Abstract
Steroidogenic factor 1 (SF-1) is essential for the development and function of steroidogenic tissues. Stable incorporation of SF-1 into embryonic stem cells (SF-1-ES cells) has been shown to prime the cells for steroidogenesis. When provided with exogenous cholesterol substrate, and after treatment with retinoic acid and cAMP, SF-1-ES cells produce progesterone but do not produce other steroids such as cortisol, estradiol, or testosterone. In this study, we explored culture conditions that optimize SF-1-mediated differentiation of ES cells into defined steroidogenic lineages. When embryoid body formation was used to facilitate cell lineage differentiation, SF-1-ES cells were found to be restricted in their differentiation, with fewer cells entering neuronal pathways and a larger fraction entering the steroidogenic lineage. Among the differentiation protocols tested, leukemia inhibitory factor (LIF) removal, followed by prolonged cAMP treatment was most efficacious for inducing steroidogenesis in SF-1-ES cells. In this protocol, a subset of SF-1-ES cells survives after LIF withdrawal, undergoes morphologic differentiation, and recovers proliferative capacity. These cells are characterized by induction of steroidogenic enzyme genes, use of de novo cholesterol, and production of multiple steroids including estradiol and testosterone. Microarray studies identified additional pathways associated with SF-1 mediated differentiation. Using biotinylated SF-1 in chromatin immunoprecipitation assays, SF-1 was shown to bind directly to multiple target genes, with induction of binding to some targets after steroidogenic treatment. These studies indicate that SF-1 expression, followed by LIF removal and treatment with cAMP drives ES cells into a steroidogenic pathway characteristic of gonadal steroid-producing cells.
Steroidogenic factor 1 (SF-1), a nuclear hormone receptor (nuclear receptor subfamily 5, group A, member 1), was discovered as a tissue-specific regulator of cytochrome P450 hydroxylase genes (1–3). SF-1 is first expressed in the adrenogonadal primordium, before the expression of Sry gene, and continues to be expressed in Leydig and Sertoli cells in the testis, granulosa and theca cells in the ovary, all three layers of the adrenal cortex, and the pituitary and hypothalamus (4, 5). In the steroidogenic tissues, SF-1 regulates steroidogenic pathways including genes encoding cytochrome P450 enzymes like CYP11A1 (6–8), CYP17A1 (9–10), CYP21A2 (11), CYP11B1 (3), 3β-hydroxysteroid dehydrogenase (3β-HSD) (12), and other proteins important in steroidogenesis. SF-1 also regulates genes involved in sex determination and development of reproductive tissues including DAX1, AMH, SOX9, SRY, and INHA (13–17). Thus, SF-1 plays a central role in development and function of the steroidogenic and reproductive system.
Mouse knockout models of SF-1 have further established the essential role of SF-1 in endocrine development (5, 18–22). SF-1−/− mice fail to develop adrenal glands or gonads. In addition, the XY SF-1−/− mice are phenotypically female because they lack gonadal steroids and antimullerian hormone (18). Because SF-1 deficiency affects multiple genetic pathways that regulate steroidogenic tissue development, as well as enzyme and hormone production, it has been challenging to dissect the mechanisms by which SF-1 regulates select pathways such as steroidogenesis. As a result, in vitro models have been used to study SF-1-mediated pathways (7, 23, 24).
Embryonic stem (ES) cells provide a potentially valuable model for studying the differentiation of the steroidogenic lineage and exploring the function of SF-1 in this process. The pluripotent ES cells can be influenced by the expression of certain lineage specific genes, and by treatment with various growth factors or chemicals, to derive a variety of differentiated cell types. For example, ES cells have been induced to differentiate into hematopoietic, neural, cardiomyoctye, and pancreatic lineages among others (25). Studies also suggest that exogenous SF-1 can induce steroidogenic cell differentiation and function. Mesenchymal stem cells have been shown to produce steroids after transfection with SF-1 (26–28). ES cells stably transfected with SF-1 produce progesterone when treated with retinoic acid (RA) and cAMP in presence of 20α-hydroxycholesterol as a substrate (29). ES cells have also been differentiated using an inducible form of SF-1 in conjunction with exposure to collagen IV and RA pulses to stimulate the development of adrenocortical-like cells capable of producing corticosterone (30, 31). These studies suggest that SF-1 initiates a genetic program that enables the ES cells to develop steroidogenic capacity. In this study, we explored various culture conditions for SF-1-expressing ES cells in an effort to develop a protocol for their efficient differentiation into cells with characteristics of the gonadal steroidogenic lineage.
Materials and Methods
ES cell culture, stable cell transfection, and hormone assays
R1 ES cells were cultured on 0.1% gelatin-coated plates in DMEM with nonessential amino acids, sodium pyruvate, 15% (vol/vol) fetal bovine serum, 2 mm l-glutamine, 0.1 mm 2-mercaptoethanol, 20 mm HEPES, 1000 U/ml murine leukemia inhibitory factor (LIF), and antibiotics. Stable ES cell lines expressing cytomegalovirus-driven SF-1 (SF-1-ES) and SF-1-ES-CYP11A1-enhanced green fluorescent protein (EGFP) were selected using 400 μg/ml of the antibiotic Geneticin (Life Technologies, Inc., Carlsbad, CA) and 20 μg/ml of the antibiotic Zeocin (InvioGen, San Diego, CA), respectively. For the biotin (Bio)-chromatin immunoprecipitation (ChIP) assays, ES cell lines expressing Simian virus 40 promoter-driven bacterial biotin ligase (Bio-ES cells) and Bio-SF-1-ES cells were created using Zeocin and geneticin selection, respectively.
For steroid induction or differentiation experiments, 25 × 104 cells were plated in 60-mm plates containing 5 ml media and treated with combinations of 1 mm 8-bromoadenosine-cAMP (Sigma, St. Louis, MO), 0.5 μm all-trans RA (Sigma), and 5 μg/ml 20α-hydroxycholesterol (Sigma) as described. Conditioned media were collected after 40 h and assayed for progesterone, testosterone, corticosterone, and estradiol by RIA performed by the University of Virginia Ligand Assay and Analysis Core (Charlottesville, VA).
Plasmid constructions
The SF-1 cDNA was cloned in vector pECFP-N1 (cyan fluorescent protein (CFP) tag removed) (BD Biosciences, Sparks, MD) and transfected into ES cells to create SF-1-ES cells. The biotin ligase gene (BirA) amplified from Escherichia coli genomic DNA was cloned into vector pZeoSV (Invitrogen, Carlsbad, CA) to generate the pZeo-BirA plasmid. For protein biotinylation, plasmid pN-BLRP was created that encoded the biotin ligase recognition peptide (BLRP; 14 amino acid sequence-GLNDIFEAQKIEWH) on the N terminal of the multiple cloning site in vector pECFP-N1 (cyan fluorescent protein tag removed). The SF-1 cDNA was cloned into pN-BLRP to create pN-BLRP-SF-1. To create pZeoCYP11A1-promoter-EGFP construct, 1.5 kb of the mouse CYP11A1 promoter was amplified from Y1 cell genomic DNA, cloned into pZeoSV replacing the Simian virus 40 promoter. The EGFP gene from vector pEGFP-N1 was then cloned downstream of the C-terminal end of the CYP11A1 promoter.
Cell proliferation assays
For dimethylthiazol diphenyltetrazolium bromide (MTT) cell proliferation assays, ES and SF-1-ES cells were plated in 24-well plates in triplicate. MTT (Sigma) stock solution (5 mg/ml in PBS) was added to the cells at indicated times and incubated for 3.5 h at 37 C. MTT was dissolved by adding solvent (4 mm HCl, 0.1% Nonidet P-40 in isopropanol) and absorbance was read (590 nm) using BioTek Powerwave-XS spectrophotometer (BioTek Instruments, Winsookie, VT).
