicates different mechanisms underlying carcinogenesis for the 2 CXPA subtypes. In CXPA-LD, the p53 mutation and growth factors/receptors such as VEGF and c-erbB-2 are suggested to participate in malignant transformation. Our results also suggest that c-kit may play a role in progression from PA to CXPA-LD. These biomarkers may be useful for detecting a carcinomatous change in the PA, and some can be utilized for therapeutic purposes in the appropriately selected cases. Hence, we believe that it is important to report the histological subtype of CXPA and to assess potential biomarkers such as p53, VEGF, c-erbB-2, c-kit, and glut-1 in diagnostic and therapeutic trials.
AUTHOR SUMMARY
Carcinoma ex Pleomorphic Adenoma of the Salivary Glands: Distinct Clinicopathologic Features and Immunoprofiles Between Subgroups According to Cellular Differentiation
Jeong Won Kim, Gui Young Kwon, Jong-Lyel Roh, Seung-Ho Choi, Soon Yuhl Nam, Sang Yoon Kim and Kyung-Ja Cho
Pathogenesis of carcinoma ex pleomorphic adenoma (CXPA) is unclear. To elucidate the clinicopathological implications of cellular differentiation in CXPA and the potential role of biomarkers in carcinogenesis, we analyzed 11 cases of CXPA with luminal differentiation (CXPA-LD) and 6 cases of CXPA with non-luminal differentiation (CXPA-NLD). The 5-yr survival rate of patients with CXPA-LD was 72.7%. p53, c-erbB-2, VEGF, and Glut-1 were more immunoreactive in carcinoma components than PAs. Also, carcinoma components frequently lacked c-kit expression. CXPAs-NLD with a larger mean tumor size (5.2 cm) than CXPAs-LD (3.3 cm, P = 0.040) remained disease-free without significant immunohistochemical expression. The immunoprofiles and clinical course of CXPA differed according to cellular differentiation. Therefore, it is important to report the histological subtype and to assess potential biomarkers.
References
- 1.Luna MA, Batsakis JG, Tortoledo ME, del Junco GW. Carcinomas ex monomorphic adenoma of salivary glands. J Laryngol Otol. 1989;103:756–759. doi: 10.1017/s0022215100109995. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001;32:596–604. doi: 10.1053/hupa.2001.25000. [DOI] [PubMed] [Google Scholar]
- 3.Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck. 2001;23:705–712. doi: 10.1002/hed.1100. [DOI] [PubMed] [Google Scholar]
- 4.Altemani A, Martins MT, Freitas L, Soares F, Araújo NS, Araújo VC. Carcinoma ex pleomorphic adenoma (CXPA): immunoprofile of the cells involved in carcinomatous progression. Histopathology. 2005;46:635–641. doi: 10.1111/j.1365-2559.2005.02157.x. [DOI] [PubMed] [Google Scholar]
- 5.Ihrler S, Weiler C, Hirschmann A, Sendelhofert A, Lang S, Guntinas-Lichius O, Arnold G, Zietz C, Harrison JD. Intraductal carcinoma is the precursor of carcinoma ex pleomorphic adenoma and is often associated with dysfunctional p53. Histopathology. 2007;51:362–371. doi: 10.1111/j.1365-2559.2007.02736.x. [DOI] [PubMed] [Google Scholar]
- 6.LiVolsi VA, Perzin KH. Malignant mixed tumors arising in salivary glands. I. Carcinomas arising in benign mixed tumors: a clinicopathologic study. Cancer. 1977;39:2209–2230. doi: 10.1002/1097-0142(197705)39:5<2209::aid-cncr2820390540>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto K, Yamamoto H, Shiratsuchi H, Nakashima T, Tamiya S, Higaki Y, Komune S, Tsuneyoshi M, Oda Y. S100P expression in ductal type of carcinoma ex pleomorphic adenoma. Am J Surg Pathol. 2011;35:346–355. doi: 10.1097/PAS.0b013e31820832a6. [DOI] [PubMed] [Google Scholar]
- 8.Cheuk W, Chan JK. Salivary gland tumors. In: Fletcher CD, editor. Diagnostic histopathology of tumors. London: Elsevier; 2007. pp. 239–326. [Google Scholar]
- 9.Tortoledo ME, Luna MA, Batsakis JG. Carcinomas ex pleomorphic adenoma and malignant mixed tumors. Histomorphologic indexes. Arch Otolaryngol. 1984;110:172–176. doi: 10.1001/archotol.1984.00800290036008. [DOI] [PubMed] [Google Scholar]
- 10.Katabi N, Gomez D, Klimstra DS, Carlson DL, Lee N, Ghossein R. Prognostic factors of recurrence in salivary carcinoma ex pleomorphic adenoma, with emphasis on the carcinoma histologic subtype: a clinicopathologic study of 43 cases. Hum Pathol. 2010;41:927–934. doi: 10.1016/j.humpath.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Demasi AP, Furuse C, Soares AB, Altemani A, Araújo VC. Peroxiredoxin I, platelet-derived growth factor A, and platelet-derived growth factor receptor alpha are overexpressed in carcinoma ex pleomorphic adenoma: association with malignant transformation. Hum Pathol. 2009;40:390–397. doi: 10.1016/j.humpath.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Barnes L, Eveson JW, Reichart P, Sidransky D. Tumours of the salivary glands. In: Barnes L, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Lyon: International Agency for Research on Cancer (IARC) Press; 2005. pp. 209–282. [Google Scholar]
