Abstract
Clinical immunolocalization has been attempted by others with an anti- Thomsen-Friedenreich antigen (TF-Ag) mAb which bound both alpha- and beta-linked TF-Ag. In this report, 124I labeled mAb JAA-F11 specific for alpha-linked TF-Ag showed higher tumor specificity in in vivo micro positron emission tomography (microPET) of the mouse mammary adenocarcinoma line, 4T1, showing no preferential uptake by the kidney. Labeled product remained localized in the tumor for at least 20 days. Glycan array analysis showed structural specificity of the antibody.
Keywords: immunolocalization, biodistribution, microPET, radiolabeled monoclonal antibody, JAA-F11, TF-Ag, breast carcinoma
Introduction
Thomsen-Friedenreich antigen (TF-Ag) is the mucin-type disaccharide Galactoseβ1–3N-acetylgalactosamine conjugated to proteins by an alpha O-serine or O-threonine linkage and is a tumor associated antigen which is found on human carcinomas of many types including those of the breast, colon, bladder and prostate. TF-Ag is a cell surface antigen, and cell surface carbohydrates and alterations in their expressions have been associated with malignant transformations (Dennis, 1992). TF-Ag is also expressed on the transplantable murine mammary cancer cell line 4T1. The murine monoclonal antibody (mAb) JAA-F11, an IgG3, is highly specific in its reaction with the alpha-linked TF-Ag which is expressed on many types of human carcinomas and their metastases (Rittenhouse-Diakun et al., 1998). Most of the monoclonal antibodies which have been produced to TF-Ag have been of the IgM isotype which is less useful in radiolocalization due to its large molecular weight. JAA-F11 has been shown to block early stages of metastasis, and has been shown to have slight but significant inhibitory effects on in vitro tumor cell growth. In recent in vivo studies, prolonged survival and a reduced number of lung metastases were seen in 4T1 tumor-bearing mice treated with mAb JAA-F11 (Heimburg et al., 2006).
Since the expression of TF-Ag has been proposed to be a tumor-marker and a clinical diagnostic tool (Takanami, 1999), we hypothesize that the mAb JAA-F11 to TF-Ag, when radiolabeled with the positron emitter 124I-Iodine, will improve detection of occult breast cancer metastatic tumors. JAA-F11 characterization using the structurally relevant saccharide, monosialo-GM1 ganglioside, showed a lack of reaction with JAA-F11, which is important because it shows that related carbohydrate structures on normal cells would not bind the JAA-F11 antibody. This point also differentiates JAA-F11 from the anti-TF-Ag antibody 170H.82 which previously underwent clinical trials only to be withdrawn (Longenecker et al., 1987; Dessureault et al., 1997). The 170H.82 antibody recognizes both the alpha- and beta-linked derivatives (Galβ1–3GalN Acα and Galβ1–3GalNAcβ) of TF-Ag and, therefore, could potentially interact with carbohydrate structures expressed on normal tissues such as asialo-GM1 (a natural killer cell marker), whereas JAA-F11 does not (Longenecker et al., 1987). No testing of the 170H.82 antibody in mice was reported, but the Longenecker group did perform in vivo tumor localization experiments with 2 other similar reagents which also bind both the TF-Ag-α and β derivatives: radiolabeled peanut lectin (PNA) (Shysh et al., 1985; Abdi et al., 1986) and radiolabeled mAb 155H.7 (Turner et al., 1988) which they reported to be similar to mAb 170H.82 (Longenecker et al., 1987). The PNA radiolocalized to the kidney both in mice (72 hours, kidney:tumor ratio of 1.3) (Shysh et al., 1985) and in humans (17 patients, only 1 in which PNA localized to a tumor) (Abdi et al., 1986). In fact in humans, the kidney localization was so dramatic, that in the discussion, the authors suggested that radiolabeled PNA might be used for renal tubular damage assessment, and found it to be concentrated in the renal tubular basement membrane (Abdi et al., 1986). In their mouse experiments with the 155H.7 antibody which has a specificity similar to 170H.82, the kidney to tumor ratio was 0.75, and the kidney was higher than any organ except the thyroid (pretreatment with cold iodine was not performed). These mouse studies were also of a limited, 72 hour, duration (Turner et al., 1988). To further understand the binding of our TF-Ag-α specific mAb JAA-F11, in vivo immunolocalization was performed in a system which would test antibody localization to a primary tumor in the mouse model and structural specificity analysis is presented herein using the printed glycan array of the Consortium for Functional Glycomics (http://www.functionalglycomics.org/static/consortium/). The glycan array tested the binding of the antibody with 200 different glycans to determine the chemical specificity of the reactivity of this mAb (Blixt et al., 2004).
Positron emission tomography (PET) is a well established modality that provides in vivo quantitative distribution of biomolecules. Iodine-124 is the only isotope of iodine which decays by positron emission with a half life of 4.2 days. This is ideal for the labeling of antibodies because of their long biological half life and also due to the ease of labeling. PET images can provide quantitative information of the biological processes over several weeks without sacrificing the animal. The biodistribution data obtained by using 124I-labeled antibodies can be useful in determining radiation dosimetry of the 131I-labeled antibodies for therapeutic use.
Materials and Methods
Monoclonal antibody
JAA-F11 is a monoclonal antibody to TF-Ag which was developed in our laboratory and is cultured in our laboratory in DMEM (Mediatech, Herndon, Va.) in 5% ultra low Ig fetal calf serum (GIBCO, Life Technologies, Grand Island, NY) with added L-glutamine, sodium pyruvate and non-essential amino acids (Mediatech, Herndon, Va.). JAA-F11 was partially purified using the ammonium sulfate precipitation technique. The supernatant was dialyzed against water and lyophilized. IgG was purified from other contaminants and collected using a Sephadex G-200 size exclusion column. Enzyme Immunoassay (EIA) was performed on the fractions using a TF-Ag-BSA coated microtiter plate, and reactive fractions were pooled, dialyzed, and lyophilized to make a final suspension of 1.2 mg/ml in 0.1 M Phosphate Buffered Saline (PBS).
Monoclonal antibody glycan analysis
JAA-F11 (produced in our laboratory) was analyzed for reactivity with 200 glycans by the Consortium for Functional Glycomics using the printed glycan array. The method used is described on the Consortium website and is detailed in Blixt (2004). Briefly, the printed array was incubated successively with the antibody, washed, and incubated with secondary antibody labeled with FITC. After washing, the image was read in a Perkin Elmer Microscanarray XL4000 and a TIFF file of the image was stored. Image analysis was performed using the Imagene V.6 image analysis software.
Radionuclide
124I was produced by the 124Te (p, n) 124I reaction (Sajjad et al., 2006). 124TeO2 (tellurium oxide) target was irradiated by 14.1 MeV protons at 24 μA current for about 4 hours in a cyclotron. The radioiodine was separated by the dry distillation method. The distilled 124I was trapped in 0.01 M NaOH. The 124I solution was analyzed on an HPLC to determine the chemical and radiochemical purity. The separation was performed using Lichrosorb RP18 analytical column (5 μm, 250 mm × 4.6 mm, Alltech). The mobile phase consisted of a mixture of 90% buffer (0.5 M potassium phosphate and 0.002 M tetrabutylammonium hydroxide, pH 7.0) and 10% acetonitrile. The flow rate was 1.0 ml/min and the UV detector was set at 225 nm wavelength.
Iodination chemistry
Two different iodination strategies were examined. The antibody was radiolabeled with 124I by using direct Iodogen method and indirect Bolton-Hunter method. The immunoreactivity of JAA-F11 was then determined using radioimmunoassay (RIA) and EIA described below. The Bolton-Hunter method was chosen for iodinating JAA-F11 based on the results obtained from these assays.
Iodogen method
JAA-F11 was labeled with 124I by using a variation of the original Iodogen method (Fraker, 1978). MAb JAA-F11 (1.2 mg/ml in PBS) was added to sodium [124I]-iodide. Iodogen coated beads (Pierce Chemical Co.) were added to the solution. The reagents were mixed in a reaction vial and incubated for 15 minutes at room temperature. [124I]-JAA-F11 was purified on an HPLC (Bio-Sil SEC-125, 300 × 7.8 mm, Bio-Rad). The mobile phase was 0.01 M PBS pH 7.4, the flow rate was 1 ml/minutes, and the UV detector was set at 254 nm wavelength.
