Abstract
Unique subpopulations of specialized metabolic sensing neurons reside in a distributed network throughout the brain and respond to alterations in ambient levels of various metabolic substrates by altering their activity. Variations in local brain substrate levels reflect their transport across the blood- and cerebrospinal-brain barriers as well as local production by astrocytes. There are a number of mechanisms by which such metabolic sensing neurons alter their activity in response to changes in substrate levels, but it is clear that these neurons cannot be considered in isolation. They are heavily dependent on astrocyte and probably tanycyte metabolism and function but also respond to hormones (e.g. leptin and insulin) and cytokines that cross the blood-brain barrier from the periphery as well as hard-wired neural inputs from metabolic sensors in peripheral sites such as the hepatic portal vein, gastrointestinal tract, and carotid body. Thus, these specialized neurons are capable of monitoring and integrating multiple signals from the periphery as a means of regulating peripheral energy homeostasis.
Specialized neurons in the brain act in concert with glial elements to form a metabolic sensing unit that senses metabolic and hormonal signals generated in the periphery as a means of informing the brain as to the metabolic status of the body. The purpose of this review is to provide a broad overview of some of the issues involved in this field with regard to the mechanisms, locations, and potential physiological functions of these sensing units.
Who are the metabolic-sensing elements of the brain?
Mayer first proposed that neurons might sense changes in blood levels of metabolic substrates (glucose) as a means of regulating food intake (1). Not until the 1960s and 1970s were such neurons that altered their activity in response to changes in ambient glucose and fatty acid levels demonstrated in the brain (2–4). Originally called glucosesensing neurons, it is now clear that such neurons respond to a diverse array of metabolic substrates, hormones such as leptin and insulin, and cytokines that are transported into the brain from the periphery or are produced locally in the brain. Thus, the terms metabolic- or nutrient-sensing neurons are often used to describe them (5). Unlike the majority of neurons that use these substrates to fuel their metabolic demands (6–8), metabolic-sensing neurons also use these same substrates to modulate their membrane potential, firing rate, transmitter and peptide release, and gene expression. Most importantly, these neurons do not act alone but also require the support of astrocytes and probably tanycytes to perform as a metabolic-sensing unit. Although not the focus of this review, this metabolic-sensing unit also receives important neural inputs from peripheral metabolic sensors, which have a major impact on its function (5).
What are the physiological functions of the metabolic sensing unit?
Despite Mayer's original hypothesis (1), it is quite likely that glucose and glucosensing neurons play little role in modulating either the onset or duration of individual meals during the normal diurnal cycle. They are, however, critical in stimulating both feeding and counterregulatory responses when glucose availability falls (9–13). Also, whereas infusions of fatty acids into the brain and/or manipulation of brain fatty acid metabolism can alter feeding, peripheral glucose metabolism, and glucose-induced insulin secretion (14–20), some of these findings likely reflect the use of nonphysiological concentrations and routes of administration of various fatty acids. Similarly, there are studies suggesting a role for amino acid sensing in the regulation of food intake, but these are based on amino acid deficient diets (21) or drug effects (22). Because metabolic substrates gain access to the brain by transport across the blood- and/or cerebrospinal fluid-brain barriers, administration of substrates directly into the brain or ventricular system runs the risk of producing nonspecific toxic or inflammatory effects that do not mimic true physiological conditions (23). Also, the assumption that such responses are due to direct effects on metabolic-sensing neurons (24) is often belied by the fact that many manipulations have their primary actions on astrocytes (25–27). Thus, until all of these caveats are addressed, it remains to be seen what the true physiological roles of metabolic-sensing neurons might be in regulating overall energy and glucose homeostasis in the body.
Where are metabolic-sensing neurons located?
