Abstract
Serum carbohydrate antigen 125 (CA-125) is a marker of ovarian cancer and obesity that is related with an increased risk of ovarian cancer. Obesity is a key factor of metabolic syndrome. We evaluated the relationship between CA-125 concentration and metabolic syndrome. The data from subjects who had any cancer and chronic infection were excluded. The data of 12,196 healthy Korean women were analyzed. After CA-125 concentration was divided by quartiles, the prevalence of metabolic syndrome and its components were compared. The lowest quartile of CA-125 compared with the highest quartile showed elevated values of most of metabolic parameters. In addition, as the quartile of CA-125 increased, metabolic derangement decreased. Increased numbers of metabolic syndrome components showed an inverse association with CA-125 levels (P < 0.001). The odds ratio (OR) for the lowest CA-125 quartile vs the highest CA-125 quartile significantly increased in the presence of metabolic syndrome (OR = 1.202, 95% Confidence Interval [CI] 1.013-1.423), elevated triglyceride (OR = 1.381, 95% CI 1.167-1.633), and low high-density lipoprotein cholesterol (OR = 1.168, 95% CI 1.039-1.312). The presence of metabolic syndrome, elevated triglyceride, or low high-density lipoprotein cholesterol negatively correlates with CA-125 concentration.
Keywords: CA-125, Metabolic Syndrome, Korean
INTRODUCTION
Carbohydrate antigen 125 (cancer antigen 125, CA-125) is a high molecular weight glycoprotein that is a serum biomarker or tumor antigen. CA-125 is elevated in the blood of some patients with specific types of cancers and is increased in some women with early stage ovarian cancer (1, 2). Therefore, it is used globally as a serological marker of malignant tumors. In post-menopausal women without ovarian cancer, the CA-125 level is influenced by a number of factors, including race/ethnicity, age, hysterectomy, smoking, and obesity (3).
Metabolic syndrome is a combination of several metabolic and physiological abnormalities in the same individual, including obesity, insulin resistance, glucose intolerance, hypertension, and dyslipidemia, and is associated with high morbidity and mortality (4, 5). Metabolic syndrome has also been identified as a causal risk factor for cardiovascular disease and several forms of cancer, such as cancers of the breast, pancreas, and colon (6-8). Obesity is the one of the most important causes of metabolic syndrome, which also can affect many gynecologic cancers, in particular ovarian and endometrial cancers (9). However, although obesity can increase the incidence of cancer, a high body mass index (BMI) does not seem to adversely influence the prognosis in patients with some gynecological malignancies (10).
There is no previous report for the relationship between serum CA-125 and metabolic syndrome. The aim of this cross-sectional study was to evaluate the relationship of serum CA-125 levels with metabolic syndrome in healthy Korean women.
MATERIALS AND METHODS
From January 2007 to May 2009, the data of 13,845 Korean women aged 20-83 years who visited the Health Promotion Center, Ajou University Hospital, Suwon, Gyeonggi-do, Republic of Korea, were included in the study. Medical history, demographics, anthropometric, and laboratory data were collected. Data on cigarette smoking were collected by a self-reported questionnaire. Subjects who, at the time of the survey, had ceased smoking for ≤ 1 month or > 1 month were considered to be, respectively, current and former smokers. Those without a smoking history were considered to be never smokers. But, in the data, there were a small number of women who were current smokers (3.65%). Of the initial 13,845 subjects, 1,649 women were excluded because of absent data for any component of metabolic syndrome, serum CA-125 levels, or smoking history. Subjects with prior diagnosis of any cancer and chronic infections such as rheumatoid arthritis, viral hepatitis, and chronic liver diseases were excluded. A total of 12,196 healthy Korean women were included in the final analyses.