A 5-bromo-2′-deoxyuridine (BrdU) incorporation assay was used to examine the cells in S phase (actively proliferating cell population). Cells were plated in differentiation media. BrdU (30 μm; Sigma) was added to the cells at indicated times and incubated for 15 min. Cells were trypsinized, washed with PBS, and fixed by adding 70% ethanol. Cells were treated with 1 n HCl/0.5% Triton X-100 to denature DNA, washed with 0.1 m Tris (pH 8), stained using anti-BrdU-fluorescein isothiocyanate-conjugated antibody and analyzed using fluorescence-activated cell sorter (FACS).
FACS analyses
The cells were trypsinized after treatment/differentiation and single-cell suspensions were analyzed using FACS. Green fluorescent protein(+) cells representing CYP11A1 promoter activation were sorted using MoFlo cell sorter (DakoCytomation), with green fluorescent protein(−) cells as control. For BrdU cell cycle analyses, cells were analyzed using FACS-Calibur flow cytometer (BD Biosciences). FACS data were analyzed using CellQuest software (BD Biosciences).
Gene expression analyses
Total RNA was isolated using Trizol (Invitrogen) and treated with ribonuclease-free deoxyribonuclease (Promega, Madison, WI). Two micrograms of total RNA were reverse transcribed using SuperScriptII reverse transcriptase (Invitrogen) and oligo deoxythymidine (Promega). PCR was performed using standard protocols with equal volumes of cDNA template and IQ-SYBR Supermix (Bio-Rad) using Bio-Rad iCycler-iQ. 18S ribosomal RNA was used as control to calculate relative expression. The amplified PCR products were separated on 2% agarose gels and visualized. The primer sequences are available upon request.
Immunofluorescent staining
Cells were fixed in 10% neutral buffered formalin, permeabilized with PBS containing 0.1%Triton X-100, and blocked with 1% BSA. The cells were immunostained using primary antibodies: rabbit antimouse 3β-HSD (Abcam, Cambridge, MA), rabbit antirat CYP11A1 (Chemicon International, Temecula, CA), and mouse monoclonal antimouse neuron specific βIII-tubulin (Abcam) and secondary antibodies: fluorescein antirabbit IgG (Vector Laboratories, Burlingame, CA) or antimouse IgG (Vector Laboratories). Cells were stained with 4′,6′-diamino-2-phenylindole solution and images were captured using an Axioskop fluorescent microscope (Carl Zeiss, New York, NY).
Embryoid body formation and differentiation
The cells were resuspended in ES cell media without LIF and β-mercaptoethanol. Drops (20 μl) containing 500 cells were placed on the lid of a petri dish and the lid was inverted over the bottom of the dish. On d 2, embryoid bodies (EB) were transferred to a 0.15% agar-coated dish and cultured further. The 4−/4+ protocol was used for neuronal differentiation (32) with 5×10−7 M RA treatment on d 4. After 8 d of culture, EB were plated onto 0.1% gelatin-coated plates, allowed to attach and grow for 2–4 more days, and treated with 250 μm cAMP where indicated.
Chromatin immunoprecipitation
Bio-ChIP assays were performed with 5 × 107 Bio-ES cells or Bio-SF-1-ES cells as described previously (33), using streptavidin beads (Invitrogen Dynabeads MyOne-Streptavidin T1). The ChIP DNA samples were cleaned using ribonuclease-A and proteinase-K, extracted with phenol-chloroform, and dissolved in water. Equal amounts of the ChIP DNA were used as template for ChIP PCR.
Microarray studies and data analysis
The Illumina (San Diego, CA) gene expression array WG-6 was used for the microarray studies. RNA samples for all cell/treatment [ES cells, SF-1-ES cells, SF-1-ES cells differentiated in LIF(−) medium and differentiated SF-1-ES cells +cAMP] were from duplicate experiments. Differentially expressed genes (DEG) among these samples were used to create a heat map. Gene clusters with significant expression change were analyzed using DAVID software (http://niaid.abcc.ncifcrf.gov/) to generate gene ontology reports.
Results
Induction of steroidogenesis in ES cells expressing SF-1
In earlier studies, ES cells stably expressing SF-1 were shown to produce progesterone after treatment with 8-bromoadenosine-cAMP, all-trans-RA, and substrate 20α-hydroxycholesterol because these cells do not have the capacity to process de novo cholesterol (29). Initially we established a similar cell system by stably transfecting R1 ES cells with the cytomegalovirus promoter-driven SF-1 gene. The ES cells were grown without a feeder cell layer, which allowed isolation of ES cell RNA without contamination by feeder cell RNA. SF-1-ES cells cultured in LIF-containing media exhibited a different morphology compared with the native ES cells (Fig. 1A). The SF-1-ES cell colonies appeared flat, and individual cells could be discerned in some of the colonies. Similar morphological changes have also been observed upon culture of the SF-1-expressing ES cells on feeder cells (29).
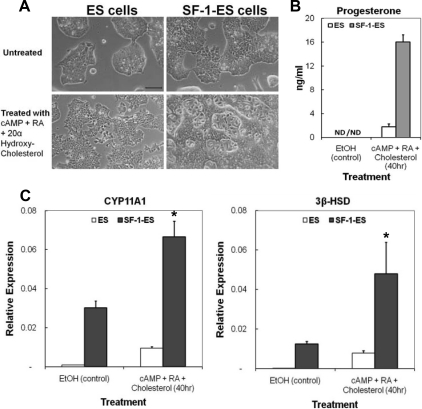
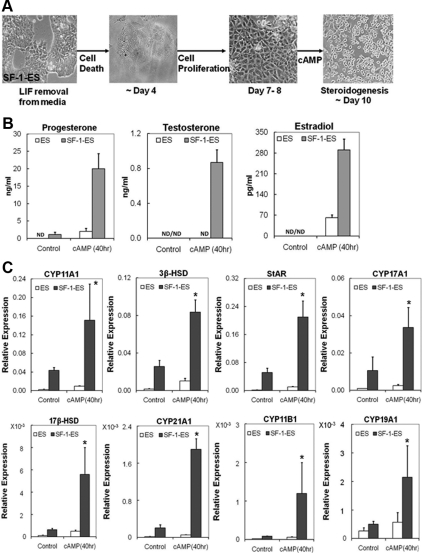
Fig. 1.
Characterization of SF-1-ES cells. A, Morphological changes seen in the ES cells after stable transfection with SF-1 to produce SF-1-ES cells and after treatment for steroidogenic induction. B, Progesterone production by ES cells and SF-1-ES cells after induction of steroidogenesis for 40 h. The results represent averages of three or more independent experiments performed at least in triplicate. Similar results were obtained when these experiments were repeated with three different SF-1-ES cell clones. C, Real-time RT-PCR of CYP11A1 and 3β-HSD mRNAs. Relative mRNA expression levels were calibrated to 18S RNA. Values are means ± sd (n = 3). *, P ≤ 0.05. ND, Not detectable.
Upon steroidogenic treatment with cAMP, RA, and cholesterol, ES cells and SF-1-ES cells showed different morphological changes. Although the native ES cells showed somewhat uniform morphology upon differentiation, SF-1-ES cells formed colonies with many cells migrating out of the colonies and rounding (Fig. 1A). The nonuniform population of SF-1-ES cells after treatment suggested that the SF-1-ES cells might be a mixed population with a subpopulation of steroidogenic cells.
