Abstract
This study examined the normal ranges and the determinants for various parameters of the short-term heart rate variability (HRV) measurements in a large Korean sample of healthy people. HRV measurements were obtained in 2,748 healthy men and 735 healthy women 18-65 yr of age. The mean total power (TP), low frequency (LF), high frequency (HF), and LF/HF ratio were 1,358.9 ± 1,840.8 ms2, 417.3 ± 807.6 ms2, 254.1 ± 414.1 ms2, and 2.4 ± 20.9 ms2 in the frequency-domain spectral analysis. The mean standard deviation of the normal-to-normal (NN) interval (SDNN) and the square root of the mean squared differences of successive NN intervals (RMSSD) were 39.6 ± 22.1 ms and 29.7 ± 18.1 ms in the time-domain analysis. The female subjects had significantly higher SDNN, RMSSD, and HF values than the male subjects. After controlling for age, there was no statistically significant difference in the SDNN. Quantile regression analysis showed that age and mean heart rate had a significant impact on short-term HRV measurement. Given that both clinicians and researchers are increasingly relying on short-term HRV assessment in measuring stress, our work suggests that age and gender should be considered as independent determinants for HRV.
Keywords: Age, Gender, Heart Rate Variability, Autonomic Nervous System, Psychophysiology
INTRODUCTION
Heart rate variability (HRV) provide a perspective on both sympathetic and parasympathetic nervous system functioning (1). HRV, which is defined as 'the amount of fluctuations from the mean heart rate', is primarily controlled by the continuous interplay of the autonomic nervous system (ANS). Two frequency components are usually distinguishable in short-term HRV: high frequency (HF) and low frequency (LF) components (2).
The number of patients visiting health-promotion centers and primary practitioners for the assessment and treatment of stress symptoms is increasing in Korea. Therefore, many hospitals and health-promotion centers measure both psychological stress and ANS function at the initial work-up and routine medical checkups to apply these pieces of information to patient care. Recently, there has been growing interest in HRV because several studies have consistently shown an association between reduced HRV and increased risk of cardiovascular morbidity and mortality (3-6), poor prognosis of many other diseases (7-9), and an association between HRV and mental disorders (10).
To apply HRV measures in monitoring physiological stress levels in clinical practice or medical checkups, normative values of HRV variables and their determinants are required. Until now, there have been a few studies on normative data of HRV in a normal population without cardiac diseases (5); one study involved standardized tests of HRV for autonomic function testing in healthy Koreans (11), but it had a limit by its relatively small sample size. A study with a larger sample size has the advantage of enabling control of other sources of variance, such as age, obesity and health-related habits.
In this study, we evaluated the normal ranges of various parameters of short-term HRV in a large Korean sample of healthy people to obtain normative data on HRV. We also analyzed the impact of gender, age, and other relevant demographic and clinical variables on short-term HRV measurements.
MATERIALS AND METHODS
Subjects
We recruited 2,748 men and 735 women who were 18-65 yr of age, from 5 health-promotion centers in Korea during their annual medical check-up. Recruitment of subject was conducted from April 1, 2003, to August 31, 2003. All participants were screened for medications and medical conditions. Psychiatric diagnoses were assessed using the Mini-International-Neuropsychiatric Interview (M.I.N.I.) (12) by an experienced psychiatric nurse and a psychiatrist. Thirty-nine participants with histories of major medical disorders and seven participants with histories of major psychiatric disorders were excluded. Before the HRV measurements, the participants answered a questionnaire on personal information and their life habits (e.g., smoking, alcohol consumption, coffee drinking, and exercise). After the measurement, 41 participants, who had an abnormally high standard deviation (SD) of the normal-to-normal interval (SDNN) in the HRV (≥ 100, 3 standard deviations), were excluded from the analysis. Finally 2,679 men and 717 women who completed the HRV measurements and questionnaires were included in the analysis.