Bolton-Hunter method
An adaptation of the Bolton-Hunter method was performed as previously described (Collingridge et al., 2002). Briefly, 124I was distilled and dried in a small reaction vial (0.3 ml, Wheaton, USA). A solution of NaI carrier (5 μl, 89.2 μM), HCl (14 μl, 100 mM), and sodium phosphate buffer (10 μl, 250 mM, pH 7.4) was added to 124I (14 μl, 370 MBq). The reaction was allowed to incubate at room temperature for 10 minutes. SHPP (N-Succinimidyl-3-[4-hydroxyphenyl]propionate in 1,4-dioxane, 2 μl, 3.8 mM) and Chloramine-T (10 μl, 22 mM) dissolved in sodium phosphate buffer (250 mM, pH 7.4) were added with mixing and the reaction was terminated immediately (~15 seconds) with a sodium phosphate buffered solution (250 mM, pH 7.4) of Na2S2O5 (10 μl, 63 mM). The reaction product was extracted with benzene/dimethylfluoride (100 μl, 40:1 v/v), dried with 30 mg Na2SO4 and transferred into another reaction vessel. The solvent was evaporated with a stream of nitrogen and JAA-F11 (10 μg/mouse, in PBS), mixed with sodium borate buffer (5 μl, pH 8.5, 100 mM), was added and incubated with [124I]-SHPP at 0°C for 1 hour. The labeled antibody was separated from low molecular weight reaction side products using Sephadex G-25. The radioiodinated antibody was injected into the animals within 2 hours of labeling.
Enzyme immunoassay
The immunoreactivity of the radiolabeled antibody was determined using EIA. EIA plates (MaxiSorp™, eBioscience) previously coated with TF-Ag-BSA conjugate in coating buffer (0.1 M Na2CO3, pH 7.6) at a concentration of 5 μg/mL were used for the assays. The coating solution was removed, 2% formaldehyde was added for 20 minutes, and the plate was washed 3 times with TBS. One hundred μl of different dilutions of radiolabeled JAA-F11 were added to the wells and incubated at 37°C for 1 hour. After washing 3 times, 100 μl of a 1:1000 dilution of anti-mouse IgG conjugated with alkaline phosphatase (Sigma) in 1%BSA-PBS buffer was added to each well for 1 hour at room temperature. After washing 3 times, 100 μl of para-Nitrophenyl Phosphate, 1 mg/ml in diethanolamine buffer was added to each well and the plate was read at 405 nm after 1 hour.
Cell cultures
The mouse mammary adenocarcinoma cell line, 4T1, was purchased from ATCC (# CRL-2539). The 4T1 cell line is an animal model for stage IV human breast cancer (Pulaski, 1998; Pulaski et al., 2000). The murine sarcoma cell line, CMS4, which expressed low levels of TF-Ag, was kindly provided by Dr. Hideho Okada (Department of Neurosurgery, University of Pittsburg, PA) (Tatsumi et al., 2003). Both the tumor cell lines were grown in RPMI 1640 medium supplemented with 10% FBS and adjusted to contain 2mM L-glutamine, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10mM HEPES, and 1.0mM sodium pyruvate.
Cell EIA
Whole cell EIA was performed to test the expression of TF-Ag on cancer cell lines. The cell lines tested were 4T1, CMS4, and Myeloma (ATCC Number: CRL-1580). Myeloma is the fusion partner for producing JAA-F11 hybridoma (Rittenhouse-Diakun et al., 1998), and was used as a TF-Ag negative control cell line. CMS4 was tested for use as an in vivo control. 4T1 and CMS4 cells were harvested by trypsinization and single-cell suspensions were made. The cells were diluted to obtain 1 × 106 cells per ml. Two hundred μl (2 ×105 cells) of the cell suspension was placed in 5 ml tubes in 5 replicates. Two hundred μl of 4% formaldehyde was added to each tube and allowed to stand for 20 minutes at room temperature to fix the cells. After centrifugation for 10 minutes at 1200 rpm, the supernatants were decanted carefully. Two hundred μl of PBS-Tween-1%BSA was added to each tube for storage. The fixed cells were placed at 4 °C overnight or up to 2 weeks. A peroxidase-linked immunoassay was performed in the tubes. Two hundred μl of 5 different JAA-F11 dilutions were added to respective tubes and incubated at 37°C for 2 hours. The tubes were washed 3 times by adding 3 ml wash buffer (1X PBS-Tween, no azide), centrifuging 10 minutes at 1200 rpm, and decanting the supernatant between each wash. Anti-mouse IgG (gamma chain specific) peroxidase conjugate was added to each tube, incubated for one hour at room temperature, and washed 3 times as before. Two hundred μl of o-phenylenediamine dihydrochloride substrate solution was added to each tube. After one hour, the reaction was stopped by adding 100 μl of stop solution (1 N H2SO4) to each tube followed by centrifugation for 10 minutes at 1200 rpm. From each tube, 200 μl of supernatant was transferred into respective wells in a microtiter plate and the plate was read at 490 nm.
Animals and tumor models
All animals in this study were housed and utilized in accordance with the Institutional Animal Care and Use Committees (IACUC) regulations in an Association for Assessment and Accreditation of Laboratory Animal Care International Accredited Facility. 4T1 and CMS4 cell lines were grown in female BALB/c mice, 8–10 weeks of age. 4T1 cells were implanted by injecting 1 × 106 cells subcutaneously under the right nipple, while 5 × 105 CMS4 cells were implanted subcutaneously in the left leg of the mice. Mice were injected with radiolabeled JAA-F11 by tail vein after day 10 to day 18 of tumor cell administration and were subjected to biodistribution and microPET imaging.
Study design
Each mouse was given a tail vein infusion of approximately 10 μg JAA-F11 labeled with approximately 3.7 MBq 124I in 0.1 ml PBS. All mice received 0.2 g/L Potassium Iodide water as a thyroid blocking regime 24 hours prior to receiving the radiotracer, and this treatment continued until the end of the study. Mice were divided in three groups. Group I (n=10) were control mice without any tumors. Group II (n=17) were mice which received 1 × 106 4T1 cells. Group III (n=3) were mice which received 1 × 106 4T1 as well as 5 × 105 CMS4 cells. Group I and group II mice received the above mentioned doses of labeled JAA-F11. Group III mice received 0.1 ml of approximately 5.55 MBq [18F]-FDG intraperitoneally 24 hours prior to receiving the above mentioned dose of JAA-F11. MicroPET imaging was performed on 3 mice each from group II and III. Biodistribution was performed on all group I, II, and III mice.
Biodistribution and tumor uptake studies
All mice were sacrificed by injecting intraperitoneally 0.1 ml sodium pentobarbital (Fatal Plus). Group I mice were sacrificed 72, 144, and 360 hours; group II mice were sacrificed at 72, 144, 240, 360, and 480 hours; and group III mice were sacrificed at 240 and 268 hours after injection of the radiotracer. At least three mice per time point were used. Blood, muscle, liver, lungs, kidneys, spleen, heart, stomach, large and small intestines, brain, and tumor tissue were weighed and radioactivity was measured using a gamma counter. Radioactivity uptake was calculated as the percentage of the injected dose per gram of tissue (%ID/g). Group III contained a smaller number of mice, and these mice bore two tumors which caused earlier morbidity leading to early sacrifice, making biodistribution at only two time points possible.
PET Imaging
The microPET camera, Focus 120® (Siemens Concorde Microsystems previously CTI Concorde Microsystems) was used for imaging. The emission scan window was set between 350 – 750 keV. The transmission scan of some mice was also performed using a Cobolt-57 point source for 9 minutes. The transmission scan window was set between 120 – 125 keV. Analysis of the acquired data and image reconstruction with filtered back projection 2D was done using the software microPET manager 2.2.4.0.
Before scanning, the mice were anesthetized with O2/isoflurane (1%-3% isoflurane) and imaged in the prone position in the gantry of the microPET scanner and the animal was scanned for 30 minutes for 124I and 15 minutes for [18F]-FDG. Three mice from group II were selected and imaged at 48, 144, 240, 360, and 480 hours after injection of the labeled tracers and were sacrificed on the last day of the study. All 3 mice of group III were imaged 90 minutes after injection of [18F]-FDG for 30 minutes. One day after FDG imaging, the above mentioned dose of [124I]-JAA-F11 was injected in 3 mice each and microPET imaging was performed. Biodistribution was performed on group III mice at 240 hours when tumor volume necessitated sacrifice.
