Abstract
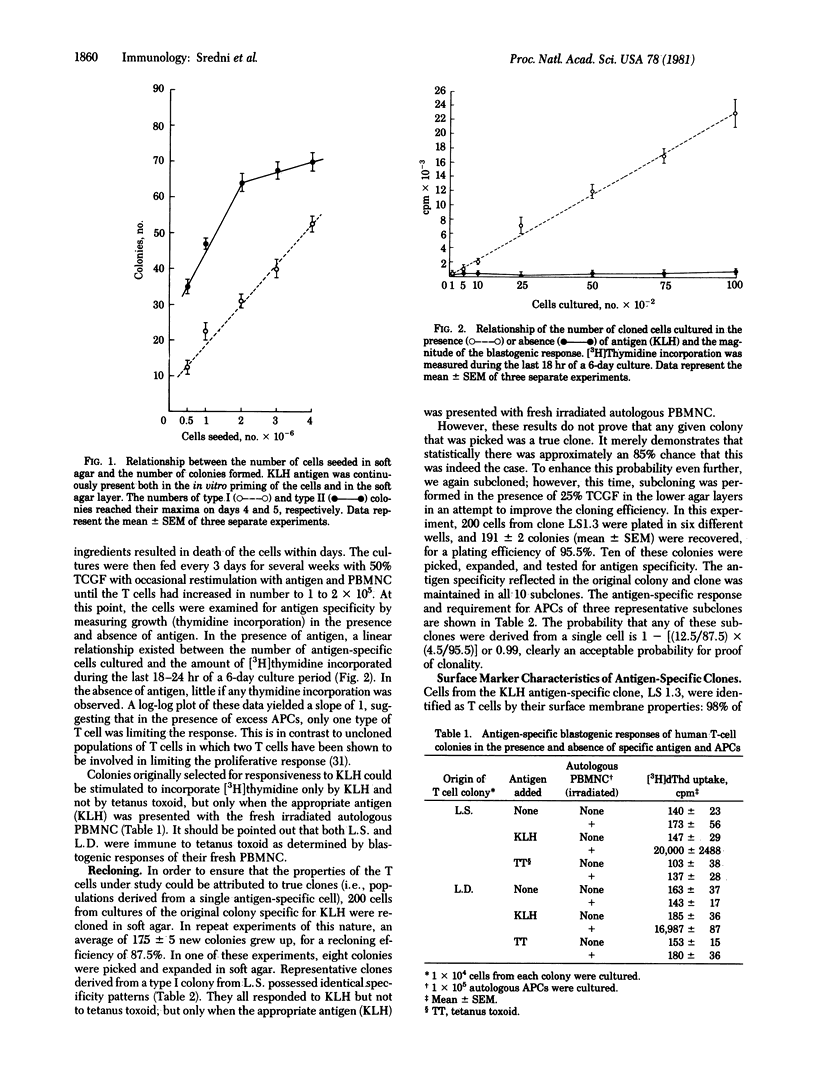
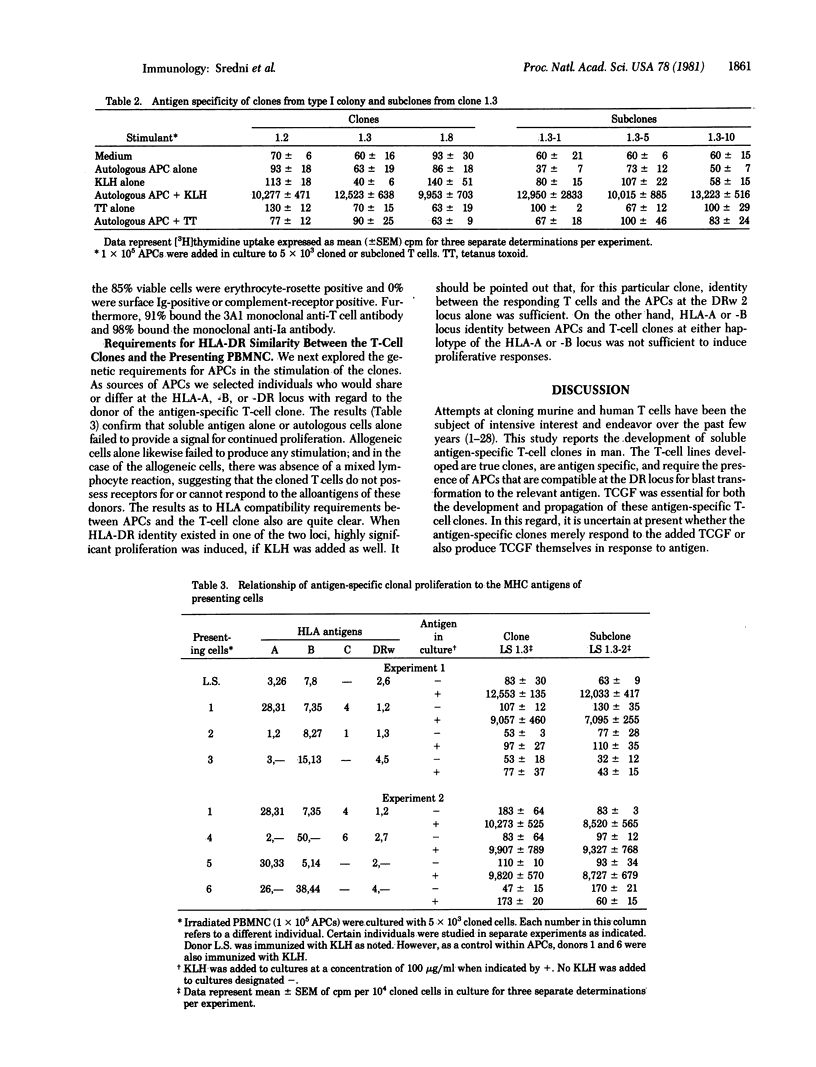
A method for cloning soluble antigen-specific proliferating human T lymphocytes directly from peripheral blood cells of individuals recently primed with the antigen is described. The soluble antigen was keyhole limpet hemocyanin. After expansion in liquid culture, these cells were shown to be antigen specific and to require HLA-DR-histocompatible presenting cells. Recloning of small numbers of these cells in soft agar under conditions of high plating efficiency yielded true clones (i.e., derived from a single cell) which retained specificity for the original stimulating antigen as shown by blastogenic responses measured by incorporation of [3H]thymidine. However, antigen-specific stimulation of clones was demonstrated only in the presence of an HLA-DR-compatible cooperating cell population together with the relevant antigen. Cells that manifested only HLA-A or B locus identity with the T-cell clone were not effective as presenting cells. Other antigens, such as tetanus toxoid, to which the donor of the clone or of the presenting cells was immune failed to stimulate the clone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin A. A., Julius M. H., Cosenza H. Antigen-specific stimulation and trans-stimulation of T cells in long-term culture. Eur J Immunol. 1979 Sep;9(9):665–670. doi: 10.1002/eji.1830090903. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Inouye H., Hank J. A., Alter B. J. Human T lymphocytes clones reactive in primed lymphocyte typing and cytotoxicity. Nature. 1979 Sep 27;281(5729):307–309. doi: 10.1038/281307a0. [DOI] [PubMed] [Google Scholar]
- Baker P. E., Gillis S., Smith K. A. Monoclonal cytolytic T-cell lines. J Exp Med. 1979 Jan 1;149(1):273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson S. Z., Paul W. E., Shevach E. M., Green I. In vitro selection and extended culture of antigen-specific T lymphocytes. II. Mechanisms of selection. J Immunol. 1975 Dec;115(6):1723–1730. [PubMed] [Google Scholar]
- Bergholtz B. O., Thorsby E. HLA-D restriction of the macrophage-dependent response of immune human T lymphocytes to PPD in vitro: inhibition by anti-HLA-DR antisera. Scand J Immunol. 1978;8(1):63–73. doi: 10.1111/j.1365-3083.1978.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Bonnard G. D., Yasaka K., Jacobson D. Ligand-activated T cell growth factor-induced proliferation: absorption of T cell growth factor by activated T cells. J Immunol. 