Abstract
Purpose of review
Estimated GFR is now commonly reported by clinical laboratories. Here we review the performance of current creatinine and cystatin C based estimating equations as well as demonstration of their utility in public health and clinical practice.
Recent findings
Lower levels of GFR are associated with multiple adverse outcomes, including acute kidney injury and medical errors. The new CKD-EPI equation improves performance and risk prediction compared to the MDRD Study equation. Current cystatin C based equations are not accurate in all populations, even in those with reduced muscle mass or chronic illness, where cystatin C would be expected to outperform creatinine. eGFR reporting has led to a greater number of referrals to nephrologists, but the increased numbers do not appear to be excessive or burdensome The MDRD Study equation appears to be able to provide drug dosage adjustments similar to the Cockcroft and Gault.
Summary
Estimated GFR and their reporting can improve and facilitate clinical practice for chronic kidney disease. Understanding strengths and limitations facilitates their optimal use. Endogenous filtration markers, alone or in combination, that less dependent on non GFR determinants of the filtration markers are necessary to lead to more accurate estimated GFR.
Keywords: glomerular filtration rate, creatinine, cystatin, prognosis
INTRODUCTION
The level of glomerular filtrate rate (GFR) is accepted as the most useful index of kidney function in health and disease. Chronic kidney disease is defined as GFR less than 60 ml/min per 1.73 m2 as well as markers of kidney damage [1, 2]. Reduction in GFR is associated with symptoms and laboratory manifestations of kidney disease [2]; as well as cardiovascular disease [3–5]; and as has more recently been demonstrated, acute kidney injury [6] and medical errors [7]. Given the centrality of GFR to CKD diagnosis, evaluation and management, the National Kidney Education Detection Program (NKDEP) [8] in the United States and organizations in other countries recommend to laboratories to report estimated GFR (eGFR) using the MDRD study or other equation whenever a serum creatinine was ordered [9–11]. The reporting of eGFR has been received by some as a landmark in public health campaign for the improvement in care and outcomes for patients with CKD, while others have raised concerns that the limitations of the equations lead to misclassification of patients with CKD [12–16]. Regardless of one’s opinion, it appears as if eGFR will be an increasingly important component of clinical practice. In the United States, recent data show that greater than 77% of laboratories report eGFR [17], and by the end of 2009 the vast majority of these laboratories will use standardized creatinine assays so as to improve the accuracy of these estimates. In addition, new recommendations from NKDEP state that eGFR calculated from the MDRD Study equation can be used for adjustment of medications based on kidney function [18]. In this review, we report on the recent literature (January 2008 to September 2009), which addresses the performance of estimating equations as well as demonstration of their utility in public health and clinical practice. Our perspective is that there are both strengths and limitations of GFR estimates, and while the introduction of eGFR reporting into widespread clinical practice has been an advance, optimal interpretation and use of GFR estimates requires attention to their limitations.
TEXT OF REVIEW
We first review the literature on the development and performance of estimating equations and then review the literature describing their use in clinical practice.
Measured GFR and rationale for estimating equations
The gold standard measurement for GFR requires urinary or plasma clearance of exogenous markers. These measurements are difficult to perform, and GFR is usually estimated from steady-state serum levels of endogenous filtration markers. Estimating equations incorporate demographic and clinical variables as surrogates of unmeasured physiologic processes that also affect the serum level [19, 20]. While equations are more accurate than the serum level of the marker, they only capture the average relationships between the marker and its non-GFR determinants, and, the relationship between the marker and its non-GFR determinants may vary across populations and over time. As such equations may be inaccurate when applied to different populations from which they were derived [20].
Table 1 [21–31] and Table 2 [23, 30–33] describe the methods and results of the studies that have evaluated the performance of creatinine and cystatin C based equations, respectively, from January 2008 to September 2009. Performance of estimating equations can be described according to bias, precision, and accuracy, where bias is defined as systematic deviation of estimated GFR compared to measured GFR using the reference (“gold”) standard; imprecision is defined as random variation (or “spread”) of estimated GFR values centered about the measured values; and accuracy incorporates both bias and imprecision [34]. The specific metrics used for each of these parameters varied across each study. In order to compare across studies, in the tables, we provide a qualitative assessment of the equation that performs best for each of bias, precision and accuracy.
Table 1.