Statistical Analysis
Comparison between data sets were determined using a two tailed unpaired t-test for two independent populations, p ≤ 0.05 was considered significant.
Results
Antibody specificity
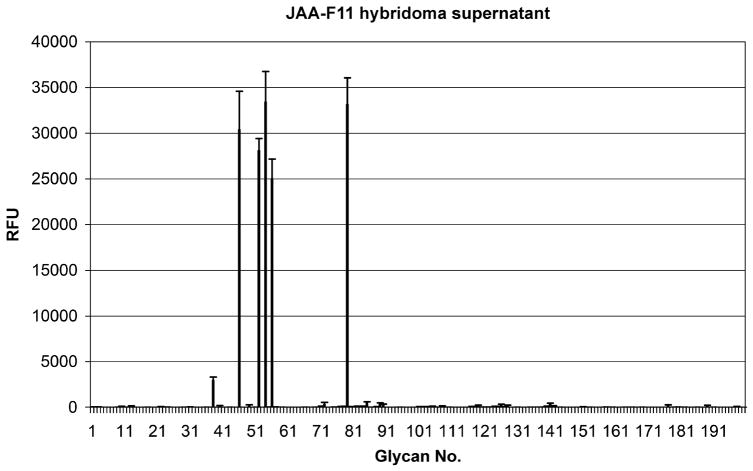
The glycan printed array analysis (Figure 1) illustrates the relative reactivity of JAA-F11 antibody to 200 glycan structures. It showed that the antibody bound to only 5 of the compounds: #56 Galβ1–3GalNAcα-Sp8 (TF-Ag), #52 Galβ1–3(GlcNAcβ1–6)GalNAcα-Sp8 (Core2), #54 Galβ1–3(Neu5Acβ2–6)GalNAcα-Sp8 (6-Sialyl TF), #79 Galβ1–4GlcNAcβ1–6GalNAcα–Sp8 (6-LacNAc-Tn), and # 46 β-D-Gal–Sp8, and bound with about 1/10 the efficiency to #38 Galα1–3GalNAcα-Sp8. The binding of JAA-F11 to the first 4 of these compounds was not significantly different from each other. JAA-F11 did not bind to the 195 other saccharide structures including related structures such as: Galβ1–3(Neu5Acα2–6)GalNAcα-Sp8 (6′-Sialyl TF), Galβ1–3(Galβ1–4GlcNAcβ1–6)GalNAcα-Sp8 (β–Galactosylated Core 2), Neu5Acα2–3Galβ1–3GalNAcα-Sp8 (α2–3Neu5Ac on Core 1), Neu5Acα2–3Galβ1–3GlcNAcβ–Sp0 (3′Sialyl Lec), GalNAcα1–3GalNAcβ–Sp8 (Forssman disaccharide-3), Galβ1–3GalNAcβ–Sp8 (Tbb), and Fucα1–2Galβ1–3GalNAcβ1–3Galα-Sp3 (part of the Globo H hexasaccharide).
Figure 1.
Analysis of JAA-F11 on 200 different glycans on a printed array indicates its specificity for the tumor antigen without much reactivity to related structures. Reactive structures are: #56 Galβ1–3GalNAcα-Sp8, #52 Galβ1–3(GlcNAcβ1–6)GalNAcα-Sp8, #54 Galβ1–3(Neu5Acβ2–6)GalNAcα-Sp8, #79 Galβ1–4GlcNAcβ1–6GalNAcα–Sp8, and # 46 β-D-Gal–Sp8, and with about 1/10 the efficiency to #38 Galα1–3GalNAcα-Sp8.
Radiolabeling and Reactivity
The average specific activity obtained by the Bolton-Hunter method was 57 MBq/ml (n=6) and the average radiolabeling yield obtained was 29.81% (SEM = 3.07, n=6). The radiolabeling yield for the Iodogen method was 19.7%. Immunoreactivity of the labeled antibody was determined by performing EIAs. Figure 2 shows that [124I]-JAA-F11 labeled with the Iodogen method is not immunoreactive with TF-Ag, whereas [124I]-JAA-F11 labeled with Bolton-Hunter method is reactive to TF-Ag. Indirect labeling by Bolton-Hunter method improved the immunoreactivity as seen in Figure 2a. Based on the above results, biodistribution and localization experiments were performed using JAA-F11 labeled by the Bolton-Hunter method.
Figure 2.
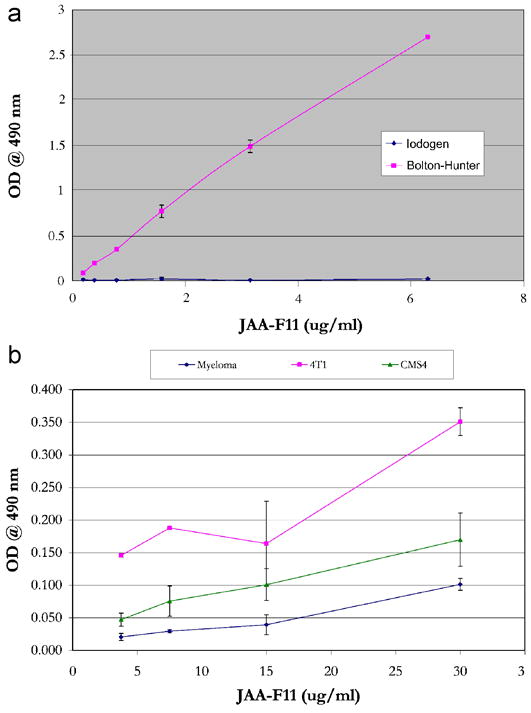
Antibody characterization. 2a. EIA of 124I-JAA-F11 labeled by Iodogen and Bolton Hunter method showing loss of immunoreactivity of the labeled antibody with the Iodogen method and preservation of JAA-F11 binding with the Bolton Hunter method. Error bars represent +/− 1 SEM. 2b. Indirect Cell EIA to test the expression of TF-Ag on cancer cell lines, showing significant JAA-F11 binding to 4T1 cells (p = 0.019 versus Myeloma cells) and not to CMS4 cells (p = 0.279 versus Myeloma cells).
Cell EIA
Whole cell EIA was performed to test the expression of TF-Ag on the cancer cell lines. The cell lines tested were 4T1, CMS4, and Myeloma (negative control). The experiment was repeated 3 times for significance, and each dilution was tested in 5 replicates. A 150 μg/ml solution of partially purified JAA-F11 in PBS was used. Students paired t-test analysis was used to compare the highest concentrations of the 3 runs for each cell line. It was observed that CMS4 cells expressed significantly less TF-Ag on the cell surface than the 4T1 cells (p = 0.019) and thus was chosen as a control cell line for in vivo experiments. Although when compared to TF-Ag negative Myeloma cells, CMS4 cells did not exhibit a significant difference (p = 0.279), the results from all experiments showed the CMS4 results to be between the Myeloma cells and the 4T1 cells, indicating a TF-Ag low expression. Figure 2b displays the average O.D. of the 5 replicates of the 3 runs with Standard Error bars. 4T1 cells gave 1.6 to 3 times the signal as the CMS4 cells. Myeloma cells do not form solid tumors in vivo so they were not selected as the in vivo negative control, and CMS4 was used as a cell line with 0.33 to 0.62 the TF-Ag expression of the 4T1 cells.