SF-1-ES cells treated for 40 h produced about 16 ng/ml progesterone, whereas progesterone levels were less than 2 ng/ml in native ES cell media (Fig. 1B), indicating that progesterone production was induced 8-fold in SF-1-ES cells relative to the native ES cells.
CYP11A1 is an enzyme that converts cholesterol into pregnenolone, which is further converted into progesterone by the enzyme 3β-HSD. RT-PCR was performed using RNA collected from the ES and SF-1-ES cells to determine the effect of steroidogenic treatment on the expression of these enzyme genes. Expression of both CYP11A1 and 3β-HSD mRNA were detectable in unstimulated SF-1-ES cells and were induced 2- to 5-fold after treatment (Fig. 1C). Of note, the control ES cells also showed low but detectable expression of both the genes after treatment. Cells were also analyzed for induction of other enzymes in the steroidogenic pathway, such as CYP17A1, 17β-hydroxysteroid dehydrogenase (17β-HSD), and CYP21A1. However, transcripts encoding these enzymes were not detected (data not shown). Additionally, corticosterone, testosterone, or estradiol was not detected in the cell medium (data not shown).
Characterization of the steroidogenic SF-1-ES cell population
EGFP expression was used to identify cell populations induced into the steroidogenic pathway. Cell lines were created by transfecting a construct containing the EGFP gene driven by 1.5 kb of the CYP11A1 promoter into the SF-1-ES cells and native ES cells (control cells). Several of the SF-1-ES-CYP11A1-EGFP lines showed EGFP expression, indicating some basal activity of the CYP11A1 promoter (Fig. 2A, top right panel). After treatment with cAMP, RA, and cholesterol, the EGFP(+) population displayed more fluorescence (Fig. 2A, bottom right panel) and increased levels of CYP11A1 mRNA in comparison with the EGFP(−) population (data not shown), confirming that the EGFP signal reflected activation of the CYP11A1 promoter.
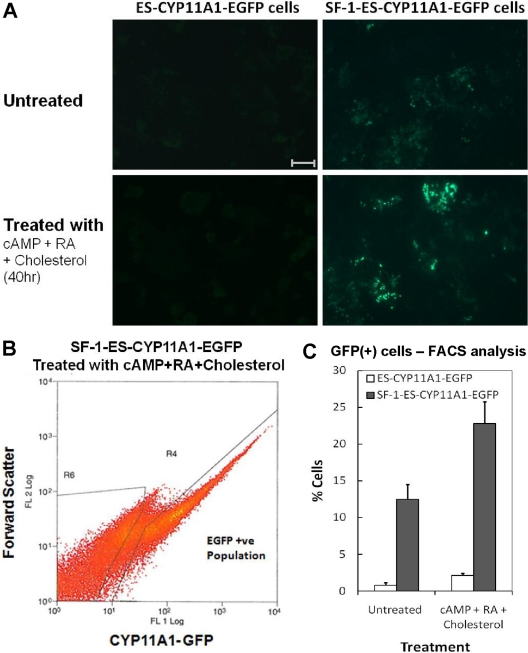
Fig. 2.
Quantitation of SF-1-ES cells expressing CYP11A1. A, EGFP fluorescence reflects CYP11A1 promoter activity in SF-1-ES cells before and after treatment with cAMP, RA, and cholesterol. B, FACS sorting of SF-1-ES-CYP11A1-EGFP (+) cells after treatment with cAMP, RA, and cholesterol. C, Quantitation of FACS analysis of SF-1-ES-CYP11A1-EGFP (+) cells. Bar, 100 μm.
FACS analysis was used to quantitate the fraction of EGFP-positive cells within the SF-1-ES-CYP11A1-EGFP population (Fig. 2, B and C). Under basal conditions, about 12% of the SF-1-ES-CYP11A1-EGFP cells were EGFP(+), whereas about 23% of the cells were EGFP(+) after treatment with cAMP, RA, and cholesterol. In contrast, the control cells showed minimal basal EGFP expression and less than 3% of the population was EGFP(+) after treatment with cAMP, RA, and cholesterol. These results indicate that upon stable transfection into ES cells, SF-1 induces CYP11A1 without additional treatment and only a subpopulation of SF-1-ES cells undergoes steroidogenic induction. Because CYP11A1 controls the rate-limiting step of the steroidogenic pathway, its expression in the ES cells is a reliable indicator of a subpopulation of ES cells being primed toward the steroidogenic differentiation path.
ES cells expressing SF-1 preferentially differentiate into steroidogenic lineage
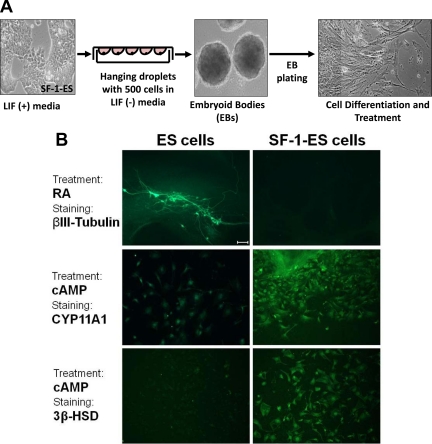
A traditional EB method was used to further assess the differentiation potential of SF-1-ES cells (32) (Fig. 3A). ES cell differentiation through EB is known to allow differentiation into multiple lineages, starting with all three germ layers (ectoderm, mesoderm, and endoderm), thus emulating the in vivo pathways that occur in early embryonic development. Marked morphological differences were observed in the differentiated ES cells compared with the differentiated SF-1-ES cells. After treatment of the EBs with RA, the native ES cells showed robust neuritic outgrowth, whereas very few neuritic structures were seen in EBs derived from SF-1-ES cells. Consistent with these morphological features, EBs derived from native ES cells showed robust neuron specific βIII-tubulin staining, indicating neuronal differentiation, but SF-1-ES cells showed little or no βIII-tubulin staining (Fig. 3B). After treatment with cAMP, most of the SF-1-ES cell EBs differentiated into cells with an epithelial-like morphology. Although very few of the native ES cells stained positively with antibodies against CYP11A1 or 3β-HSD, many SF-1-ES cells showed staining for these steroidogenic markers (Fig. 3B). EBs from SF-1-ES cells also showed increased mesendodermal gene expression (Kdr and Foxa2, data not shown), the lineage that ultimately gives rise to steroidogenic tissues. In parallel experiments, reduced differentiation into cardiomyocytes was observed in the SF-1-ES cell EBs when compared with the ES cell EBs (data not shown). These results demonstrate that SF-1 not only induces the steroidogenic program in ES cells but may also restrict the ES cells from differentiating into other lineages, such as the neuronal lineage.
Fig. 3.
Differentiation of SF-1-ES cells after the formation of EBs. A, Schematic illustration of the protocol for differentiation of cells through EB formation. B, ES cells and SF-1-ES cells were differentiated as EBs and then treated with RA for neuronal differentiation or with cAMP and stained with neuronal (βIII-tubulin) or with steroidogenic markers (CYP11A1; 3β-HSD). Bar, 100 μm.