There is a great heterogeneity of cell types, functions, and locations among metabolic-sensing neurons. Glucosensing neurons have been identified in a number of brain sites including the hypothalamus, medulla, basal ganglia, and amygdala and are loosely connected within a distributed network (28). Among the best characterized are those involved in the regulation of energy homeostasis such as the proopiomelanocortin and neuropeptide Y/agouti-related peptide neurons in the arcuate hypothalamic nucleus (ARC) and orexin neurons of the lateral hypothalamic area (29–32). The general lack of specific markers for metabolic-sensing neurons means that most of them must be identified by physiological studies in each candidate area. One exception is glucokinase (GK), a low-affinity hexokinase that initiates glycolysis in pancreatic β- and α-cells and appears to be a specific marker for glucosensing neurons (29, 33–37). GK mRNA and/or protein are localized in discrete hypothalamic, diencephalic, basal ganglia, amygdalar, and various hindbrain areas, many of which have physiologically identified glucosensing neurons (4, 35, 38–41). Much less is known about any specific characteristics of the astrocytes and tanycytes that provide metabolic and trophic support to these metabolic-sensing neurons. However, it is clear that astrocyte characteristics do vary markedly as a function of their anatomical location and physiological functions (42).
How do neurons sense metabolic substrates?
Most substances enter the brain by facilitated transport across the blood-brain barrier, which is composed of vascular endothelial cells separated by tight junctions and astrocytic foot processes that abut these vessels (43). The requirement for transport means that extracellular brain glucose levels are 10–25% of plasma levels, depending on the brain area and the physiological state of the animal. When plasma levels fall to 4–5 mm during fasting, brain levels fall to approximately 0.4–0.7 mm. They rise to 1.5–2.5 mm after a large carbohydrate meal (11, 44, 45). Importantly, even in structures that lie adjacent to the median eminence, which lacks a blood-brain barrier, tanycyte processes lining the base of the third ventricle prevent diffusion from the median eminence to neurons in the ARC and ventromedial (VMN) hypothalamic nuclei (11, 46). On the other hand, substances that enter cerebrospinal fluid by crossing the blood-choroid plexus barrier can potentially reach metabolic-sensing neurons in the ventromedial hypothalamus by diffusion through the fenestrations separating individual tanycytes lining the base of the third ventricle (46).
Glucosensing neurons
There are two types of glucosensing neurons that are either excited (GE) or inhibited (GI) as ambient glucose levels rise (28) (Fig. 1). These responses occur either as a result of intracellular glucose metabolism (33, 36, 37, 47, 48) or by propagation of an electrogenic potential generated by interaction of glucose with a glucose transporter or channel (49, 50). The best-characterized metabolic pathway by which glucose excites neurons involves the transport of glucose into the neuron, predominantly by glucose transporter (Glut) 3, and phosphorylation by GK. This initiates glycolysis, the oxidative production of ATP, the elevation of the ATP to ADP ratio with the inactivation of an ATP-sensitive K+ (KATP) channel, the depolarization of the cell membrane causing calcium influx via a voltage-dependent calcium channel, and the propagation of an action potential (28, 48) (Fig. 1). Up to 65% of GE and 45% of GI VMN neurons express GK (36), whereas 54 and 42% of medial amygdalar GE and GI neurons express GK, respectively (38).
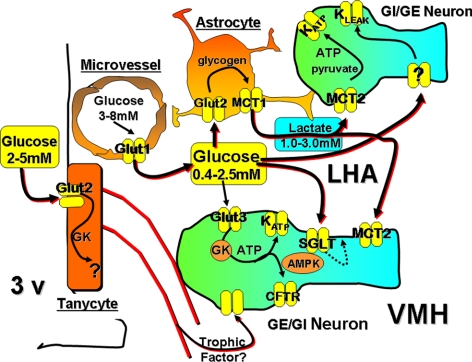
Fig. 1.