Studies evaluating creatinine based GFR estimating equations*
| Study description | GFR | GFR estimates | Results * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Type | Population or Subgroup | N | Marker | GFR (mean ± SD or range) | Scr calibration | MDRD | CG | Other | Bias | Precision | Accuracy |
| Levey [21] | CP, RS | General Population | 8254 DDS, 3896 EVS | 125I-Iothalamate (U) | 68 ± 40 | Y | Y | N | CKD-EPI | CKD-EPI | CKD-EPI | CKD-EPI |
| <60 | CKD-EPI | CKD-EPI | CKD-EPI | |||||||||
| >60 | CKD-EPI | CKD-EPI | CKD-EPI | |||||||||
|
| ||||||||||||
| Botev [22] | CP | European | 2208 | Inulin (U) | 72.4 ± 39.0 | Indirect | Y | Y | MCQ | CG | MDRD | Similar |
| <60 | 32.4 ± 15.0 | MDRD | MDRD | MDRD | ||||||||
| 60–90 | 75.5 ± 9.0 | CG | MDRD | MDRD | ||||||||
| >90 | 114.5 ± 17.7 | CG | MDRD | CG | ||||||||
| Rigalleau [23] | CP | Diabetes | 124 | 51 Cr-EDTA (U, P) | 10–121 | N | Y | N | NR | MCQ=MDRD | MDRD | |
|
| ||||||||||||
| Fontsere [24] | CP | Diabetes | 118 | 125I-Iothalamate (U) | 96.3 ± 50.9 | N | Y | Y | MCQ | NR | NR | NR |
| Hyperfiltration | 26 | 159.5 ± 18.8 | MCQ | NR | NR | |||||||
| Normal | 56 | 115.6 ± 14.1 | MCQ | NR | NR | |||||||
| CKD stage 3–4 | 36 | 31.2± 10.8 | MCQ | NR | NR | |||||||
|
| ||||||||||||
| Chudleigh [25] | CP | Diabetes | 293 | 51 Cr-EDTA (P) | 114.9 ± 22.4 | Y | Y | N | MDRD IDMS | IDMS | Similar | IDMS |
| GFR<90 | 37 | 83.8 ± 4.3 | IDMS | Similar | Similar | |||||||
| GFR>90 | 256 | 119.4 ± 20.2 | IDMS | Similar | IDMS | |||||||
|
| ||||||||||||
| Sebasky [26] | CP | Kidney donors | 255 | Iohexol (P) | 71.8 ± 11.8 | Y | Y | Y | MCQ | MDRD | MCQ=MDRD | MDRD |
|
| ||||||||||||
| Saleem [27] | CP | General pop (NZ) | 601 | 82.85 (3.91–213.74) | N | Y | MCQ | MDRD | NR | MDRD | ||
| <90 | MDRD | NR | MDRD | |||||||||
| >90 | MDRD | NR | MDRD | |||||||||
|
| ||||||||||||
| Matsuo et al. [28] | CP | Japanese | 350 | Inulin (U) | 57.2 ± 34.7 | Y | N | N | Japanese | Japanese | NR | Japanese |
|
| ||||||||||||
| Li et al. [29] | CP | Chinese | 1415 | 99m Tc-DTPA (NR) | 59.1 ± 36.3 | N | Y | - | Chinese, Japanese modifications | Chinese | NR | NR |
| <30 | 77 | 17.9 ± 7.9 | MDRD | NR | MDRD | |||||||
| 30–59 | 72 | 43.4 ± 8.5 | MDRD | NR | MDRD | |||||||
| 60–89 | 77 | 74.6 ± 8.9 | Chinese | Chinese | ||||||||
| ≥ 90 | 57 | 113.6 ± 18.3 | Chinese | Chinese | ||||||||
|
| ||||||||||||
| Delanaye [30] | CP | Anorexia nervosa | 27 | 51 Cr-EDTA (P) | 67.5 ± 23.1 | Y | Y | Y | - | CG | CG | NR |
|
| ||||||||||||
| Beringer et al. [31] | CP | Cystic Fibrosis | 19 | 125I-Iothalamate (U) | 104 ± 32 | NR | Y | Y | - | MDRD | CG | CG |
| Healthy volunteers | 19 | MDRD | MDRD | MDRD | ||||||||
Includes studies published between January 2008 to September 2009
P: Plasma, U: Urine; NR: Not Reported; CP-Clinical Population; Cr, creatinine; MDRD, Modification of Diet in Renal Disease study equation; MCQ; Mayo Clinic Equation; CG, Cockcroft and Gault equation
Table 2.
Studies evaluating cystatin C based glomerular filtration rate estimating equations*
| Study description | Measured GFR | Equations | Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Type | Population | N | Method | Mean ± SD or range | Cr Calibration | Cys | Cr | Cys and Cr | Bias | Precision | Accuracy |
| Rigalleau [23] | CP | Diabetes | 124 | Cr 51-EDTA (P, U) | 10–121 | N | MDRD; MCQ | Rule KI; composite (cys±cr) | composite (cys±cr) | composite (cys±cr) | composite (cys±cr) | |
| Delanaye [30] | CP | Anorexia nervosa | 27 | Cr 51-EDTA (P) | 67.5 ± 23.1 | Y | Rule[74] Larsson[75] Stevens [43] | CG, MDRD | Stevens | eGFRcys (Rule) | All similar except MDRD | NR |
| Beringer [31] | CP | Cystic Fibrosis | 19 | 125I-Iothalamate (U) | 104 ± 32 | NR | Tidman [76] | CG, MDRD | eGFRcys | eGFRcys | eGFRcys | |
| Healthy volunteers | 19 | 125I-Iothalamate (U) | 105 ± 30 | eGFRcr (MDRD) | eGFRcys | eGFRcr (MDRD) | ||||||
| Sterner [32] | CP | non renal transplant-CKD | 425 | Iohexol (P) | 42(8–115) | Y | Grubb [77] | MDRD | eGFRcys | Similar | eGFRcys | |
| Willems et al. [33] | CP | Diabetes | 67 | Cr 51-EDTA (NR) | 110 (44–328) | Y | Cys C level | MDRD | NR | NR | Similar | |
| I. | Healthy volunteers | 19 | 125I-Iothalamate (U) | 105 ± 30 | eGFRcr (MDRD) | eGFRcys | eGFRcr (MDRD) | |||||
Includes studies published between January 2008 to September 2009
P: Plasma, U: Urine; NR: Not Reported; CP-Clinical Population; Cr, creatinine, Cys, cystatin C, eGFRcys, estimated GFR from cysatin C; eGFRcr, estimated GFR from creatinine
Creatinine
Eleven studies described the development or evaluation of creatinine based equations (Table 1) [21–31] Of those, 6 studies (55%) used standardized creatinine, which is an increase from our prior review where 28% of the studies used appropriate creatinine methods [35]. The three creatinine equations used in these studies were the MDRD Study, Cockroft-Gault and the Mayo Clinic equations [21, 36, 37].