Biodistribution
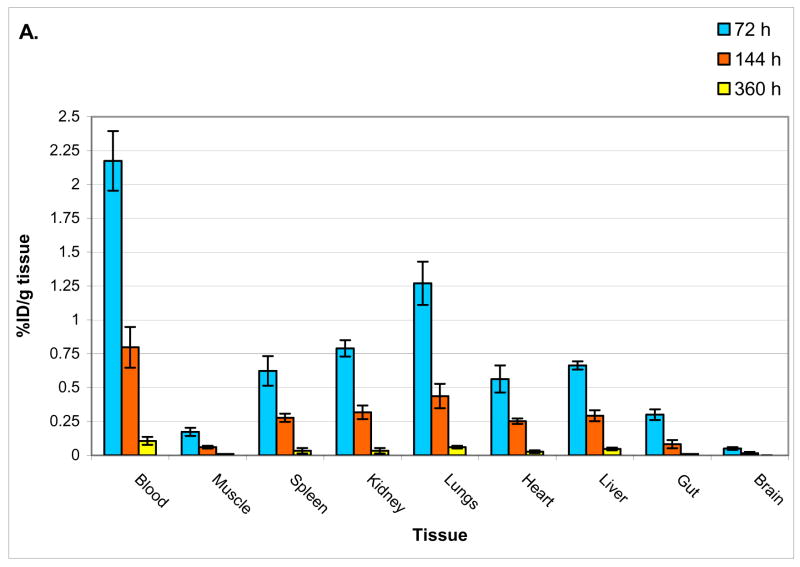
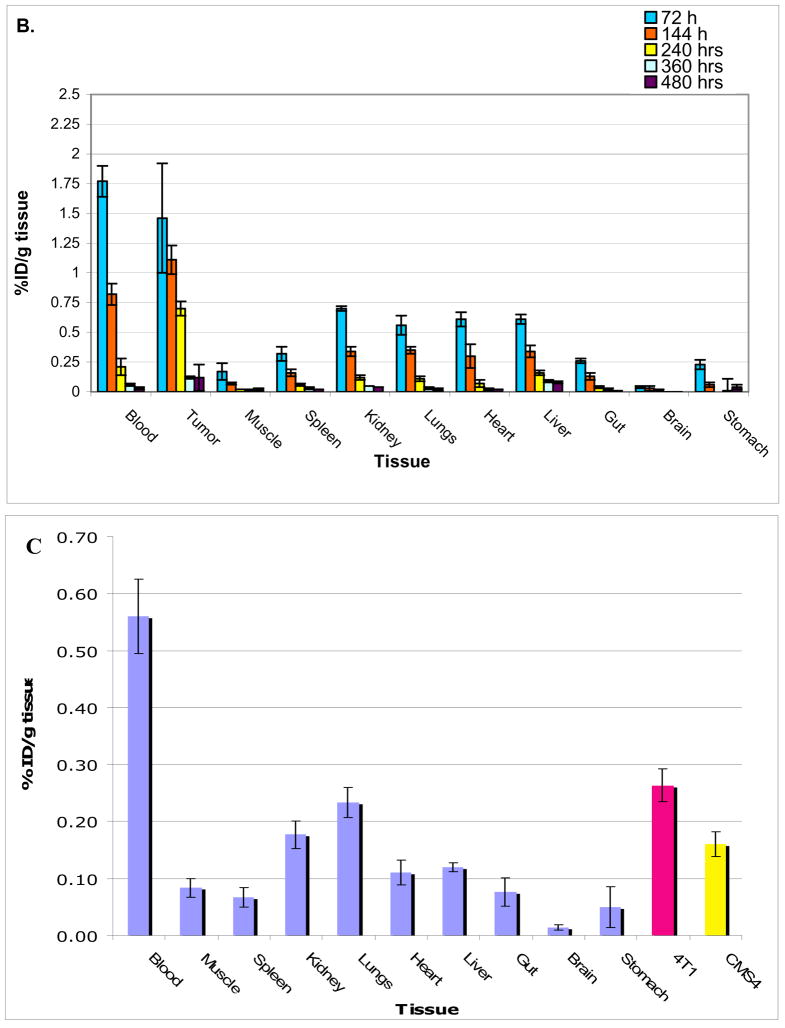
Figures 3a and 3b show biodistribution of [124I]-JAA-F11 in tissues of mice without any tumors and mice bearing TF-Ag positive 4T1 tumors. Care was taken to rinse the blood off the organs before counting them on the gamma counter. The biodistribution results in mice without a tumor (group I) were found to be normal, with no concentration in any organ. The biodistribution analysis of mice with TF-Ag positive tumor (group II) showed that there is preferential uptake of the radiolabeled antibody in the TF-Ag positive 4T1 tumor. As expected, activity in the blood was highest in the earliest time point and decreased overtime, and the biodistribution of group II mice showed high specific uptake in 4T1 tumors compared to other organs. The 4T1 tumor retained around 6 times more [124I]-JAA-F11 than blood on day 20 after injection of the radiotracer (tumor = 0.19 %ID/g; Blood = 0.03 %ID/g). The biodistribution in mice with a TF-Ag positive and a TF-Ag low to negative tumor was also encouraging (Figure 3c), with 4T1 tumor retaining 1.63 times more [124I]-JAA-F11 than CMS4 on day 10 after injection of the drug (4T1 tumor = 0.26 %ID/g; CMS4 tumor = 0.16 %ID/g). Unpaired t-test analysis was performed and the difference in the biodistribution of [124I]-JAA-F11 in 4T1 and CMS4 in mice was found to be significant (p = 0.015). This preferential uptake in vivo matched well with the EIA results on these two tumor lines. In no case was preferential kidney uptake seen with JAA-F11. The higher variability in radiolabel retention in tumor at 72 hours as compared to other tissues is due to the fact that intact Ab is still in circulation, and this, combined with the fact that tumor size, vascularity, and necrosis are variable between animals, contributes to the high variability at this time point.
Figure 3.
Biodistribution of 124I-JAA-F11. 3a. mice without tumor, 3b. mice bearing TF-Ag positive 4T1 tumor, 3c. mice bearing 4T1 and CMS4 tumors imaged at 240 hours.
PET Imaging
MicroPET imaging of the mice was performed at 48, 144, 240, 360, and 480 hours. Figure 4 shows the coronal view of the serial images obtained over the 20 days of the mice bearing 4T1 tumors injected with [124I]-JAA-F11. As seen in Figure 4, [124I]-JAA-F11 was taken up by the 4T1 tumor as early as 48 hours. The blood pool activity remained until 144 hours (Figure 4). The radiolabeled JAA-F11 was seen to be specifically localized in the tumor until 20 days. Transmission scans were done in mice at later time points. This helped in obtaining outlines of the mice for visualization, since after 144 hours the blood pool activity is cleared and it was difficult to observe the outlines of the mouse, and only the tumor was visible. With transmission scan overlapped on the emission scan, the position of the tumor was apparent (Figure 4, 240 and 480 hours).
Figure 4.