LIF withdrawal is sufficient to induce steroidogenic lineage differentiation in SF-1-ES cells
In an effort to develop a simplified protocol for ES cell differentiation toward the steroidogenic lineage, we tested different culture conditions that allowed differentiation. These conditions included the presence or absence of RA, cAMP, cholesterol, and LIF for different periods of time, followed by analysis of progesterone production (Fig. 4). In the media containing LIF, cAMP treatment for 7 d caused SF-1-ES cells to differentiate into a morphologically nonuniform population. Although many colonies were observed in the culture, some SF-1-ES cells migrated out of the colonies. cAMP treatment of SF-1-ES cells induced detectable progesterone production, whereas the native ES cells showed no steroid production (note text in image). These results indicated that in the presence of SF-1, long-term exposure to cAMP was capable of differentiating ES cells into steroid producing cells, even in the absence of exogenous cholesterol as a substrate. The persistence of SF-1-ES cell colony structures suggested that the cells had not undergone complete differentiation.
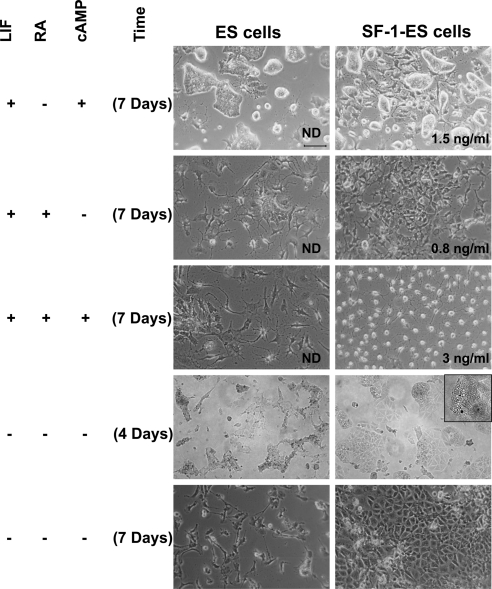
Fig. 4.
Effects of different treatment protocols on the differentiation of SF-1-ES cells. ES cells and SF-1-ES cells were differentiated in modified culture media as indicated at the left side of the panel. Progesterone production was measured in case of the cells differentiated in presence of LIF and absence of an external source of cholesterol. The amount of progesterone produced is indicated in the bottom right of the corresponding figures. Progesterone production in the absence of LIF is described in Fig. 5. ND, Not detected.
Long-term treatment with RA for 7 d in the presence of LIF caused the native ES cells to differentiate into a heterogeneous population with a few colonies and some cells resembling neuronal cells. SF-1-ES cells, on the other hand, showed more uniform differentiation with few cells showing neuronal-like and more of the cells showing epithelial-like morphology. These cells also produced detectable levels of progesterone, whereas the native ES cells did not (Fig. 4).
Upon treatment with cAMP and RA for 7 d in the presence of LIF, more ES cells had a neuronal morphology. However, the SF-1-ES cells became round, which has also been seen in other cell lines upon steroidogenic induction (34–36). These cells showed increased progesterone production compared with the individual treatments.
Differentiation was also induced by the removal of LIF. By d 4, the ES cells in LIF(−) media differentiated into a heterogeneous population, with most cells undergoing apoptosis (Fig. 4). Many of the SF-1-ES cells also underwent apoptosis. However, by 4 d most of the surviving SF-1-ES cells showed an angular epithelial-like morphology and droplet accumulation (shown in higher magnification in Fig. 4, insert). By d 7, the SF-1-ES cells became a more homogeneous population with epithelial-like morphology, whereas the native ES cells showed progressive apoptosis and few cells proliferated after 7 d.
SF-1-ES cells differentiated in LIF(−) media for 7 d were also treated with cAMP for 3–4 d. The overall differentiation protocol is depicted schematically in Fig. 5A. This produced a 6-fold higher amount of progesterone than any of the previous differentiation protocols (Fig. 5B). In this protocol, the cells also showed significant staining for CYP11A1 and 3β-HSD compared with the ES cells along with characteristic cell rounding (data not shown).
Fig. 5.
Expression of steroidogenic pathway genes and steroids in SF-1-ES cells differentiated in the absence of LIF. In presence of SF-1, ES cells differentiate in LIF(−) media to form a proliferating cell population capable of effective steroidogenesis upon cAMP induction. A, Schematic illustration of the protocol for differentiation of SF-1-ES cells into a steroidogenic cell population. B, Steroidogenesis in the differentiated SF-1-ES cells after cAMP treatment as assessed by progesterone, testosterone, and estradiol production. C, Real-time RT-PCR analysis characterizing steroidogenic pathway gene induction in differentiated SF-1-ES cells after cAMP treatment. Relative mRNA expression levels were calibrated to 18S RNA. Values are means ± sd (n = 3). *, P ≤ 0.05. ND, Not detectable.
LIF removal, combined with cAMP treatment, induces multiple steroidogenic pathways in SF-1-ES cells
The SF-1-ES cells were allowed to differentiate beyond d 7 in LIF(−) media and then treated with cAMP at different time intervals. With cAMP treatment for 4 d starting on d 8 of differentiation in LIF(−) culture media, the cells were found to produce testosterone and estradiol on d 12 (Fig. 5B). CYP17A1, 17β-HSD, CYP21A1, CYP11B1, and CYP19A1 were also induced in the SF-1-ES cells (Fig. 5C). This differentiation protocol therefore advanced the SF-1-ES cells further down the steroidogenic lineage in comparison with the protocols previously described. Increased expression of the steroidogenic enzymes shows that the cells have the capacity to process multiple steroid hormone intermediates and produce terminal pathway steroids (testosterone, estradiol). These steroids were produced without the addition of exogenous substrate cholesterol, thus allowing cells to use de novo cholesterol to produce multiple steroids.
SF-1 affects ES cell proliferation in growing and differentiating cell populations
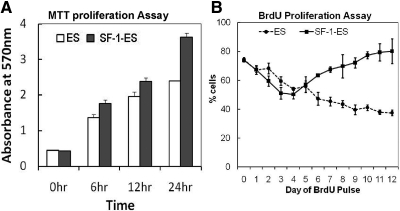
In LIF-containing media, SF-1-ES cells were observed to have a higher doubling rate than the native ES cells, and this observation was assessed further in MTT proliferation assays. Beginning with an equal number of cells, SF-1-ES cells showed a 1.5-fold higher proliferation compared with ES cells at 24 h in LIF(+) media (Fig. 6A). During differentiation in LIF(−) medium, the ES cells and SF-1-ES cells showed different cell death and proliferation patterns. In the BrdU cell proliferation assay, the native ES cells showed a constant decrease in cell proliferation (Fig. 6B) upon differentiation in LIF(−) media. By comparison, SF-1-ES cells showed a decrease in proliferation during the first 4 d of differentiation, but the number of cells incorporating BrdU increased thereafter. After about 10 d of differentiation in LIF(−) media, BrdU incorporation in SF-1-ES cells had recovered almost to the level seen before LIF removal. These results indicate that after the initial crisis after LIF removal, differentiated SF-1-ES cells recover proliferative capacity.
Fig. 6.
Effect of SF-1 expression on proliferation of ES cells in the presence and absence of LIF. A, Proliferation of ES cells and SF-1-ES cells grown in media containing LIF, as analyzed by the MTT assay. B, Proliferation of native ES cells and SF-1-ES cells during differentiation in LIF(−) culture media as analyzed by BrdU incorporation.