CA-125 was determined with an electrochemoiluminescence immunoassay (Roche Modular Analytics E170; Roche Diagnostics, Mannheim, Germany). Blood pressure (BP) was measured using a standard mercury manometer with the participant in a sitting position for 5 min prior to measurement; the average of two measurements was recorded. Circumferential measurements of the waist at the umbilicus were performed with the patients in a standing position. Fasting blood specimens were used for measuring lipids, glucose, and CA-125. Metabolic syndrome was defined as three or more of the following criteria, according to the National Cholesterol Education Program Third Adult Treatment Panel guidelines (NCEP ATP III) (11): waist circumference (WC) ≥ 102 cm for males and ≥ 88 cm for females, triglyceride (TG) level ≥ 150 mg/dL, high-density lipoprotein cholesterol (HDLC) < 50 mg/dL, BP ≥ 130/85 mmHg or the use of BP medications, and fasting glucose level ≥ 110 mg/dL. Abdominal girth cut-off to determine WC was defined according to the NCEP guidelines specified for Asia (≥ 90 cm for males and ≥ 85 cm for females). Subjects were grouped by smoking status, the sum of individual metabolic components (0-5), and the presence or absence of metabolic syndrome (yes or no). BMI was calculated as weight (kg) divided by height squared (m2).
Statistics
ANOVA was used to compare subjects' characteristics according to serum CA-125 quartiles such as metabolic syndrome components and smoking status. ANOVA trend analysis using polynomial contrasts was adapted to perform tests for trend. Multivariate logistic and linear regression analyses were performed to examine whether clinical parameters and smoking status would significantly contribute to changes in serum CA-125 levels. Results of group data and CA-125 levels are expressed as mean ± standard deviation (SD). All statistical analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). P values < 0.05 were considered statistically significant.
Ethics statement
The institutional review board of Ajou University Hospital approved this study conduction (AJIRB-MED-OBS-10-415). The board waived informed consent from subjects.
RESULTS
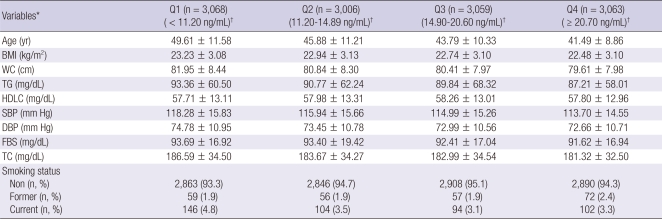
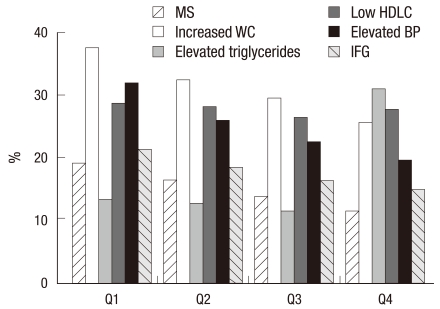
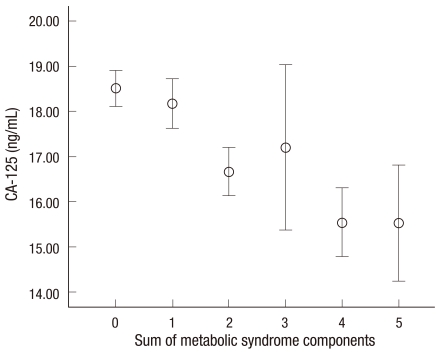
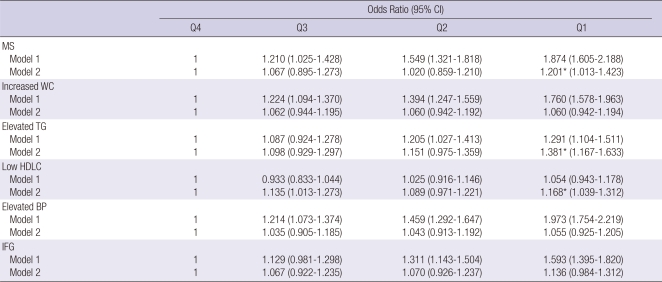
The characteristics of 12,196 subjects according to the quartiles of CA-125 are summarized in Table 1. The overall proportion of metabolic syndrome was 11.5%. Interestingly, most of the metabolic markers in the lowest quartile (Q1) of CA-125 showed higher values compared with the highest quartile (Q4). Numbers of current cigarette smokers were not so high because the data were from women. We compared the prevalence of abnormal metabolic syndrome parameters such as elevated WC, elevated TG, elevated BP, elevated fasting blood sugar and decreased HDLC according to the quartile of CA-125 concentration. Fig. 1 presents the patterns of the abnormal metabolic parameters. The prevalence of metabolic syndrome and its all components showed a significant decrease according to the quartile of serum CA125 concentration increase (P < 0.05). We also calculated the mean CA-125 concentration by the numbers of metabolic syndrome. The mean concentration of CA-125 showed the trend of decrease as the numbers of metabolic syndrome increased. Especially, the mean concentration of CA-125 significantly decreased above the two parameters of metabolic syndrome components (Fig. 2). We conducted logistic regression analysis of metabolic syndrome and its components as independent variables and CA-125 quartiles as dependent variables. As the concentration of CA-125 decreased as the quartile increased, we regarded the highest quartile as the reference group. The odds ratios (ORs) for the lowest CA-125 quartile compared with the highest CA-125 quartile were significant in the presence of metabolic syndrome and elevated TG and low HDLC before and after age-adjustment (Table 2).