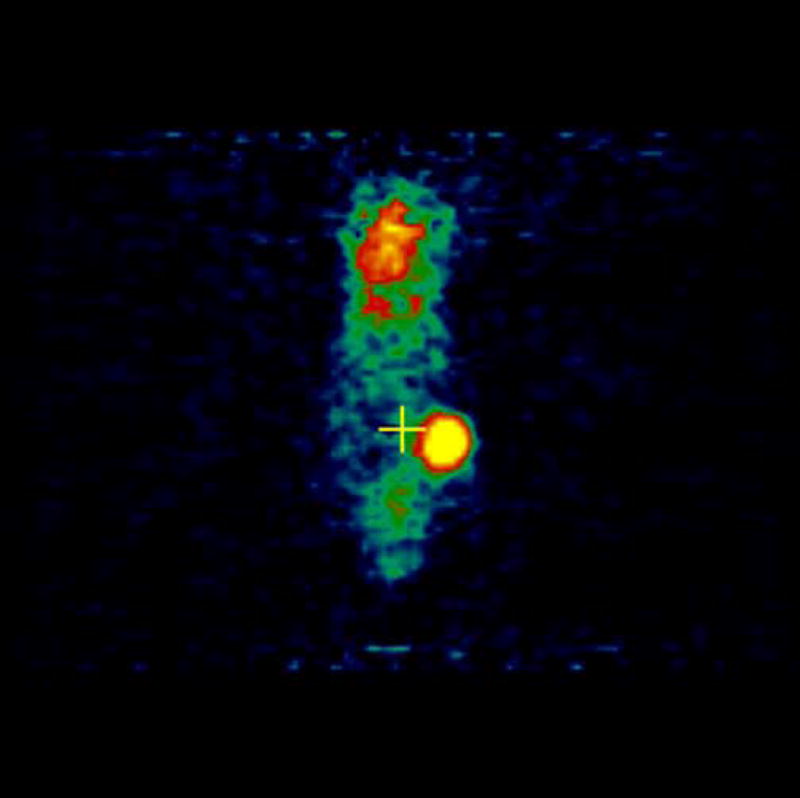
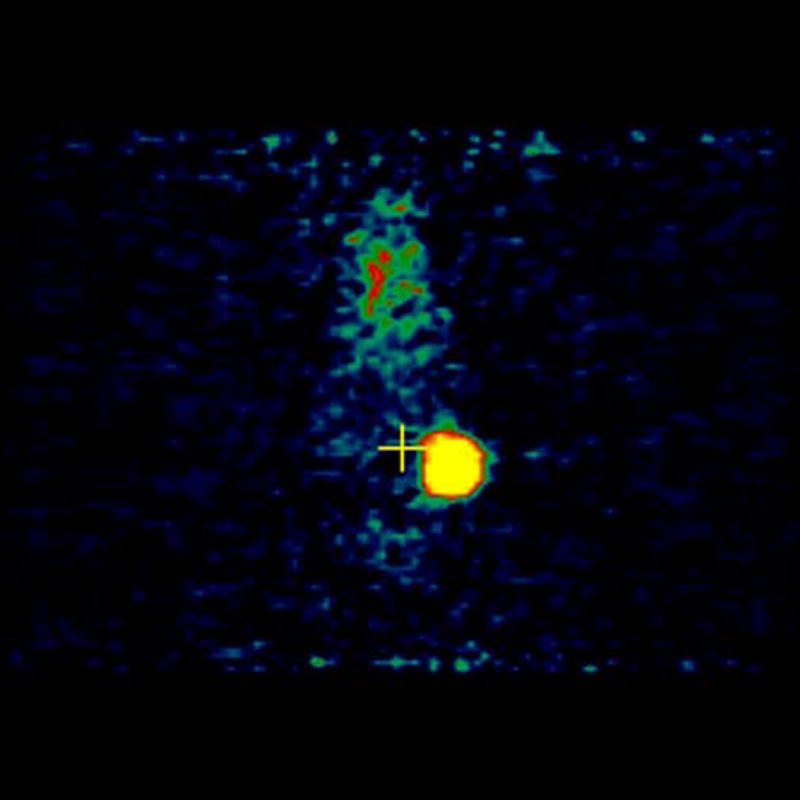
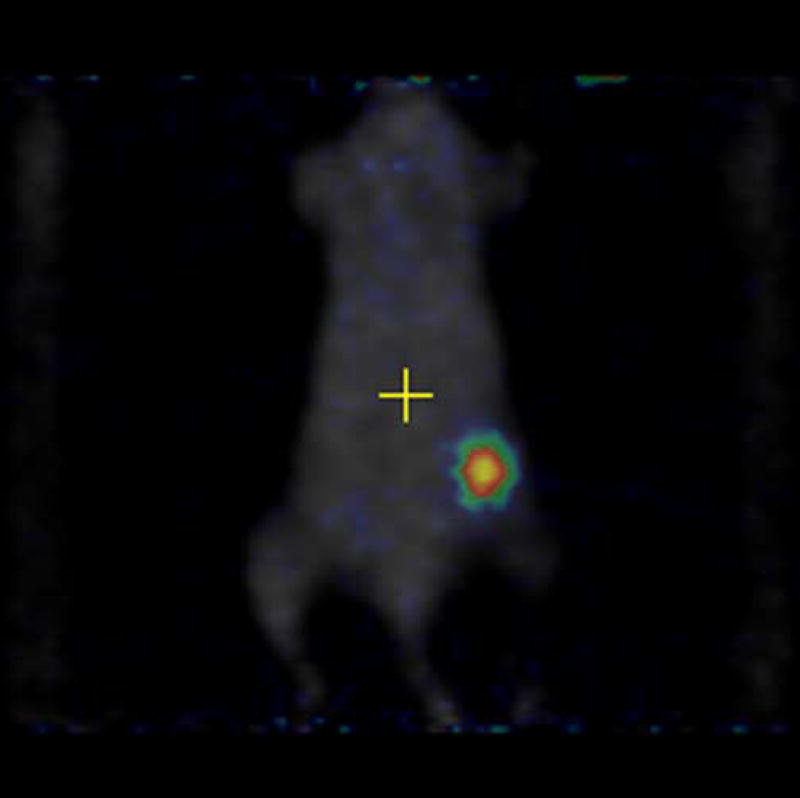
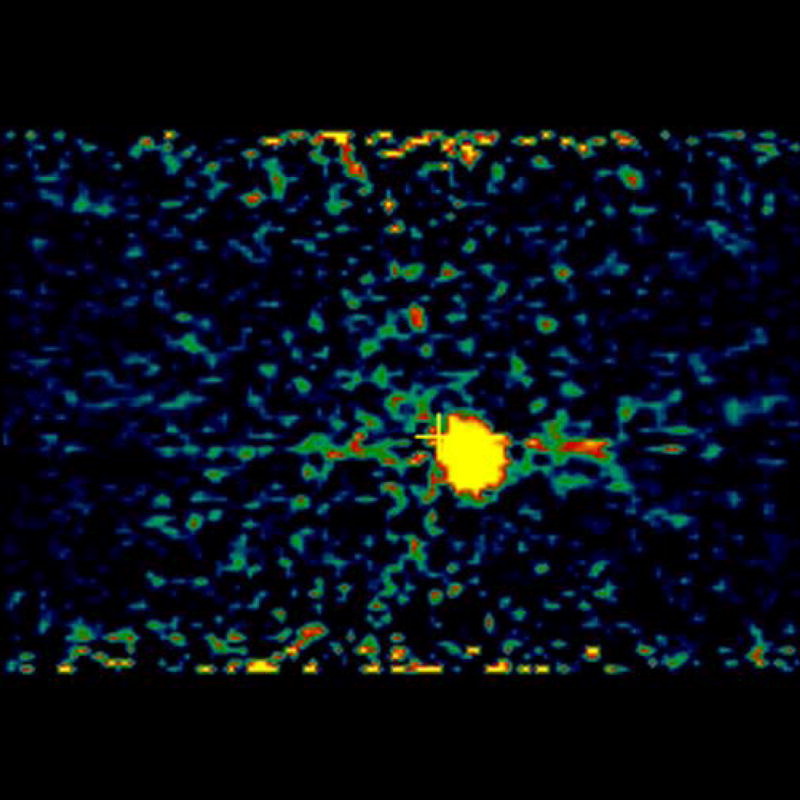
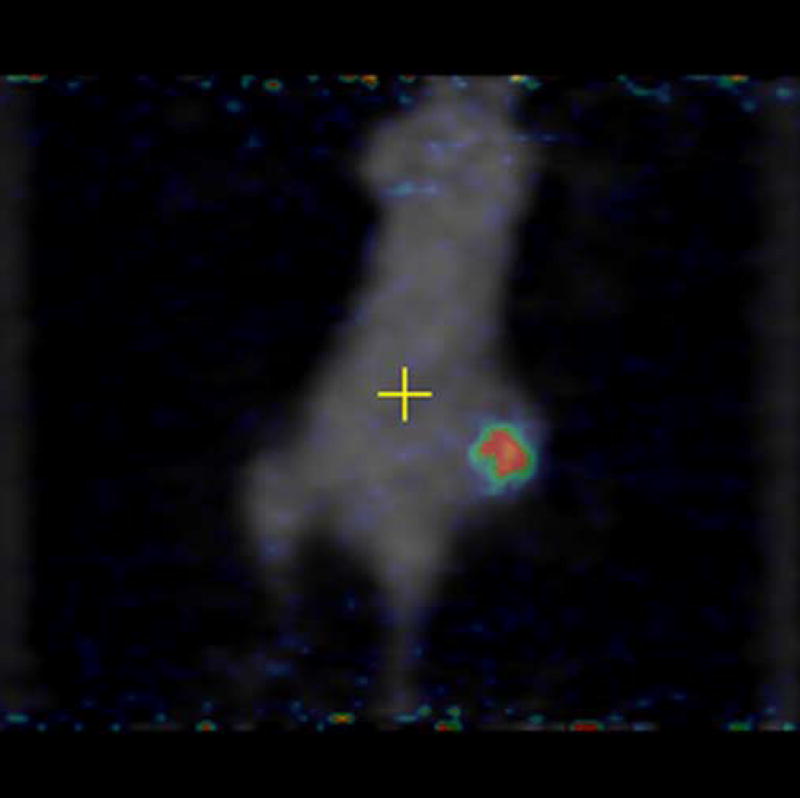
microPET images showing immunolocalization of 124I-JAA-F11 in mice with 4T1 tumors imaged for 48, 144, 240, 360, and 480 hours. Before scanning, the mice were anesthetized with O2/isoflurane (1%–3% isoflurane) and imaged in the prone position in the gantry of the microPET scanner and the animal was scanned for 30 minutes for 124I.