- 13.Zietz C, Rössle M, Haas C, Sendelhofert A, Hirschmann A, Sturzl M, Löhrs U. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Pathol. 1998;153:1425–1433. doi: 10.1016/S0002-9440(10)65729-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeRoche TC, Hoschar AP, Hunt JL. Immunohistochemical evaluation of androgen receptor, HER-2/neu, and p53 in benign pleomorphic adenomas. Arch Pathol Lab Med. 2008;132:1907–1911. doi: 10.5858/132.12.1907. [DOI] [PubMed] [Google Scholar]
- 15.Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16:2266–2274. doi: 10.1158/1078-0432.CCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ettl T, Schwarz S, Kleinsasser N, Hartmann A, Reichert TE, Driemel O. Overexpression of EGFR and absence of C-KIT expression correlate with poor prognosis in salivary gland carcinomas. Histopathology. 2008;53:567–577. doi: 10.1111/j.1365-2559.2008.03159.x. [DOI] [PubMed] [Google Scholar]
- 17.Han H, Silverman JF, Santucci TS, Macherey RS, d'Amato TA, Tung MY, Weyant RJ, Landreneau RJ. Vascular endothelial growth factor expression in stage I non-small cell lung cancer correlates with neoangiogenesis and a poor prognosis. Ann Surg Oncol. 2001;8:72–79. doi: 10.1007/s10434-001-0072-y. [DOI] [PubMed] [Google Scholar]
- 18.Mori Y, Tsukinoki K, Yasuda M, Miyazawa M, Kaneko A, Watanabe Y. Glucose transporter type 1 expression are associated with poor prognosis in patients with salivary gland tumors. Oral Oncol. 2007;43:563–569. doi: 10.1016/j.oraloncology.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Gnepp DR, Brandwein-Gensler MS, El-Naggar AK, Nagao T. Carcinoma ex pleomorphic adenoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Lyon: IARC; 2005. pp. 242–243. [Google Scholar]
- 20.Sato M, Hayashi Y, Yoshida H, Yanagawa T, Yura Y, Nitta T. Search for specific markers of neoplastic epithelial duct and myoepithelial cell lines established from human salivary gland and characterization of their growth in vitro. Cancer. 1984;54:2959–2967. doi: 10.1002/1097-0142(19841215)54:12<2959::aid-cncr2820541225>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761–774. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Deguchi H, Hamano H, Hayashi Y. c-myc, ras p21 and p53 expression in pleomorphic adenoma and its malignant form of the human salivary glands. Acta Pathol Jpn. 1993;43:413–422. doi: 10.1111/j.1440-1827.1993.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto Y, Kishimoto Y, Wistuba II, Virmani AK, Vuitch F, Gazdar AF, Albores-Saavedra J. DNA analysis at p53 locus in carcinomas arising from pleomorphic adenomas of salivary glands: comparison of molecular study and p53 immunostaining. Pathol Int. 1998;48:265–272. doi: 10.1111/j.1440-1827.1998.tb03904.x. [DOI] [PubMed] [Google Scholar]
- 24.Müller S, Vigneswaran N, Gansler T, Gramlich T, DeRose PB, Cohen C. c-erbB-2 oncoprotein expression and amplification in pleomorphic adenoma and carcinoma ex pleomorphic adenoma: relationship to prognosis. Mod Pathol. 1994;7:628–632. [PubMed] [Google Scholar]
- 25.Nagler RM, Kerner H, Ben-Eliezer S, Minkov I, Ben-Itzhak O. Prognostic role of apoptotic, Bcl-2, c-erbB-2 and p53 tumor markers in salivary gland malignancies. Oncology. 2003;64:389–398. doi: 10.1159/000070298. [DOI] [PubMed] [Google Scholar]
- 26.Sugano S, Mukai K, Tsuda H, Hirohashi S, Furuya S, Shimosato Y, Ebihara S, Takeyama I. Immunohistochemical study of c-erbB-2 oncoprotein overexpression in human major salivary gland carcinoma: an indicator of aggressiveness. Laryngoscope. 1992;102:923–927. doi: 10.1288/00005537-199208000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Sharon E, Kelly RJ, Szabo E. Sustained response of carcinoma ex pleomorphic adenoma treated with trastuzumab and capecitabine. Head Neck Oncol. 2010;2:12. doi: 10.1186/1758-3284-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swelam W, Ida-Yonemochi H, Maruyama S, Ohshiro K, Cheng J, Saku T. Vascular endothelial growth factor in salivary pleomorphic adenomas: one of the reasons for their poorly vascularized stroma. Virchows Arch. 2005;446:653–662. doi: 10.1007/s00428-005-1219-1. [DOI] [PubMed] [Google Scholar]
- 29.Andreadis D, Epivatianos A, Poulopoulos A, Nomikos A, Papazoglou G, Antoniades D, Barbatis C. Detection of C-KIT (CD117) molecule in benign and malignant salivary gland tumours. Oral Oncol. 2006;42:57–65. doi: 10.1016/j.oraloncology.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Horiuchi C, Tsukuda M, Taguchi T, Ishiguro Y, Okudera K, Inoue T. Correlation between FDG-PET findings and GLUT1 expression in salivary gland pleomorphic adenomas. Ann Nucl Med. 2008;22:693–698. doi: 10.1007/s12149-008-0162-z. [DOI] [PubMed] [Google Scholar]