Abstract
Systemic lupus erythematosus is an autoimmune disease characterized by autoantibodies and systemic inflammation that results in part from dendritic cell activation by nucleic acid containing immune complexes. There are many mouse models of lupus, some spontaneous and some induced. We have been interested in an induced model in which estrogen is the trigger for development of a lupus-like serology. The R4A transgenic mouse expresses a transgene-encoded H chain of an anti-DNA Ab. This mouse maintains normal B cell tolerance with deletion of high-affinity DNA-reactive B cells and maturation to immunocompetence of B cells making nonglomerulotropic, low-affinity DNA-reactive Abs. When this mouse is given estradiol, normal tolerance mechanisms are altered; high-affinity DNA-reactive B cells mature to a marginal zone phenotype, and the mice are induced to make high titers of anti-DNA Abs. We now show that estradiol administration also leads to systemic inflammation with increased B cell-activating factor and IFN levels and induction of an IFN signature. DNA must be accessible to B cells for both the production of high-affinity anti-DNA Abs and the generation of the proinflammatory milieu. When DNase is delivered to the mice at the same time as estradiol, there is no evidence for an abrogation of tolerance, no increased B cell-activating factor and IFN, and no IFN signature. Thus, the presence of autoantigen is required for positive selection of autoreactive B cells and for the subsequent positive feedback loop that occurs secondary to dendritic cell activation by DNA-containing immune complexes.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of multiple autoantibodies (1, 2). Anti-DNA Abs are among the most significant because they are common, they are essentially diagnostic of SLE, their titers fluctuate with disease activity (3), and they contribute to tissue injury in the kidney and probably brain as well (4–6). For these reasons, they have been studied extensively.
There are several ways to induce the production of anti-DNA Abs in nonspontaneously autoimmune mice. DNA has been coupled to a protein carrier to induce production of anti-DNA Abs (7–10). In these studies, the protein is foreign and, therefore, immunogenic to T cells and the DNA functions as a hapten to activate DNA-reactive B cells. Peptide mimetopes of DNA have been exploited to induce a T cell-dependent anti-DNA response (11–13). In some studies, apoptotic cells have been administered to mice and shown to induce production of an anti-DNA response (14–18). It has been demonstrated that many perturbations of the immune system that diminish the clearance of apoptotic debris can stimulate the production of anti-DNA Abs (19–23). The apoptotic material contains endogenous ligands for TLRs, and so may transform tolerogenic dendritic cells (DCs) into immunogenic DCs and trigger an immune response to self Ag (24–28). Once anti-DNA Abs are present, they form DNA-containing immune complexes that, when internalized by DCs, can activate TLR9 and excite production of B cell-activating factor (BAFF) and IFN-α (29, 30). When the immune complexes are internalized by DNA-specific B cells, the B cells can be activated through TLR9 engagement to secrete pathogenic Ab.
We have been studying the induction of anti-DNA Abs in the R4A transgene (Tg) BALB/c mouse that harbors an IgG2b H chain of an anti-DNA Ab (31, 32). Approximately 90% of B cells express an endogenous H and L chain, and 10% express the R4A Tg H chain with a spectrum of L chains. Tg-expressing B cells undergo a normal maturation program in the bone marrow (33). Under normal conditions, B cell tolerance is maintained (33–36). B cells expressing the R4A H chain along with a L chain that produces high-affinity DNA binding are deleted at both the immature stage in the bone marrow and the transitional stage in the spleen (35, 36). Many B cells expressing the R4A H chain along with a L chain producing a low-affinity DNA binding are allowed to mature to immunocompetence, as are B cells expressing the R4A H chain along with a L chain that confers no DNA binding (33, 34, 37).
When R4A Tg BALB/c mice are given estradiol (E2) pellets to achieve a sustained serum concentration of 75–100 pg/ml, the R4A-expressing high-affinity DNA-reactive B cells survive negative selection and mature to immunocompetence as marginal zone (MZ) B cells (38). Our studies have shown that E2 causes a decrease in BCR signaling strength and reduced BCR-mediated apoptosis of immature and transitional B cells (36, 39). In this study, we asked whether the E2-induced lupus-like serology was accompanied by other features of SLE, such as elevated serum BAFF levels and an IFN signature. We further asked whether Ag was needed for the proinflammatory milieu and the positive selection and activation of high-affinity DNA-reactive B cells. We demonstrate that Ag is critical to the generation of the proinflammatory milieu. It is also required for positive selection of pathogenic autoreactive B cells; the diminished negative selection alone that is secondary to reduced BCR signaling is not alone sufficient for the development of pathogenic autoreactivity. These observations have important clinical implications.
Materials and Methods
Mice, hormone treatment, and therapeutic regimens
R4A Tg BALB/c mice, described previously (31), were bred and maintained at the Feinstein Institute for Medical Research. All animal studies were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee. Sixty-day time-release pellets (Innovative Research of America) containing E2 (0.18 mg) or placebo (P; vehicle control) were implanted beneath the skin of 8- to 10-wk-old female mice. The E2 pellets maintain serum E2 concentrations of 75–100 pg/ml (34). To avoid the problem of fluctuations in the endogenous E2 levels that occur in P-treated mice, all mice were ovariectomized prior to implantation of pellets. For experimental studies, mice were divided into four groups, as follows: P, E2, E2 plus DNase, and E2 plus heat-inactivated (HI) DNase. DNase treatment of mice was performed as reported by Macanovic et al. (40). Briefly, mice were injected i.p. daily with 450 μg bovine pancreatic DNase (Sigma-Aldrich) or HI enzyme (68°C for 15 min) in 200 μl saline for 6 wk. Before the start of treatment, and at weekly intervals until 6 wk, animals were bled by retro-orbital puncture. Urine was collected at both the beginning and the end of the experiment to examine the level of proteinuria.
Flow cytometry
Splenocytes from R4A Tg mice treated with P, E2, E2 plus DNase, and E2 plus HI DNase were isolated, Fc blocked, and stained with PerCP-labeled anti-B220, FITC-labeled anti-CD21/CD35 Ab, PE-Cy7–labeled anti-CD23 Ab, Pacific blue-labeled anti-CD24 Ab, PE-labeled anti-IgG2b Ab (BD Pharmingen), and allophycocyanin-labeled anti-AA4.1 Ab (eBioscience) at 4°C for 30 min and washed with PBS. The stained cells were analyzed by flow cytometry using an LSRII instrument (BD Biosciences), and the data were analyzed using Flowjo software (Tree Star).
Measurement of dsDNA in plasma
dsDNA was measured in the plasma of the experimental mice using picogreen dsDNA reagent (Invitrogen), according to the manufacturer’s instructions. Different dilutions of plasma in 100 μl Tris-EDTA buffer were incubated with 100 μl picogreen assay reagent. The mixture was incubated for 5 min at room temperature, the fluorescence was measured using a fluorescence microplate reader, and standard fluorescein wavelengths (excitation ~480 nm, emission ~520 nm) were used. The dsDNA in the samples was quantitated by means of a standard curve using lambda DNA (25 pg/ml to 25 ng/ml) as the standard. Appropriate controls such as plasma from BALB/c mice and plasma from New Zealand Black/White (NZB/W) young and sick mice were used to validate the picogreen assay.
