Abstract
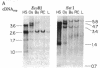
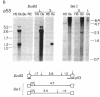
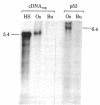
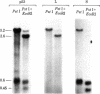
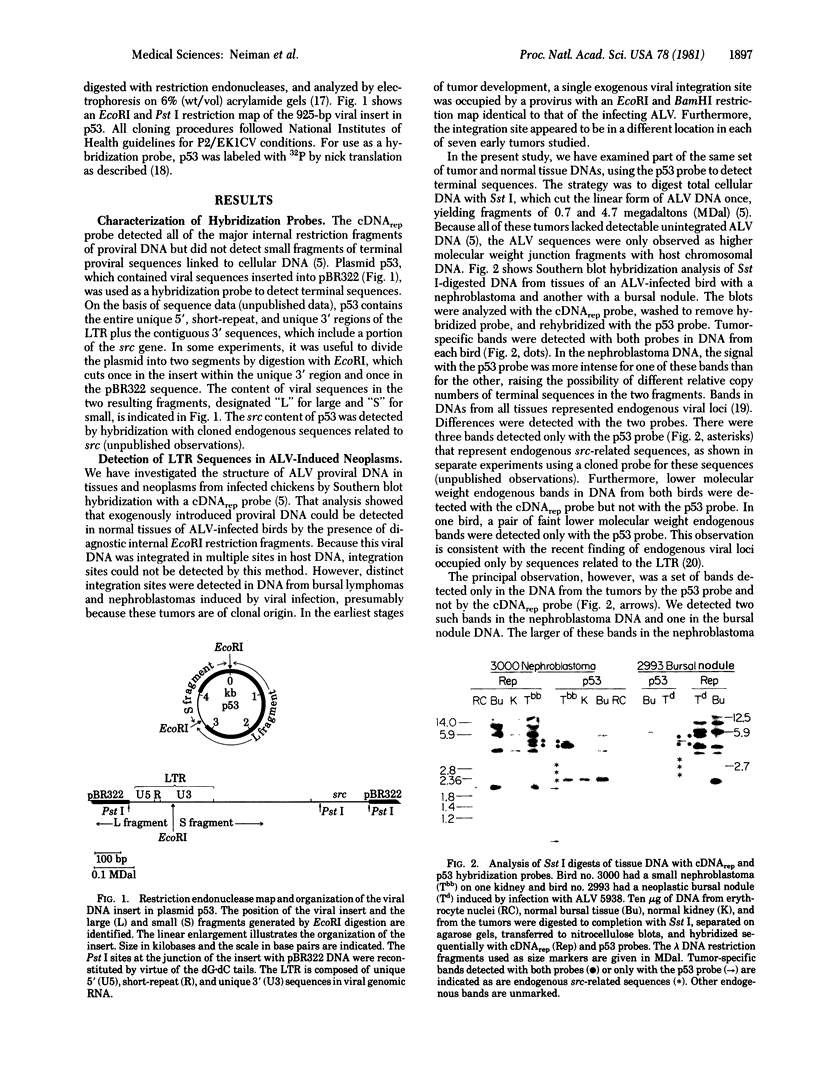
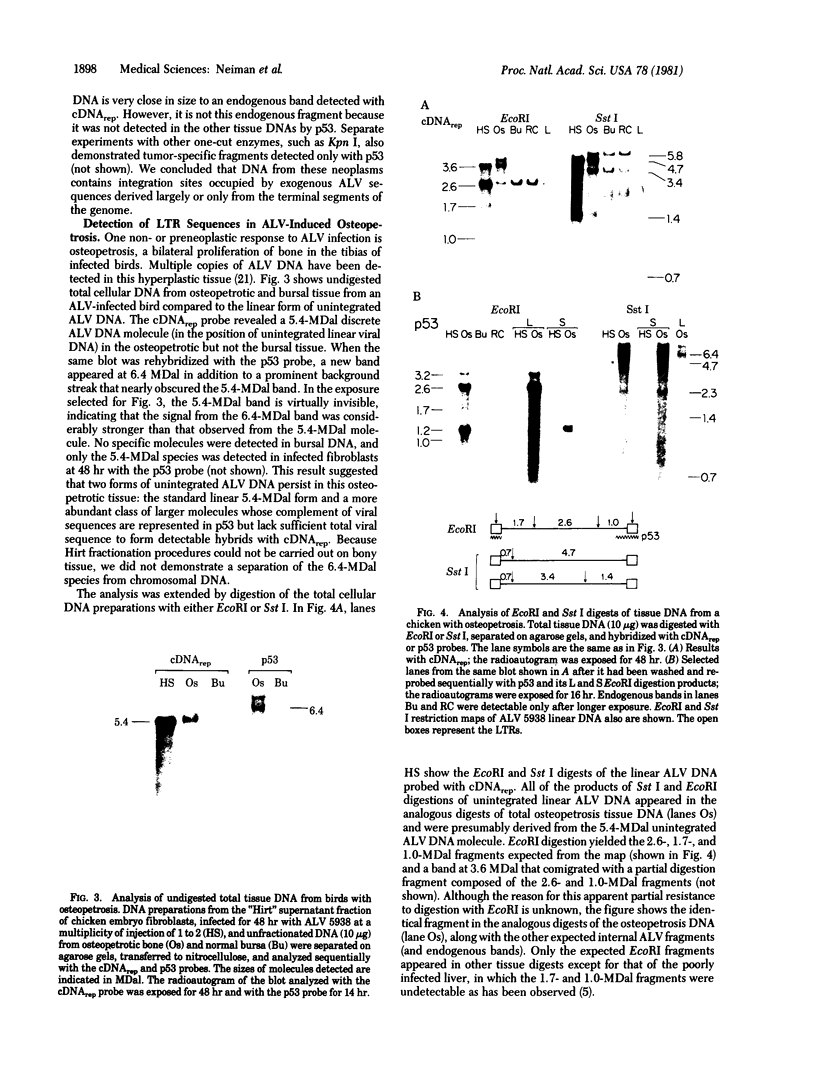
A cDNA transcript of Rous sarcoma virus, which contained the long terminal repeat (LTR) and some additional 3'-terminal sequences, was inserted into the plasmid pBR322. This recombinant plasmid, p53, was then used as a hybridization probe to detect viral terminal sequences in DNA from a number of tissues of birds with a variety of avian leukosis virus (ALV)-induced proliferative diseases. Using restriction endonuclease digestion and blot hybridization analysis, we detected, in addition to standard ALV genomes, viral terminal sequences linked to host DNA and not to viral genes. In DNA from bursal lymphomas and nephroblastomas, we observed small numbers of integration sites occupied by sequences in p53 and lacking most or all of the remainder of the viral genome. In DNA from osteopetrosis, we observed apparently multiple copies of molecules containing host DNA linked to viral LTR sequences. Some of these structures were contained in discrete, probably unintegrated, DNA molecules. We concluded that viral LTR sequences can be inserted as independent elements during recombination with host DNA in some forms of interaction between exogenous retroviruses and host cells. Because the LTRs have been implicated in integration and transcription of viral genes, the possibility that translocation or activation, or both, of host genes may occur as a consequence of viral infection is reinforced by these observations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M. Endogenous viral genes of the White Leghorn chicken: common site of residence and sites associated with specific phenotypes of viral gene expression. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5941–5945. doi: 10.1073/pnas.75.12.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Payne L. N., Dent P. B., Burmester B. R., Good R. A. Pathogenesis of avian lymphoid leukosis. I. Histogenesis. J Natl Cancer Inst. 1968 Aug;41(2):373–378. