Abstract
Imperatoxin A (IpTxa) is known to modify the gating of skeletal ryanodine receptor (RyR1). In this paper, the ability of charged aa residues of IpTxa to induce substate of native RyR1 in HSR was examined. Our results show that the basic residues (e.g., Lys19, Lys20, Lys22, Arg23, and Arg24) are important for producing substate of RyR1. In addition, other basic residues (e.g., Lys30, Arg31, and Arg33) near the C-terminus and some acidic residues (e.g., Glu29, Asp13, and Asp2) are also involved in the generation of substate. Residues such as Lys8 and Thr26 may be involved in the self-regulation of substate of RyR1, since alanine substitution of the aa residues led to a drastic conversion to the substate. The modifications of the channel gating by the wild-type and mutant toxins were similar in purified RyR1. Taken together, the specific charge distributions on the surface of IpTxa are essential for regulation of the channel gating of RyR1.
1. Introduction
In striated muscles, depolarization of the cell surface membranes leads to activation of ryanodine receptors (RyRs) in the junctional sarcoplasmic reticulum (SR) [1–5]. A number of endogenous RyR modulators such as calmodulin, FK506-binding proteins, calsequestrin, triadin, and HRC have been identified [6–8]. Exogenous modulators of RyRs such as toxins and peptides have also been reported [9, 10].
Imperatoxin A (IpTxa) from the African scorpion Pandinus imperator is a high-affinity modulator of skeletal RyR (RyR1). It greatly increases open probability (Po) and [3H]ryanodine binding to RyR1 at nanomolar concentration [11, 12]. Moreover, binding of IpTxa to RyRs reconstituted in planar lipid bilayers generates marked occurrence of long-lasting openings in subconductance state (substate) [13]. Confocal imaging of skeletal muscle fibers to monitor IpTxa-induced Ca2+ sparks demonstrate that the toxin induces long-duration and low-amplitude local Ca2+ release consistent with the observation of the prolonged substate in the presence of IpTxa [14]. Another structurally related scorpion toxin, maurocalcine (MCa), and one specific small fragment of II-III loop region of skeletal DHPR (Peptide A) also bind to RyR1 and modify channel activity [15–18]. MCa also strongly enhances [3H]ryanodine binding to RyR1 and induces long-lasting substate with the current amplitude of 48% of the full conductance [16, 18]. Peptide A could bind to RyR1 and could either activate or inhibit the activity of RyR1. It could also induce the long-lasing substate [15, 17, 19].
A comparison of the amino acid (aa) sequences of these RyR1-modifing probes shows a common basic aa domain and the C-terminal hydroxyl-containing side chain. The aa sequences may also contribute to the essential structure for activating RyR1 [15, 16]. Especially, a cluster of positively charged aa residues on the surface of the peptide A is critical for activation of RyR1 [19, 20]. The mutations of the specific basic residues of MCa and IpTxa have failed to induce long-lasting substate and to potentiate [3H]ryanodine binding [20, 21]. To date, the ability of a single aa residue of IpTxa to control the substate of RyR1 is not fully understood. Recently we have found that several basic aa residues of IpTxa (e.g., Lys19, Arg23, and Arg33) are necessary for increasing open probability and inducing substate in rabbit skeletal RyR1 [22].
In the present study, to evaluate the roles of the charged aa residues of IpTxa in modifying the RyR1 gating, synthetic wild-type and alanine-scanning mutants of IpTxa were tested on planar lipid bilayer-incorporated RyR1. The basic aa mutants (e.g., K19A, K20A, K22A, R23A, and R24A) resulted in a significant loss of production of substate in RyR1, consistent with the previous suggestion that the critical basic domain of toxin determines its binding to the channel [15, 16, 22]. The effective domain encompassing these basic residues involved in producing substate is structurally conserved with both MCa and Peptide A [19, 23]. This suggests a common role of the highly clustered positive charges for their action on RyR1 channel gating. The mutations of some acidic residues (e.g., Asp2, Asp13, and Glu29) and basic residues within C-terminal region of IpTxa (e.g., Lys30, Arg31, and Arg33) also led to a significant inhibition on the gating. When Lys8 and Thr26 were replaced by alanine, the substate was predominant indicating that these two residues are essential for the functions of the toxin. In addition, the effects of the wild-type and mutant toxins on the gating behavior of RyR1 are strikingly similar when the native RyR1 in SR and the purified RyR1 are used for the incorporation into bilayers, suggesting that generation of the substate is due to a direct binding of the toxin to RyR1.
2. Materials and Methods
2.1. Materials
Porcine brain phosphatidylethanolamine and phosphatidylserine were purchased from Avanti Polar Lipids, Inc. All other reagents were from Sigma.
2.2. Chemical Synthesis of Wild-Type and Mutant IpTxa Peptides
The peptide synthesis was conducted by a peptide synthesizer (Applied Biosystems model 433A). The linear precursors of wild-type and mutant IpTxa were synthesized by solid-phase Fmoc chemistry starting from Fmoc-Arg (2,2,5,7,8-pentamethylchroman-6-sulphonyl)-Alko or Fmoc-Ala-Alko resin using a variety of blocking groups for amino acid protection. After cleavage by trifluoroacetic acid, crude linear peptides were extracted with 2 M ethanoic acid, diluted to final peptide concentration of 25 μM in a solution of 1 M ammonium acetate and 2.5 mM reduced/0.25 mM oxidized glutathione adjusted to pH 7.8 with aqueous NH4OH, and stirred slowly at 4°C for 2-3 days. In the redox buffer system, oxidized glutathione acts as oxidase and assists in the formation of disulfide bonds whereas reduced glutathione functions as disulfide isomerase and facilitates formation of correct disulfide bonds by promoting rapid reshuffling of incorrect disulfide parings. A 10 : 1 mixture of reduced and oxidized glutathione was suggested to be an efficient redox buffer system for producing disulfide bonds in IpTxa [21]. The folding reactions were monitored by HPLC. The crude oxidized products were purified by successive chromatography with CM-cellulose CM-52 and preparative HPLC with C18 silica columns. The purity of all analogues were in the range of 60 to 95% as measured by analytical HPLC and MALDI-TOF-MS (matrix-assisted laser desorption ionization-time-of-flight MS) measurements (see Supplementary Figure 1 (in Supplementary material available online at doi: 10.1155/2011/386384)).