SF-1 binds directly to select target genes in ES cells during steroidogenic induction
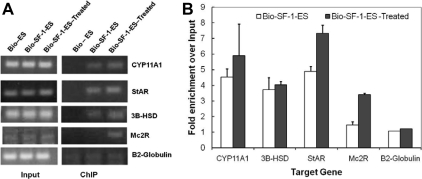
A Bio-ChIP assay was used to assess SF-1 binding to candidate target genes. Cells expressing biotin ligase (BirA) and biotin-tagged SF-1 (Bio-SF-1-ES cells) were shown to produce biotinylated SF-1 (Bio-SF-1) protein that could be isolated from cell lysates with streptavidin beads and detected by Western blot analysis (data not shown). ES cells expressing only BirA (Bio-ES cells) were used as a control. ChIP assays were performed using Bio-ES cells and Bio-SF-1-ES cells without treatment or after steroidogenic induction with cAMP, RA, and cholesterol. SF-1 binding to known control regions of target genes was analyzed (Fig. 7A) and the results were represented as fold enrichment relative to input (Fig. 7B). The ChIP assays confirmed Bio-SF-1 binding to the steroidogenic acute regulatory protein, CYP11A1, and 3B-HSD promoter regions. SF-1 binding to melanocortin 2 receptor promoter was detected only when the cells were treated with cAMP, RA, and cholesterol.
Fig. 7.
SF-1 binds the control regions of steroidogenic target genes in ES cells. A, ChIP-PCR analysis of known SF-1 target regions shows selective enrichment of SF-1 bound DNA elements in SF-1-ES cells compared with the ES cells. B, Quantitation of SF-1 binding to target sequences after treatment to induce steroidogenesis in SF-1-ES cells.
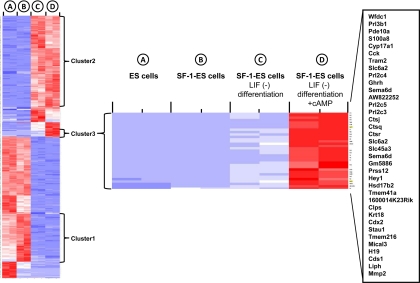
Microarray analysis of SF-1 differentiated ES cells
Microarray analysis was performed using RNA collected from ES cells and from undifferentiated and differentiated SF-1-ES cells. The results corroborated the expression pattern of select genes measured by semiquanititative RT-PCR (data not shown). Among the genes with significant changes in expression, several clusters were identified (Fig. 8). Of particular interest were genes up-regulated in SF-1-ES cells compared with native ES cells (cluster 1), genes up-regulated in SF-1-ES cells after differentiation (cluster 2), and genes up-regulated only in differentiated SF-1-ES cells treated with cAMP (cluster 3). These gene clusters were analyzed using DAVID software (http://niaid.abcc.ncifcrf.gov/) and showed enrichment of genes that belonged to various gene ontology categories. Cluster 1included genes related to cell growth (e.g. CDC20 and MCM5) and stem cell and mesodermal cell differentiation (e.g. TCF15 and WNT3A). Many of these genes were down-regulated upon differentiation of the SF-1-ES cells, which included Oct4 and Nanog. Cluster 2 included genes involved in apoptosis (e.g. BCL10, Caspase6, and Caspase8), angiogenesis (e.g. VEGF-A, VEGFR-1, and NPR1), and mesodermal lineage and tissue (heart and muscle) development (e.g. FHL2, CXADR, PITX2, and CD164). Cluster 3, the steroidogenic cell population, included genes involved in hormone /steroid biosynthetic processes (e.g. HSD17b2 and CYP17a1). The gene ontology report for this cluster is provided in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Fig. 8.
Microarray gene expression profiles in SF-1-ES cells and differentiated SF-1-ES cells. The figure shows heat map for differentially expressed genes among the samples: ES cells, SF-1-ES cells, SF-1-ES cells differentiated in LIF(−) medium and differentiated SF-1-ES cells + cAMP. With the microarray probe signals, principal component analysis was performed to assess sample variability. The search for genes varying among the conditions was performed with the bioconductor package limma to construct an F-like test. The moderated F-statistic P values derived above were further adjusted for multiple testing by Benjamini and Hochberg's method to control false discovery rate (FDR). The FDR cutoff of less than 0.1% was used to obtain the list of DEG among the conditions. Hierarchical clustering analysis and a heat map were generated after the signals of the DEG were standardized with mean = 0 and sd = 1. Cluster 1: SF-1-ES cells compared with native ES cells; cluster 2: genes up-regulated in SF-1-ES cells after differentiation; cluster 3: genes up-regulated only in differentiated SF-1-ES cells treated with cAMP (steroidogenic cell population). The heat diagram represents expression of individual genes relative to the mean of the four different cell/treatment samples. Blue indicates down-regulation and red indicates up-regulation of a gene.
Discussion
SF-1 plays a pivotal role in the establishment and development of the steroidogenic lineages. The adrenal glands and the gonads fail to develop in SF-1 null mice and the progenitors of the adrenogonadal tissue undergo apoptosis during early embryonic development (5, 37, 38). Thus, SF-1 plays a role in progenitor cell proliferation and survival as well as acting directly on multiple steroidogenic target genes (24). Based on these features, SF-1 has been characterized as a master regulator of steroidogenesis. However, a challenge with loss of function studies, including tissue-specific, Cre-mediated deletion of SF-1 is that the tissues do not develop, thereby precluding functional analyses of SF-1 target genes. As an alternative, steroidogenic cell lines, such as Y1 adrenal cells (23) or MA-10 Leydig cells (7), as well as other cell lines transfected with SF-1 (24) have been used to identify target genes, signaling pathways and synergistic factors that act in concert with SF-1 (17). The SF-1 targets identified in these and other studies include many steroidogenic enzymes as well as other transcription factors that control the various biochemical steps required for steroidogenesis (1, 10, 39–42). However, these models remain somewhat limited because the cell lines are incompletely differentiated, with steroid synthesis limited to progesterone production.
Although transiently transfected SF-1 is capable of stimulating target gene promoter activity in differentiated cell lines such as human embryonic kidney 293 or TSA201 cells (43), stably expressed SF-1 is not capable of activating endogenous steroid synthesis (31, 44). These findings indicate that although the nuclear receptor subfamily 5, group A, nuclear receptors, SF-1 and LRH-1, can replace OCT-4 in the reprogramming of somatic cells (45), they are not sufficient to redirect differentiated cells into a steroidogenic pathway. An alternative approach has been to induce steroidogenic lineage development in pluripotential ES or mesenchymal stem cells (26–31, 46), analogous to approaches used for other tissue types (25). Crawford et al. (29) stably expressed SF-1 in murine ES cells. After treatment with RA and cAMP, these cells produced progesterone, albeit with a requirement for an exogenous membrane-permeable source of cholesterol. Other groups (31) found that SF-1 expression in ES cells was toxic, leading to cell death after removal of LIF. Consequently, SF-1 has been introduced into bone marrow-derived adult mesenchymal stem cells using stable transfection (31, 44) or using adenovirus mediated delivery (26–28). These studies show that SF-1 induces both murine and human mesenchymal stem cells to differentiate into a steroidogenic lineage, with further differentiation after treatment with cAMP. The murine lines differentiated largely into the glucocorticoid pathway (44), whereas the human lines exhibited features of both gonadal and adrenal lineages (28). Of note, other transcription factors involved in adrenal or gonadal development, including Wilms' tumor 1, dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1, C-terminal domain-2, PBX-1, and WNT4 failed to induce steroidogenesis, suggesting that this property is relatively unique to SF-1 (28).
Initially we replicated the findings by Crawford et al. (29) by stably transfecting murine ES cells with SF-1and inducing steroidogenesis with RA, cAMP, and cholesterol. These SF-1-ES cells showed morphological changes and induction of CYP11A1 and 3β-HSD and produced progesterone (Fig. 1), confirming that SF-1 induces the rate-limiting steps of the steroidogenic cascade. However, these cells did not produce other steroid products, nor did they show further steroidogenic pathway gene induction. Given the relatively limited steroidogenesis associated with this protocol, we developed a series of alternative approaches to enhance cellular differentiation. Broadly these strategies included: 1) cultures in the presence of LIF with variations in the order and length of treatment with RA and cAMP; 2) use of EB to allow differentiation to occur before plating and various treatments; and 3) removal of LIF to induce differentiation followed by various treatments. Although the LIF withdrawal protocol proved most effective for inducing steroidogenesis, each of these protocols provided insights into SF-1-mediated differentiation of ES cells.