General or diverse populations
Three studies evaluated equations in general or diverse population samples (Table 1). One study compare the MDRD study equation to the Mayo Clinic equation in the general population in New Zealand and showed the MDRD Study equation performed better across all three measures of performance This is inconsistent with the fact that Mayo clinic equation was developed in a population with and without CKD, whereas the MDRD Study equation was developed in populations with CKD. Another study evaluated the Cockroft-Gault and the MDRD Study equations in 2208 Europeans and showed lesser bias with the Cockroft-Gault equation but greater precision with MDRD Study equation [22]. In May 2009, a new equation, the CKD-EPI equation, was developed in a pooled dataset from 10 studies that included participants of diverse clinical characteristics, with and without kidney disease, and validated in a separate dataset pooled from 16 additional studies [21]. In the 16 studies used for its validation, the CKD-EPI equation was more accurate than the MDRD Study equation with lower bias especially at an estimated GFR greater than 60 ml/min per 1.73 m2; however precision was not substantially improved compared to the MDRD Study equation [21].
Special Populations
Diabetes
Three studies evaluated the performance of the estimating equations in patients with diabetes [23–25]. Overall, there does not appear to be a clear consensus that one equation is better, but most studies did not use standardized creatinine and therefore optimal comparisons were not possible.
Kidney Donors
One study compared the performance of the Cockroft-Gault, MDRD Study and Mayo Clinic equations in 255 kidney donors [26]. The MDRD Study equation performed better than the Mayo Clinic and Cockroft-Gault equations, consistent with the findings from the study in the general population in New Zealand described above [27].
Asians
Matsuo and colleagues modified the MDRD Study equation for use in the Japanese population and standardized creatinine in 413 participants and validated in 350 participants[28]. The modification leads to a lower GFR estimate for the same level of creatinine. The new equation led to improved performance compared to the original MDRD Study equation as well as their prior modification of the MDRD Study equation for use with non-standardized creatinine methods [38].
Another study evaluated the performance of original MDRD Study equation and the MDRD Study equation modified with the Chinese and Japanese coefficients in a Chinese population [29]. In contrast to the Japanese coefficient, the Chinese coefficient leads to a higher GFR estimates for the same level of creatinine. In patients with eGFR greater than 90 ml/min per 1.73 m2, bias and accuracy improved with the use of the Chinese coefficient compared to the original MDRD Study; however, in the GFR range 30–59 ml/min per 1.73 m2, the use of the Chinese coefficient lead to worse bias and accuracy compared to the MDRD Study equation.
In the Chinese population, the Japanese coefficient had greater bias across all GFR levels. This has been posited to be due to differences in GFR measurement methods, creatinine calibration, or true differences in the study populations [39].
Cystatin C
Cystatin C is under investigation as a replacement for serum creatinine in estimating the GFR [40–42]. Prior studies demonstrated that the combination of cystatin C and creatinine provides the best estimate [43]. A convergence of evidence now suggests that, in contrast to early reports, there are non GFR determinants of its serum level [44, 45]. Nevertheless, these data also suggest that cystatin C is less dependent upon muscle mass than creatinine, and should provide more accurate GFR estimates particularly in populations with differences in muscle mass. We identified 5 studies that evaluated cystatin C based equations (Table 2) [23, 30–33], two of which tested the performance in populations with decreased muscle mass.
CKD population
Sterner and colleagues compared the performance of MDRD Study equation to the Grubb cystatin C based equation [32, 46]. Cystatin C based equation performed superior in terms of bias; although accuracy was similar with the MDRD Study equation.
Diabetes
Willems and colleagues found the MDRD Study equation and the serum level of Cystatin C were similarly accurate [33]. Whereas, Rigalleau et al. showed that the composite equation with both cystatin C and creatinine performed better than any of the creatinine based equations [23].
Anorexia Nervosa
Delanaye and colleagues studied 27 patients with anorexia nervosa [30]. For eGFR less than 60 ml/min per 1.73 m2, the Rule cystatin C equation had the lowest bias. For all other levels of GFR, the Cockcroft and Gault equation had the lowest bias among all cystatin C and creatinine based equations. The improvement with Cockcroft and Gault over other creatinine based equations may be expected given the inclusion of weight in the numerator that leads to more accurate estimates in this extremely underweight population. However, the greater accuracy compared to cystatin C based equations is surprising and may suggest an independent relationship between weight and cystatin C.
Cystic Fibrosis
Beringer and colleagues compared the performance of cystatin based equations to the Cockroft-Gault and MDRD Study equations in patients with cystic fibrosis [31]. The cystatin C based equation outperformed the other equations in terms of precision and accuracy, however, bias was not substantially different among the three equations.
Use of Estimating Equations
GFR estimating equations can be used for detection, evaluation and management of kidney disease. Here, we describe the literature on their use for detection, assessment of change over time of kidney function, referral to nephrologist, drug dosage adjustment and cardiovascular disease prognosis.
Detection of Chronic Kidney Disease
Detection of CKD is important in studies of CKD prevalence and in screening populations at increased risk to detect patients for early treatment. Numerous studies now document the high prevalence of CKD around the world. Several recent studies highlight the difficulty in detecting CKD using estimating equations.