Measurement of serum DNase
The levels of DNase protein in the serum of mice were measured by a sandwich ELISA. ELISA plates (Costar) were coated with anti-DNase Ab (1:1000 dilution). Serum samples at different dilutions were added to the plates subsequent to blocking with 1.0% BSA/PBS, followed by the addition of biotinylated anti-DNase Ab. DNase protein levels were detected using streptavidin coupled to alkaline phosphatase (AP; 1:1000 dilution). A standard curve with purified DNase was generated to calculate the concentration of DNase in serum.
Anti-DNase Abs in serum
Abs to DNase in mice sera were assayed by ELISA after 6 wk of treatment with P, E2, E2 plus DNase, or E2 plus HI DNase. Bovine pancreatic DNase-coated plates (10 μg/ml) were blocked with 1.0% BSA/PBS, followed by the addition of serum (1:250 dilution) from R4A Tg mice treated with P, E2, E2 plus DNase, and E2 plus HI DNase for 6 wk. The plates were probed with AP-conjugated anti-mouse IgG (1:1000 dilution), developed with AP substrate, and measured at 405 nm.
Analysis of Vκ-Jκ L chain genes by single-cell RT-PCR
Splenocytes from three mice in each experimental group were stained with Abs specific for B220, IgG2b, and AA4.1, and the mature (B220+IgG2b+ AA4.1−) and immature (B220+IgG2b+AA4.1+) Tg+ B cells were sorted as single cells into 96-well plates using a FACS Aria cell sorter (BD Biosciences). cDNA was prepared from the single cells, and the Vκ-Jκ L chain genes were amplified by two rounds of PCR, as previously described (41), using the following primers: universal Vκ, 5′-GGCTGCAGSTT-CAGTGGCAGTGGRTCWGGRAC-3′ plus C region primer (Cκ) (first round), 5′-TGGATGGGTGGGAAGATG-3′ and Cκ (second round), 5′-AAGATGGATACAGTTGGT-3′. Sequence analysis of the PCR products was performed using the second-round Cκ primer (Genewiz) subsequent to exo-shrimp alkaline phosphatase treatment (USB Biochemicals).
Real-time PCR
Splenocyte total RNA was isolated using the RNeasy kit from Qiagen, and cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was performed with a Roche 480 light cycler using Roche 480 master mix (Roche Applied Science) and TaqMan primer/probe sets (Applied Biosystems). The relative expression of BAFF, type I IFNs α and β, and the IFN-regulated genes mx-1 and ifi202b was determined in comparison with polymerase (RNA) II (DNA-directed) polypeptide A (polr2a). Data were analyzed using the Pfaffl method (42).
Measurement of serum BAFF
An ELISA was performed to determine serum BAFF levels, as described previously (41). Ninety-six–well plates were coated with 5 μg/ml anti-mouse BAFF mAb (clone 5A8; Apotech) overnight at 4°C and blocked with 5% BSA/PBS. Serial dilutions of mouse sera or mouse rBAFF (Apotech) were added to the wells, followed by 10 μg/ml biotinylated anti-mouse BAFF mAb (clone 1C9; Apotech) and HRP-labeled streptavidin. The plates were developed with HRP substrate, and the OD was measured at 450 nm.
Anti-dsDNA Ab ELISA
Serum anti-DNA Ab levels were determined, as previously described (43). Immulon 2HB 96-well plates (Thermo LabSystems) were coated with 100 μl/ml sonicated calf thymus DNA that had been passed through a nitro-cellulose filter to remove ssDNA. Mouse serum at different dilutions was added to plates after blocking with 1.0% BSA/PBS. IgG2b+ anti-DNA Abs were detected using AP-labeled anti-mouse IgG2b Ab (Southern Bio-technology). Purified R4A mAb was used (1–50 μg/ml) to generate a standard curve for calculating the concentration of anti-dsDNA Abs in the sera.
DNA inhibition ELISAs
The linear range of DNA reactivity was determined by the generation of dilution curves for serum samples from three each E2-, E2 plus DNase-, and E2 plus HI-inactivated DNase-treated R4A Tg mice. Serum samples were diluted (1:100) and preincubated with various concentrations of sonicated DNA (~2.0 kb in length) for 2 h at 37°C, and the remaining DNA reactivity was measured by DNA ELISA. The range of relative affinities of the anti-DNA Abs present in the sera was calculated, as previously described (44).
Serum treatment of DCs
Splenic DCs isolated from 20 10-wk-old BALB/c mice using CD11c-coated microbeads (Miltenyi Biotec) were resuspended in RPMI 1640 complete medium containing 10% FBS. The cell purity was >85%, as assessed by flow cytometry. A total of 7.5 × 105 cells was plated in 48-well tissue culture plates (Costar) containing 500 μl RPMI 1640 complete medium and stimulated with 5 μl (1% final concentration) serum from R4A Tg mice treated with P, E2, E2 plus DNase, or E2 plus HI DNase (three in each group) for 16 h. The cells were harvested and RNA isolated using RNeasy kit (Qiagen).
Renal pathology
Kidneys from the different experimental groups of R4A Tg mice (three per group) described above were fixed in formalin, paraffin embedded, sectioned (10 μm thickness), stained with biotinylated anti-mouse IgG, and developed with an AP ABC detection kit (Vector Laboratories). Glomerular IgG deposition in kidney sections was visualized under a Zeiss microscope at original magnifications ×5 and ×20. The number of glomeruli present in three different microscopic fields for each sample was determined. Three mice in each group were analyzed, and the mean percentage of positive glomeruli is shown. The investigator was blinded to the origin of the kidneys.
Analysis of proteinuria
Proteinuria was measured using Bayer reagent strips (Bayer), according to the manufacturer’s instructions, as well as by measuring total protein in the urine using the Coomassie blue reagent (Pierce).
Studies of renal pathogenicity of anti-dsDNA Ab-containing serum
Sera (100 μl) from R4A Tg mice treated with P, E2, E2 plus DNase, or E2 plus HI DNase were administered i.p. to 8-wk-old SCID mice (Jackson ImmunoResearch Laboratories). After 24 h, kidneys from the SCID mice were analyzed for glomerular IgG deposition, as described above. Purified R4A (75 μg), which has previously been demonstrated to deposit in kidneys of SCID mice, was used as a positive control (45).
Statistical analysis
Statistical analysis was performed using unpaired Student’s t test, the exact Kruskal-Wallis test, and Fisher’s exact tests, used as appropriate. A p value <0.05 was considered statistically significant.