Measurement of HRV
All subjects were instructed to avoid alcohol or caffeinated beverages after 10 p.m. (22:00) on the night before the HRV measurements. In addition, they refrained from smoking 1 hr before the measurements. To control for diurnal variation, HRV was measured between 8 a.m. (08:00) and 12 a.m. (12:00) by using the SA-2000E model (Medi-core, Seoul, Korea). Each subject was comfortably seated on a chair, electrodes were placed on their wrists and left foot to derive HRV for 5 min, and the subject was guided to breathe according to their usual rate during the HRV measurement.
The following HRV parameters were measured by frequency domain spectral analysis (13): total power (TP), LF, and HF. TP is the variance of the normal-to-normal interval (NN) over a temporal segment. The LF/HF ratio was calculated because it considered to reflect sympatho-vagal balance or sympathetic modulation (2). The standard deviation of the normal-to-normal interval (SDNN) was used to estimate long-term components of the HRV. It is calculated by statistical time domain measurements. The square root of the mean squared differences of successive NN intervals (RMSSD) was also calculated by statistical time domain measurements.
Statistical analysis
All HRV variables were expressed as the mean ± SD and were tested by the Kolmogorov-Smirnov test for normal distribution. All HRV variables were then compared between age-matched groups. ANOVA was used to compare age and life habits with the HRV variables. Quantile regression models were constructed to identify the association between dependent variables (SDNN, RMSSD, TP) and the independent variables (mean heart rate, age, body mass index [BMI], and systolic blood pressure) analyzed. This models enable the effect of independent variables on different points of the distribution of the dependent variables which were asymmetric. All HRV variables were described as percentiles. The quantiles were based on the distribution of HRV values where the 10th, 25th, 50th, 75th, and 90th percentiles were considered, and the median is the 50th quantile. All statistical analyses were performed by using the SPSS 12.0 for Windows (SPSS Korea Datasolution Inc.).
Ethics statement
All participants provided informed consent after receiving a complete explanation regarding the study.
RESULTS
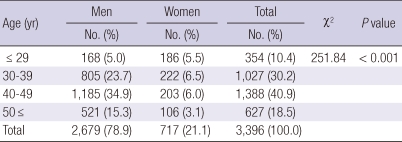
The mean age of the subjects was 41.4 ± 9.00 yr (range, 18-65 yr); the male and female subjects had mean ages of 42.3 ± 8.3 and 38.0 ± 10.5 yr, respectively. Educationally, subjects who were high school graduates and had higher qualifications were 3,022 (89%). Smokers were 1,956 (57.6%) and subjects who reported drinking alcohol more than once a week were 1,681 (49.5%). Subjects who reported exercising more than once a week were 2,211 (65.1%), and subjects who consumed coffee regularly were 2,649 (78.0%). Their demographic characteristics are presented in Table 1.
Table 1.
The demographic characteristics of participants
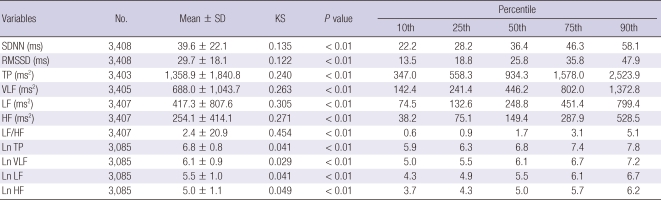
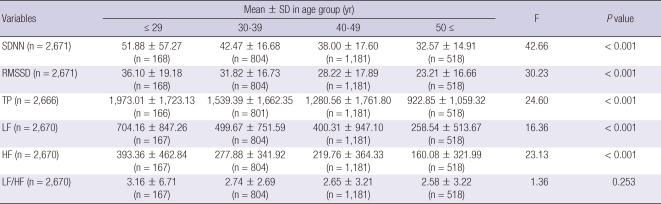
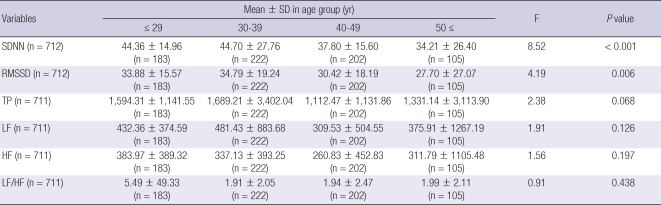
The mean TP, LF, HF, and LF/HF ratio were 1,358.9 ± 1,840.8 ms2, 417.3 ± 807.6 ms2, 254.1 ± 414.1 ms2, and 2.4 ± 20.9, respectively, in the frequency-domain spectral analysis. The mean SDNN and RMSSD were 39.6 ± 22.1 ms and 29.7 ± 18.1 ms, respectively, in the time-domain analysis. The results of the 5-min HRV resting measurements in the normal population are presented in Table 2. In men, the SDNN, RMSSD, TP, LF, and HF significantly decreased with increasing age (F = 42.66, P < 0.001; F = 30.23, P < 0.001; F = 24.60, P < 0.001; F = 16.36, P < 0.001; F = 23.13, P < 0.001, respectively), as shown in Table 3. In female, the SDNN and RMSSD significantly decreased with increasing age (F = 8.52, P < 0.001; F = 4.19, P = 0.006) as shown in Table 4.