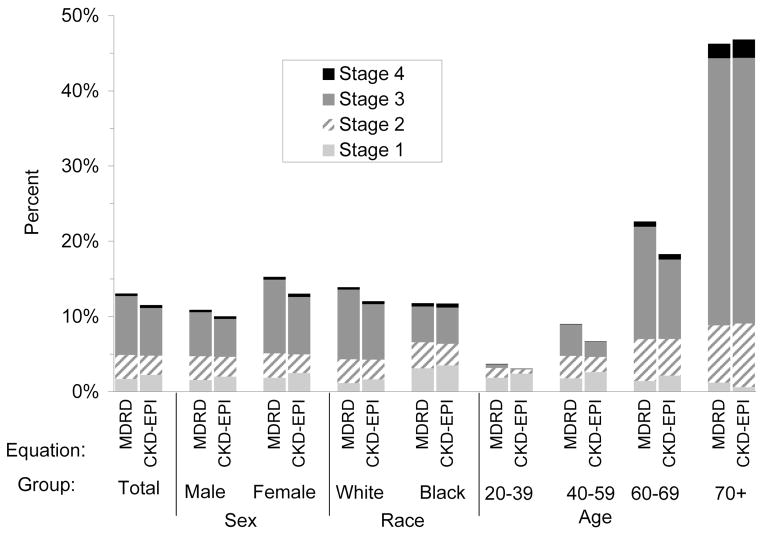
Levey and colleagues compared the distribution of estimated GFR and prevalence of CKD in US adults using the MDRD Study and CKD-EPI creatinine equations as applied to NHANES 1988–2006 [21]. As expected, due to the lesser bias of the CKD-EPI equation compared to the MDRD Study equation, the median eGFR for the population was higher (94.5 vs. 85.0 ml/min/1.73 m2) (Figure 1) and the overall prevalence was lower (11.5 vs. 13.1%, respectively).
Figure 1. Comparison of distribution of estimated GFR and Chronic Kidney Disease (CKD) prevalence by age (NHANES 1999–2004).
Prevalence of CKD by age. CKD stages were categorized based on the classification system established by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Previously Published in Levey AS, Stevens LA, Schmid CH, et al., A new equation to estimate glomerular filtration rate. Ann Intern Med, 2009. 150(9): p. 604-12.
Astor and colleagues compared the distribution of eGFR and prevalence of CKD using the MDRD Study and CKD-EPI cystatin C equations in NHANES 1988–1994 [43, 47]. Compared to the MDRD Study equation, use of cystatin C alone or in combination with creatinine led to lower eGFR distributions and higher prevalence estimates of CKD. Foley and colleagues compared trends over time in the distribution of estimated GFR using creatinine and cystatin C in NHANES 1988–84 and 1999–2002 [48]. They found a decrease in eGFR based on creatinine, as had been reported previously [49], but not in eGFR based on cystatin C. Possible explanations for this discrepancy are differences over time in assay calibration of creatinine or cystatin C, or changes over time in the distribution of non-GFR determinants of these markers.
Monitoring Progression of Chronic Kidney Disease
All currently used estimating equations have been developed from cross-sectional databases. Prior analyses of the African American Study of Hypertension and Kidney Disease (AASK) demonstrated that eGFR leads to estimates of a slower but more precise slope of GFR decline than measured GFR. In this review period 3 additional studies evaluated the performance of estimating equations to estimate changes over time in measured GFR.
In the MDRD Study, there was a 28% slower mean rate of decline in eGFR vs. measured GFR and only dietary protein affected the difference between estimated and measured GFR slope [50]. Nonetheless, differences in slope estimates were greater than 2.0 ml/min/173 m2 per year in 41% of patients, due either to differences in non-GFR determinants of serum creatinine or imprecision in measured GFR. A second study compared eGFR using several equations with measured GFR over time in 155 patients in Australia [51]. Over time, the mean difference between estimated and measured GFR was relatively stable, especially in patients with lower GFR. Finally, Abraham and colleagues developed an equation to estimate longitudinal change in GFR from prior measured GFR and changes over time in covariates in children participating in the Chronic Kidney Disease in Children Cohort Study (CKiDS) [52]. Changes in height and serum creatinine were the most important covariates. Notably, when initial GFR measurement was not used, addition of changes in covariates did not add to the accuracy of estimates based on a cross-sectional equation.
Referral to Nephrologists
Three recent reports have described the impact of eGFR reporting on referral to nephrologists. After implementation of eGFR reporting in Ontario, Canada, non-urgent nephrology referrals increased from 134 per nephrologist per year to 156 per nephrologist per year [53, 54]. The 22 additional consults per nephrologist per year translates into an increase in the rate of referral by 2.9 consults per 100,000 population, with a greater increase in women and the elderly, consistent with bias in these groups by serum creatinine. In an evaluation of eGFR reporting in Australia, monthly referrals increased by 40% following the introduction of eGFR reporting [55]. The appropriateness of nephrology referrals fell criteria, although a greater number of CKD patients were appropriately referred [56]. In a UK study, there was an initial increase in the number of referrals following institution of eGFR reporting, which was reversed by the introduction of a referral management program.
Use of GFR estimating equations for drug dosage adjustment
In its Guidance to Industry, the FDA states that the method for assessment of kidney function that is most widely used in clinical practice ought to be the method used for adjustment of drug dosages [57]. At the time, the Cockcroft and Gault was widely used and the FDA provided this equation as an example an estimate that could be used. Since then, the MDRD Study equation is now more widely reported and creatinine assays are standardized to gold standard methods [17].
Several studies have compared the two equations for drug dosing purposes. Table 3 lists the studies that have compared the two equations for this purpose since January 2006 [58–66]. There is substantial heterogeneity in the methods used among the studies, which, complicates comparison of the two equations. First, most studies used Cockcroft and Gault as the gold standard by which to compare the drug dosages. However, since the value determined from the equation is dependent upon the creatinine assay used, this is an inappropriate gold standard [18, 67]. Prior to availability of standardized creatinine assays, there was substantial variation in the creatinine assay used, which caused differences in dosing recommendations resulting from pharmacokinetic studies, even for drugs with the same pharmacokinetics. For the same medications, variation in creatinine assays among clinical laboratories caused differences in drug exposure among patients, even if drug dosing recommendations are followed. Therefore, even if the same equation used in the pharmacokinetic studies was used to assign a drug dosage, the assigned drug dosage would likely be different than intended. Only two studies compared the estimates to the gold standard of measured GFR. Second, three studies mentioned whether creatinine method used was standardized and of those, only two used standardized creatinine value. Third, five studies expressed GFR in ml/min (as appropriate for drug dosing). Finally, of the eight studies, five used actual body weight in the Cockcroft Gault equation, whereas five used ideal body weight.