Results
Generation of a proinflammatory milieu by E2 administration
R4A Tg mice harbor the H chain of the nephritogenic R4A anti-DNA Ab (31). These mice normally maintain B cell tolerance; upon exposure to increased levels of E2, they display an altered B cell repertoire with enhanced survival and activation of high-affinity DNA-reactive B cells. Elevated anti-dsDNA Ab levels, immune complex deposition in kidneys, and subsequent proteinuria can be observed, peaking ~6 wk after initiation of treatment and remaining high for months thereafter (33) (J. Venkatesh, E. Peeva, and B. Diamond, unpublished observations). The mice exhibit minimal inflammation in the kidney despite the presence of IgG deposition, presumably because they lack the genetic background necessary for renal inflammation. Hence, the R4A Tg mouse model is a useful model system to study some downstream effects of anti-DNA Abs in a host devoid of pre-existing immunologic abnormalities.
Studies in SLE have shown that DNA-containing immune complexes can activate DCs in vitro to produce both BAFF and IFN-α (29, 30), leading to the increased expression of multiple IFN-inducible genes, termed the IFN signature (46–49). Other studies have suggested that RNA-containing immune complexes are more contributory to DC activation and the induction of an IFN signature (50). Still another study performed in humans has suggested a genetic predisposition to increased type 1 IFN production that may precede autoantibody production (51). We have previously shown increased BAFF mRNA in E2-treated R4A Tg mice (36). In this study, we asked whether the induction of R4A-encoded anti-DNA Abs was sufficient to induce inflammatory features of SLE.
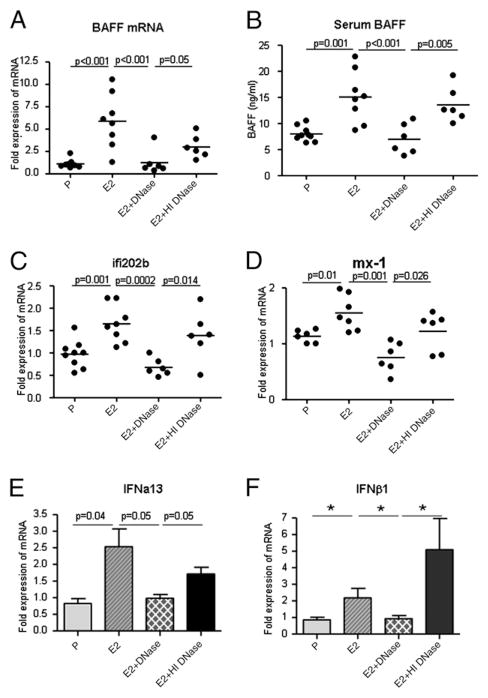
Serum BAFF levels were measured by ELISA. Mice receiving E2 pellets exhibited an increase in BAFF mRNA in splenocytes, as previously shown (Fig. 1A), and increased serum BAFF levels (Fig. 1B). It is known that B cell lymphopenia leads to increased BAFF levels (52). We, therefore, ascertained that there was no decrease in total B cell number secondary to the E2 administration (P, 586,352 ± 56,301; E2, 509,414 ± 14,049 B cells per 106 splenocytes), although we have previously shown a decrease in transitional B cells in the spleen (38) and others have shown an E2-induced decrease in B cell lymphopoiesis in the bone marrow (53). We also assayed for expression of type 1 IFNs (IFN-α,β) in splenic DCs treated with serum from P- and E2-treated R4A Tg mice, as well as ifi202b and mx-1, two prominent genes in the IFN signature. An increase in the mRNA of the IFN-inducible genes ifi202b and mx-1 in splenocytes from E2-exposed R4A Tg mice was observed (Fig. 1C, 1D). Cultured splenic DCs treated with serum from E2-exposed R4A Tg mice displayed an upregulation in the transcription of IFN-α and IFN-β genes (Fig. 1E, 1F).
FIGURE 1.
Administration of DNase diminishes BAFF induction and leads to abrogation of an E2-induced increase in type 1 IFNs and IFN-inducible genes in R4ATg mice. DNase treatment of R4ATg mice restores E2-induced BAFF mRNA levels (A) as well as serum BAFF levels (B) to that of levels observed in P-treated mice after 5 wk of treatment, whereas treatment with HI DNase did not affect E2-induced BAFF levels. C–F, An upregulation of type 1 IFNs (IFN-α,β), as well as the IFN-inducible genes ifi202 and mx1, was observed in E2-treated R4ATg mice. Administration of DNase, but not HI DNase, resulted in diminution of IFN-α, IFN-β, ifi202, and mx1 transcription to levels comparable to P-treated mice. RNA from splenocytes was analyzed for expression of BAFF and the IFN-inducible genes ifi202 and mx1, whereas RNA from mouse splenic DCs treated with serum from P, E2, E2 plus DNase, or E2 plus HI DNase R4A Tg mice was analyzed for IFN-α and IFN-β expression by real-time PCR. Unpaired t test was used to analyze the statistical differences in BAFF, ifi202, mx1, and IFN-α, and the exact Kruskal-Wallis test to determine the statistical significance in IFN-β between groups (*p < 0.04). Six to nine mice were studied per treatment group. For IFN-α and IFN-β assay, n = 3–4.
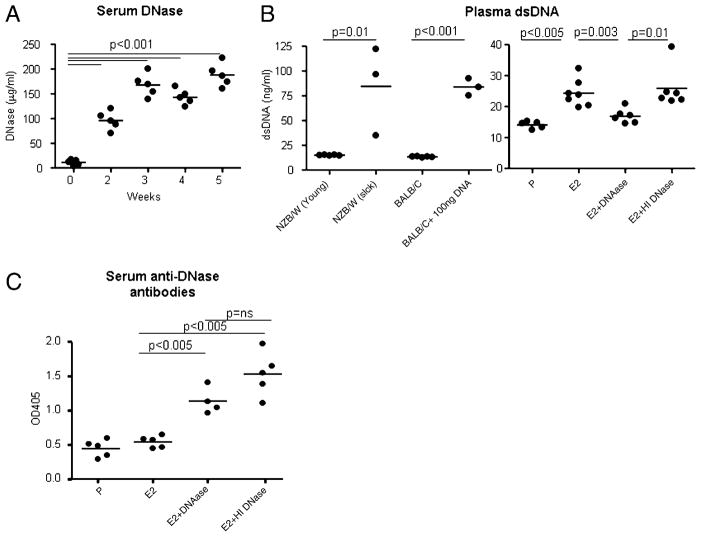
To determine whether E2 was directly responsible for the induction of a proinflammatory milieu or whether the production of proinflammatory cytokines was secondary to the presence of DNA-containing immune complexes, R4A Tg mice were injected with 450 μg bovine pancreatic DNase daily i.p. for 5 wk during the period of treatment with E2 to reduce the availability of DNA. To confirm that the exogenous DNase altered serum levels of DNase, a DNase ELISA was performed. An increase in serum DNase levels was observed throughout the treatment period, stabilizing by 3 wk (Fig. 2A). Furthermore, bovine pancreatic DNase was biologically active in mouse circulation, as determined by a decrease in plasma DNA detectable by the dsDNA-specific pico-green assay (Fig. 2B). Administration of HI DNase did not increase plasma dsDNA levels. This was observed as early as 2 wk following initiation of treatment. Because the DNase was active, we could investigate the effects of lowering the concentration of endogenous DNA in B cell selection and development of a lupus-like serology. As the source of DNase was bovine pancreas, there was a possibility that mice would mount an Ab response to DNase itself. Treatment with bovine pancreatic DNase, both native and HI, induced an anti-DNase response in R4A mice (Fig. 2C). However, bovine pancreatic DNase decreased endogenous DNA levels in mouse serum; thus, there was residual DNase activity not neutralized by anti-DNase Abs.