Model of the interrelationship among various types of glucosensing neurons, cerebral microvessels, astrocytes, and tanycytes within the ventromedial (VMH) and lateral hypothalamic area (LHA). GE neurons increase their activity in response to increasing glucose levels by the following: 1) glucose transport via Glut3 transporters, phosphorylation by GK, production of ATP by mitochondrial oxidation, and inactivation of the KATP channel leading to membrane depolarization; 2) interaction with the sodium glucose co-transporter (SGLT) and metabolism-independent generation of an electrogenic potential; and 3) use of astrocyte-derived lactate, which is transported into neurons by monocarboxylate transporter 2 (MCT2) with conversion to pyruvate for oxidative production of ATP and inactivation of the KATP channel. GI neurons are activated by the following: 1) low levels of glucose and GK-modulated phosphorylation leading to increased AMP levels, activation of AMPK, and closure of a chloride channel (possibly cystic fibrosis transmembrane receptor); and 2) activation of a K+ leak channel generating a metabolism-independent electrogenic potential. Tanycytes contain GK and Glut2 and may provide trophic support for glucosensing neurons. 3v, Third ventricle.
Other GE neurons requiring glucose metabolism use AMP-activated protein kinase (AMPK) (51) and a KATP-independent channel, which is activated at high, likely nonphysiological (>5 mm) glucose levels (52). Additionally, some lateral hypothalamic orexin/hypocretin neurons are activated by astrocyte-derived lactate (53) (Fig. 1). On the other hand, some ventromedial hypothalamic GE neurons are activated by the interaction of high levels of glucose (3–15 mm) with a sodium-glucose cotransporter, a process that propagates an electrogenic potential leading to membrane depolarization without the requirement for intracellular glucose metabolism (49).
Activation of GI neurons at low glucose levels is also mediated by either intracellular glucose metabolism or nonmetabolic pathways (Fig. 1). About 45% of VMN GI neurons use GK as a gatekeeper (36, 37) and then recruit AMPK-regulated generation of nitric oxide to inhibit a chloride channel, possibly the cystic fibrosis transmembrane receptor (Fig. 1), causing membrane depolarization and activation (54). Additionally, approximately 50% of the inhibition of GI neurons at high glucose levels is modulated by the formation of reactive oxygen species during glucose oxidation (39). On the other hand, activation of some lateral hypothalamic orexin/hypocretin GI neurons at low glucose occurs by closure of a leak-like K+ channel of unknown type, a process that does not require intracellular metabolism of glucose (50), (Fig. 1).
Fatty acid-sensing neurons
Up to 70% of ARC and VMN neurons are either excited or inhibited by long-chain fatty acids such as oleic acid (39–41). Within the VMN, 90% of the glucosensing neurons also have their activity altered by fatty acids. In a large percentage of these neurons, glucose and fatty acids have opposing effects on neuronal activity, much as they do on intracellular metabolism in many other cells (55). Neuronal fatty acid-sensing mechanisms include activation of the KATP channel by long-chain fatty acid acyl coenzyme A (56) or inactivation by generation of ATP or reactive oxygen species during mitochondrial β-oxidation (39–41, 57). Many fatty acid-sensing neurons are activated by interaction of long-chain fatty acids with the fatty acid transporter/receptor, FAT/CD36, presumably by activation of store-operated calcium channels by a mechanism that is independent of fatty acid metabolism (39). Importantly, most neurons use fatty acids primarily for membrane production rather than as a metabolic substrate (58, 59), and only nanomolar concentrations of fatty acid are required to alter the activity of fatty acid sensing neurons in the absence of astrocytes (39). Although cerebral lipids are both produced in the brain and transported into it from the periphery (58, 59), the mechanism of this transport and the actual levels of various fatty acids in the extracellular space in the brain remain largely unknown.