Table 3.
Summary of Studies Comparing Kidney Function Estimates for Drug Dosage Adjustment
| Study | Methods | Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Patient type | N | BSA | Calibrated Scr | MGFR | eGFR MDRD | eCrCl CG | eCrCl CG-I | eCrCl A | Age | Wt | Summary of Conclusions |
| Stevens [58] | Outpatients or participants in research studies | 5504 | N | Y | Y | 69 | 75 | 62 | N | 47 | 82 | 88–89% for specific drugs; 75–78% for FDA Kidney function categories |
| Lemos [59] | Outpatients with gynecological cancers | 96 | N | N | Y | 79 | 74 | 85 | N | 60 | 62 | MDRD more accurate for carboplantin dosing |
| Bookstaver [60] | ICU patients | 71 | Y | N | N | 69 | NA | 83 | N | 52 | 78 | MDRD more accurate for aminoglycoside AUC |
| Wargo [61] | Inpatients with CKD | 409 | N | N | N | 40 | NA | 35 | N | 74 | 55–73 | 64–80% concordance for specific drugs |
| Gill [62] | Nursing home residents | 180 | Both | Y | N | 72 | 58 | NA | N | 85 | 57.4 | 37% concordance for CKD stages; 73–80% for specific drugs |
| Golik [63] | Inpatients with GFR < 90 | 207 | N | N | N | 69 | 72* | 52* | Y | 64 | 83 | 67–78% concordance for specific drugs |
| Melloni [64] | Inpatients admitted with ACS and enrolled in clinical trial | 46942 | N | D | 66 | NA | 53 | N | 49–82 | 73–100 | 80% concordance for CKD Stages | |
| Moranville [65] | Inpatients with CKD | 4698 | Y | N | N | NP | NP | NP | N | 65–71 | 81–85 | 86–99% concordance for eGFR target thresholds |
| Hermsen [66] | Inpatients with AKD or CKD | 372 | N | N | N | 47 | NA | 34 | Y | 72 | 62 | 64% for specific drugs |
Includes studies published between January 2006 to September 2009
BSA, body surface area; Scr, serum creatinine; mGFR, measured GFR; eGFR, estimated GFR; MDRD, Modification of Diet in Renal Disease study equation; CG, Cockcroft and Gault equation; eCrCl, estimated Creatinine Clearance; CG-I, Cockcroft and Gault equation adjusted for ideal body weight; eCrClA, estimated creatinine clearance using adjusted serum creatinine values; Wt, weight
Studies also differences in the metric used for comparison of the equations. Four studies compared concordance for drug specific dosing levels, with concordance rates ranged from 64 to 89%. Four studies also compared the equations according to predefined CKD stages or eGFR target levels, with reported concordance rates of 37 to 99%. In the two studies that compared estimated GFR to measured GFR, both showed that the MDRD Study equation had greater concordance with measured GFR than the Cockcroft and Gault [58, 59]. One study of inpatients receiving aminoglycoside or vancomycin compared the area under the curve for actual drug levels to the eGFR and showed greater precision for the MDRD Study equation [60]. Importantly, no study looked at adverse outcomes, side effects of ineffective doses.
Prognosis
A number of studies have shown a J-shaped relationship of GFR estimated from serum creatinine and total mortality in studies of the general population [68, 69]. This is likely due to confounding by chronic diseases associated with malnutrition and inflammation causing low creatinine generation and overestimation of measured GFR [70]. These results are especially important in evaluating the risk associated with eGFR <60 ml/min/1.73 m2, since the risk in the reference group is not uniform [71]. When evaluating risk of eGFR 45–59 ml/min/1.73 m2, it is more appropriate to use a more narrow reference group, such as eGFR of 75–89 or 90–104 ml/min/1.73 m2, rather than eGFR >60 ml/min/1.73 m2.
Two recent studies compared the risk of eGFR 45–59 using general population studies. The Atherosclerosis Risk in Communities (ARIC) and the Australian Diabetes, Obesity and Lifestyle study (AusDiab) using the CKD-EPI equation rather than the MDRD Study equation, due to reclassification of low risk patients to higher eGFR when using the CKD-EPI equation [72, 73].
Astor and colleagues compared risk prediction for all cause and cardiovascular disease mortality in NHANES 1999–1994 based on CKD-EPI cystatin C equations and MDRD Study equation [47]. GFR estimates using cystatin C or cystatin C plus creatinine provided more accurate predictions than the MDRD Study equation. In particular, the risk relationship of estimated GFR computed using either cystatin C or cystatin C plus creatinine to mortality was more steep at eGFR >60 ml/min/1.73 m2 compared to the MDRD Study equation.