Table 2.
The results of the 5-minutes HRV resting measures in a normal population
SD, standard deviation; KS, Kolmogorov-Smirnov test; SDNN, Standard deviation of normal-to-normal interval; RMSSD, the square root of the mean squared differences of successive NN intervals; TP, total power; VLF, very low frequency; LF, low frequency; HF, high frequency; Ln, Logarithmic.
Table 3.
The differences of HRV variables in men by age group
SD, standard deviation; SDNN, Standard deviation of normal-to-normal interval; RMSSD, The square root of the mean squared differences of successive NN intervals; TP, total power; LF, low frequency; HF, high frequency.
Table 4.
The differences of HRV variables in women by age group
SD, standard deviation; SDNN, Standard deviation of normal-to-normal interval; RMSSD, The square root of the mean squared differences of successive NN intervals; TP, total power; LF, low frequency; HF, high frequency.
The female subjects had significantly higher SDNN, RMSSD, and HF values than the male subjects (F = 4.74, P = 0.030; F = 19.09, P < 0.001; F = 26.09, P < 0.001, respectively). After controlling for age, there was no statistically significant difference in the SDNN. However, the RMSSD, LF/HF ratio, logarithmic (Ln) TP, Ln LF, and Ln HF were significantly different between genders (F = 4.38, P = 0.037; F = 40.59, P < 0.001; F = 3.86, P = 0.005; F = 27.19, P < 0.001; and F = 6.78, P = 0.009, respectively).
There were no significant differences in the HRV variables between the smokers and the non-smokers. However, the SDNN, RMSSD and HF of the participants who drank alcohol more than once a week were lower than those of the participants who drank less than once a week (F = 3.74, P = 0.053; F = 3.92, P = 0.048; F = 3.40, P = 0.065, respectively). The Ln TP, Ln LF and Ln HF values were significantly higher in the participants who consumed coffee regularly than in those did not (F = 6.55, P = 0.011; F = 4.78, P = 0.029; F = 4.49, P = 0.034). Regarding exercise, the SDNN, RMSSD, TP, LF, and HF values were significantly increased with the exercise frequency (F = 8.47, P < 0.001; F = 13.42, P < 0.001; F = 4.97, P = 0.002; F = 4.14, P = 0.006; and F = 4.70, P = 0.003, respectively).
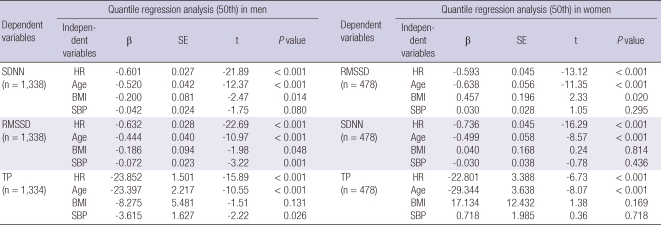
Quantile regression analysis identified several HRV parameters that were significantly associated with other demographic or clinical variables. Age and mean heart rate seemed to be the two most important determinants of the SDNN, RMSSD, and TP (Table 5).