Table 1.
Baseline characteristics of study subjects according to the quartile of serum CA125 concentration
*P < 0.05; All values showed statistical significance in the comparisons of variables according to the quartile of serum CA125 concentration by ANOVA test. †Data are presented as CA125 range. Data are shown as mean ± SD. BMI, body mass index; WC, waist circumference; TG, triglyceride; HDLC, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar, TC; total cholesterol; Non, non-smoker; Former, former smoker; Current, current smoker.
Fig. 1.
Prevalence of metabolic syndrome and its components according to the quartile of serum CA125 concentration. Prevalence of metabolic syndrome and its all components showed a significant decrease according to the quartile of serum CA125 concentration (P < 0.05). WC, waist circumference; HDLC, high-density lipoprotein cholesterol; BP, blood pressure; IFG, impaired fasting glucose. Q1; 1st quartile; Q2, 2nd quartile; Q3, 3rd quartile; Q4, 4th quartile.
Fig. 2.
The relationship between CA125 level and the sum of metabolic syndrome components. Vertical bars indicate 95% confidence interval. Circles denote the mean. Lower and upper bars indicate 95% confidence interval (P trend < 0.001).
Table 2.
Logistic regression analysis of the metabolic syndrome and its components as independent variables and CA125 quartile as a dependent variable
*P < 0.05. MS, metabolic syndrome; BMI, body mass index; WC, waist circumference; TG, triglyceride; HDLC, high-density lipoprotein cholesterol; BP, blood pressure; IFG, impaired fasting glucose; Q2, 2nd quartile; Q3, 3rd quartile; Q4, 4th quartile. CI, confidence interval; Reference group, no metabolic syndrome and normal metabolic components. Model 1; before adjustment, Model 2; after adjustment for age.
DISCUSSION
In this cross-sectional observational study, an inverse correlation was evident between serum CA-125 concentration and metabolic syndrome, and its components such as TG and HDLC. The presence of metabolic syndrome, elevated TG, or low HDLC negatively correlated with CA-125 concentration.
Over the past two decades, the prevalence of metabolic syndrome has sharply increased worldwide, and is associated with the global epidemic of obesity and diabetes (5, 12, 13). Metabolic syndrome is associated with the increased risks of cardiovascular disease and various cancers (6, 7, 14). Obesity is a key factor of metabolic syndrome and it is a risk factor for ovarian cancer because of its relationship to sex steroid hormones (15). There have been many studies on obesity and ovarian cancer. One study evaluated the impact of obesity on ovarian cancer risk using BMI as the measure of obesity (16). Another study using the new guidelines and a larger sample confirmed the association between obesity and increased risk for ovarian cancer at 70% (17). Obesity was associated with clear cell cancers, where a 2-fold increased risk was observed (OR 2.2, 95% confidence interval [CI] 1.2-4.1) (18). There was no association with BMI at age 20, or weight gain for any of the histological subtypes of ovarian cancer. These results add to the current evidence that obesity increases a woman's risk of developing distinct histological subtypes of ovarian cancer (19). Increasing BMI has been associated with an increased incidence of ovarian cancer (relative risk = 1.14, 95% CI 1.03-1.27) (20). In a meta-analysis, 24 of 28 studies reported a positive association between obesity and ovarian cancer (9). Overexpression of some lipid metabolic enzymes is also found in ovarian cancer (21).