The reagents of differentiation used here, RA and cAMP, are both known to play important roles in development and lineage establishment in vivo and in vitro. RA has been used to differentiate ES cells into multiple lineages such as cardiomyocytes (47), adipocytes (48), vascular muscle cells (49), and neuronal lineage cell types (50). The cAMP signaling pathway plays a critical role in the acute steroidogenic response of adrenal and gonadal cells upon tropic hormone stimulation (51) and is also known to be involved in SF-1-mediated expression of target genes including CYP11A1 (7) and INHA (17). In the presence of LIF, either RA or cAMP is capable of stimulating steroidogenesis, although the combination is more effective (Fig. 4), suggesting that multiple pathways must be activated, in addition to SF-1, for effective steroidogenesis. The EB experiments demonstrated that SF-1-ES cells are relatively resistant to neuronal differentiation after RA treatment (Fig. 3B), suggesting that SF-1 restricts some differentiation pathways and enhances the steroidogenic pathway. In contrast, cAMP treatment of plated EB induced an epithelial-like morphology and markedly enhanced the expression of steroidogenic enzyme genes. RNA from differentiated SF-1-ES cells derived from EB showed the up-regulation of mesendodermal marker genes (data not shown), suggesting that SF-1 may foster this differentiation pathway. During development in vivo, the urogenital mesoderm is known to give rise to the adrenogonadal primordium (38).
Removal of LIF from ES cells induces apoptotic cell death as well as P38/MAPK activation and differentiation of surviving cells along various lineages (52, 53). We hypothesized that LIF withdrawal in the presence of SF-1 expression might drive the ES cells farther down the steroidogenic pathway. As expected, LIF withdrawal initially resulted in massive cell death. However, after this initial crisis, a subpopulation of SF-1-expressing cells emerged and developed distinct morphologic features including droplet formation at d 3–4 after LIF withdrawal. The cells developed an epithelial-like morphology after 7–8 d of differentiation and growth. Surprisingly, these cells ultimately recovered proliferative capacity (Fig. 6). Others have reported that SF-1-expressing ES cells do not survive after LIF withdrawal (31). The reason for this difference is not clear but could be caused by relative levels of SF-1 expression or the culture conditions. It seems likely that the ES cells must undergo significant reprogramming after LIF withdrawal. This is reflected in the gene expression profiles and the recovery of proliferative potential. Although there is not much evidence that SF-1 is directly involved in cell proliferation or survival in mammals, SF-1 is thought to promote cell proliferation through the activation of cyclin D1 transcription during ovarian development in chick embryos (54). In our studies, the presence of SF-1 increased the cell doubling times during routine culture and was documented further in MTT and BrdU proliferation assays (Fig. 6). It is plausible that SF-1 plays distinct roles in cell proliferation and differentiation pathways, depending on the presence of other transcription factors and signaling pathways.
The combination of LIF withdrawal and prolonged treatment with cAMP resulted in the greatest amount of progesterone production (Fig. 5B). Moreover, SF-1-ES cells treated with this protocol were the most fully differentiated along the steroidogenic lineage as evidenced by up-regulation of multiple steroidogenic genes and the production of progesterone, testosterone, and estradiol (Fig. 5, B and C). Importantly, these cells were differentiated enough to be capable of using de novo cholesterol as a substrate for steroid production. In this differentiation protocol, exposure to cAMP plays an important role, affecting both the morphology and the extent of differentiation. cAMP is also known mediate the action of tropic hormones that stimulate steroidogenesis (e.g. ACTH, LH, FSH) and to influence SF-1 action on many target genes including CYP11A1, CYP11B1, CYP21, and STAR (55).
Microarray studies confirmed that each step in this protocol was associated with significant changes in gene expression profiles (Fig. 8). For example, Oct4 was up-regulated in SF-1-ES cells and was down-regulated upon differentiation of these cells. Consistent with these results, Oct4 has recently been shown to be up-regulated by SF-1 in ES cells (56). As expected, LIF removal causes major alterations in gene expression, up-regulating many pathways and down-regulating others. Of particular note, cAMP treatment after LIF withdrawal alters expression of a number of genes, constituting cluster 3 in Fig. 8. The up-regulated genes include several steroidogenic enzyme genes along with a variety of other signaling molecules and secreted proteins. Clearly more detailed analyses of these pathways will yield insight into how SF-1 directs differentiation.
Differentiation of the SF-1-ES cells using LIF withdrawal and cAMP treatment appears to favor a gonadal cell type lineage. The differentiated cells produced testosterone and estradiol with robust induction of CYP17A1 and 17β-HSD. RT-PCR results indicated up-regulation of the desert hedgehog receptor, Patched1, in the differentiated cells (data not shown), consistent with a fetal Leydig cell-like pathway (57, 58). Of note, LH receptors were not detected in either the microarrays or by RT-PCR (data not shown). In the developing testis, the fetal Leydig cells are known to arise from SF-1-positive somatic progenitor cells (59). Recent studies have suggested that the testicular interstitial progenitor cells may contain multiple cell types expressing different cell surface markers like v-maf musculoaponeurotic fibrosarcoma oncogene homolog B, c-maf proto-oncogene, and vascular cell adhesion protein 1, even before the establishment of the Leydig cell lineage or the testis cord architecture (60–62). In addition, vascular-mesenchymal signaling through Vegf and Pdgf (60) and Notch (62) may also influence interstitial cell development through cell-cell interactions. Ultimately it should be possible to characterize these, and other, pathways during various stages of SF-1 mediated in vitro differentiation. In addition to the interstitial cell pathway genes, other genes important for gonadal/testicular development and function were also up-regulated after SF-1-ES cell differentiation. For example, Sox17 is thought to be transcriptional activator in premeiotic and postmeiotic testicular germ cells (63). Bcl-w is a prosurvival factor of Sertoli cells and testicular germ cells (64), and coxsackie and adenovirus receptor is important in the establishment of Sertoli and germ cell adhesion (65). Although CYP21A1 and CYP11B1 were also expressed, corticosterone was not produced at detectable levels. These findings contrast with those of Yazawa et al. (44), who expressed SF-1 in mesenchymal stem cells or used a tetracycline-inducible system to activate SF-1 expression after differentiating ES cells along the mesenchymal pathway by culturing them on collagen IV-coated dishes (31). Under these conditions, the cells preferentially produce corticosterone. These comparisons are of great interest because they suggest that the further manipulation of differentiation conditions may allow the selection of specific steroidogenic lineages with characteristics of Leydig, granulosa, or various types of adrenocortical cells (reticularis, fasciculata, glomerlosa).
In summary, we demonstrate that ES cells can be directed along the gonadal cell steroidogenic lineage through expression of SF-1 and differentiation by LIF withdrawal and cAMP treatment. The differentiation protocol described here provides an effective in vitro method to study the molecular mechanisms of steroidogenic tissue development.
Supplementary Material
Acknowledgments
We thank Dr. Jeff Weiss for his advice throughout the work.