The metabolic-sensing unit
Astrocytes
Astrocytes are essential for both neuronal metabolism and metabolic sensing and are a critical component of the so-called tripartite synapse, which also includes the pre- and postsynaptic processes of neurons (60). Astrocytes take up glutamate released by neurons, use it for their own cellular metabolism, and recycle the resultant glutamine for neuronal metabolism (27). They also take up glucose, metabolize, and release it as lactate or store it as glycogen (27). Although the exact degree to which neuronal metabolism is dependent on astrocyte-derived lactate is still controversial (61), it is clear that neurons can take up lactate via monocarboxylate transporters (62) and convert it to pyruvate for oxidative production of ATP (27). Because lactate bypasses most regulatory glucosensing pathways, alterations in astrocyte lactate production by transmitters such as norepinephrine, dopamine, serotonin, glutamate, and γ-aminobutyric acid (63) can override the effects of glucose on neuronal glucosensing (53, 64). Perhaps the most important function of astrocyte lactate production from glycogen is to provide an energy reserve to support neuronal function during hypoglycemia (65).
Finally, the majority of fatty acid oxidation in the brain occurs in astrocytes (6). Astrocytes can also produce ketone bodies (66), which are exported and taken up by neuronal monocarboxylate transporters to serve as an alternate energy source for neuronal metabolism. AMPK is a major regulator of astrocyte ketone production (67) so that studies in which AMPK activity or other fatty acid metabolic pathways are altered may produce physiological effects primarily be altering astrocyte rather than neuronal metabolism.
Tanycytes
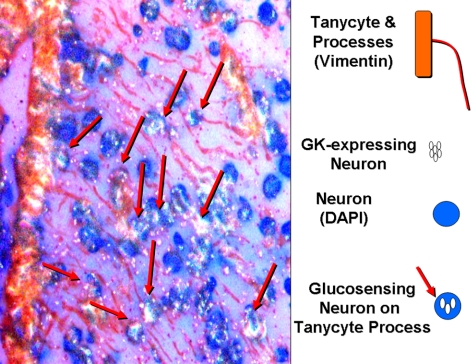
Little is known about the function of tanycytes in supporting neuronal metabolism. However, processes of tanycytes lining the ventral third cerebral ventricle divide the ARC and VMN into compartments and effectively prevent diffusion of substances such as glucose and larger molecules from the median eminence, which lacks a blood-brain barrier, into the ARC (11, 46). Presumptive GK-expressing glucosensing neurons line up along these processes suggesting a supportive role of tanycytes in metabolic sensing (Fig. 2). Also, tanycytes express both Glut2 and GK, which makes them potentially glucosensing themselves (68). Transient destruction of third ventricular tanycytes markedly impairs the counterregulatory response to glucoprivation, and this is reversed when they regenerate. Clearly much work is required to further elucidate the role of these intriguing cells as members of the metabolic sensing unit.
Fig. 2.
Vimentin-expressing tanycytes line the lower third of the third ventricle and send processes into the ARC and VMN. Presumptive GK-expressing glucosensing neurons lie in close approximation to these processes, suggesting a supportive role in neuronal glucosensing. DAPI, 4′,6′-Diamidino-2-phenylindole.
In summary, select neurons throughout the brain use metabolic substrates from the periphery to alter their activity as a means of sensing and possibly regulating the metabolic status of the body. The function of these metabolic-sensing neurons is dependent on the support of neighboring astrocytes and tanycytes. Although much is known, conflicting results derived from sometimes ambiguous study designs mean that we still have a long way to go to understand the role of the metabolic-sensing unit in the regulation of energy homeostasis in the body.
Acknowledgments
Disclosure Summary: B.E.L. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DKRO1 30066 and DK RO1 53181 and the Juvenile Diabetes Research Foundation for some of the work included in this review. The other authors have nothing to declare.
Footnotes
- AMPK
- AMP-activated protein kinase
- ARC
- arcuate hypothalamic nucleus
- GE
- excited glucosensing neurons
- GI
- inhibited glucosensing neurons
- Glut
- glucose transporter
- GK
- glucokinase
- KATP
- ATP-sensitive K+
- VMN
- ventromedial hypothalamic nucleus.