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Toyoshima K., Bishop J. M., Varmus H. E. Organization of the endogenous proviruses of chickens: implications for origin and expression. Virology. 1981 Jan 15;108(1):189–207. doi: 10.1016/0042-6822(81)90538-9. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P. E. Mapping by competitive hybridization of sequences which differ between endogenous and exogenous chicken leukosis viruses. Virology. 1978 Mar;85(1):9–16. doi: 10.1016/0042-6822(78)90407-5. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., McMillin-Helsel C., Cooper G. M. Specific restriction of avian sarcoma viruses by a line of transformed lymphoid cells. Virology. 1978 Sep;89(2):360–371. doi: 10.1016/0042-6822(78)90178-2. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Purchase H. G., Okazaki W. Chicken leukosis virus genome sequences in DNA from normal chick cells and virus-induced bursal lymphomas. Cell. 1975 Apr;4(4):311–319. doi: 10.1016/0092-8674(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Neiman P., Payne L. N., Weiss R. A. Viral DNA in bursal lymphomas induced by avian leukosis viruses. J Virol. 1980 Apr;34(1):178–186. doi: 10.1128/jvi.34.1.178-186.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Iverson L. Nucleotide sequences from the 3'-ends of vesicular stomatitis virus mRNA's as determined from cloned DNA. J Virol. 1979 Nov;32(2):404–411. doi: 10.1128/jvi.32.2.404-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Davids L. J., Neiman P. E. Comparison of an avian osteopetrosis virus with an avian lymphomatosis virus by RNA-DNA hybridization. J Virol. 1975 Jan;17(1):160–167. doi: 10.1128/jvi.17.1.160-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Verma I. M. Genome organization of RNA tumor viruses. I. In vitro synthesis of full-genome-length single-stranded and double-stranded viral DNA transcripts. J Virol. 1978 Jun;26(3):615–629. doi: 10.1128/jvi.26.3.615-629.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]