FIGURE 2.
Bovine pancreatic DNase is biologically active in vivo. A, A detectable increase in serum DNase levels was observed upon administration of 450 μg bovine pancreatic DNase in R4ATg mice as measured by ELISA. B, A significant decrease in circulating plasma dsDNA levels was observed in E2-treated R4A Tg mice administered DNase, but not HI DNase compared with E2-treated R4A Tg mice. As controls for the picogreen assay, plasma dsDNA levels were measured in BALB/c mice and young and sick NZB/W mice. C, Treatment with bovine pancreatic DNase resulted in the generation of anti-DNase Abs in R4A Tg mice. R4A Tg mice were treated with P, E2, E2 plus DNase, and E2 plus HI DNase for 6 wk. After 6 wk, anti-DNase Abs were detected in serum by ELISA using serum at 1:250 dilution. Four to seven mice per group were used for the studies.
Both the increased BAFF and the induction of type 1 and type 2 IFNs as well as IFN signature were reversed by administration of DNase, but not by HI DNase, demonstrating that E2 did not by itself cause these effects (Fig. 1). The elimination of BAFF overexpression by exogenous DNase strongly suggests that DNA-containing immune complexes were responsible for this feature of SLE and that E2 does not directly upregulate BAFF. DNase treatment also caused a decline in the expression of IFN-inducible genes to baseline levels. Thus, E2 does not directly and by itself regulate expression of IFNs as well as IFN-inducible genes ifi202b and mx-1. Rather, DNA, presumably in TLR9-activating immune complexes, is responsible for establishing the proinflammatory milieu induced by E2 exposure. These observations suggest that DNA-containing immune complexes can activate DCs sufficiently in vivo to establish a proinflammatory milieu.
DNase treatment results in preferential elimination of high-affinity anti-DNA MZ B cells induced by E2
Previously, we demonstrated that the strength of the BCR signaling is diminished by E2 exposure (36). Engagement of the BCR following E2 exposure results in a lower calcium flux and decreased ERK phosphorylation (36). This is associated with an expansion of transgene-expressing B cells and more high-affinity DNA-reactive B cells with a MZ phenotype (35, 38). Because we know the repertoire of L chains that fails to generate DNA binding, the repertoire that generates low-affinity DNA binding, and the repertoire that generates high-affinity DNA binding (Table I) (33, 34), we can examine L chain usage in transgene-expressing B cells and determine how different manipulations of the mice alter B selection. Usually, B cells expressing L chains such as Vκ1A-Jκ1, Vκ1A-Jκ4, and Vκ10-Jκ5 that generate high-affinity DNA-reactive B cells, with apparent affinities of 10−8 to 10−9 M, are eliminated by deletion in R4A mice as immature B cells in the bone marrow and at the transitional stage of B cell maturation in the spleen, whereas B cells expressing L chains such as Vκ1A-Jκ5, Vκ21-Jκ1, and Vκ21-Jκ2 L chains that generate low-affinity DNA-reactive B cells (Table I) are less susceptible to negative selection, and many of these latter B cells, with apparent affinities of 10−6 to 10−7 M, survive and are selected into the mature, immunocompetent repertoire (35).
Table I.
Relative affinities of DNA-reactive B cells from R4A Tg mice
Because essentially all IgG2b-producing B cells express the R4A transgene (41), we can analyze L chain expression in transgene-expressing B cells by focusing on IgG2b+ B cells. Using single-cell PCR analysis, we have demonstrated in earlier studies that E2 treatment of R4A Tg mice leads to a shift in the DNA-reactive B cell repertoire, with an increase in high-affinity DNA-reactive B cells in both the transitional and mature B cell repertoire and a decrease in low-affinity DNA-reactive B cells in the mature B cell subset (35). Because we demonstrated that DNase led to an elimination of the effects of DNA-containing immune complexes, we were interested in ascertaining whether DNase treatment also led to an alteration in the E2-induced shift in the B cell repertoire in R4A Tg mice or merely led to a reduction in the amount of Ag available to form immune complexes. L chain sequences were, therefore, determined in both transitional and mature Tg+ B cells isolated from P-, E2-, E2 plus DNase-, or E2 plus HI DNase-treated R4A Tg mice (three in each group, yielding a total of 110, 114, 135, and 114 sequences). We identified 23 different Vκ-Jκ L chains in all experimental groups, 10 of which were commonly expressed in all the groups. In R4A mice, Vκ4/5 L chains predominated (40%), followed by Vκ1 (22%) and Vκ21 (12%) L chains. In contrast, E2 treatment resulted in a predominant Vκ1 L chain usage (45%), followed by Vκ21 (21%) and Vκ9/10 (10%) L chains, as identified in our previous studies (35). Interestingly, DNase treatment shifted the B cell repertoire toward that observed in P-treated mice with predominant usage of Vκ4/5 L chains, followed by Vκ1 and Vκ21 L chains.
The transitional and mature R4A-expressing B cells in E2-treated mice expressed L chains that generate high-affinity DNA reactivity at a frequency of 23 and 29%, respectively, whereas only 12 and 8%, respectively, of immature and transitional Tg+ B cells in P-treated mice expressed L chains that give rise to high-affinity DNA-reactive B cells. Upon DNase administration, E2-treated R4A Tg mice expressed L chains that confer high-affinity DNA reactivity in 13 and 11% of transitional and mature B cells, respectively (Table II). HI DNase treatment did not alter the E2-induced repertoire, demonstrating that active DNase was required for the reversion of the B cell repertoire to that present in P-treated mice.
Table II.