Table 5.
The results of quantile regression analysis (50th)
SE, standard error; SDNN, Standard deviation of normal-to-normal interval; RMSSD, The square root of the mean squared differences of successive NN intervals; TP, total power; HR, mean heart rate; BMI, body mass index; SBP, systolic blood pressure.
DISCUSSION
In this study, we examined the normal ranges of various parameters of short-term HRV and the impact of gender and age on the values in a large Korean sample of healthy people. First, we suggest mean TP, LF, HF and LF/HF values of 1,358.9 ± 1,840.8 ms2, 417.3 ± 807.6 ms2, 254.1 ± 414.1 ms2, and 2.4 ± 20.9 by frequency domain analysis and the mean SDNN and RMSSD values of 39.6 ± 22.1 ms and 29.7 ± 18.1 ms by time-domain analysis. Tsuji et al. (14) suggested the normative values for all eight HRV measurements. Their Ln-LF and Ln-HF values are higher than those derived in our study, and may be explained by the higher average age and ethnic difference in their study. Park et al. (11) suggested mean TP, SDNN, and RMSSD values of 1,106.9 ± 1,109.1 ms2, 35.9 ± 15.5 ms, and 27.3 ± 15.6 ms, respectively, by time-domain analysis, and mean LF, HF, and LF/HF values of 287.5 ± 384.1 ms2, 227.0 ± 284.4 ms2, and 2.2 ± 3.4, respectively, by frequency-domain analysis in healthy Koreans. Although their findings are similar to our results, they are based on a relatively small sample size and thus cannot provide further information on the impact of demographic and clinical determinants.
Then, we analyzed whether the various demographic or clinical variables (gender, age, BMI, heart rate, blood pressure) influence HRV measurements. There were significant differences in HRV parameters between the genders, and after controlling for age, the statistical significance of those differences was changed slightly. Some studies have shown that HRV measurements are affected not only by age (14-17), but also by gender (17, 18). Especially, Ramaekers et al. (19) suggested that estrogens have a cardioprotective effect in women. Huikuri et al. (20) reported higher parasympathetic activity in postmenopausal women given hormonal replacement therapy; generally increased parasympathetic activity in women may be reflected by higher average HF and RMSSD values and the resulting lower LF/HF ratio.
As the 50th quantile regression analysis, the changes in the RMSSD depended on BMI in men but not in women, which is similar to previous study showing gender differences in BMI (18). Obviously, most HRV parameters depend on heart rate in both genders because HRV-estimating methods are based on heart rate. Muneta et al. (21) reported that approximately 80% of their patients with isolated systolic hypertension (ISH) showed a significant positive correlation between blood pressure and heart rate. These results suggest that blood pressure in patients with ISH is susceptible to fluctuations in ANS activity. In this study, the RMSSD and TP of the male participants depend on systolic blood pressure, which may be explained by the fact that the RMSSD and TP reflect the reactivity of cardiac rhythm.
Lastly, we analyzed whether various life habits influence HRV measurements. In this study, the SDNN, RMSSD and HF of the participants who habitually drank alcohol were significantly lower than those of the participants who did not. Further, the SDNN, RMSSD, TP, LF and HF values significantly increased with the exercise frequency. These findings are consistent with the reports of previous studies showing that exercise improve HRV by increasing the vagal tone (22) and habitual intake of alcohol negatively influences HRV by increasing autonomic instability (23).
Cagirci et al. (24) reported that the LF and LF/HF ratio are significantly higher and SDNN, RMSSD, and HF values are significantly lower in heavy smokers. Cigarette smoking induces functional changes in the cardiovascular system and these may reflect changes in a different organ system (e.g., the ANS influencing HRV) (25). We did not find the significant differences in the HRV parameters between the smokers and the non-smokers, which may be inconsistent with the results of previous studies. A possible reason for the lack of difference is that the history of smoking was not strictly controlled.