CONCLUSION
In the current era, there are advances in our understanding of the performance and utilization of GFR estimation. First, the new CKD-EPI equation developed in people with and without kidney disease, and that which uses the same four variables as the MDRD Study equation, but which improves bias and risk prediction, without a decline in accuracy in people with CKD, is an important step forward. The CKD-EPI equation should replace the MDRD Study equation for routine clinical use. Second, there is now recognition that there are non GFR determinants of cystatin C, and therefore while a better predictor of risk, is not necessarily a more accurate estimate of GFR, even in populations with low muscle mass. Indeed, the two studies that evaluated estimating equations in populations with reduced muscle mass, did not clearly show cystatin C to an improvement over creatinine based equations. New GFR estimates that are less 14 dependent upon nonGFR determinants are required to improve GFR estimates across populations as well as within populations over time. Third, the availability of GFR reports has a substantial effect on clinical practice. However, the impact needs to be further studied and education programs as to use these estimates needs to be developed.
Acknowledgments
Sponsorship statements
Lesley Stevens: Research support from the NIH, National Kidney Foundation and Gilead Inc
Smita Padala: none declared
Andrew Levey: Research support from the NIH, National Kidney Foundation, Gilead Inc and Amgen
We are grateful to Aghogho Okparavero MBBS, MPH for his assistance in preparation of the manuscript
Footnotes
Conflict of Interest: none declared
References
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
**of outstanding interest
- 1.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67(6):2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2):S1–266. [PubMed] [Google Scholar]
- 3.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–65. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Astor B, Sarnak M. Evidence for increased cardiovascular disease risk in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. 2004;13(1):73–81. doi: 10.1097/00041552-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 6*.Hsu CY, Ordonez JD, Chertow GM, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008:101–7. doi: 10.1038/ki.2008.107. This study assesses the risk for dialysis-requiring acute renal failure among hospitalized adult members of Kaiser Permanente Northern California. A very strong relationship was seen between the level of GFR and risk for acute renal failure, with the increase in risk observed for GFR < 60 ml/min per 1.73 m2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Seliger SL, Zhan M, Hsu VD, et al. Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol. 2008;19(12):2414–9. doi: 10.1681/ASN.2008010022. This study describes the risk for post surgical adverse safety events, associated with chronic kidney disease in hospitalized patients in the Veteran’s Health Administration. Patients with CKD had a higher risk for errors, even after case-mix adjustment. There was a significant trend for greater association of adverse events with decreasing eGFR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 9.Australian and New Zealand Society of Nephrology (ANZSN) Website. 2008 December 19; Available from: www.nephrology.edu.au.
- 10.La Caisse nationale d'assurance maladie des professions independantes. . Avenant a la convention nationale des directeurs de laboratoire prive d'analyses medicales. 2004 [cited 2008 December 22]; Available from: http://www.admi.net/jo/20030227/SANS0320604X.html.
- 11.Mathew TH. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust. 2005;183(3):138–41. doi: 10.5694/j.1326-5377.2005.tb06958.x. [DOI] [PubMed] [Google Scholar]
- 12.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009 doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Stevens LA, Levey AS. Chronic kidney disease is common: what do we do next? Nephrol Dial Transplant. 2008;23(4):1122–5. doi: 10.1093/ndt/gfn117. [DOI] [PubMed] [Google Scholar]
- 14.Glassock RJ, Winearls C. An epidemic of chronic kidney disease: fact or fiction? Nephrol Dial Transplant. 2008;23(4):1117–21. doi: 10.1093/ndt/gfn086. [DOI] [PubMed] [Google Scholar]
- 15.Glassock RJ, Winearls C. CKD--fiction not fact. Nephrol Dial Transplant. 2008;23(8):2695–6. doi: 10.1093/ndt/gfn331. author reply 2696–9. [DOI] [PubMed] [Google Scholar]
- 16.Eckardt KU, Berns JS, Rocco MV, et al. Definition and classification of CKD: the debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53(6):915–20. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 17*.Miller WG. Estimating glomerular filtration rate. Clin Chem Lab Med. 2009;47(9):1017–9. doi: 10.1515/CCLM.2009.264. This editorial provides new figures on the percentage of clinical laboratories reporting estimated GFR in the United States, which is up to 77 from 75% in the previous report. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Disease Education Program. Estimation of Kidney Function for Medication Dosage Prescriptions in Adults. 2009 [cited 2009 August 4]; Available from: http://www.nkdep.nih.gov/
- 19.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20(11):2305–13. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 21**.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. The CKD-EPI equation is a new equation to estimate GFR from serum creatinine. It was developed in a diverse dataset of people with and without kidney disease and validated in a several set of studies. It shows improved performance compared to MDRD study equation, especially at higher levels of GFR. Its use leads to a lower prevalence of CKD in the United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botev R, Mallie JP, Couchoud C, et al. Estimating glomerular filtration rate: Cockcroft-Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol. 2009;4(5):899–906. doi: 10.2215/CJN.05371008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigalleau V, Beauvieux MC, Le Moigne F, et al. Cystatin C improves the diagnosis and stratification of chronic kidney disease, and the estimation of glomerular filtration rate in diabetes. Diabetes Metab. 2008;34(5):482–9. doi: 10.1016/j.diabet.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Fontsere N, Bonal J, Salinas I, et al. Is the new Mayo Clinic Quadratic equation useful for the estimation of glomerular filtration rate in type 2 diabetic patients? Diabetes Care. 2008;31(12):2265–7. doi: 10.2337/dc08-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chudleigh RA, Ollerton RL, Dunseath G, et al. Performance of the revised '175' Modification of Diet in Renal Disease equation in patients with type 2 diabetes. Diabetologia. 2008;51(9):1714–8. doi: 10.1007/s00125-008-1086-9. [DOI] [PubMed] [Google Scholar]