2.3. Preparation of Junctional SR Vesicles from Rabbit Skeletal Muscle
A heavy fraction of fragmented SR vesicles (HSR) containing junctional SR was prepared from rabbit fast-twitch back and leg muscles as described previously [24].
2.4. Planar Lipid Bilayers
Single-channel recordings of rabbit skeletal RyR1 incorporated into planar lipid bilayers were carried out as described previously [25–27]. Lipid bilayers, consisting of brain tissue phosphatidylethanolamine and phosphatidylserine (1 : 1) in decane (20 mg/mL) were formed across a hole of approximately 200 μm diameter. Thinning of the bilayer was monitored by bilayer capacitance. The basic composition of the cis/trans solution consisted of 300 mM cesium methanesulfonate, 10 mM Tris/Hepes (pH 7.2), 2 mM EGTA, and 1.998 mM CaCl2 ([Ca2+]free = 10 μM) [27]. [Ca2+]free was calculated using the “Chelator” program (Theo Schoenmaker). Cs+ was selected as the charge carrier to ensure precise control of free [Ca2+], to increase the channel conductance, and to avoid any contribution from potassium channels present in the SR membrane [12]. Chloride channels were inhibited by using the impermeant anion methanesulfonate [12]. Incorporation of ion channels was carried out as described by Miller and Racker [25] and confirmed by recording the characteristically high single-channel conductance of RyRs [27, 28]. The trans side was maintained at ground and the cis side was clamped at −30 mV relative to the ground. After addition of the IpTxa to the cis chamber, the single channel data were collected at −30 mV for 2–5 min. The channel activity was recorded on a DTR-1204 Digital Recorder (Biologic Science Instrument) and displayed on a Tektronix TDS 340A oscilloscope. Recordings were filtered with an 8-pole low-pass Bessel filter at 1 kHz and digitalized through a Digidata 1200 series interface (Axon Instruments). Data acquisition and analysis were done with the Axon Instruments software, pClamp v7.0.
Data were analyzed using the Hill equation described previously [12, 13, 15]:
| (1) |
where (Po, max ) is the Po observed at saturating concentrations of IpTxa, EC50 is the IpTxa concentration for which 50% of Po, max is obtained and nH is the Hill coefficient. Also, Psubstate, max is the Psubstate observed at saturating concentrations of IpTxa, EC50 is the IpTxa concentration for which 50% of Psubstate, max is obtained, and nH is the Hill coefficient. The probability of full open state of the channel (Po) was defined as the ratio of the time spent in the open state to the total time exclusive of time spent in the substate. The probability to obtain substate (Psubstate) was calculated as the time spent in the substate divided by a given total recording time. The durations of the substate were obtained by manual positioning of the cursors and constructing all point histograms. Mean duration of substate was measured by total substate time divided by total substate frequencies.
2.5. Statistical Analysis
Results are given as means ± SE. Significant differences were analyzed using Student's t-test. Differences were considered to be significant when P < 0.05. The fitting of the data to the graphs were carried out using the software, Origin v7.
3. Results
3.1. Effects of IpTxa on Single-Channel Gating Properties of RyR1
To examine how IpTxa modifies RyR1 activity, RyR1 in HSR incorporated in planar lipid bilayers was tested in the presence or absence of synthetic wild-type IpTxa. The chamber solutions for both cis (cytosolic) and trans (luminal) sides included 300 mM cesium methanesulfonate and 10 mM Tris-Hepes (pH 7.2). 10 μM free Ca2+ was added to the cis side to activate the incorporated RyR1. After an addition of IpTxa to the cis chamber, the single channel gating properties were recorded at a holding potential of −30 mV for over 2 min at each toxin concentration. Figure 1(a) shows traces from continuous recordings in the absence and in the presence of 6, 15, 30, 50, and 100 nM IpTxa. When the toxin concentration increased, the occurrence of substate of RyR1 was remarkably increased. To examine the effects of IpTxa on full open state, we calculated the probability to obtain the full open state (Po) as the time spent in the open state divided by the total time exclusive of the time spent in the substate. The time for recorded Po was in the range of 30 s–2 min. Figure 1(b) shows a plot of Po versus IpTxa concentration at 10 μM Ca2+. The steady-state Po was 0.18 ± 0.02 at 10 μM Ca2+. When the concentration of IpTxa was increased in cis chamber, Po increased markedly to 0.7, suggesting that the cytosolic IpTxa enhanced the channel activity by increasing open probability in a dose-dependent manner. Using the Hill equation (1) the parameters such as Po, max , EC50, and Hill coefficient (nH) were calculated. The calculated Po, max and EC50 for Po were 0.72 ± 0.02 and 17.35 ± 4.67 nM, respectively. The Hill coefficient (nH) for Po was 1.14. Application of IpTxa to the trans (SR lumen) side of chamber did not show any effect (data not shown), suggesting that increase of the full opening events of the RyR1 channel is due to interaction between the toxin and the cytosolic region of the channel.
Figure 1.