Frequency of high-affinity and low-affinity DNA-reactive B cells in R4A Tg mice treated with E2 with or without DNase
| Placebo (%) | E2 (%) | E2 + DNase (%) | E2 + HI DNase (%) | |
|---|---|---|---|---|
| Transitional | 6/50 (12) | 12/52 (23)* | 8/63 (12.6)ns | 13/47 (29.7)* |
| Mature | ||||
| High affinity | 5/60 (8.3) | 18/62 (27.7)* | 8/72 (11.1)ns | 13/57 (22.8)* |
| Low affinity | 11/60 (18) | 5/62 (8)* | 18/72 (25) | 4/57 (7)* |
A significant increase in high-affinity anti-DNA B cells in R4A Tg mice treated with E2 was observed and was abrogated by treatment with DNase, but not HI DNase. Fisher’s exact test was used to analyze significance between the various treatment groups compared with the P-treated group.
p < 0.05.
ns, Not significant.
As previously reported, there was no effect of E2 on the frequency of low-affinity transitional DNA-reactive B cells. In E2-treated mice, there were fewer mature Tg-expressing B cells with L chains that generate low-affinity DNA-reactive B cells than in P-treated mice (Table II). We have previously reported this and believe it reflects a competition for Ag with fewer low-affinity DNA-reactive B cells when high-affinity B cells are present. DNase treatment, but not HI DNase treatment, resulted in a restoration of low-affinity DNA-reactive B cells in the mature Tg+ B cells. Just as the decrease in the frequency of low-affinity DNA-reactive B cells in E2-treated R4A Tg mice probably reflects a failure of these low-affinity DNA-reactive B cells to compete for entrance into follicular niches when high-affinity DNA-reactive B cells escape tolerance, the increase in low-affinity DNA-reactive B cells probably occurs when high-affinity B cells do not survive (35). Taken together, these data suggest that DNase treatment of E2-exposed R4A Tg mice causes preferential elimination of high-affinity DNA-reactive B cells and restoration of low-affinity DNA-reactive B cell population. Moreover, it suggests that the low BCR signal strength is not by itself sufficient to change the B cell repertoire; rather, Ag is required to mediate positive selection.
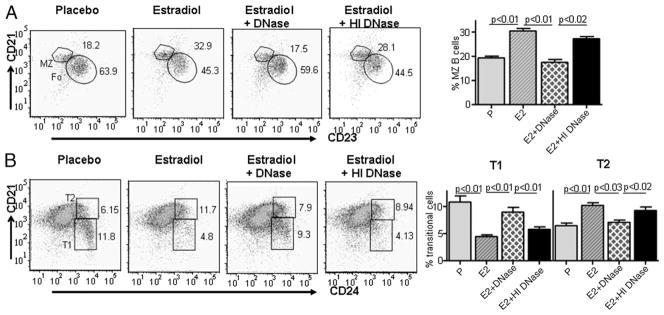
We have demonstrated previously that E2 exposure of R4A Tg mice displayed decreased number of transitional B cells and a shift in T1:T2 ratio, with more T2 cells (38). An increase in the mature B cell population that is comprised of MZ and follicular B cells was observed and the percentage of MZ B cells was doubled (38). We wanted to determine whether DNase treatment could abrogate E2-induced changes in peripheral B cell development. Interestingly, treatment with DNase, but not HI DNase, diminished the 2-fold increase in the MZ B cells seen in E2-treated R4A Tg mice and the transitional T1 and T2 B cells were restored to that observed in the placebo group (Fig. 3).
FIGURE 3.
DNase treatment abrogates E2-induced changes in B cell development in R4A Tg mice. Administration of DNase resulted in reversal of E2-induced increase in MZ B cells (A) and transitional B cells (B). However, HI DNase did not affect E2-induced changes in MZ and transitional B cells. MZ B cells were identified as CD21highCD23negCD24low, and transitional B cells were identified as CD21lowCD24high (T1) and CD21highCD24hgh (T2). Five mice were used in each group.
Serum titers of anti-DNA Abs
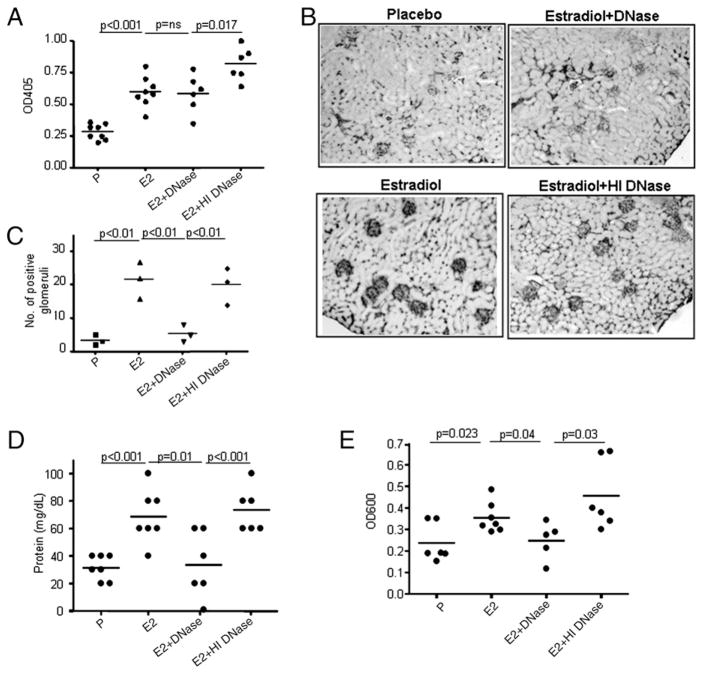
Surprisingly, administration of DNase to E2-treated R4A Tg mice did not decrease the serum titers of anti-DNA Ab to baseline levels (Fig. 4A). Because we knew that the DNase-treated mice harbored few high-affinity DNA-reactive B cells, we reasoned that the Ab titers reflected low-affinity Abs that were not bound to DNA in serum. To further ascertain that administration of DNase to E2-treated R4A mice led to a loss of high-affinity DNA binding, we measured the apparent affinities of the anti-dsDNA Abs in the sera of E2-, E2 plus DNase-, and E2 plus HI DNase-treated R4A Tg mice. The parental R4A mAb has an affinity of ~3.6 ×10−8 M. Sera from E2-treated R4A Tg mice have an apparent affinity of 2.6–5.4 × 10−8 M. Interestingly, sera from E2 plus DNase-treated R4A Tg mice displayed an apparent affinity of 1.4–3.2 × 10−7 M. However, the apparent affinity of sera from R4A Tg mice administered E2 plus HI DNase was comparable to that observed in sera from E2-treated R4A Tg mice (3.7–6.3 × 10−8 M). Thus, high-affinity DNA-reactive B cells present in E2-treated mice were secreting Ab into serum.
FIGURE 4.