References
- 1. Mayer J. 1953. Glucostatic mechanism of regulation of food intake. N Engl J Med 249:13–16 [DOI] [PubMed] [Google Scholar]
- 2. Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. 1964. Activity of single neurons in the hypothalamus feeding centers: effect of glucose. Am J Physiol 207:1146–1154 [DOI] [PubMed] [Google Scholar]
- 3. Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. 1964. Reciprocal activities of the ventromedial and lateral hypothalamic area of cats. Science 143:484–485 [DOI] [PubMed] [Google Scholar]
- 4. Oomura Y, Nakamura T, Sugimori M, Yamada Y. 1975. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol Behav 14:483–486 [DOI] [PubMed] [Google Scholar]
- 5. Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. 2004. Neuronal glucosensing: what do we know after 50 years? Diabetes 53:2521–2528 [DOI] [PubMed] [Google Scholar]
- 6. Edmond J. 1992. Energy metabolism in developing brain cells. Can J Physiol Pharmacol 70(Suppl):S118–S129 [DOI] [PubMed] [Google Scholar]
- 7. Sokoloff L, Reivich M, Kennedy C, DesRosiers MH, Patlak CS, Pettigrew O, Sakaruda O, Shinohara M. 1977. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 23:897–916 [DOI] [PubMed] [Google Scholar]
- 8. Pellerin L, Pellegri G, Martin JL, Magistretti PJ. 1998. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci USA 95:3990–3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith GP, Epstein AN. 1969. Increased feeding in response to decreased glucose utilization in the rat and monkey. Am J Physiol 217:1083–1087 [DOI] [PubMed] [Google Scholar]
- 10. Gilbert M, Magnan C, Turban S, André J, Guerre-Millo M. 2003. Leptin receptor-deficient obese Zucker rats reduce their food intake in response to a systemic supply of calories from glucose. Diabetes 52:277–282 [DOI] [PubMed] [Google Scholar]
- 11. Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. 2009. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci 29:7015–7022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. 1995. Local ventromedial hypothalamic glucopenia triggers counterregulatory hormone release. Diabetes 44:180–184 [DOI] [PubMed] [Google Scholar]
- 13. Ritter S, Bugarith K, Dinh TT. 2001. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol 432:197–216 [DOI] [PubMed] [Google Scholar]
- 14. Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. 2002. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51:271–275 [DOI] [PubMed] [Google Scholar]
- 15. Migrenne S, Magnan C, Cruciani-Guglielmacci C. 2007. Fatty acid sensing and nervous control of energy homeostasis. Diabetes Metab 33:177–182 [DOI] [PubMed] [Google Scholar]
- 16. Ross RA, Rossetti L, Lam TK, Schwartz GJ. 2010. Differential effects of hypothalamic long chain fatty acid infusions on suppression of hepatic glucose production. Am J Physiol Endocrinol Metab 299:E633–E639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. López M, Lage R, Saha AK, Pérez-Tilve D, Vázquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodríguez-Cuenca S, Deoliveira RM, Castañeda T, Datta R, Dong JZ, Culler M, Sleeman MW, Alvarez CV, Gallego R, Lelliott CJ, Carling D, Tschöp MH, Diéguez C, Vidal-Puig A. 2008. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 7:389–399 [DOI] [PubMed] [Google Scholar]
- 18. Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, Rossetti L. 2006. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 116:1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cruciani-Guglielmacci C, Hervalet A, Douared L, Sanders NM, Levin BE, Ktorza A, Magnan C. 2004. β Oxidation in the brain is required for the effects of non-esterified fatty acids on glucose-induced insulin secretion in rats. Diabetologia 47:2032–2038 [DOI] [PubMed] [Google Scholar]
- 20. Wang H, Astarita G, Taussig MD, Bharadwaj KG, DiPatrizio NV, Nave KA, Piomelli D, Goldberg IJ, Eckel RH. 2011. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metab 13:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang CX, Erecius LF, Beverly JL, 3rd, Gietzen DW. 1999. Essential amino acids affect interstitial dopamine metabolites in the anterior piriform cortex of rats. J Nutr 129:1742–1745 [DOI] [PubMed] [Google Scholar]