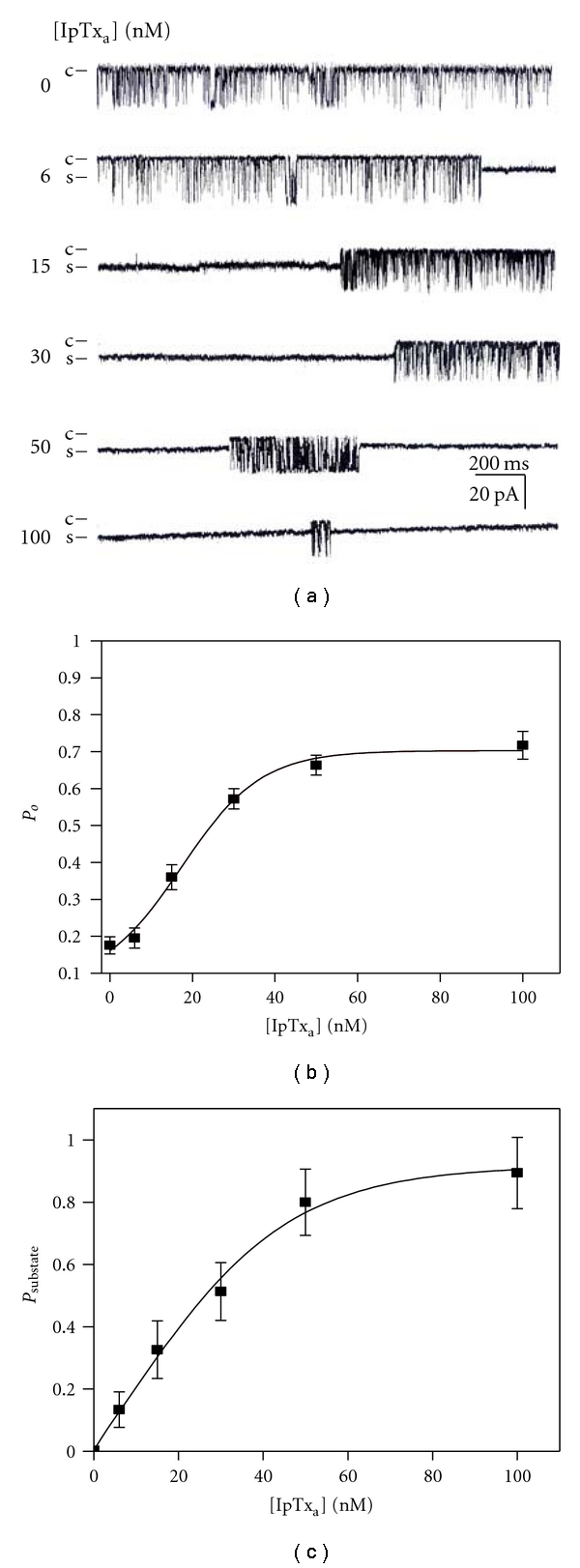
Properties of single channel gating in IpTxa-modified native RyR1. (a) Channel current traces of a single skeletal RyR1 in planar lipid bilayers activated by various concentrations of IpTxa. IpTxa at 6–100 nM (final) was added to the cis solution to activate the channel. Channel openings are shown as downward deflections. Channel activities were recorded at a holding potential of −30 mV. (b) A plot of Po versus concentration of IpTxa to activate rabbit skeletal RyR1. Data points are means ± SE of ten experiments. (c) A plot of Psubstate versus IpTxa concentration for rabbit skeletal RyR1. Data points are means ± SE of 9 experiments.
In light of the evidence that IpTxa could induce substate both in cardiac and skeletal RyRs [13], the effects of synthetic IpTxa on the occurrence of substate were tested using native RyR1 in rabbit skeletal HSR. Figure 1(a) shows that an addition of IpTxa to the cis side of RyR1 channel could induce the substate. The probability to obtain substate (Psubstate) increased, when the concentration of IpTxa increased from 6 to 100 nM in the cis chamber (Figure 1(c)). The calculated Psubstate, max and EC50 for Psubstate were 0.92 ± 0.06 and 23.27 ± 2.37 nM, respectively. The Hill coefficient (nH) for Psubstate was 1.24, suggesting that IpTxa and RyR1 do not have the cooperative bindings in the concentration range.
3.2. Effects of Alanine Scanning Mutants of IpTxa on RyR1
In the previous studies [21, 22], it was proposed that specific basic amino acid residues on the surface of IpTxa are required for the electrostatic interaction of the toxin with RyR1. Particularly, Gurrola et al. [15] suggested the structural domain composed of Lys19-Arg24 followed by Thr26 is responsible for the IpTxa-RyR1 binding. A possible molecular interaction between IpTxa and RyR1 was further investigated in the present study by producing various alanine scanning mutants at charged aa. We first tested the effects of IpTxa mutants on substate of RyR1 at a holding potential of –30 mV.
The single channel traces of RyR1 activated at 30 nM wild type or mutant IpTxa are shown in Figure 2(a). Ability of the different mutant toxins to induce substate of RyR1was further tested using different toxin concentrations. Interestingly, both K8A and T26A mutants displayed a significantly increased substate lifetime, indicating a negative role of these residues in modifying RyR1 gating. Psubstae, max of K8A or T26A reached almost to 1, and EC50 (nM) values were shifted from 23.27 ± 0.37 (wild type) to 5.70 ± 2.86 (K8A) or to 9.98 ± 1.92 (T26A; Figure 2(b) and Table 1). The mutations of the basic aa (Lys19, Lys20, Lys22, Arg23, Arg24, Lys30, Arg31, and Arg33) significantly reduced the probability to obtain substate (Psubstate), compared with the wild-type toxin (Figure 2(b) and Table 1). The effect of D15A mutant was similar to that of wild-type toxin, suggesting no major involvement of Asp15 in the binding between the toxin and RyR1. On the other hand, the substitutions of some acidic aa such as Asp2, Asp13, and Glu29 by alanine alter the probability to obtain substate significantly (Figure 2 and Table 1). These results suggest that the charged aa distributed on the surface of IpTxa contribute to the stimulatory action of the toxin and to the interaction between IpTxa and RyR1.
Figure 2.