Monda et al. (26) recently reported that espresso coffee influences parasympathetic activity in the supine position and Mattioli (27) reported the beneficial effects of coffee consumption due to anti-inflammatory actions mediated by antioxidants in the beverage. Rauh et al. (28) suggested that modest consumption of caffeine does not reveal negative or positive effects on HRV parameters in young and healthy habitual caffeine consumers. In our study, Ln-TP, Ln-LF, and Ln-HF values were significantly higher in the habitual coffee consumers. Further research is necessary to determine the effects of caffeine on HRV in habitual caffeine users.
This study has some limitations. First, the subjects were not randomly selected from the general population and there were more men than women. Approximately three-fourths of the subjects were permanent workers who underwent annual checkups regularly, which are mandatory in Korea. As more men are employed in full-time positions, the gender disparity may reflect the reality. Second, the psychometric instruments were administered by only self-reports, and there is a possibility of missing past medical and psychiatric disorders. However, the possibility of selection bias is quite low because the subjects were healthy.
In conclusion, using a very large sample of healthy people, we have reported the normal ranges of HRV variables. Given that both clinicians and researchers are increasingly relying on short-term HRV assessment in measuring stress, our work suggest that age and gender should be considered as independent determinants for HRV.
AUTHOR SUMMARY
Determinants for Heart Rate Variability in a Normal Korean Population
Gyung-Mee Kim and Jong-Min Woo
In this study, we reported the normal ranges of short-term heart rate variability (HRV) variables in healthy Korean people, and analyzed whether the various demographic or clinical variables influence HRV measurements. There were significant differences in HRV parameters between genders and after controlling for age, the statistical significance of those differences was changed. Given that both clinicians and researchers are increasingly relying on short-term HRV assessment in measuring stress, our work suggests that age and gender should be considered as independent determinants for HRV.
References
- 1.Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biol Psychol. 1998;49:303–323. doi: 10.1016/s0301-0511(98)00051-9. [DOI] [PubMed] [Google Scholar]
- 2.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 3.Steeds R, Fletcher J, Smith M, West J, Channer K, Townend J. Prognostic significance of early short-term measurements of heart rate variability following acute myocardial infarction. Am J Cardiol. 2004;94:1275–1278. doi: 10.1016/j.amjcard.2004.07.111. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Anker SD, Chua TP, Szelemej R, Piepoli M, Adamopoulos S, Webb-Peploe K, Harrington D, Banasiak W, Wrabec K, Coats AJ. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79:1645–1650. doi: 10.1016/s0002-9149(97)00215-4. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 6.Bae YP, Yi BD, Kim BG, Park JH, Kwon YS, Park JY, Lee CW, Kim BH, Jang JS. Relationships between cardiac autonomic neuropathy and the brachial-ankle pulse wave velocity in patients with yype 2 diabetes. Endocrinol Metab. 2011;26:44–52. [Google Scholar]
- 7.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 8.Rechlin T, Orbes I, Weis M, Kaschka WP. Autonomic cardiac abnormalities in alcohol-dependent patients admitted to a psychiatric department. Clin Auton Res. 1996;6:119–122. doi: 10.1007/BF02291234. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Kim JA, Choi YS, Kim SH, Lee JY, Kim YE. Heart rate variability and length of survival in hospice cancer patients. J Korean Med Sci. 2010;25:1140–1145. doi: 10.3346/jkms.2010.25.8.