1979 Dec;123(6):2704–2708. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Click R. E., Benck L., Alter B. J. Immune responses in vitro. I. Culture conditions for antibody synthesis. Cell Immunol. 1972 Feb;3(2):264–276. doi: 10.1016/0008-8749(72)90165-7. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. II. Cellular interactions in the PFC response of human tonsillar and peripheral blood B lymphocytes to polyclonal activation by pokeweed mitogen. J Immunol. 1976 Dec;117(6):2100–2104. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Glasebrook A. L., Fitch F. W. T-cell lines which cooperate in generation of specific cytolytic activity. Nature. 1979 Mar 8;278(5700):171–173. doi: 10.1038/278171a0. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner H., Fathman C. G. Clones of alloreactive T cells. I. A. unique homozygous MLR-stimulating determinant present on B6 stimulators. Immunogenetics. 1980;10(2):175–184. doi: 10.1007/BF01561566. [DOI] [PubMed] [Google Scholar]
- Kurnick J. T., Altevogt P., Lindblom J., Sjöberg O., Danneus A., Wigzell H. Long-term maintenance of HLA-D restricted T cells specific for soluble antigens. Scand J Immunol. 1980;11(2):131–136. doi: 10.1111/j.1365-3083.1980.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Kurnick J. T., Grönvik K. O., Kimura A. K., Lindblom J. B., Skoog V. T., Sjöberg O., Wigzell H. Long term growth in vitro of human T cell blasts with maintenance of specificity and function. J Immunol. 1979 Apr;122(4):1255–1260. [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. II. Antigen-mediated physical interactions between immune guinea pig lymph node lymphocytes and syngeneic macrophages. J Exp Med. 1975 Jan 1;141(1):138–154. doi: 10.1084/jem.141.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M. T., Strausser J. L., Rosenberg S. A. In vitro growth of cytotoxic human lymphocytes. II. Use of T cell growth factor (TCGF) to clone human T cells. J Immunol. 1980 Jun;124(6):2972–2978. [PubMed] [Google Scholar]
- Macdonald H. R., Engers H. D., Cerottini J. C., Brunner K. T. Generation of cytotoxic T lymphocytes in vitro. II. Effect of repeated exposure to alloantigens on the cytotoxic activity of long-term mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):718–730. doi: 10.1084/jem.140.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Rozenszajn L. A., Shoham D., Kalechman I. Clonal proliferation of PHA-stimulated human lymphocytes in soft agar culture. Immunology. 1975 Dec;29(6):1041–1055. [PMC free article] [PubMed] [Google Scholar]