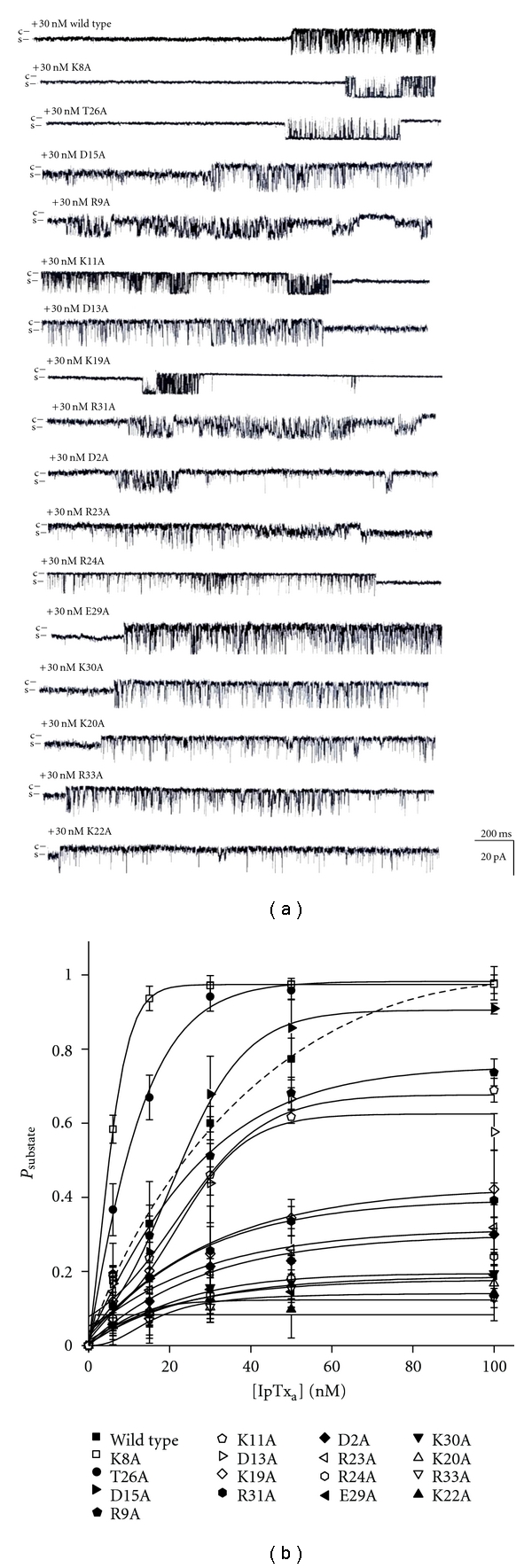
Single channel current traces of RyR1 modified by various alanine scanning IpTxa mutants. (a) Single channel current traces of skeletal RyR1 activated in the presence of 30 nM wild-type or various mutant IpTxa (cis side) at −30 mV holding potential. Single channel opening is shown as a downward deflection. (b) The plots of Psubstate of RyR1 versus concentration of IpTxa are shown. The data points are means ± SE from 3–5 independent experiments.
Table 1.
Effects of the mutant IpTxa on Psubstate of native RyR1 in SR. Psubstate, max and EC50 for Psubstate, max were calculated using (1) as described in “Section 2.” Values are means ± SE of 3–9 experiments. The average lengths of the substate events were determined at 30 nM concentration. Asterisks indicate significant differences from the wild-type toxin for each parameter (Student t-test, P < 0.05). ND: not determined.
| Psubstate | Mean duration of substate (s) | ||
|---|---|---|---|
| Psubstate, max | EC50 (nM) | ||
| Wild type | 0.92 ± 0.06 | 23.27 ± 2.37 | 4.56 ± 0.42 |
| K8A | 0.97 ± 0.01 | 5.70 ± 2.86* | ND |
| T26A | 0.98 ± 0.05 | 9.98 ± 1.92* | 3.66 ± 0.40* |
| D15A | 0.91 ± 0.05 | 21.47 ± 1.92 | 4.50 ± 0.51 |
| R9A | 0.75 ± 0.05* | 19.91 ± 3.16 | 5.86 ± 0.52* |
| K11A | 0.70 ± 0.07* | 21.76 ± 3.49 | 4.35 ± 1.07 |
| D13A | 0.62 ± 0.07* | 21.72 ± 7.92 | 2.76 ± 0.25* |
| K19A | 0.43 ± 0.07* | 21.84 ± 6.47 | 1.94 ± 0.43* |
| R31A | 0.40 ± 0.03* | 19.24 ± 4.65 | 3.42 ± 0.27* |
| D2A | 0.38 ± 0.08* | 28.63 ± 14.02 | 2.96 ± 0.60* |
| R23A | 0.32 ± 0.09* | 19.54 ± 9.36 | 2.50 ± 0.26* |
| R24A | 0.26 ± 0.08* | 26.03 ± 7.01 | 1.55 ± 0.94* |
| E29A | 0.19 ± 0.03* | 6.07 ± 4.28* | 1.75 ± 0.29* |
| K30A | 0.19 ± 0.02* | 16.66 ± 2.86* | 1.72 ± 0.39* |
| K20A | 0.18 ± 0.05* | 18.53 ± 8.17 | 3.64 ± 0.78* |
| R33A | 0.14 ± 0.03* | 16.03 ± 5.65* | 1.97 ± 0.19* |
| K22A | 0.12 ± 0.02* | 18.49 ± 2.88 | 3.31 ± 0.15* |
3.3. Mean Duration of IpTxa-Induced Substate
To investigate the causes for the decreased Psubstate by the mutant toxins, the average length of substate at 30 nM wild type or mutant of IpTxa was calculated as total substate time divided by the toral frequencies of substate. The recording time in each concentration was 2 min. Despite the marked increase in Psubstate as IpTxa was increased from 6 nM to 100 nM, the mean duration of the IpTxa induced-substate of RyR1 appeared to be similar at different [IpTxa] (Figure 4). The average mean duration of substate of the mutants (D2A, D13A, K19A, K22A, R23A, R24A, E29A, K30A, and R33A) was significantly less than that of wild-type IpTxa (Table 1). Some mutants (K19A, R24A, E29A, and K30A) showed more marked reduction in mean duration of substate (<2 s). The decreased mean duration of substate in the mutant toxins is due probably to the loss of their ability to induce long-lasting substate.
Figure 4.
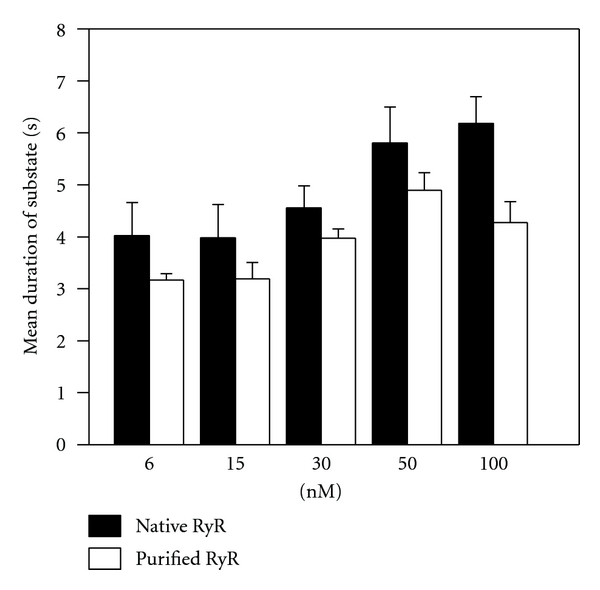
Effects of IpTxa on the mean durations of substate of native and purified RyR1. Mean durations of the substate events in native RyR1 versus purified RyR1 were determined in the presence of 6–100 nM IpTxa. Data points are means ± SE for 10 experiments.