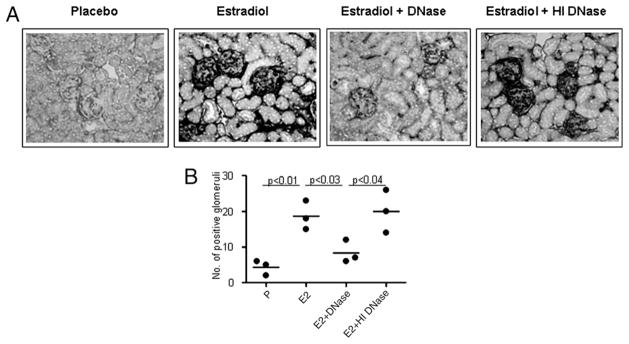
Treatment with DNase alleviates E2-induced target organ damage despite persistently elevated serum anti-dsDNA Ab titers. A, Serum anti-dsDNA Ab levels in R4A Tg mice treated with E2, E2 plus DN-ase, and E2 plus HI DNase for 6 wk. A significant increase in anti-dsDNA Ab levels in sera of R4A Tg mice was observed after implantation with E2 pellets and was unaltered by DNase administration. B, Glomerular IgG deposition in R4A Tg mice following administration of E2, E2 plus DNase, or E2 plus HI DNase. C, The number of positive glomeruli was counted in three different microscopic fields in each section. The average number of positive glomeruli in three individual mice in each group is represented. DNase treatment, but not HI DNase treatment of E2-treated R4A Tg mice resulted in a marked decrease in Ab deposition in the kidney. A representative of five mice per group is shown at original magnification ×5. Proteinuria was measured in five P-, E2-, E2 plus DNase-, and E2 plus HI DNase-treated R4A Tg mice using reagent strips (D) and by the Coomassie blue reagent (E). Proteinuria was increased in E2-treated R4ATg mice and was diminished upon administration of DN-ase. Treatment with HI DNase did not affect the E2-induced increase in proteinuria levels.
Previously, we have shown that E2-treated R4A Tg mice display immune complex deposition in the kidneys and that only high-affinity Abs deposit in the kidney (33). DNase treatment of E2-treated R4A Tg mice resulted in a marked decrease in Ab deposition in the kidney (Fig. 4B, 4C) that correlated with a decrease in proteinuria (Fig. 4D, 4E); HI DNase failed to affect immune complex deposition (Fig. 4B, 4C). Whereas these data were consistent with the observation that the anti-DNA Abs in E2 plus DNase-treated mice were of low affinity, they might also reflect an enzymatic removal of accessible Ag from the glomeruli. We, therefore, asked directly whether the serum of E2-exposed DNase-treated mice had glomerulotropic potential.
The parental R4A Ab with an affinity of 10−8 has been shown to deposit in the glomeruli of the kidneys in SCID mice when administered i.p. (44). This approach permits a study of the potential pathogenicity of anti-DNA Abs. To confirm that the administration of DNase to E2-treated R4A Tg mice resulted in the accumulation of low-affinity anti-DNA Abs that are nonglomerulotropic, the serum from E2-treated R4A Tg mice given DNase was assayed for glomerular deposition in SCID mice. As shown in Fig. 5, serum from E2-treated R4A Tg mice bound strongly to glomeruli; however, the serum from E2 plus DNase-treated R4A Tg mice did not deposit in kidneys of SCID mice. IgG in serum from E2 plus HI DNase-treated R4A Tg mice bound to glomeruli similar to IgG in serum from E2-treated R4A Tg mice (Fig. 5). These data corroborate the repertoire analysis and the analysis of serum Ab affinity.
FIGURE 5.
Serum from E2-treated, but not E2 plus DNase-treated R4A Tg mice leads to glomerular IgG deposition in SCID mice. SCID mice were injected i.p. with 100 μl serum from P-, E2-, E2 plus DNase-, or E2 plus HI DNase-treated R4A Tg mice. Mice were sacrificed 24 h later, and the kidneys were stained for IgG deposition (A). The IgG-positive glomeruli were counted in three different microscopic fields for each section. The average number of IgG-positive glomeruli in three individual mice in each group is represented (B). Serum from E2-treated, but not E2 plus DNase-treated R4A Tg mice resulted in glomerular deposition. A representative section from each group is shown at original magnification ×20. IgG from E2-treated R4A Tg mice deposits in kidneys; however, IgG from E2 plus DNase-treated mice does not deposit in glomeruli. HI DNase does not prevent IgG deposition in E2-treated R4A Tg mice.
Discussion
It is of considerable interest that the presence of anti-DNA Abs is sufficient to increase BAFF levels in the serum and to induce an IFN signature in splenocytes. That DNA-containing immune complexes can induce increased BAFF expression in DCs has been shown in in vitro studies (54). The data reported in this study demonstrate that a proinflammatory milieu can be generated in a non-spontaneously autoimmune host just by virtue of inducing high-affinity anti-DNA Abs. From our studies it is apparent that E2 alone did not directly cause the proinflammatory milieu, as administration of DNase to E2-treated mice totally abrogated the inflammatory response. This observation also confirms that some anti-DNA Abs exist in immune complexes and that the availability of DNA in a mouse with no apparent defect in clearance of apoptotic debris is sufficient to form proinflammatory immune complexes. Furthermore, it supports the hypothesis that TLR9 activation can lead to the upregulation of inflammatory cytokines. This is a contentious issue, as some, but not all, lupus-prone strains of mouse display improvement with a deletion of TLR9 (55–58). Similarly, studies of human SLE have been contradictory, with some investigators suggesting that DNA-containing immune complexes induce the IFN signature and others arguing that R4A-containing immune complexes, which activate TLR7, are more important (25, 26, 59). It has also been shown that relatives of patients with SLE exhibit high serum levels of type 1 IFN, suggesting that there is a predisposition to enhanced IFN production in SLE patients (51). This is consistent with an IFN regulatory factor 5 susceptibility allele in this disease, which has been identified in genome-wide scans (60). This study, however, shows that elevated serum titers of high-affinity anti-DNA Abs are necessary to induce DC activation in a host with unimpaired clearance of apoptotic debris and no pre-existing overexpression of inflammatory cytokines. Because it now seems that the systemic immune activation present in SLE may contribute to accelerated atherosclerosis (61), the fact that anti-DNA Abs alone can initiate an inflammatory cascade may inform therapeutic strategies. It should be noted that E2-treated mice given either DNase or HI DNase might have circulating immune complexes composed of enzyme and anti-DNase Ab. These complexes did not appreciably alter the inflammatory milieu as E2- and E2 plus HI DNase-treated mice appear similar.
It is apparent from these studies that the production of high-affinity DNA-reactive Abs triggers a positive feedback loop. The ensuing elevation in BAFF can function to facilitate the survival and maturation to immunocompetence of more autoreactive B cells. Such a model has clearly been demonstrated in mouse studies and is highly likely to apply in humans as well (62). This would explain the high BAFF levels even in patients who are not B cell lymphopenic.
It was perhaps most surprising that the enhanced number of high-affinity DNA-reactive B cells in the transitional and mature B cell compartments of E2-treated mice depends on the presence of DNA. Having previously shown that E2 exposure diminishes the strength of BCR signaling (36), we assumed that there would be less negative selection of high-affinity DNA-reactive B cells independent of the presence of Ag. The data reported in this study, however, strongly suggest that differentiation to a mature state requires positive selection. Thus, in the absence of an adequate exposure to DNA, high-affinity DNA-reactive B cells did not mature to immunocompetence, despite a lower BCR signaling capacity and reduced negative selection.