This work was supported by National Institutes of Health Grants P01 HD021921 and R01 HD044801, the Northwestern University Genomics Core, and Cancer Center Support Grant NCI CA060553.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Bio
- Biotin
- BirA
- Bio ligase gene
- BLRP
- Bio ligase recognition peptide
- BrdU
- 5-bromo-2′-deoxyuridine
- CFP
- cyan fluorescent protein
- ChIP
- chromatin immunoprecipitation
- DEG
- differentially expressed gene
- EB
- embryoid body
- EGFP
- enhanced green fluorescent protein
- ES
- embryonic stem
- FACS
- fluorescence-activated cell sorter
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- 17β-HSD
- 17β-hydroxysteroid dehydrogenase
- LIF
- leukemia inhibitory factor
- MTT
- dimethylthiazol diphenyltetrazolium bromide
- RA
- retinoic acid
- SF-1
- steroidogenic factor 1
- SF-1-ES
- incorporation of SF-1 into ES cells.
References
- 1. Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. 1993. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol 7:852–860 [DOI] [PubMed] [Google Scholar]
- 2. Morohashi K, Honda S, Inomata Y, Handa H, Omura T. 1992. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem 267:17913–17919 [PubMed] [Google Scholar]
- 3. Morohashi K, Zanger UM, Honda S, Hara M, Waterman MR, Omura T. 1993. Activation of CYP11A and CYP11B gene promoters by the steroidogenic cell-specific transcription factor, Ad4BP. Mol Endocrinol 7:1196–1204 [DOI] [PubMed] [Google Scholar]
- 4. Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. 1995. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol 9:478–486 [DOI] [PubMed] [Google Scholar]
- 5. Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J. 1995. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu MC, Hsu NC, Pai CI, Wang CK, Chung Bc. 2001. Functions of the upstream and proximal steroidogenic factor 1 (SF-1)-binding sites in the CYP11A1 promoter in basal transcription and hormonal response. Mol Endocrinol 15:812–818 [DOI] [PubMed] [Google Scholar]
- 7. Chau YM, Crawford PA, Woodson KG, Polish JA, Olson LM, Sadovsky Y. 1997. Role of steroidogenic-factor 1 in basal and 3′,5′-cyclic adenosine monophosphate-mediated regulation of cytochrome P450 side-chain cleavage enzyme in the mouse. Biol Reprod 57:765–771 [DOI] [PubMed] [Google Scholar]
- 8. Huang Y, Hu M, Hsu N, Wang CL, Chung B. 2001. Action of hormone responsive sequence in 2.3 kb promoter of CYP11A1. Mol Cell Endocrinol 175:205–210 [DOI] [PubMed] [Google Scholar]
- 9. Sewer MB, Waterman MR. 2002. Transcriptional complexes at the CYP17 CRS. Endocr Res 28:551–558 [DOI] [PubMed] [Google Scholar]
- 10. Bakke M, Lund J. 1995. Mutually exclusive interactions of two nuclear orphan receptors determine activity of a cyclic adenosine 3′,5′-monophosphate-responsive sequence in the bovine CYP17 gene. Mol Endocrinol 9:327–339 [DOI] [PubMed] [Google Scholar]
- 11. Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. 1996. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 273:1219–1221 [DOI] [PubMed] [Google Scholar]
- 12. Leers-Sucheta S, Morohashi K, Mason JI, Melner MH. 1997. Synergistic activation of the human type II 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase promoter by the transcription factor steroidogenic factor-1/adrenal 4-binding protein and phorbol ester. J Biol Chem 272:7960–7967 [DOI] [PubMed] [Google Scholar]
- 13. Kawabe K, Shikayama T, Tsuboi H, Oka S, Oba K, Yanase T, Nawata H, Morohashi K. 1999. Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol 13:1267–1284 [DOI] [PubMed] [Google Scholar]
- 14. De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P. 1998. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol Cell Biol 18:6653–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sekido R, Lovell-Badge R. 2008. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453:930–934 [DOI] [PubMed] [Google Scholar]
- 16. de Santa Barbara P, Méjean C, Moniot B, Malclès MH, Berta P, Boizet-Bonhoure B. 2001. Steroidogenic factor-1 contributes to the cyclic-adenosine monophosphate down-regulation of human SRY gene expression. Biol Reprod 64:775–783 [DOI] [PubMed] [Google Scholar]
- 17. Ito M, Park Y, Weck J, Mayo KE, Jameson JL. 2000. Synergistic activation of the inhibin α-promoter by steroidogenic factor-1 and cyclic adenosine 3′,5′-monophosphate. Mol Endocrinol 14:66–81 [DOI] [PubMed] [Google Scholar]
- 18. Luo X, Ikeda Y, Parker KL. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- 19. Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, Morohashi K, Li E. 1995. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn 204:22–29 [DOI] [PubMed] [Google Scholar]
- 20. Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. 2001. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development 128:147–154 [DOI] [PubMed] [Google Scholar]
- 21. Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL. 2004. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol 18:1610–1619 [DOI] [PubMed] [Google Scholar]
- 22. Zhao L, Kim KW, Ikeda Y, Anderson KK, Beck L, Chase S, Tobet SA, Parker KL. 2008. Central nervous system-specific knockout of steroidogenic factor 1 results in increased anxiety-like behavior. Mol Endocrinol 22:1403–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schimmer BP, Cordova M, Cheng H, Tsao A, Goryachev AB, Schimmer AD, Morris Q. 2006. Global profiles of gene expression induced by adrenocorticotropin in Y1 mouse adrenal cells. Endocrinology 147:2357–2367 [DOI] [PubMed] [Google Scholar]
- 24. Schimmer BP, White PC. 2010. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol 24:1322–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murry CE, Keller G. 2008. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132:661–680 [DOI] [PubMed] [Google Scholar]
- 26. Gondo S, Okabe T, Tanaka T, Morinaga H, Nomura M, Takayanagi R, Nawata H, Yanase T. 2008. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology 149:4717–4725 [DOI] [PubMed] [Google Scholar]
- 27. Gondo S, Yanase T, Okabe T, Tanaka T, Morinaga H, Nomura M, Goto K, Nawata H. 2004. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells 9:1239–1247 [DOI] [PubMed] [Google Scholar]
- 28. Tanaka T, Gondo S, Okabe T, Ohe K, Shirohzu H, Morinaga H, Nomura M, Tani K, Takayanagi R, Nawata H, Yanase T. 2007. Steroidogenic factor 1/adrenal 4 binding protein transforms human bone marrow mesenchymal cells into steroidogenic cells. J Mol Endocrinol 39:343–350 [DOI] [PubMed] [Google Scholar]
- 29. Crawford PA, Sadovsky Y, Milbrandt J. 1997. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol 17:3997–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyamoto K, Yazawa T, Mizutani T, Imamichi Y, Kawabe SY, Kanno M, Matsumura T, Ju Y, Umezawa A. 2011. Stem cell differentiation into steroidogenic cell lineages by NR5A family. Mol Cell Endocrinol 336:123–126 [DOI] [PubMed] [Google Scholar]
- 31. Yazawa T, Kawabe S, Inaoka Y, Okada R, Mizutani T, Imamichi Y, Ju Y, Yamazaki Y, Usami Y, Kuribayashi M, Umezawa A, Miyamoto K. 2011. Differentiation of mesenchymal stem cells and embryonic stem cells into steroidogenic cells using steroidogenic factor-1 and liver receptor homolog-1. Mol Cell Endocrinol 336:127–132 [DOI] [PubMed] [Google Scholar]