3.4. Effects of IpTxa on Purified RyR1
To determine whether the activation of native RyR1 in SR by IpTxa was due to a direct activation of RyR1 or due to an indirect activation through other proteins associated to RyR1, we added IpTxa to purified RyR1 incorporated into planar lipid bilayers (Figure 3(a)). 30 nM IpTxa increased Psubstate both in native and purified RyR1 in a similar extent (Figures 1 and 3). Figure 4 shows that the mean durations of substate of purified RyR1 are similar to native RyR1 at the tested concentration range, suggesting that the response of RyR1 to IpTxa was not mediated by other RyR1-associated proteins. Purity of RyR1 at the final purification step was verified by Coomassie blue staining (Supplementary Figure 2).
Figure 3.
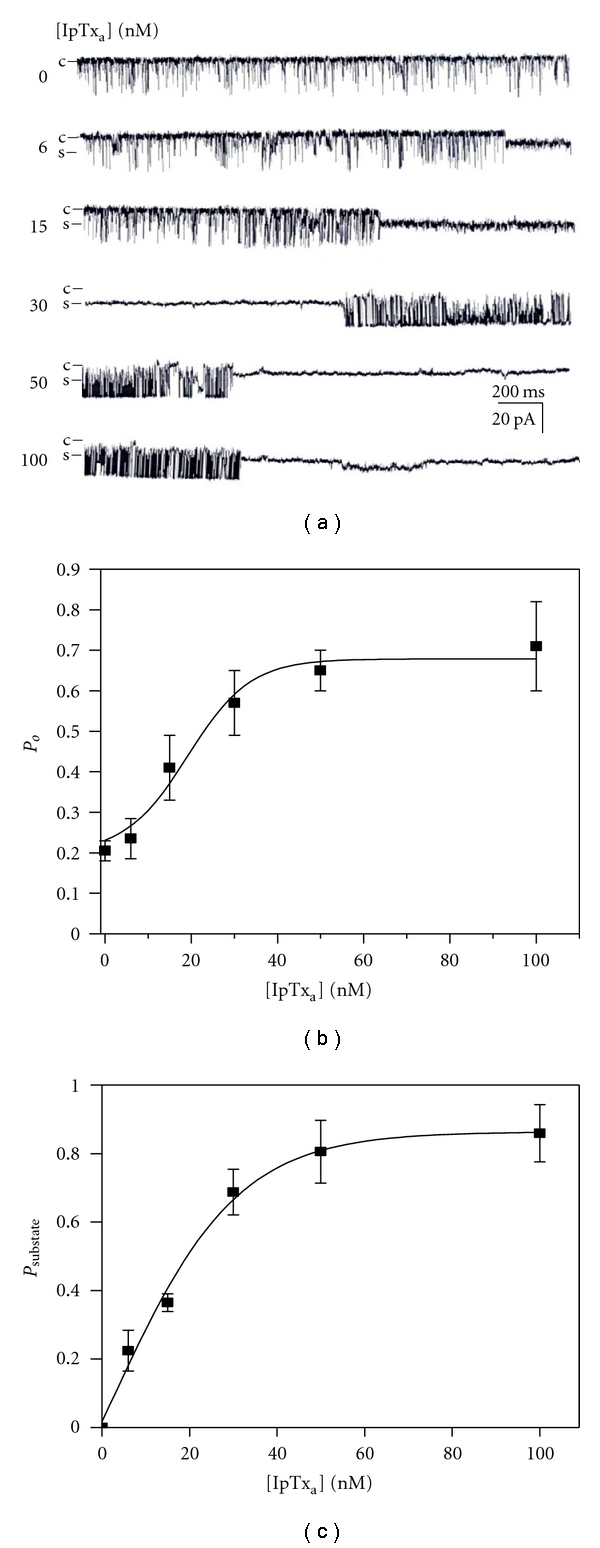
Effects of IpTxa on purified rabbit RyR1 incorporated in planar lipid bilayers. (a) Single channel activity of purified RyR1 incorporated in planar lipid bilayers at 10 μM Ca2+ with or without IpTxa (6–100 nM) was recorded. Single channel currents are shown as downward deflections from the closed level (indicated by c). The holding potential was −30 mV. (b) The plot of Po of purified RyR1 versus various concentrations of IpTxa is shown. (c) The plot of Psubstate of purified RyR1 versus various concentrations of IpTxa is shown. The results are shown as the means ± SE for 7 experiments.
3.5. Effects of IpTxa Mutants on Purified RyR1
We further tested the effects of mutant IpTxa (K8A, K30A, R31A, or R33A) on Psubstate of purified RyR1. Channel activity was monitored for 2 min in the presence of 6 to 100 nM mutant toxins (Figure 5(a)). The plots of Psubstate versus [IpTxa] showed that the mutant toxins (K30A, R31A, and R33A) led to much smaller Psubstate (0.16 ± 0.05, 0.15 ± 0.02, and 0.18 ± 0.06, resp.) than that of wild-type IpTxa(0.86 ± 0.05) without a significant change of EC50 (Figure 5(b) and Table 2). K8A led to similar Psubstate, max (0.85 ± 0.02) to wild-type toxin (Figure 5(b)). K30A and R33A produced less than 2 s of mean duration of substate (Table 2).
Figure 5.