A search of the literature revealed over 10,000 articles on the ovarian cancer. Thousands of scientific studies have reported on metabolic syndrome. It is impossible to review all these papers. However, in stark contrast, little data has been published concerning the association of the concentration of CA-125 and metabolic syndrome. As described above, CA-125 is a marker of ovarian cancer and obesity is both related with the increase of ovarian cancer risk and is a key factor of metabolic syndrome. By simple assumption, CA-125 would be expected to have a positive relationship with obesity and metabolic syndrome. However, presently, CA-125 was inversely correlated with the presence of metabolic syndrome and the numbers of metabolic syndrome components. We are unsure why this pattern was apparent. One possible mechanism is that subjects with a greater BMI have larger plasma volumes, which could decrease the serum concentrations of soluble tumor markers-a phenomenon known as hemodilution (22). In addition, increased BMI has a reported negative association with CA-125 concentration (3). In the latter study, the mean concentration of CA-125 in the obese group of patients decreased minimally compared to the normal and overweight groups. Furthermore, the OR of obese patients having an elevated CA-125 was significantly decreased compared to the normal weight group. Therefore, a serum concentration of CA-125 was negative correlated with obesity, which was also correlated with metabolic syndrome. Consequently, women who have metabolic syndrome may have a low level of serum CA-125.
CA-125 is best-known as a marker for ovarian cancer, but it may also elevated in other benign medical conditions such as endometrial, fallopian tube, lung, breast and gastrointestinal tract problems (1). It also tends to be elevated in the presence of any inflammation condition in the abdominal area, be it cancerous or benign (23). However, with the current clinical approach, it is difficult to clarify the mechanism of the negative association between CA-125 and metabolic syndrome, because there have not been any clinical or experimental reports on the relationship between CA-125 and metabolic syndrome. Thus, it is necessary to investigate the relationship between CA-125 and metabolic syndrome at the cellular and molecular levels, or by a prospective intervention study to evaluate the change of CA-125 according to the changes of BMI or improvement of metabolic syndrome.
There are some limitations with this study. First, because the study was cross-sectional, we could not determine whether there was a causal or resultant relationship between the individual and the combined components of metabolic syndrome and the elevation of serum CA-125. Second, it is not clear whether the analysis of this restricted patient group introduced a selection bias, and these findings may or may not reflect the situation in the overall population. Third, we analyzed the data of all ages in the health promotion center not of high risk or postmenopausal women. Finally, we could not adjust for all possible confounding factors. Nevertheless, our study is the first report on the relationship of the CA-125 and metabolic syndrome and its parameters.
In conclusion, the presence of metabolic syndrome or elevated TG or low HDLC is negatively correlated with CA-125 concentration in this cross-sectional observational study.
Footnotes
This research is supported by the Ubiquitous Computing and Network (UCN) Project, Ministry of Knowledge and Economy (MKE) Knowledge and Economy Frontier R&D Program in Korea, and the Korea Breast Cancer Foundation.
AUTHOR SUMMARY
Serum CA125 Concentration has Inverse Correlation with Metabolic Syndrome
Nam-Seok Joo, Kyu-Nam Kim and Kyung Soo Kim
The data of 12,196 healthy Korean women were analyzed. After CA-125 concentration was divided by quartiles, the prevalence of metabolic syndrome and its components were compared. Increased numbers of metabolic syndrome components showed an inverse association with CA-125 levels. The presence of metabolic syndrome, elevated triglyceride or low high-density lipoprotein cholesterol negatively correlates with CA-125 concentration.