- 32. Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. 1995. Embryonic stem cells express neuronal properties in vitro. Dev Biol 168:342–357 [DOI] [PubMed] [Google Scholar]
- 33. Kim J, Cantor AB, Orkin SH, Wang J. 2009. Use of in vivo biotinylation to study protein-protein and protein-DNA interactions in mouse embryonic stem cells. Nat Protoc 4:506–517 [DOI] [PubMed] [Google Scholar]
- 34. Lawrence TS, Ginzberg RD, Gilula NB, Beers WH. 1979. Hormonally induced cell shape changes in cultured rat ovarian granulosa cells. J Cell Biol 80:21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maizels ET, Cottom J, Jones JC, Hunzicker-Dunn M. 1998. Follicle stimulating hormone (FSH) activates the p38 mitogen-activated protein kinase pathway, inducing small heat shock protein phosphorylation and cell rounding in immature rat ovarian granulosa cells. Endocrinology 139:3353–3356 [DOI] [PubMed] [Google Scholar]
- 36. Whitehouse BJ, Gyles SL, Squires PE, Sayed SB, Burns CJ, Persaud SJ, Jones PM. 2002. Interdependence of steroidogenesis and shape changes in Y1 adrenocortical cells: studies with inhibitors of phosphoprotein phosphatases. J Endocrinol 172:583–593 [DOI] [PubMed] [Google Scholar]
- 37. Luo X, Ikeda Y, Lala DS, Baity LA, Meade JC, Parker KL. 1995. A cell-specific nuclear receptor plays essential roles in adrenal and gonadal development. Endocr Res 21:517–524 [DOI] [PubMed] [Google Scholar]
- 38. Ikeda Y, Shen WH, Ingraham HA, Parker KL. 1994. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8:654–662 [DOI] [PubMed] [Google Scholar]
- 39. Clemens JW, Lala DS, Parker KL, Richards JS. 1994. Steroidogenic factor-1 binding and transcriptional activity of the cholesterol side-chain cleavage promoter in rat granulosa cells. Endocrinology 134:1499–1508 [DOI] [PubMed] [Google Scholar]
- 40. Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T. 1993. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem 268:7494–7502 [PubMed] [Google Scholar]
- 41. Lynch JP, Lala DS, Peluso JJ, Luo W, Parker KL, White BA. 1993. Steroidogenic factor 1, an orphan nuclear receptor, regulates the expression of the rat aromatase gene in gonadal tissues. Mol Endocrinol 7:776–786 [DOI] [PubMed] [Google Scholar]
- 42. Michael MD, Kilgore MW, Morohashi K, Simpson ER. 1995. Ad4BP/SF-1 regulates cyclic AMP-induced transcription from the proximal promoter (PII) of the human aromatase P450 (CYP19) gene in the ovary. J Biol Chem 270:13561–13566 [DOI] [PubMed] [Google Scholar]
- 43. Ito M, Yu R, Jameson JL. 1997. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol 17:1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Umezawa A, Miyamoto K. 2006. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology 147:4104–4111 [DOI] [PubMed] [Google Scholar]
- 45. Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, Orlov YL, Huss M, Yang L, Lufkin T, Lim B, Ng HH. 2010. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell 6:167–174 [DOI] [PubMed] [Google Scholar]
- 46. Yanase T, Gondo S, Okabe T, Tanaka T, Shirohzu H, Fan W, Oba K, Morinaga H, Nomura M, Ohe K, Nawata H. 2006. Differentiation and regeneration of adrenal tissues: an initial step toward regeneration therapy for steroid insufficiency. Endocr J 53:449–459 [DOI] [PubMed] [Google Scholar]
- 47. Wobus AM, Kaomei G, Shan J, Wellner MC, Rohwedel J, Ji G, Fleischmann B, Katus HA, Hescheler J, Franz WM. 1997. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol 29:1525–1539 [DOI] [PubMed] [Google Scholar]
- 48. Dani C, Smith AG, Dessolin S, Leroy P, Staccini L, Villageois P, Darimont C, Ailhaud G. 1997. Differentiation of embryonic stem cells into adipocytes in vitro. J Cell Sci 110(Pt 11):1279–1285 [DOI] [PubMed] [Google Scholar]
- 49. Drab M, Haller H, Bychkov R, Erdmann B, Lindschau C, Haase H, Morano I, Luft FC, Wobus AM. 1997. From totipotent embryonic stem cells to spontaneously contracting smooth muscle cells: a retinoic acid and db-cAMP in vitro differentiation model. FASEB J 11:905–915 [DOI] [PubMed] [Google Scholar]
- 50. Gajović S, St. Onge L, Yokota Y, Gruss P. 1997. Retinoic acid mediates Pax6 expression during in vitro differentiation of embryonic stem cells. Differentiation 62:187–192 [DOI] [PubMed] [Google Scholar]
- 51. Epstein LF, Orme-Johnson NR. 1991. Regulation of steroid hormone biosynthesis. Identification of precursors of a phosphoprotein targeted to the mitochondrion in stimulated rat adrenal cortex cells. J Biol Chem 266:19739–19745 [PubMed] [Google Scholar]
- 52. He Z, Li JJ, Zhen CH, Feng LY, Ding XY. 2006. Effect of leukemia inhibitory factor on embryonic stem cell differentiation: implications for supporting neuronal differentiation. Acta Pharmacol Sin 27:80–90 [DOI] [PubMed] [Google Scholar]
- 53. Duval D, Malaisé M, Reinhardt B, Kedinger C, Boeuf H. 2004. A p38 inhibitor allows to dissociate differentiation and apoptotic processes triggered upon LIF withdrawal in mouse embryonic stem cells. Cell Death Differ 11:331–341 [DOI] [PubMed] [Google Scholar]
- 54. Ishimaru Y, Komatsu T, Kasahara M, Katoh-Fukui Y, Ogawa H, Toyama Y, Maekawa M, Toshimori K, Chandraratna RA, Morohashi K, Yoshioka H. 2008. Mechanism of asymmetric ovarian development in chick embryos. Development 135:677–685 [DOI] [PubMed] [Google Scholar]
- 55. Sewer MB, Dammer EB, Jagarlapudi S. 2007. Transcriptional regulation of adrenocortical steroidogenic gene expression. Drug Metab Rev 39:371–388 [DOI] [PubMed] [Google Scholar]
- 56. Yang HM, Do HJ, Kim DK, Park JK, Chang WK, Chung HM, Choi SY, Kim JH. 2007. Transcriptional regulation of human Oct4 by steroidogenic factor-1. J Cell Biochem 101:1198–1209 [DOI] [PubMed] [Google Scholar]
- 57. Yao HH, Whoriskey W, Capel B. 2002. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16:1433–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park SY, Tong M, Jameson JL. 2007. Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult Leydig cell development. Endocrinology 148:3704–3710 [DOI] [PubMed] [Google Scholar]
- 59. Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HH. 2009. Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev Biol 329:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cool J, DeFalco TJ, Capel B. 2011. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci USA 108:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. DeFalco T, Takahashi S, Capel B. 2011. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol 352:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tang H, Brennan J, Karl J, Hamada Y, Raetzman L, Capel B. 2008. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development 135:3745–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kanai Y, Kanai-Azuma M, Noce T, Saido TC, Shiroishi T, Hayashi Y, Yazaki K. 1996. Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis. J Cell Biol 133:667–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yan W, Samson M, Jégou B, Toppari J. 2000. Bcl-w forms complexes with Bax and Bak, and elevated ratios of Bax/Bcl-w and Bak/Bcl-w correspond to spermatogonial and spermatocyte apoptosis in the testis. Mol Endocrinol 14:682–699 [DOI] [PubMed] [Google Scholar]
- 65. Wang CQ, Mruk DD, Lee WM, Cheng CY. 2007. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res 313:1373–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.