- 22. Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. 2006. Hypothalamic mTOR signaling regulates food intake. Science 312:927–930 [DOI] [PubMed] [Google Scholar]
- 23. Sanders NM, Dunn-Meynell AA, Levin BE. 2004. Third ventricular alloxan reversibly impairs glucose counterregulatory responses. Diabetes 53:1230–1236 [DOI] [PubMed] [Google Scholar]
- 24. Lam TK. 2010. Neuronal regulation of homeostasis by nutrient sensing. Nat Med 16:392–395 [DOI] [PubMed] [Google Scholar]
- 25. Guzmán M, Blázquez C. 2001. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab 12:169–173 [DOI] [PubMed] [Google Scholar]
- 26. Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J. 1987. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 18:551–561 [DOI] [PubMed] [Google Scholar]
- 27. Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. 2007. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262 [DOI] [PubMed] [Google Scholar]
- 28. Levin BE, Kang L, Sanders NM, Dunn-Meynell AA. 2006. Role of neuronal glucosensing in the regulation of energy homeostasis. Diabetes 55(Suppl 2):S122–S130 [Google Scholar]
- 29. Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. 2002. Glucokinase is the likely mediator of glucosensing in both glucose excited and glucose inhibited central neurons. Diabetes 51:2056–2065 [DOI] [PubMed] [Google Scholar]
- 30. Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. 2003. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 144:1331–1340 [DOI] [PubMed] [Google Scholar]
- 31. Burdakov D, Luckman SM, Verkhratsky A. 2005. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci 360:2227–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fioramonti X, Contié S, Song Z, Routh VH, Lorsignol A, Pénicaud L. 2007. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: Integration in NPY and POMC networks? Diabetes 56:1219–1227 [DOI] [PubMed] [Google Scholar]
- 33. Yang XJ, Kow LM, Funabashi T, Mobbs CV. 1999. Hypothalamic glucose sensor. Similarities to and differences from pancreatic β-cell mechanisms. Diabetes 48:1763–1772 [DOI] [PubMed] [Google Scholar]
- 34. Lynch RM, Tompkins LS, Brooks HL, Dunn-Meynell AA, Levin BE. 2000. Localization of glucokinase gene expression in the rat brain. Diabetes 49:693–700 [DOI] [PubMed] [Google Scholar]
- 35. Levin BE, Routh VH, Sanders NM, Kang L, Dunn-Meynell AA. 2004. Anatomy, physiology and regulation of glucokinase as a brain glucosensor. In: Matschinsky FM, Magnuson MA. eds. Glucokinase and glycemic disease: from basics to normal therapeutics. Basel: Karger; 301–312 [Google Scholar]
- 36. Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. 2004. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53:549–559 [DOI] [PubMed] [Google Scholar]
- 37. Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. 2006. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55:412–420 [DOI] [PubMed] [Google Scholar]
- 38. Zhou L, Podolsky N, Sang Z, Ding Y, Fan X, Tong Q, Levin BE, McCrimmon RJ. 2010. The medial amygdalar nucleus: a novel glucose-sensing region that modulates the counterregulatory response to hypoglycemia. Diabetes 59:2646–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Le Foll C, Irani BG, Magnan C, Dunn-Meynell AA, Levin BE. 2009. Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am J Physiol Regul Integr Comp Physiol 297:R655–R664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jo YH, Su Y, Gutierrez-Juarez R, Chua S., Jr 2009. Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J Neurophysiol 101:2305–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Migrenne S, Cruciani-Guglielmacci C, Kang L, Wang R, Rouch C, Lefevre AL, Ktorza A, Routh VH, Levin BE, Magnan C. 2006. Fatty acid signaling in the hypothalamus and the neural control of insulin secretion. Diabetes 55(Suppl 2):S139–S144 [Google Scholar]
- 42. Qian JA, Bull MS, Levitt P. 1992. Target-derived astroglia regulate axonal outgrowth in a region-specific manner. Dev Biol 149:278–294 [DOI] [PubMed] [Google Scholar]
- 43. Rodríguez EM, Blázquez JL, Guerra M. 2010. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31:757–776 [DOI] [PubMed] [Google Scholar]