Experiments involving mice with 4T1 as well as negative control tumor CMS4 were performed. [18F]-FDG was used as a positive control and was injected in mice with 4T1 and CMS4 tumors. Localization of [18F]-FDG was observed in high metabolic areas in the body, including the two tumors. Figures 5a and 5b show microPET slices of [18F]-FDG highlighting 4T1 and CMS4 tumors in the mice, respectively. The same mice received [124I]-JAA-F11 one day after they received [18F]-FDG. Figure 6a and 6b shows microPET slice highlighting 4T1 tumor and CMS4 respectively.
Figure 5.
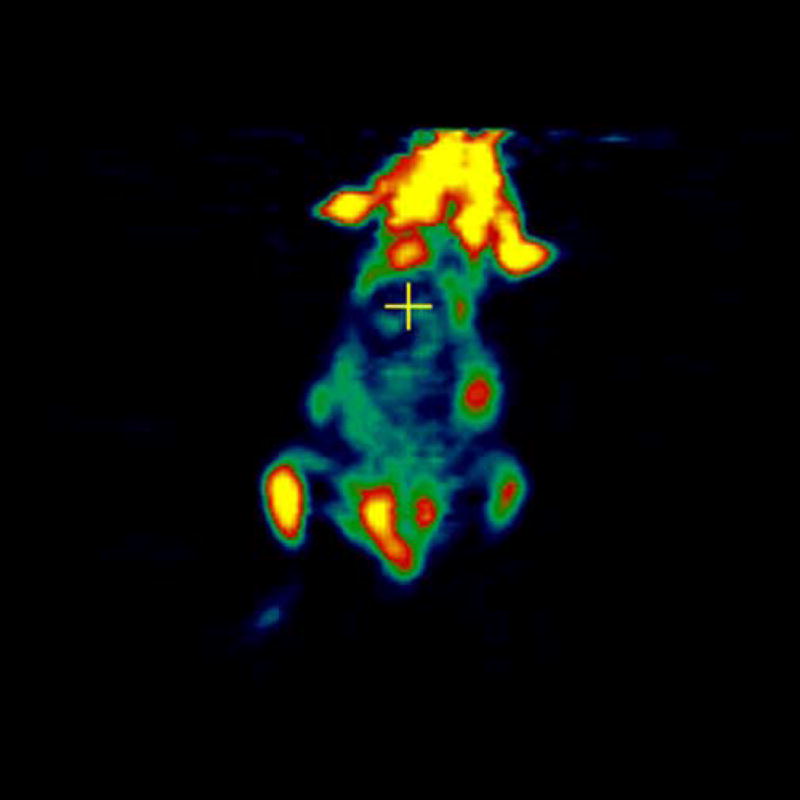
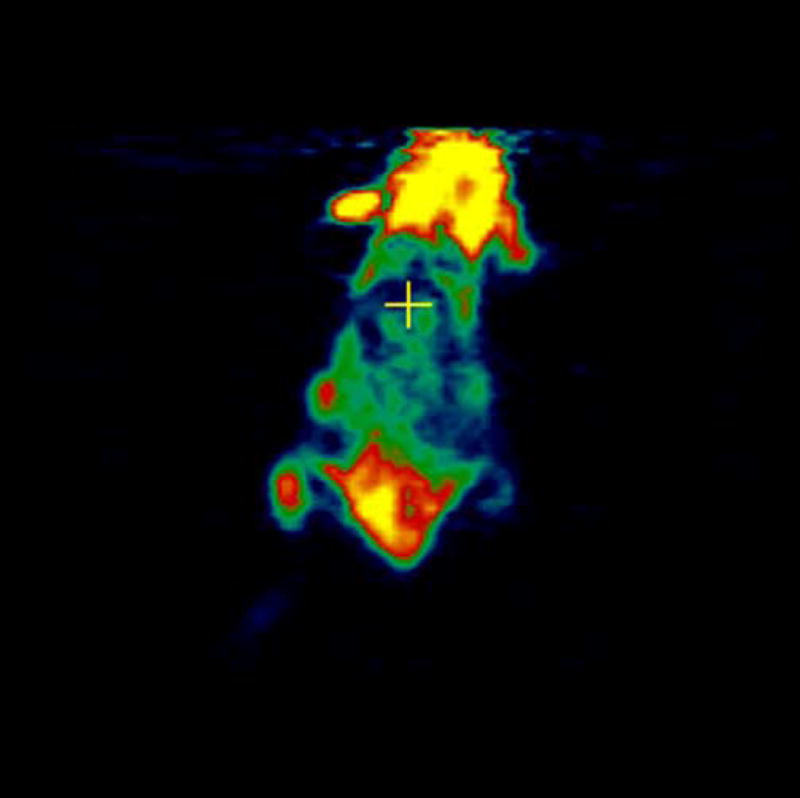
microPET images showing immunolocalization of 18F-FDG in mice with 4T1 and CMS4 tumors. 5a. the planar slice which showed maximum 4T1 localization is shown. 5b. the planar slice which showed maximum CMS4 localization is shown. Tumors were not on the same plane, therefore 2 images were used. Before scanning, the mice were anesthetized with O2/isoflurane (1%-3% isoflurane) and imaged in the prone position in the gantry of the microPET scanner and the animal was scanned for 15 minutes for [18F]-FDG.
Figure 6.
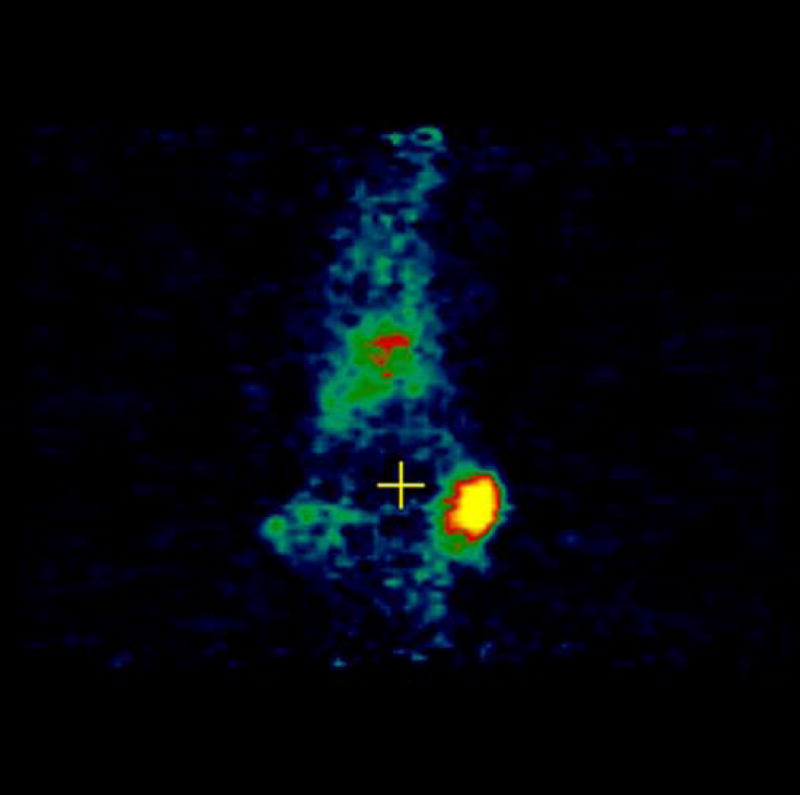
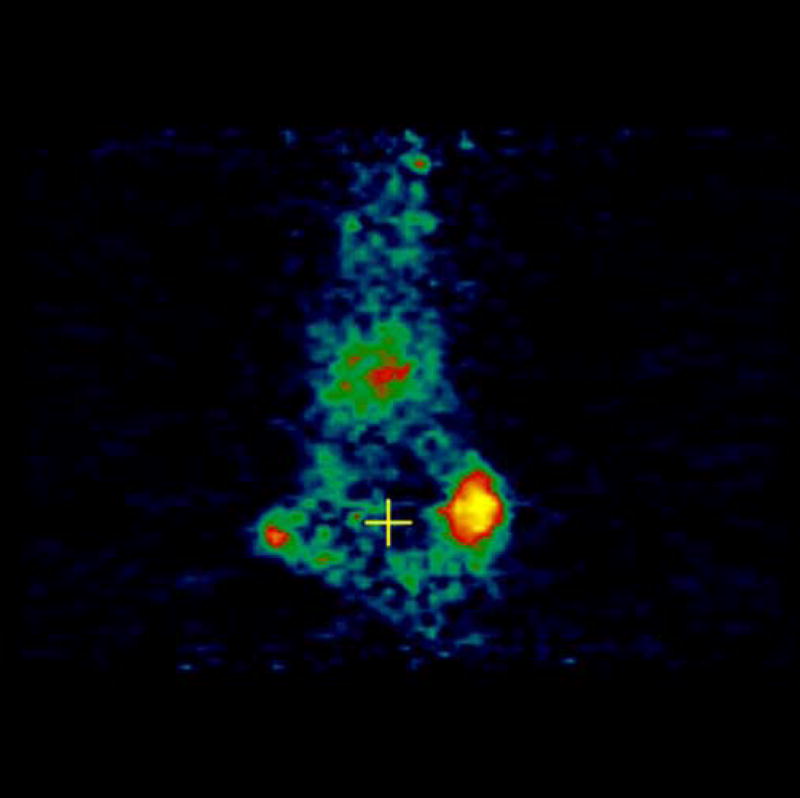
microPET images showing immunolocalization of [124I]-JAA-F11 in mice with 4T1 and CMS4 tumors. 6a. image with maximum 4T1 tumor localization, 6b. image with maximum CMS4 tumor localization. Tumors were not on same plane, therefore 2 images were used. Before scanning, the mice were anesthetized with O2/isoflurane (1%–3% isoflurane) and imaged in the prone position in the gantry of the microPET scanner and the animal was scanned for 30 minutes for 124I.