Low-affinity anti-DNA Abs do not initiate renal inflammation and do not form immune complexes that activate TLR9. It is also possible that the low-affinity Abs are not present in immune complexes, whereas the high-affinity Abs form immune complexes in plasma, and that these differences may contribute to the difference in glomerular deposition. In parallel, it is clear that some individuals with high titers of anti-DNA Abs do not develop renal disease (63). These individuals may have primarily low-affinity Abs. These data suggest that it may be important to screen patients for the presence of high-affinity anti-DNA Abs to determine appropriate treatment and response to therapy. The current ELI-SAs used in most clinical assays do not distinguish between high-and low-affinity Abs.
These studies also suggest that DNase might be an effective therapy in SLE. Whereas we cannot know that active DNase functions only to decrease Ag load, it clearly led to a reduction in plasma DNA. The DNase1-deficient mouse develops a SLE-like disease (64). A few patients with SLE have been shown to be DNase1 deficient (65), and some patients have been reported to have Abs to DNase (66). One study in NZB/W mice showed a short-term delay in disease onset when DNase treatment was begun prior to disease onset and even showed reduced renal pathology if therapy is begun after onset of disease (39). A second study failed to replicate a reduction in renal disease, but did show a decrease in DNA-reactive B cells, similar to what was seen in the data reported in this study (67). It is possible that the limited success of DNase treatment reflected the production of Abs to exogenous DNase.
The first trial of DNase in patients with SLE was reported in 1961 (68). Eight patients were treated with bovine enzyme, which was highly immunogenic and led to some severe Arthus reactions and early termination of the study. Almost 40 years later, a second clinical trial was initiated (69). Clinical measurements included serum cytokines, serum anti-DNA Abs, and anti-DNA–secreting B cells in peripheral blood. None of these parameters was significantly affected, but there was no detectable increase in serum DNase activity in the patients studied. Thus, it remains unresolved whether DNase therapy might be a nonimmunosuppressive therapeutic strategy in SLE. The studies reported in this work strongly support the need to revisit this question.
Acknowledgments
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to C.M.G.) and the Department of Defense (to B.D.); the National Institutes of Health; a Career Development award from the Systemic Lupus Erythematosus Foundation (to J.V.); and a fellowship from the Arthritis Foundation (to D.K.).
We thank Sylvia Jones for expert secretarial assistance.
Abbreviations used in this article
- AP
alkaline phosphatase
- BAFF
B cell-activating factor
- DC
dendritic cell
- E2
estradiol
- HI
heat-inactivated
- MZ
marginal zone
- NZB/W
New Zealand Black/White
- P
placebo
- SLE
systemic lupus erythematosus
- Tg
transgene
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Plotz PH. The autoantibody repertoire: searching for order. Nat Rev Immunol. 2003;3:73–78. doi: 10.1038/nri976. [DOI] [PubMed] [Google Scholar]
- 2.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 3.ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus: a long-term, prospective study. Arthritis Rheum. 1990;33:634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 4.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 5.Waldman M, Madaio MP. Pathogenic autoantibodies in lupus nephritis. Lupus. 2005;14:19–24. doi: 10.1191/0961203305lu2054oa. [DOI] [PubMed] [Google Scholar]
- 6.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 7.Desai DD, Krishnan MR, Swindle JT, Marion TN. Antigen-specific induction of antibodies against native mammalian DNA in non-autoimmune mice. J Immunol. 1993;151:1614–1626. [PubMed] [Google Scholar]
- 8.Petrakova N, Gudmundsdotter L, Yermalovich M, Belikov S, Eriksson L, Pyakurel P, Johansson O, Biberfeld P, Andersson S, Isaguliants M. Autoimmunogenicity of the helix-loop-helix DNA-binding domain. Mol Immunol. 2009;46:1467–1480. doi: 10.1016/j.molimm.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Cerutti ML, Zarebski LM, de Prat Gay G, Goldbaum FA. A viral DNA-binding domain elicits anti-DNA antibodies of different specificities. Mol Immunol. 2005;42:327–333. doi: 10.1016/j.molimm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Moens U, Mathiesen I, Ghelue MV, Rekvig OP. Green fluorescent protein modified to bind DNA initiates production of anti-DNA antibodies when expressed in vivo. Mol Immunol. 2002;38:505–514. doi: 10.1016/s0161-5890(01)00086-4. [DOI] [PubMed] [Google Scholar]
- 11.Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dryden DT, Tock MR. DNA mimicry by proteins. Biochem Soc Trans. 2006;34:317–319. doi: 10.1042/BST20060317. [DOI] [PubMed] [Google Scholar]
- 13.Sibille P, Ternynck T, Nato F, Buttin G, Strosberg D, Avrameas A. Mimotopes of polyreactive anti-DNA antibodies identified using phage-display peptide libraries. Eur J Immunol. 1997;27:1221–1228. doi: 10.1002/eji.1830270525. [DOI] [PubMed] [Google Scholar]
- 14.Bach JF, Koutouzov S, van Endert PM. Are there unique auto-antigens triggering autoimmune diseases? Immunol Rev. 1998;164:139–155. doi: 10.1111/j.1600-065x.1998.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 15.Isenberg D, Rahman MA, Ravirajan CT, Kalsi JK. Anti-DNA antibodies: from gene usage to crystal structures. Immunol Today. 1997;18:149–153. doi: 10.1016/s0167-5699(97)84659-2. [DOI] [PubMed] [Google Scholar]
- 16.van Venrooij WJ, Pruijn GJ. Ribonucleoprotein complexes as autoantigens. Curr Opin Immunol. 1995;7:819–824. doi: 10.1016/0952-7915(95)80054-9. [DOI] [PubMed] [Google Scholar]
- 17.Desai DD, Marion TN. Induction of anti-DNA antibody with DNA-peptide complexes. Int Immunol. 2000;12:1569–1578. doi: 10.1093/intimm/12.11.1569. [DOI] [PubMed] [Google Scholar]
- 18.Voynova EN, Tchorbanov AI, Todorov TA, Vassilev TL. Breaking of tolerance to native DNA in nonautoimmune mice by immunization with natural protein/DNA complexes. Lupus. 2005;14:543–550. doi: 10.1191/0961203305lu2165oa. [DOI] [PubMed] [Google Scholar]
- 19.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Díaz M, Gallardo G, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 23.Gaipl US, Voll RE, Sheriff A, Franz S, Kalden JR, Herrmann M. Impaired clearance of dying cells in systemic lupus erythematosus. Autoimmun Rev. 2005;4:189–194. doi: 10.1016/j.autrev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Lövgren T, Eloranta ML, Båve U, Alm GV, Rönnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 25.Lövgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Rönnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjögren’s syndrome autoantigen-associated RNA. Arthritis Rheum. 2006;54:1917–1927. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- 26.Eloranta ML, Lövgren T, Finke D, Mathsson L, Rönnelid J, Kastner B, Alm GV, Rönnblom L. Regulation of the interferon-alpha production induced by RNA-containing immune complexes in plasmacytoid dendritic cells. Arthritis Rheum. 2009;60:2418–2427. doi: 10.1002/art.24686. [DOI] [PubMed] [Google Scholar]