- 44. de Vries MG, Arseneau LM, Lawson ME, Beverly JL. 2003. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes 52:2767–2773 [DOI] [PubMed] [Google Scholar]
- 45. Silver IA, Erecińska M. 1994. Extracellular glucose concentrations in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14:5068–5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mullier A, Bouret SG, Prevot V, Dehouck B. 2010. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol 518:943–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. 1974. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature 247:284–286 [DOI] [PubMed] [Google Scholar]
- 48. Rowe IC, Treherne JM, Ashford ML. 1996. Activation by intracellular ATP of a potassium channel in neurones from rat basomedial hypothalamus. J Physiol 490:97–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Malley D, Reimann F, Simpson AK, Gribble FM. 2006. Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes 55:3381–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. 2008. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci USA 105:11975–11980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. 2007. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117:2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fioramonti X, Lorsignol A, Taupignon A, Pénicaud L. 2004. A New ATP-sensitive K+ channel-independent mechanism is involved in glucose-excited neurons of mouse arcuate nucleus. Diabetes 53:2767–2775 [DOI] [PubMed] [Google Scholar]
- 53. Parsons MP, Hirasawa M. 2010. ATP-Sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal. J Neurosci 30:8061–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH. 2009. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol 297:C750–C758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Randle PJ, Priestman DA, Mistry S, Halsall A. 1994. Mechanisms modifying glucose oxidation in diabetes mellitus. Diabetologia 37(Suppl 2):S155–S161 [DOI] [PubMed] [Google Scholar]
- 56. Gribble FM, Proks P, Corkey BE, Ashcroft FM. 1998. Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA. J Biol Chem 273:26383–26387 [DOI] [PubMed] [Google Scholar]
- 57. Wang R, Cruciani-Guglielmacci C, Migrenne S, Magnan C, Cotero VE, Routh VH. 2006. Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. J Neurophysiol 95:1491–1498 [DOI] [PubMed] [Google Scholar]
- 58. Rapoport SI, Chang MC, Spector AA. 2001. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res 42:678–685 [PubMed] [Google Scholar]
- 59. Smith QR, Nagura H. 2001. Fatty acid uptake and incorporation in brain: studies with the perfusion model. J Mol Neurosci 16:167–172; discussion 215–221 [DOI] [PubMed] [Google Scholar]
- 60. Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22:208–215 [DOI] [PubMed] [Google Scholar]
- 61. Gandhi GK, Cruz NF, Ball KK, Dienel GA. 2009. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem 111:522–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ. 2000. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 100:617–627 [DOI] [PubMed] [Google Scholar]
- 63. Uehara T, Sumiyoshi T, Itoh H, Kurata K. 2008. Lactate production and neurotransmitters; evidence from microdialysis studies. Pharmacol Biochem Behav 90:273–281 [DOI] [PubMed] [Google Scholar]
- 64. Song Z, Routh VH. 2005. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 54:15–22 [DOI] [PubMed] [Google Scholar]
- 65. Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR. 2000. Astrocytic glycogen influences axon function and survival during glucose deprivation in central with matter. J Neurosci 20:6804–6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Auestad N, Korsak RA, Morrow JW, Edmond J. 1991. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem 56:1376–1386 [DOI] [PubMed] [Google Scholar]
- 67. Blázquez C, Woods A, de Ceballos ML, Carling D, Guzmán M. 1999. The AMP-activated protein kinase is involved in the regulation of ketone body production by astrocytes. J Neurochem 73:1674–1682 [DOI] [PubMed] [Google Scholar]
- 68. García MA, Millán C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H, Reinicke K, Zúñiga F, Vera JC, Oñate SA, Nualart F. 2003. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J Neurochem 86:709–724 [DOI] [PubMed] [Google Scholar]