- Rozenszajn L. A., Sredni B. In vitro growth of colonies of mitogen-stimulated mouse T lymphocytes: I. Conditions affecting colony formation; II. Structure of colonies and component cells. Exp Hematol. 1980 Apr;8(4):494–505. [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Schreier M. H., Tees R. Clonal induction of helper T cells: conversion of specific signals into nonspecific signals. Int Arch Allergy Appl Immunol. 1980;61(2):227–237. doi: 10.1159/000232437. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Skidmore B. J., Kurnick J. T., Goldstine S. N., Chiller J. M. Propagation of antigen-specific T cell helper function in vitro. J Immunol. 1979 Dec;123(6):2525–2531. [PubMed] [Google Scholar]
- Schwartz R. H., Jackson L., Paul W. E. T lymphocyte-enriched murine peritoneal exudate cells. I. A reliable assay for antigen-induced T lymphocyte proliferation. J Immunol. 1975 Nov;115(5):1330–1338. [PubMed] [Google Scholar]
- Schwartz R. H., Paul W. E. T-lymphocyte-enriched murine peritoneal exudate cells. II. Genetic control of antigen-induced T-lymphocyte proliferation. J Exp Med. 1976 Mar 1;143(3):529–540. doi: 10.1084/jem.143.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sredni B., Kalechman Y., Michlin H., Rozenszajn L. A. Development of colonies in vitro of mitogen-stimulated mouse T lymphocytes. Nature. 1976 Jan 15;259(5539):130–132. doi: 10.1038/259130a0. [DOI] [PubMed] [Google Scholar]
- Sredni B., Tse H. Y., Schwartz R. H. Direct cloning and extended culture of antigen-specific MHC-restricted, proliferating T lymphocytes. Nature. 1980 Feb 7;283(5747):581–583. doi: 10.1038/283581a0. [DOI] [PubMed] [Google Scholar]
- Tse H. Y., Schwartz R. H., Paul W. E. Cell-cell interactions in the T cell proliferative response. I. Analysis of the cell types involved and evidence for nonspecific T cell recruitment. J Immunol. 1980 Aug;125(2):491–500. [PubMed] [Google Scholar]
- Watanabe T., Fathman C. G., Coutinho A. Clonal growth of T cells in vitro: preliminary attempts to a quantitative approach. Immunol Rev. 1977;35:3–37. doi: 10.1111/j.1600-065x.1977.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Hengartner H., Nabholz M., Lernhardt W., Schreier M. H., Haas W. Fine specificity of a continuously growing killer cell clone specific for H-Y antigen. Eur J Immunol. 1979 Aug;9(8):592–597. doi: 10.1002/eji.1830090804. [DOI] [PubMed] [Google Scholar]