References
- 1.Bast RC, Jr, Xu FJ, Yu YH, Barnhill S, Zhang Z, Mills GB. CA 125: the past and the future. Int J Biol Markers. 1998;13:179–187. doi: 10.1177/172460089801300402. [DOI] [PubMed] [Google Scholar]
- 2.Verheijen RH, von Mensdorff-Pouilly S, van Kamp GJ, Kenemans P. CA 125: fundamental and clinical aspects. Semin Cancer Biol. 1999;9:117–124. doi: 10.1006/scbi.1998.0114. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CC, Kessel B, Riley TL, Ragard LR, Williams CR, Xu JL, Byus SS Prostate, Lung, Colorectal and Ovarian Cancer Project Team. The epidemiology of CA-125 in women without evidence of ovarian cancer in the Prostate, Lung, Colorectal and ovarian cancer (PLCO) Screening Trial. Gynecol Oncol. 2008;110:383–389. doi: 10.1016/j.ygyno.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 5.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 6.Furberg AS, Veierød MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004;96:1152–1160. doi: 10.1093/jnci/djh216. [DOI] [PubMed] [Google Scholar]
- 7.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:385–391. [PubMed] [Google Scholar]
- 8.Michaud DS, Liu S, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Dietary sugar, glycemic load, and pancreatic cancer risk in a prospective study. J Natl Cancer Inst. 2002;94:1293–1300. doi: 10.1093/jnci/94.17.1293. [DOI] [PubMed] [Google Scholar]
- 9.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systemic review and mea-analysis. Eur J Cancer. 2007;43:690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Bray F, Dos Santos Silva I, Moller H, Weiderpass E. Endometrial cancer incidence trends in Europe: underlying determinants and prospects for prevention. Cancer Epidemiol Biomarkers Prev. 2005;14:1132–1142. doi: 10.1158/1055-9965.EPI-04-0871. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Forcd Consensus Group. The metabolic syndrome: a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim MH, Kim MK, Choi BY, Shin YJ. Prevalence of the metabolic syndrome and its association with cardiovascular diseases in Korea. J Korean Med Sci. 2004;19:195–201. doi: 10.3346/jkms.2004.19.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryu SY, Kweon SS, Park HC, Shin JH, Rhee JA. Obesity and the metabolic syndrome in Korean adolescents. J Korean Med Sci. 2007;22:513–517. doi: 10.3346/jkms.2007.22.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, Fraumeni JF, Adam HO. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 15.McLemore MR, Miaskowski C, Aouizerat BE, Chen LM, Dodd MJ. Epidemiological and genetic factors associated with ovarian cancer. Cancer Nurs. 2009;32:281–288. doi: 10.1097/NCC.0b013e31819d30d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez C, Calle EE, Fakhrabadi-Shokoohi D, Jacobs EJ, Thun MJ. Body mass index, height, and the risk of ovarian cancer mortality in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:822–828. [PubMed] [Google Scholar]
- 17.Runnebaum IB, Stickeler E. Epidemiological and molecular aspects of ovarian cancer risk. J Cancer Res Clin Oncol. 2001;127:73–79. doi: 10.1007/s004320000153. [DOI] [PubMed] [Google Scholar]
- 18.Nagle CM, Olsen CM, Webb PM, Jordan SJ, Whiteman DC, Green AC Australian Cancer Study Group; Australian Ovarian Cancer Study Group. Endometrioid and clear cell ovarian cancers: a comparative analysis of risk factors. Eur J Cancer. 2008;44:2477–2484. doi: 10.1016/j.ejca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Olsen CM, Nagle CM, Whiteman DC, Purdie DM, Green AC, Webb PM Australian Cancer Study (Ovarian Cancer) and Australian Ovarian Cancer Study Group. Body size and risk of epithelial ovarian and related cancers: a population-based case-control study. Int J Cancer. 2008;123:450–456. doi: 10.1002/ijc.23509. [DOI] [PubMed] [Google Scholar]
- 20.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tania M, Khan MA, Song Y. Association of lipid metabolism with ovarian cancer. Curr Oncol. 2010;17:6–11. doi: 10.3747/co.v17i5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vollmer RT, Humphrey PA. Tumor volume in prostate cancer and serum prostate-specific antigen. Analysis from a kinetic viewpoint. Am J Clin Pathol. 2003;119:80–89. doi: 10.1309/UNAQ-JTFP-B1RQ-BQD4. [DOI] [PubMed] [Google Scholar]
- 23.Sarandakou A, Protonotariou E, Rizos D. Tumor markers in biological fluids associated with pregnancy. Crit Rev Clin Lab Sci. 2007;44:151–178. doi: 10.1080/10408360601003143. [DOI] [PubMed] [Google Scholar]