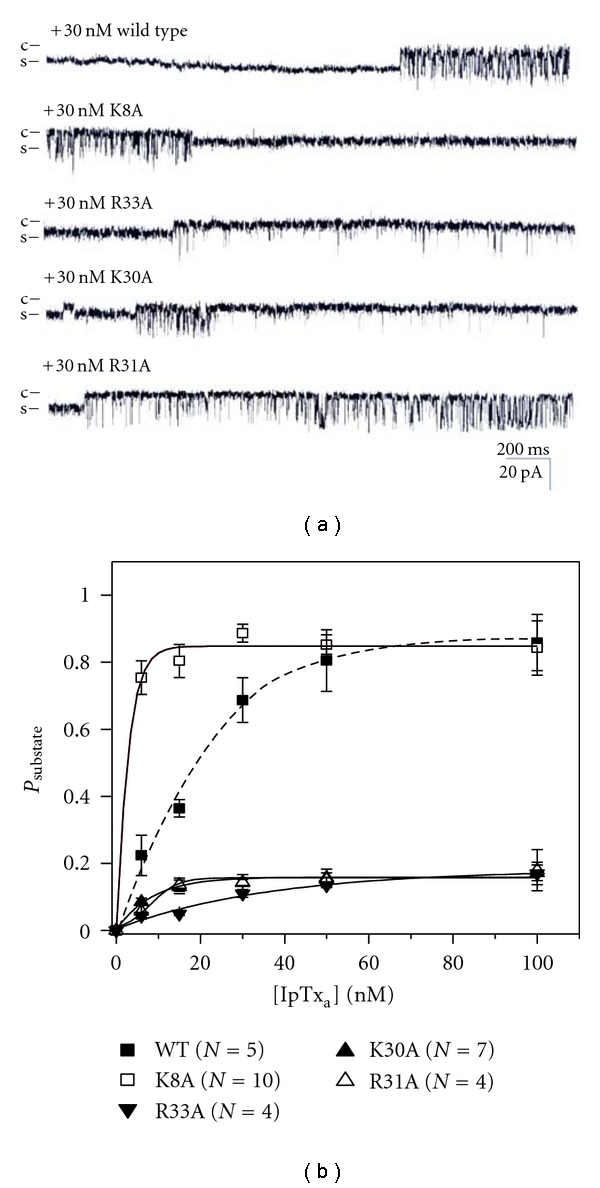
Properties of single channel gating in wild-type and mutant IpTxa-modified purified RyR1. (a) Single channel currents of purified RyR1 activated in the presence of wild-type or mutant IpTxa were measured at −30 mV holding potential. The single channel opening is shown as a downward deflection. The recordings of single channel currents were measured at 30 nM wild-type or mutant IpTxa in the cis solution. (b) The plots of Psubstate of purified RyR1 versus wild-type or mutant IpTxa at various concentrations.
Table 2.
Effects of the mutant IpTxa on Psubstate of purified RyR1. Psubstate, max and EC50 for Psubstate of purified RyR1 were calculated as described in the legend to Table 1. Values are means ± SE of 4–10 experiments. The average lengths of the substate events were determined at 30 nM concentration. Asterisks indicate significant differences from the wild type for each parameter (Student t-test, P < 0.05). ND: not determined.
| Psubstate | Mean duration of substate (s) | ||
|---|---|---|---|
| Psubstate, max | EC50 (nM) | ||
| Wild type | 0.86 ± 0.05 | 17.08 ± 2.31 | 3.97 ± 0.18 |
| K8A | 0.85 ± 0.02 | 5.29 ± 4.84* | ND |
| R33A | 0.18 ± 0.06* | 19.25 ± 1.05 | 1.43 ± 0.09* |
| K30A | 0.16 ± 0.05* | 15.66 ± 5.99 | 1.88 ± 0.22* |
| R31A | 0.15 ± 0.02* | 18.23 ± 1.65 | 3.15 ± 0.10* |
4. Discussion
4.1. Effects of IpTxa Mutants on Substate of RyR1
The highly positive charges of the basic residues of IpTxa could contribute to the formation of its functional surface area having uniquely oriented charge distribution [15, 19, 21–23]. In the present study we tested the hypothesis that electrostatic force mediates the IpTxa-RyR1 interaction by studying the effects of alanine scanning mutations of charged aa residues in IpTxa on RyR1 functions.
Single point mutations of charged residues in IpTxa generally affected the probability of occurring substate (Psubstate) in RyR1 (Figure 2 and Table 1). Previously, it was shown that mutations in a cluster of basic residues (Lys19-Arg24) decreased the ability of the toxin to activate [3H]ryanodine binding to RyR1 [15, 21]. The recombinant mutant toxins (e.g., K19A, R23A, and R24A) were less effective to increase open probability (Po) and to induce substate of the channel [22]. Our present results demonstrate that a cluster of the basic residues 19–24 is necessary for inducing substate of RyR1, confirming the functional importance of the clustered basic residues. In addition, replacing other basic residues located in C-terminal region of IpTxa with alanine (e.g., K30A, R31A, and R33A) also reduced the effects of the toxin on channel modification (Figure 2(b)). As described previously, the C-terminal basic residues (Lys22, Arg23, and Arg24) are aligned in the central domain of IpTxa and possibly are responsible for activating RyR1 [21]. Our present findings agree with the previous suggestion that the positively charged region within the C-terminus is involved in the interaction with RyR1.
K8A and T26A, the two mutated analogs of IpTxa, showed dramatically decreased EC50 of Psubstate compared to that of wild-type toxin, indicating higher binding affinity to RyR1 (Figures 2(b) and 5(b)). Although the ability of K8A to increase the substate lifetime of RyR1 was previously studied using recombinant IpTxa mutant [22], the effect of K8A on [3H]ryanodine binding to RyR1 is controversial [15, 21]. T26A mutant was reported to reduce toxin-activated ryanodine binding to RyR1 [15]. The inconsistency between occurrence of substate and ryanodine binding to RyR1 affected by K8A or T26A suggests multiple independent actions of IpTxa on different modes of channel gating. In fact, Dulhunty et al. [17] proposed an existence of multiple toxin binding sites within RyR1 including the transient activation site and substate site [17].
4.2. The Effects of IpTxa and Peptide A on RyR1 Gating
Marked functional similarity of the three peptides, IpTxa, MCa, and Peptide A has been proposed on the basis of their primary structural homology of a specific domain consisting of basic amino acids (Lys19-Arg23 of IpTxa or MCa, and Arg681-Lys685 of Peptide A) [15, 17, 18, 23, 29]. Stretches of these positively charged residues tend to adopt different secondary structures such as α-helical structure for Peptide A and β-sheet structure for IpTxa and MCa. However, their orientation on the surface of the peptides could be similar [19, 21]. Peptide A was shown to share the common binding site on RyR1 with IpTxa and MCa and mimicked the toxin effects on RyR1 gating [15, 23, 29]. However, evidence for noncompetitive binding of MCa and peptide A to RyR1 was shown by [3H]ryanodine binding and real-time Surface Plasmon resonance (SPR) studies. MCa and peptide A induced distinct modification of channel gating in an additive, but not competitive, manner. This indicates the possibility of an existence of independent binding sites for the two peptides on RyR1 [16]. These different results have been further understood by the hypothesis that the toxins and peptide A binding sites within RyR have both common activation site and independent substate sites [17]. Although the present results show that the mutations within the structural motif shared by Peptide A inhibited substate induction (Figure 2), it is hard to find direct evidence of competitive function in the common region of RyR1. Even though our investigations were undertaken without Peptide A, it could be suggested that the prolonged substate opening triggered by IpTxa is independent of the action of Peptide A [17]. Further study will be necessary to clarify whether the structurally conserved domains of IpTxa and Peptide A compete for the induction of substate in RyR1.