- 26.Sebasky M, Kukla A, Leister E, et al. Appraisal of GFR-estimating equations following kidney donation. Am J Kidney Dis. 2009;53(6):1050–8. doi: 10.1053/j.ajkd.2009.01.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saleem M, Florkowski CM, George PM. Comparison of the Mayo Clinic Quadratic Equation with the Modification of Diet in Renal Disease equation and radionuclide glomerular filtration rate in a clinical setting. Nephrology (Carlton) 2008;13(8):684–8. doi: 10.1111/j.1440-1797.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- 28*.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92. doi: 10.1053/j.ajkd.2008.12.034. This paper describes a new equation to estimate GFR for Japanese using standardized creatinine assays. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Zhang X, Xu G, et al. Determination of reference intervals for creatinine and evaluation of creatinine-based estimating equation for Chinese patients with chronic kidney disease. Clin Chim Acta. 2009;403(1–2):87–91. doi: 10.1016/j.cca.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 30*.Delanaye P, Cavalier E, Radermecker RP, et al. Estimation of GFR by different creatinine- and cystatin-C-based equations in anorexia nervosa. Clin Nephrol. 2009;71 (5):482–91. doi: 10.5414/cnp71482. This paper is one of the first to evaluate GFR estimating equations in populations expected to have reduced muscle mass. Contrary to a priori hypotheses, cystatin C did not perform substantially better than creatinine, with the best equation being the Cockcroft and Gault. [DOI] [PubMed] [Google Scholar]
- 31*.Beringer PM, Hidayat L, Heed A, et al. GFR estimates using cystatin C are superior to serum creatinine in adult patients with cystic fibrosis. J Cyst Fibros. 2009;8(1):19–25. doi: 10.1016/j.jcf.2008.07.004. This paper is one of the first to evaluate GFR estimating equations in populations with chronic illness. The bias was similar among creatinine and cystatin C based equations, although precision and overall accuracy was substantially better. In the matched controls who were healthy volunteers, the MDRD Study equation provided the best estimates. [DOI] [PubMed] [Google Scholar]
- 32.Sterner G, Bjork J, Carlson J, et al. Validation of a new plasma cystatin C-based formula and the Modification of Diet in Renal Disease creatinine-based formula for determination of glomerular filtration rate. Scand J Urol Nephrol. 2009;43(3):242–9. doi: 10.1080/00365590902800738. [DOI] [PubMed] [Google Scholar]
- 33.Willems D, Wolff F, Mekhali F, et al. Cystatin C for early detection of renal impairment in diabetes. Clin Biochem. 2009;42(1–2):108–10. doi: 10.1016/j.clinbiochem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol. 2008;21(6):797–807. [PMC free article] [PubMed] [Google Scholar]
- 35.Coresh J, Stevens LA. Kidney function estimating equations: where do we stand? Curr Opin Nephrol Hypertens. 2006;15(3):276–84. doi: 10.1097/01.mnh.0000222695.84464.61. [DOI] [PubMed] [Google Scholar]
- 36.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 37.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 38.Imai E, Horio M, Nitta K, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11(1):41–50. doi: 10.1007/s10157-006-0453-4. [DOI] [PubMed] [Google Scholar]
- 39.Rule AD, Teo BW. GFR estimation in Japan and China: what accounts for the difference? Am J Kidney Dis. 2009;53(6):932–5. doi: 10.1053/j.ajkd.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 41.Grubb AO. Cystatin C--properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. doi: 10.1016/S0065-2423(01)35015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madero M, Sarnak MJ, Stevens LA. Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens. 2006;15(6):610–6. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 43.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65(4):1416–21. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 45*.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–60. doi: 10.1038/ki.2008.638. This report compares the association of serum cystatin and serum creatinine to multiple predictor variables after adjusting for GFR. Important variables that predicted level of cystatin C after adjustment for GFR were diabetes, higher C-reactive protein and white blood cell count and lower serum albumin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grubb A, Bjork J, Lindstrom V, et al. A cystatin C-based formula without anthropometric variables estimates glomerular filtration rate better than creatinine clearance using the Cockcroft-Gault formula. Scand J Clin Lab Invest. 2005;65(2):153–62. doi: 10.1080/00365510510013596. [DOI] [PubMed] [Google Scholar]
- 47*.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20:2214–2222. doi: 10.1681/ASN.2008090980. This study evaluated the risk of cardiovascular mortality in the National Health and nutrition examination survey 1988–1994. Compared to the MDRD Study equation, use of cystatin C alone led to lower eGFR distributions and higher prevalence estimates of CKD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foley RN, Wang C, Snyder JJ, et al. Cystatin C Levels in U.S. Adults, 1988–1994 Versus 1999–2002: NHANES. Clin J Am Soc Nephrol. 2009 doi: 10.2215/CJN.05281008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: Approaches and initiatives - A position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 50.Xie D, Joffe M, Brunelli S, et al. A comparison of change in measured and estimated glomerular filtration rate in patients with nondiabetic kidney disease. Clin J Am Soc Nephrol. 2008;3:1332–1338. doi: 10.2215/CJN.05631207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee D, Levin A, Roger SD, et al. Longitudinal analysis of performance of estimated glomerular filtration rate as renal function declines in chronic kidney disease. Nephrol Dial Transplant. 2009;24(1):109–16. doi: 10.1093/ndt/gfn477. [DOI] [PubMed] [Google Scholar]