Discussion
JAA-F11 antibody bound to Galβ1–3GalNAc-alpha but not to Galβ1–3GalNAc-beta linked structures, indicating that the antibody would bind to tumor tissue and not to the central nervous system GM1 ganglioside, asialo-GM1 of NK cells, or GD1 of glycolipids. Its lack of binding with Galβ1–3(Neu5Acα2–6)GalNAcα-Sp8 indicates that this antibody would not be lost to the human plasma Gc protein which is a transporter or reservoir of vitamin D (Matsuura et al., 2004). Its lack of binding with Fucα1–2Galβ1–3GalNAcβ1–3Galα-Sp3 indicates lack of binding with Globo H derivatives. The binding to β-D-Gal–Sp8 was not seen in solution and may be due to the reported increase in affinity to monosaccharides seen when polyvalent solid phase presentation occurs (Mortell, 1996; Liang et al., 1997) and thus may not be biologically relevant. This type of analysis is consistent with the specificity seen in the imaging analysis, and supports the idea that this antibody will provide better imaging results than other anti-TF-Ag antibodies.
Radioiodination of JAA-F11 was first attempted using the direct iodination method since this is the most commonly used method. It was observed by EIA that the directly labeled mAb was no longer immunoreactive with TF-Ag. Analysis of the amino acid sequence of the mAb showed that there are seven tyrosine groups in the Complementarity Determining Regions (CDRs) of JAA-F11 (data not shown). Since the direct Iodogen method labels tyrosine residues in the proteins, this can alter the CDR of JAA-F11 which is the region of the antibody that binds to TF-Ag. An indirect iodination method, the Bolton-Hunter method, was utilized. This reagent reacts with lysine residues in the antibody through the formation of stable amide bonds. The radiolabeling yields obtained were of good efficiency (29.81%), and the labeled mAb was found to be immunoreactive with TF-Ag by EIA.
The biodistribution results in mice without a tumor showed no concentration to any organ, unlike the kidney localization seen by others with reagents that bound both the alpha- and beta-linked TF-Ag (Turner et al., 1988). The biodistribution results in mice with only a TF-Ag positive tumor were promising, with 4T1 tumors still retaining around 6 times more [124I]-JAA-F11 than blood on day 20 after injection of the drug (tumor = 0.19 %ID/g; Blood = 0.03 %ID/g). The biodistribution analysis showed that there is preferential uptake of the radiolabeled antibody in the TF-Ag positive tumor and that this radioactivity remains for 20 days, a fact that bodes well for future immunotherapeutic protocols. At 20 days radioactivity remains in the tumor, and this is promising, however, radioactivity as a ratio to tumor has increased in the liver at this time point. At earlier time points much higher radioactivity is in the tumor than in the liver, at 20 days, although the liver/tumor ratio is higher, the total amount of the radioactivity that the liver retains is small compared to the tumor dose received at earlier time points, thus the dose to the tumor is much higher than the dose to the liver. In future studies liver Fc receptor blocking experiments with cold non-specific antibody will be attempted to decrease liver dose. Results by others (Yakaki et al., 2001, Ravn et al., 2007) have shown faster clearance time with fragments, but increased liver and/or kidney binding, so this may not be an appropriate change unless conjugated with Polyethylene Glycol as in the method of Li L et al., 2006. Use of a cold unrelated antibody may block Fc receptor binding sites and block some Fc mediated uptake in the liver that occurs at later time points
A TF-Ag low to negative control cell line was also investigated. To examine the expression of TF-Ag on CMS4, an indirect cellular EIA was performed, to compare to 4T1 cells. The difference was found to be significant (p<0.05) according to the results of a paired Student’s t-test. With TF-Ag signal 1.6 to 3 times higher on the 4T1 cells, CMS4 was thus chosen as a TF-Ag low cell line for the in vivo experiments. When injected in mice, CMS4 was observed to grow faster than 4T1. The biodistribution in mice with a 4T1 and CMS4 were also encouraging, with 4T1 tumor retaining 1.63 times more 124I-JAA-F11 than CMS4 on day 10 after injection of the drug (4T1 tumor = 0.26 %ID/g; CMS4 tumor = 0.16 %ID/g). The difference was found to be significant (p = 0.015).
High uptake of [124I]-JAA-F11 was observed in 4T1 tumors by microPET imaging. This proved the specificity of JAA-F11 for TF-Ag expressed on 4T1, which is in agreement with the results obtained in the in vitro indirect cellular EIA experiments. Although, after dissection, metastasis in the lungs of the mice were observed, radiolabeled Ab was injected at an early time point (day 10 to day 18 after tumor cell injection) to prevent excess morbidity prior to completion of the MicroPET studies. At this early time point the metastatic foci would have been very small and unable to take up enough Ab for later visualization. Future experiments will involve primary tumor removal, and longer time for metastatic focus to develop prior to radiolabeled Ab administration. Such experiments with a different Ab resulted in visualization of metastasis and it is anticipated that these experiments will also allow visualization of metastasis in our system. Immunohistochemistry on the lung metastasis (data not shown) showed reactivity with the JAA-F11 antibody.
Transmission scans were done in mice at later time points. This helped in obtaining an outline of the mouse for visualization, since after 144 hours the blood pool activity is cleared and only the tumor was visible. With transmission scan overlapped on the emission scan, the position of the tumor was apparent. The kidney uptake of JAA-F11 was less (kidney:tumor ratio of 0.48 at 72 hours) compared to the 0.75 ratio seen with the 155H.7 antibody (Turner 1988) which bound both the Galβ1–3GalNAc-α and the Galβ1–3GalNAc-β derivatives.
For further investigations, using Ab fragments, pretargeting, imaging in nude or SCID mice bearing TF-Ag positive and negative human breast cancer xenografts, humanization (chimerization) of JAA-F11, and ultimately radioimmunotherapy with [131I]-JAA-F11 (murine JAA-F11 as well as humanized JAA-F11) should be performed.
