Abstract
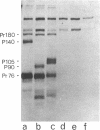
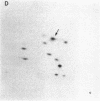
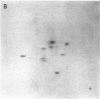
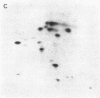
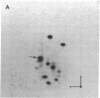
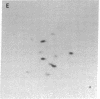
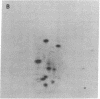
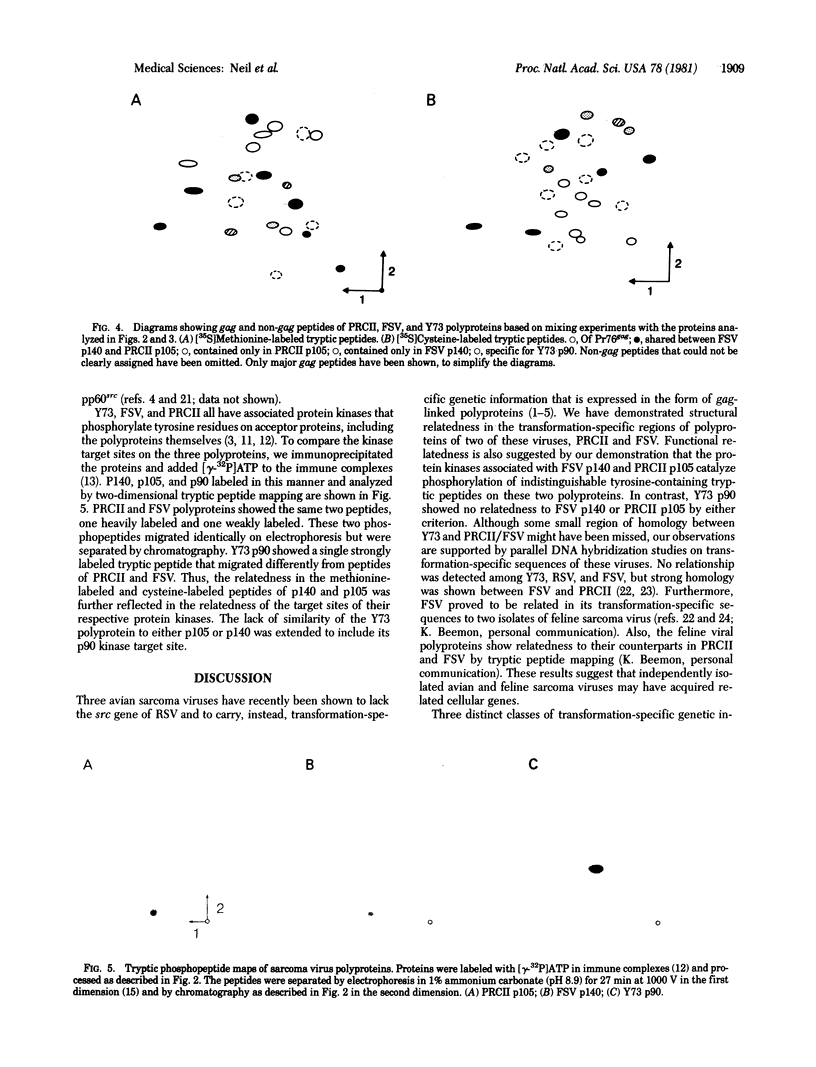
The gag-linked transformation-specific proteins (polyproteins( of PRCII, Fujinami, and Y73 avian sarcoma viruses have been compared by tryptic peptide mapping. In addition to shared gag peptides, PRCII polyprotein p105 and FSV polyprotein p140 were found to have seven methionine-containing and five cysteine-containing tryptic peptides in common. These represent the majority of the non-gag peptides for each virus. In contrast, no overlap was detected with the non-gag peptides of the Y73 polyprotein p90. Examination of the tryptic phosphopeptides of p105, p140, and p90 labeled by their associated protein kinases gave similar results. Although the major phosphopeptides of p105 and p140 comigrated, they were distinct from the phosphopeptide of p90. Three classes of transformation-specific proteins can now be identified among known avian sarcoma viruses. After the pp60src of Rous sarcoma virus and B77 virus, the proteins of PRCII and Fujinami virus form a second class and Y73--currently the only representative--characterizes the third. Despite their structural differences, these viruses may share a common mechanism of transformation, effected by their associated protein kinases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bister K., Duesberg P. H. Structure and specific sequences of avian erythroblastosis virus RNA: evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Vogt P. K. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Lai M. M., Vogt P. K. Genome of avian myelocytomatosis virus MC29: analysis by heteroduplex mapping. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1265–1268. doi: 10.1073/pnas.76.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Breitman M. L., Vogt P. K. Characterization of a 105,000 molecular weight gag-related phosphoprotein from cells transformed by the defective avian sarcoma virus PRCII. Virology. 1981 Jan 15;108(1):98–110. doi: 10.1016/0042-6822(81)90530-4. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Temperature-sensitive transformation by Rous sarcoma virus and temperature-sensitive protein kinase activity. J Virol. 1980 Jan;33(1):220–229. doi: 10.1128/jvi.33.1.220-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Varmus H. E., Bishop J. M. Nucleotide sequences related to the transforming gene of avian sarcoma virus are present in DNA of uninfected vertebrates. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4102–4106. doi: 10.1073/pnas.75.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Vigne R., Neil J. C., Breitman M. L., Vogt P. K. Recovered src genes are polymorphic and contain host markers. Virology. 1980 Aug;105(1):71–85. doi: 10.1016/0042-6822(80)90157-9. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kawai S., Toyoshima K. Unifected avian cells contain structurally unrelated progenitors of viral sarcoma genes. Nature. 1980 Oct 16;287(5783):653–654. doi: 10.1038/287653a0. [DOI] [PubMed] [Google Scholar]