- 27.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 28.Rönnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17:394–399. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulé MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–4491. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 31.Offen D, Spatz L, Escowitz H, Factor S, Diamond B. Induction of tolerance to an IgG autoantibody. Proc Natl Acad Sci USA. 1992;89:8332–8336. doi: 10.1073/pnas.89.17.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shefner R, Kleiner G, Turken A, Papazian L, Diamond B. A novel class of anti-DNA antibodies identified in BALB/c mice. J Exp Med. 1991;173:287–296. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci USA. 2000;97:2703–2708. doi: 10.1073/pnas.040577497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spatz L, Saenko V, Iliev A, Jones L, Geskin L, Diamond B. Light chain usage in anti-double-stranded DNA B cell subsets: role in cell fate determination. J Exp Med. 1997;185:1317–1326. doi: 10.1084/jem.185.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17 beta-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol. 2006;176:2703–2710. doi: 10.4049/jimmunol.176.5.2703. [DOI] [PubMed] [Google Scholar]
- 36.Venkatesh J, Peeva E, Xu X, Diamond B. Cutting edge: hormonal milieu, not antigenic specificity, determines the mature phenotype of autoreactive B cells. J Immunol. 2006;176:3311–3314. doi: 10.4049/jimmunol.176.6.3311. [DOI] [PubMed] [Google Scholar]
- 37.Bynoe MS, Spatz L, Diamond B. Characterization of anti-DNA B cells that escape negative selection. Eur J Immunol. 1999;29:1304–1313. doi: 10.1002/(SICI)1521-4141(199904)29:04<1304::AID-IMMU1304>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 39.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macanovic M, Sinicropi D, Shak S, Baughman S, Thiru S, Lachmann PJ. The treatment of systemic lupus erythematosus (SLE) in NZB/W F1 hybrid mice; studies with recombinant murine DNase and with dexamethasone. Clin Exp Immunol. 1996;106:243–252. doi: 10.1046/j.1365-2249.1996.d01-839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawabata D, Venkatesh J, Ramanujam M, Davidson A, Grimaldi CM, Diamond B. Enhanced selection of high affinity DNA-reactive B cells following cyclophosphamide treatment in mice. PLoS One. 2010;5:e8418. doi: 10.1371/journal.pone.0008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ray SK, Putterman C, Diamond B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci USA. 1996;93:2019–2024. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieto A, Gaya A, Jansa M, Moreno C, Vives J. Direct measurement of antibody affinity distribution by hapten-inhibition enzyme immunoassay. Mol Immunol. 1984;21:537–543. doi: 10.1016/0161-5890(84)90070-1. [DOI] [PubMed] [Google Scholar]
- 45.Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc Natl Acad Sci USA. 1997;94:1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crow MK, Wohlgemuth J. Microarray analysis of gene expression in lupus. Arthritis Res Ther. 2003;5:279–287. doi: 10.1186/ar1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, Panoskaltsis-Mortari A, Gregersen PK, Behrens TW, Baechler EC. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 2009;60:3098–3107. doi: 10.1002/art.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Båve U, Alm GV, Rönnblom L. The combination of apoptotic U937 cells and lupus IgG is a potent IFN-alpha inducer. J Immunol. 2000;165:3519–3526. doi: 10.4049/jimmunol.165.6.3519. [DOI] [PubMed] [Google Scholar]
- 51.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoek KL, Carlesso G, Clark ES, Khan WN. Absence of mature peripheral B cell populations in mice with concomitant defects in B cell receptor and BAFF-R signaling. J Immunol. 2009;183:5630–5643. doi: 10.4049/jimmunol.0901100. [DOI] [PubMed] [Google Scholar]
- 53.Erlandsson MC, Jonsson CA, Islander U, Ohlsson C, Carlsten H. Oestrogen receptor specificity in oestradiol-mediated effects on B lymphopoiesis and immunoglobulin production in male mice. Immunology. 2003;108:346–351. doi: 10.1046/j.1365-2567.2003.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K, Tsukada J, Nomura Y, Mimata H, Kubo M, Yoshimura A. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 55.Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–342. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- 56.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and Toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harley JB, Alarcón-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, et al. International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN) Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sherer Y, Zinger H, Shoenfeld Y. Atherosclerosis in systemic lupus erythematosus. Autoimmunity. 2010;43:98–102. doi: 10.3109/08916930903374527. [DOI] [PubMed] [Google Scholar]
- 62.Kalled SL. The role of BAFF in immune function and implications for autoimmunity. Immunol Rev. 2005;204:43–54. doi: 10.1111/j.0105-2896.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 63.Manson JJ, Ma A, Rogers P, Mason LJ, Berden JH, van der Vlag J, D’Cruz DP, Isenberg DA, Rahman A. Relationship between anti-dsDNA, anti-nucleosome and anti-alpha-actinin antibodies and markers of renal disease in patients with lupus nephritis: a prospective longitudinal study. Arthritis Res Ther. 2009;11:R154. doi: 10.1186/ar2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Möröy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 65.Yasutomo K, Horiuchi T, Kagami S, Tsukamoto H, Hashimura C, Urushihara M, Kuroda Y. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet. 2001;28:313–314. doi: 10.1038/91070. [DOI] [PubMed] [Google Scholar]
- 66.Yeh TM, Chang HC, Liang CC, Wu JJ, Liu MF. Deoxyribonuclease-inhibitory antibodies in systemic lupus erythematosus. J Biomed Sci. 2003;10:544–551. doi: 10.1159/000072382. [DOI] [PubMed] [Google Scholar]
- 67.Jacob M, Napirei M, Ricken A, Dixkens C, Mannherz HG. Histopathology of lupus-like nephritis in Dnase1-deficient mice in comparison to NZB/W F1 mice. Lupus. 2002;11:514–527. doi: 10.1191/0961203302lu242oa. [DOI] [PubMed] [Google Scholar]
- 68.Lachmann PJ. Allergic reactions, connective tissue, and disease. Sci Basis Med Annu Rev. 1967;1967:36–58. [PubMed] [Google Scholar]
- 69.Davis JC, Jr, Manzi S, Yarboro C, Rairie J, Mcinnes I, Averthelyi D, Sinicropi D, Hale VG, Balow J, Austin H, et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus. 1999;8:68–76. doi: 10.1191/096120399678847380. [DOI] [PubMed] [Google Scholar]