This investigation has led to several important conclusions about the localization potential of JAA-F11 and its biodistribution in mice. High specificity and localization of the labeled mAb greater than 20 days was obtained, which is very promising from an immunotherapy point of view, the ultimate goal. These results are a step forward towards developing [131I]-JAA-F11 as a potential clinically useful radioimmunotherapy drug against TF-Ag positive breast cancer metastasis. The 170H.82 antibody which bound both alpha- and beta-linked TF-Ag showed promise in breast cancer immunolocalization, withdrawn only after phase II trials (1997), and JAA-F11 shows a more restricted structural specificity and less kidney association. Further studies are warranted to determine if this finding will translate to specificity and sensitivity in human localization, and ultimately, therapeutic efficacy.
A study on utilization of multivalent single chain fragment variable (scFvs) for immunolocalization experiments was recently published (Ravn et al., 2007). These scFvs were selected from a phage library and are also TF-Ag-alpha specific, as is JAA-F11. Unfortunately, unlike our whole antibody, these fragments showed higher kidney binding than tumor binding, which the authors felt was due to filtration entrapment in the kidney of this size multimeric fragment. The future experiments with JAA-F11 will use blocking with cold unrelated Ab to block Fc binding sites, (Fab′)2, and humanized antibody and polyethylene glycol DOTA conjugated diabodies (Li L. 2006) rather than the scFvs multimeric fragments as the polyethylene glycol diabodies do not become entrapped in the kidney the way the scFvs do.
Acknowledgments
The glycan-array analysis was conducted by the Protein-Carbohydrate Interaction Core H of The Consortium for Functional Glycomics funded by the National Institute of General Medical Sciences grant GM62116. We also thank Director Richard Alvarez, Coordinator Richard Cummings, and Angela Lee for assistance with the glycan array analysis.
This work was supported by NIH R15AI49210–01, Peptide Mimics for Anti-Carbohydrate Response and UB STOR Product Development Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biomira Annual Report http://www.biomira.com/documents/annualReports/97-Ann-Rep.pdf. “1997.” Retrieved September 2006.
- Abdi EA, Kamitomo VJ, McPherson TA, Catz Z, Boniface G, Longenecker BM, Noujaim AA. Radioiodinated peanut lectin: clinical use as a tumour-imaging agent and potential use in assessing renal-tubular function. Eur J Nucl Med. 1986;11(9):350–4. doi: 10.1007/BF00253300. [DOI] [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101(49):17033–8. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge DR, Carroll VA, Glaser M, Aboagye EO, Osman S, Hutchinson OC, Barthel H, Luthra SK, Brady F, Bicknell R, Price P, Harris AL. The development of [(124)I]iodinated-VG76e: a novel tracer for imaging vascular endothelial growth factor in vivo using positron emission tomography. Cancer Res. 2002;62(20):5912–9. [PubMed] [Google Scholar]
- Dennis JW. Changes in glycosylation associated with malignant transformation and tumor progression. In: Fukuda M, editor. Cell Surface Carbohydrates and Cell Development. 1992. pp. 161–194. [Google Scholar]
- Dessureault S, Koven I, Reilly RM, Couture J, Schmocker B, Damani M, Kirsh J, Ichise M, Sidlofsky S, McEwan AJ, Boniface G, Stern H, Gallinger S. Pre-operative assessment of axillary lymph node status in patients with breast adenocarcinoma using intravenous 99mtechnetium mAb-170H.82 (Tru-Scint AD) Breast Cancer Res Treat. 1997;45(1):29–37. doi: 10.1023/a:1005878113826. [DOI] [PubMed] [Google Scholar]
- Fraker PJ, Speck JC., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978;80(4):849–57. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Heimburg J, Yan J, Morey S, Glinskii OV, Huxley VH, Wild L, Klick R, Roy R, Glinsky VV, Olson-Rittenhouse K. Inhibition of Spontaneous Breast Cancer Metastasis by Anti-Thomsen-Friedenreich Antigen Monoclonal Antibody JAA-F11. Neoplasia. 2006 November;8(11):939–948. doi: 10.1593/neo.06493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yazaki PJ, Anderson AL, Crow D, Colcher D, Wu AM, et al. Improved biodistribution and radioimmunoimaging with poly(ethylene glycol)-DOTA-conjugated anti-CEA diabody. Bioconjug Chem. 2006;17(1):68–76. doi: 10.1021/bc0502614. [DOI] [PubMed] [Google Scholar]
- Liang R, Loebach J, Horan N, Ge M, Thompson C, Yan L, Kahne D. Polyvalent binding to carbohydrates immobilized on an insoluble resin. Proc Natl Acad Sci U S A. 1997;94(20):10554–9. doi: 10.1073/pnas.94.20.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker BM, Willans DJ, MacLean GD, Selvaraj S, Suresh MR, Noujaim AA. Monoclonal antibodies and synthetic tumor-associated glycoconjugates in the study of the expression of Thomsen-Friedenreich-like and Tn-like antigens on human cancers. J Natl Cancer Inst. 1987;78(3):489–96. [PubMed] [Google Scholar]
- Matsuura T, Uematsu T, Yamaoka M, Furusawa K. Effect of salivary gland adenocarcinoma cell-derived alpha-N-acetylgalactosaminidase on the bioactivity of macrophage activating factor. Int J Oncol. 2004;24(3):521–8. [PubMed] [Google Scholar]
- Mortell KHWR, Kiessling LL. Recognition specificity of neoglycopolymers prepared by ring-opening metathesis polymerization. J Am Chem Soc. 1996;118:2297–2298. [Google Scholar]
- Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58(7):1486–93. [PubMed] [Google Scholar]
- Pulaski BA, Terman DS, Khan S, Muller E, Ostrand-Rosenberg S. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, and CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res. 2000;60(10):2710–5. [PubMed] [Google Scholar]
- Ravn P, Stahn R, Danielczyk A, Faulstich D, Karsten U, Goletz S. The Thomsen-Friedenreich disaccharide as antigen for in vivo tumor targeting with multivalent scFvs. Cancer Immunol Immunother. 2007 doi: 10.1007/s00262-007-0292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Diakun K, Xia Z, Pickhardt D, Morey S, Baek MG, Roy R. Development and characterization of monoclonal antibody to T-antigen: (gal beta1-3GalNAc-alpha-O) Hybridoma. 1998;17(2):165–73. doi: 10.1089/hyb.1998.17.165. [DOI] [PubMed] [Google Scholar]
- Sajjad M, Bars E, Nabi HA. Optimization of 124I production via 124Te(p,n)124I reaction. Appl Radiat Isot. 2006;64(9):965–70. doi: 10.1016/j.apradiso.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Shysh A, Eu SM, Noujaim AA, Suresh MR, Longenecker BM. In vivo localization of radioiodinated peanut lectin in a murine TA3/Ha mammary carcinoma model. Eur J Nucl Med. 1985;10(1–2):68–74. doi: 10.1007/BF00261767. [DOI] [PubMed] [Google Scholar]
- Takanami I. Expression of Thomsen-Friedenreich antigen as a marker of poor prognosis in pulmonary adenocarcinoma. Oncol Rep. 1999;6(2):341–4. doi: 10.3892/or.6.2.341. [DOI] [PubMed] [Google Scholar]
- Tatsumi T, Huang J, Gooding WE, Gambotto A, Robbins PD, Vujanovic NL, Alber SM, Watkins SC, Okada H, Storkus WJ. Intratumoral delivery of dendritic cells engineered to secrete both interleukin (IL)-12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity. Cancer Res. 2003;63(19):6378–86. [PubMed] [Google Scholar]
- Turner CJ, Sykes TR, Longenecker BM, Noujaim AA. Comparative radiolabeling and distribution of a tumour-directed monoclonal antibody. Int J Rad Appl Instrum B. 1988;15(6):701–6. doi: 10.1016/0883-2897(88)90064-5. [DOI] [PubMed] [Google Scholar]
- Yazaki PJ, Wu AM, Tsai SW, Williams LE, Ikler DN, Wong JY, et al. Tumor targeting of radiometal labeled anti-CEA recombinant T84.66 diabody and t84.66 minibody: comparison to radioiodinated fragments. Bioconjug Chem. 2001;12(2):220–8. doi: 10.1021/bc000092h. [DOI] [PubMed] [Google Scholar]