- 52.Abraham AG, Schwartz GJ, Furth S, et al. Longitudinal formulas to estimate GFR in children with CKD. Clin J Am Soc Nephrol. 2009;4(11):1724–30. doi: 10.2215/CJN.01860309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Jain AK, McLeod I, Huo C, et al. When laboratories report estimated glomerular filtration rates in addition to serum creatinines, nephrology consults increase. Kidney Int. 2009 doi: 10.1038/ki.2009.158. This paper describes the impact of implementation of eGFR reporting in Ontario, Canada and showed that non-urgent nephrology referrals increased from 134 per nephrologist per year to 156 per nephrologist per year. [DOI] [PubMed] [Google Scholar]
- 54.Stevens LA, Levey AS. Impact of reporting estimated glomerular filtration rate: it's not just about us. Kidney Int. 2009;76(3):245–7. doi: 10.1038/ki.2009.143. [DOI] [PubMed] [Google Scholar]
- 55.Noble E, Johnson DW, Gray N, et al. The impact of automated eGFR reporting and education on nephrology service referrals. Nephrol Dial Transplant. 2008;23(12):3845–50. doi: 10.1093/ndt/gfn385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richards N, Harris K, Whitfield M, et al. Primary care-based disease management of chronic kidney disease (CKD), based on estimated glomerular filtration rate (eGFR) reporting, improves patient outcomes. Nephrol Dial Transplant. 2008;23(2):549–55. doi: 10.1093/ndt/gfm857. [DOI] [PubMed] [Google Scholar]
- 57.Food and Drug Administration. Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function Study Design, Data Analysis, and Impact on Dosing and Labeling. U.S. Department of Health and Human Services; Rockville: 1998. [Google Scholar]
- 58*.Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009;54(1):33–42. doi: 10.1053/j.ajkd.2009.03.008. This report compares the MDRD Study equation, Cockcroft and Gault equation and measured GFR for adjustment of medications for the level of kidney function. The MDRD study equation provides the most similar dosing adjustments compared to the gold standard measured GFR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Lemos ML, Hsieh T, Hamata L, et al. Evaluation of predictive formulae for glomerular filtration rate for carboplatin dosing in gynecological malignancies. Gynecol Oncol. 2006;103(3):1063–9. doi: 10.1016/j.ygyno.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 60.Bookstaver PB, Johnson JW, McCoy TP, et al. Modification of Diet in Renal Disease and modified Cockcroft-Gault formulas in predicting aminoglycoside elimination. Ann Pharmacother. 2008;42(12):1758–65. doi: 10.1345/aph.1L144. [DOI] [PubMed] [Google Scholar]
- 61.Wargo KA, Eiland EH, 3rd, Hamm W, et al. Comparison of the modification of diet in renal disease and Cockcroft-Gault equations for antimicrobial dosage adjustments. Ann Pharmacother. 2006;40(7–8):1248–53. doi: 10.1345/aph.1G635. [DOI] [PubMed] [Google Scholar]
- 62.Gill J, Malyuk R, Djurdjev O, et al. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group--a cautionary tale. Nephrol Dial Transplant. 2007;22(10):2894–9. doi: 10.1093/ndt/gfm289. [DOI] [PubMed] [Google Scholar]
- 63.Golik M, Lawrence K. Comparison of dosing recommendations for antimicrobial drugs based on two methods for assessing kidney function: cockcroft-gault and modification of diet in renal disease. Pharmacotherapy. 2008;28:1125–32. doi: 10.1592/phco.28.9.1125. [DOI] [PubMed] [Google Scholar]
- 64.Melloni C, Peterson ED, Chen AY, et al. Cockcroft-Gault versus modification of diet in renal disease: importance of glomerular filtration rate formula for classification of chronic kidney disease in patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. 2008;51(10):991–6. doi: 10.1016/j.jacc.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 65.Moranville MP, Jennings HR. Implications of using modification of diet in renal disease versus Cockcroft-Gault equations for renal dosing adjustments. Am J Health Syst Pharm. 2009;66(2):154–61. doi: 10.2146/ajhp080071. [DOI] [PubMed] [Google Scholar]
- 66.Hermsen ED, Maiefski M, Florescu MC, et al. Comparison of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for dosing antimicrobials. Pharmacotherapy. 2009;29(6):649–55. doi: 10.1592/phco.29.6.649. [DOI] [PubMed] [Google Scholar]
- 67.Stevens LA, Levey AS. Use of the MDRD study equation to estimate kidney function for drug dosing. Clin Pharmacol Ther. 2009;86(5):465–7. doi: 10.1038/clpt.2009.124. [DOI] [PubMed] [Google Scholar]
- 68.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41(1):47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 69.Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63(3):1121–9. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 70.Stevens LA, Levey AS. Chronic kidney disease in the elderly--how to assess risk. N Engl J Med. 2005;352(20):2122–4. doi: 10.1056/NEJMe058035. [DOI] [PubMed] [Google Scholar]
- 71.Coresh J. CKD prognosis: beyond the traditional outcomes. Am J Kidney Dis. 2009;54(1):1–3. doi: 10.1053/j.ajkd.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Matsushito K, Selvin E, Bash L, et al. Risk implications of the new CKD-EPI equations as compared to the MDRD Study equation for estimated GFR: The ARIC Study. Am J Kidney Dis. 2010 doi: 10.1053/j.ajkd.2009.12.016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White S, Polkinghorne K, Atkins R, et al. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD-EPI and MDRD Study GFR estimating equations: The AusDiab Study. Am J Kidney Dis. 2010 doi: 10.1053/j.ajkd.2009.12.011. (in press) [DOI] [PubMed] [Google Scholar]
- 74.Rule AD, Bergstralh EJ, Slezak JM, et al. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 75.Larsson A, Malm J, Grubb A, et al. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/min. Scand J Clin Lab Invest. 2004;64(1):25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 76.Tidman M, Sjostrom P, Jones I. Plasma cystatin C for estimating residual GFR (rGFR) in dialysis patients. Nephrol Dial Transplant. 2008;23(3):1072–3. doi: 10.1093/ndt/gfm771. author reply 1073. [DOI] [PubMed] [Google Scholar]
- 77.Grubb A, Nyman U, Bjork J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51(8):1420–31. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]