4.3. Comparison of the Active Sites with MCA
IpTxa and MCa share 82% aa identity in their primary structures. In addition to the similar β-sheet structure of the common stretch of the basic residues (Lys19-Arg24), the solution structures of IpTxa and MCa exhibit similar overall molecular folding [21, 30]. Because of this structural homology, these two toxins share functional similarities. Both IpTxa and MCa strongly induce SR Ca2+ release and activate ryanodine binding to skeletal RyR. In addition, both peptides have the ability to induce reversible transition of RyR1 gating mode between substate and fast full open gating [12, 13, 15–17]. Previously, it was demonstrated that mutations of each basic residue within Lys19-Thr26 and mutation of Lys8 of MCa decreased the ability of the toxin to induce Ca2+ release and potentiate [3H]ryanodine binding in the SR [29]. Moreover, the occurrence of long-lasting substate was markedly prevented by mutations of the basic residues within Lys20-Arg24 of MCa [29]. This inhibitory effect was reduced, if the mutation was farther from Lys24 while alanine replacement completely inhibited the substate event of skeletal RyR [17, 29]. Therefore, the previous results showing the effects of MCa mutants are partially coherent with our observations of the effects of IpTxa mutants on RyR1 substate. In the present study, IpTxa mutant, R24A, was the most effective in decreasing mean duration of substate of RyR1 (Figure 3 and Table 1). R24A mutant showed comparable Bmax value of Psubstate with those of other mutants, R23A, K22A, and K20A, although the values were significantly less than that of wild-type IpTxa (Figure 3 and Table 1). This suggests that the common domain clustered by positively charged residues (Lys20-Arg24) are responsible for the actions of two scorpion toxins to induce long-lasting substate opening of skeletal RyR1.
In spite of the high sequence identity, a significant functional difference between IpTxa and MCa has been observed. Two toxins induced different degree of substate of RyR1 at +40 mV holding potential (28% and 48% of full conductance state for IpTxa and MCa, resp.) [10, 13]. In addition, comparison of 3D structures of two peptides showed significantly different structural motifs near the N-terminal regions, where MCa but not IpTxa has a β-strand and makes the hydrophobic core by connecting to the side chains of four cysteine residues, Cys10, Cys16, Cys21, and Cys32 [21]. Thus, the difference in the functional effect between IpTxa and MCa on RyR1 gating appears to be due to the subtle change in the local charge distribution or a structural dissimilarity. Here we further suggest and it appears that both acidic (e.g., Asp2, Asp13, and Glu29) and basic residues of C-terminal region of IpTxa (e.g., Lys30, Arg31, and Arg33) are involved in functional interaction with RyR1 in the case of IpTxa (Figure 2(b)). To our knowledge, to date there is no report regarding to the involvement of the acidic aa residues of IpTxa are related to the occurrence of Psubstate of RyR1.
5. Conclusions
In this study, the ability of charged aa residues of IpTxa to induce substate of RyR1 was examined in detail. Both basic and acidic aa residues are involved in producing substate of RyR1 supporting the hypothesis that the structural domain constituting a local cluster of charged aa is important for modifying the mode of channel gating [15, 16, 21]. Residues such as Lys8 and Thr26 of IpTXa are important in terms of their inhibitory role in producing substate of RyR1. The modified channel gating properties induced by wild-type and mutant toxins were found both in native and purified RyR1. Taken together, the specific charge distributions on the surface of IpTxa may directly regulate the gating behavior of RyR1.
Supplementary Material
Supplementary Figure 1: Characterization of synthesized wild type and mutant IpTxa.
Supplementary Figure 2: Coomassie blue stained HSR and purified RyR1.
Acknowledgments
The authors thank Dr. M. Samso at Virginia Commonwealth University for providing the purified rabbit RyR1. This paper was supported by the Korea MEST NRF Grant (20110002144), the 2011 GIST Systems Biology Infrastructure Establishment Grant and KISTI-KREONET. I. R. Seo, D. E. Kang, and D. W. Song equally contributed to this paper.