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. Am Heart J. 2000;140(4 Suppl):77–83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- 11.Park SB, Lee BC, Jeong KS. Standardized tests of heart rate variability for autonomic function tests in healthy Koreans. Int J Neurosci. 2007;117:1707–1717. doi: 10.1080/00207450601050097. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 13.Slaap BR, Boshuisen ML, Van Roon AM, den Boer JA. Heart rate variability as predictor of nonresponse to mirtazapine in panic disorder: a preliminary study. Int Clin Psychopharmacol. 2002;17:69–74. doi: 10.1097/00004850-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Determinants of heart rate variability. J Am Coll Cardiol. 1996;28:1539–1546. doi: 10.1016/s0735-1097(96)00342-7. [DOI] [PubMed] [Google Scholar]
- 15.Agelink MW, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, Ziegler D. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin Auton Res. 2001;11:99–108. doi: 10.1007/BF02322053. [DOI] [PubMed] [Google Scholar]
- 16.Jensen-Urstad K, Storck N, Bouvier F, Ericson M, Lindblad LE, Jensen-Urstad M. Heart rate variability in healthy subjects is related to age and gender. Acta Physiol Scand. 1997;160:235–241. doi: 10.1046/j.1365-201X.1997.00142.x. [DOI] [PubMed] [Google Scholar]
- 17.Parati G, Di Rienzo M. Determinants of heart rate and heart rate variability. J Hypertens. 2003;21:477–480. doi: 10.1097/00004872-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Kuch B, Hense HW, Sinnreich R, Kark JD, von Eckardstein A, Sapoznikov D, Bolte HD. Determinants of short-period heart rate variability in the general population. Cardiology. 2001;95:131–138. doi: 10.1159/000047359. [DOI] [PubMed] [Google Scholar]
- 19.Ramaekers D, Ector H, Aubert AE, Rubens A, Van de Werf F. Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur Heart J. 1998;19:1334–1341. doi: 10.1053/euhj.1998.1084. [DOI] [PubMed] [Google Scholar]
- 20.Huikuri HV, Pikkujämsä SM, Airaksinen KE, Ikäheimo MJ, Rantala AO, Kauma H, Lilja M, Kesäniemi YA. Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation. 1996;94:122–125. doi: 10.1161/01.cir.94.2.122. [DOI] [PubMed] [Google Scholar]
- 21.Muneta S, Murakami E, Sumimoto T, Iwata T, Hiwada K, Sato Y, Imamura Y. Blood pressure and heart rate variability in elderly patients with isolated systolic hypertension. J Hum Hypertens. 1991;5:393–398. [PubMed] [Google Scholar]
- 22.Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26:303–312. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata K, Araki S, Yokoyama K, Sata F, Yamashita K, Ono Y. Autonomic neurotoxicity of alcohol assessed by heart rate variability. J Auton Nerv Syst. 1994;48:105–111. doi: 10.1016/0165-1838(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 24.Cagirci G, Cay S, Karakurt O, Eryasar N, Kaya V, Canga A, Yesilay AB, Kilic H, Topaloglu S, Aras D, Demir AD, Akdemir R. Influence of heavy cigarette smoking on heart rate variability and heart rate turbulence parameters. Ann Noninvasive Electrocardiol. 2009;14:327–332. doi: 10.1111/j.1542-474X.2009.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unverdorben M, von Holt K, Winkelmann BR. Smoking and atherosclerotic cardiovascular disease: part III: functional biomarkers influenced by smoking. Biomark Med. 2009;3:807–823. doi: 10.2217/bmm.09.69. [DOI] [PubMed] [Google Scholar]
- 26.Monda M, Viggiano A, Vicidomini C, Viggiano A, Iannaccone T, Tafuri D, De Luca B. Espresso coffee increases parasympathetic activity in young, healthy people. Nutr Neurosci. 2009;12:43–48. doi: 10.1179/147683009X388841. [DOI] [PubMed] [Google Scholar]
- 27.Mattioli AV. Effects of caffeine and coffee consumption on cardiovascular disease and risk factors. Future Cardiol. 2007;3:203–212. doi: 10.2217/14796678.3.2.203. [DOI] [PubMed] [Google Scholar]
- 28.Rauh R, Burkert M, Siepmann M, Mueck-Weymann M. Acute effects of caffeine on heart rate variability in habitual caffeine consumers. Clin Physiol Funct Imaging. 2006;26:163–166. doi: 10.1111/j.1475-097X.2006.00663.x. [DOI] [PubMed] [Google Scholar]