References
- 1.Tsugorka A, Rios E, Blatter LA. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995;269(5231):1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- 2.Rios E, Ma J, Gonzalez A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. Journal of Muscle Research and Cell Motility. 1991;12(2):127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- 3.Cheng W, Altafaj X, Ronjat M, Coronado R. Interaction between the dihydropyridine receptor Ca2+ channel β-subunit and ryanodine receptor type 1 strengthens excitation-contraction coupling. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(52):19225–19230. doi: 10.1073/pnas.0504334102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern MD, Lakatta EG. Excitation-contraction coupling in the heart: the state of the question. FASEB Journal. 1992;6(12):3092–3100. doi: 10.1096/fasebj.6.12.1325933. [DOI] [PubMed] [Google Scholar]
- 5.Wier WG, Egan TM, López-López JR, Balke CW. Local control of excitation-contraction coupling in rat heart cells. Journal of Physiology. 1994;474(3):463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. Journal of Molecular and Cellular Cardiology. 2004;37(2):417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Mackrill JJ. Ryanodine receptor calcium channels and their partners as drug targets. Biochemical Pharmacology. 2010;79(11):1535–1543. doi: 10.1016/j.bcp.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Song DW, Lee JG, Youn HS, Eom SH, Kim DH. Ryanodine receptor assembly: a novel systems biology approach to 3D mapping. Progress in Biophysics and Molecular Biology. 2011;105(3):145–161. doi: 10.1016/j.pbiomolbio.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 9.El-Hayek R, Antoniu B, Wang J, Hamilton SL, Ikemoto N. Identification of calcium release-triggering and blocking regions of the II-III loop of the skeletal muscle dihydropyridine receptor. The Journal of Biological Chemistry. 1995;270(38):22116–22118. doi: 10.1074/jbc.270.38.22116. [DOI] [PubMed] [Google Scholar]
- 10.Fajloun Z, Kharrat R, Chen L, et al. Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca2+ release channel/ryanodine receptors. FEBS Letters. 2000;469(2-3):179–185. doi: 10.1016/s0014-5793(00)01239-4. [DOI] [PubMed] [Google Scholar]
- 11.Valdivia HH, Kirby MS, Lederer WJ, Coronado R. Scorpion toxins targeted against the sarcoplasmic reticulum Ca2+- release channel of skeletal and cardiac muscle. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(24):12185–12189. doi: 10.1073/pnas.89.24.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Hayek R, Lokuta AJ, Arevalo C, Valdivia HH. Peptide probe of ryanodine receptor function: imperatoxin A, a peptide from the venom of the scorpion Pandinus imperator, selectively activates skeletal-type ryanodine receptor isoforms. The Journal of Biological Chemistry. 1995;270(48):28696–28704. doi: 10.1074/jbc.270.48.28696. [DOI] [PubMed] [Google Scholar]
- 13.Tripathy A, Resch W, Xu LE, Valdivia HH, Meissner G. Imperatoxin A induces subconductance states in Ca2+ release channels (ryanodine receptors) of cardiac and skeletal muscle. Journal of General Physiology. 1998;111(5):679–690. doi: 10.1085/jgp.111.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shtifman A, Ward CW, Wang J, Valdivia HH, Schneider MF. Effects of imperatoxin A on local sarcoplasmic reticulum Ca2+ release in frog skeletal muscle. Biophysical Journal. 2000;79(2):814–827. doi: 10.1016/S0006-3495(00)76338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurrola GB, Arévalo C, Sreekumar R, Lokuta AJ, Walker JW, Valdivia HH. Activation of ryanodine receptors by imperatoxin A and a peptide segment of the II-III loop of the dihydropyridine receptor. The Journal of Biological Chemistry. 1999;274(12):7879–7886. doi: 10.1074/jbc.274.12.7879. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Estève E, Sabatier JM, et al. Maurocalcine and peptide A stabilize distinct subconductance states of ryanodine receptor type 1, revealing a proportional gating mechanism. The Journal of Biological Chemistry. 2003;278(18):16095–16106. doi: 10.1074/jbc.M209501200. [DOI] [PubMed] [Google Scholar]
- 17.Dulhunty AF, Curtis SM, Watson S, Cengia L, Casarotto MG. Multiple actions of imperatoxin A on ryanodine receptors: interactions with the II-III loop “A” fragment. The Journal of Biological Chemistry. 2004;279(12):11853–11862. doi: 10.1074/jbc.M310466200. [DOI] [PubMed] [Google Scholar]
- 18.Lukacs B, Sztretye M, Almassy J, et al. Charged surface area of maurocalcine determines its interaction with the skeletal ryanodine receptor. Biophysical Journal. 2008;95(7):3497–3509. doi: 10.1529/biophysj.107.120840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casarotto MG, Green D, Pace SM, Curtis SM, Dulhunty AF. Structural determinants for activation or inhibition of ryanodine receptors by basic residues in the dihydropyridine receptor II-III loop. Biophysical Journal. 2001;80(6):2715–2726. doi: 10.1016/S0006-3495(01)76240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Hayek R, Ikemoto N. Identification of the minimum essential region in the II-III loop of the dihydropyridine receptor α1 subunit required for activation of skeletal muscle-type excitation-contraction coupling. Biochemistry. 1998;37(19):7015–7020. doi: 10.1021/bi972907o. [DOI] [PubMed] [Google Scholar]
- 21.Lee CW, Lee EH, Takeuchi K, et al. Molecular basis of the high-affinity activation of type 1 ryanodine receptors by imperatoxin A. Biochemical Journal. 2004;377(2):385–394. doi: 10.1042/BJ20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo IR, Choi MR, Park CS, Kim DH. Effects of recombinant imperatoxin A (IpTxa) mutants on the rabbit ryanodine receptor. Molecules and Cells. 2006;22(3):328–335. [PubMed] [Google Scholar]
- 23.Green D, Pace S, Curtis SM, et al. The three-dimensional structural surface of two β-sheet scorpion toxins mimics that of an α-helical dihydropyridine receptor segment. Biochemical Journal. 2003;370(2):517–527. doi: 10.1042/BJ20021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DH, Ohnishi ST, Ikemoto N. Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. The Journal of Biological Chemistry. 1983;258(16):9662–9668. [PubMed] [Google Scholar]
- 25.Miller C, Racker E. Ca2+ induced fusion of fragmented sarcoplasmic reticulum with artificial planar bilayers. Journal of Membrane Biology. 1976;30(1):283–300. doi: 10.1007/BF01869673. [DOI] [PubMed] [Google Scholar]
- 26.Smith JS, Coronado R, Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum: activation by Ca2+ and ATP and modulation by Mg2+ Journal of General Physiology. 1986;88(5):573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee EH, Meissner G, Kim DH. Effects of quercetin on single Ca2+ release channel behavior of skeletal muscle. Biophysical Journal. 2002;82(3):1266–1277. doi: 10.1016/S0006-3495(02)75483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai FA, Erickson HP, Rousseau E, Liu QY, Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- 29.Estève E, Smida-Rezgui S, Sarkozi S, et al. Critical amino acid residues determine the binding affinity and the Ca 2+ release efficacy of maurocalcine in skeletal muscle cells. The Journal of Biological Chemistry. 2003;278(39):37822–37831. doi: 10.1074/jbc.M305798200. [DOI] [PubMed] [Google Scholar]
- 30.Mosbah A, Kharrat R, Fajloun Z, et al. A new fold in the scorpion toxin family, associated with an activity on a ryanodine-sensitive calcium channel. Proteins. 2000;40(3):436–442. doi: 10.1002/1097-0134(20000815)40:3<436::aid-prot90>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Characterization of synthesized wild type and mutant IpTxa.
Supplementary Figure 2: Coomassie blue stained HSR and purified RyR1.


