Abstract
A long-standing assumption in the cognitive aging literature is that performance on working memory (WM) tasks involving serial recall is relatively unaffected by aging, whereas tasks that require the rearrangement of items prior to recall are more age-sensitive. Previous neuroimaging studies of WM have found age-related increases in neural activity in frontoparietal brain regions during simple maintenance tasks, but few have examined whether there are age-related differences that are specific to rearranging WM items. In the current study, older and younger adults' brain activity was monitored using functional magnetic resonance imaging (fMRI) as they performed WM tasks involving either maintenance or manipulation (letter–number sequencing). The paradigm was developed so that performance was equivalent across age groups in both tasks, and the manipulation condition was not more difficult than the maintenance condition. In younger adults, manipulation-related increases in activation occurred within a very focal set of regions within the canonical brain WM network, including left posterior prefrontal cortex and bilateral inferior parietal cortex. In contrast, older adults showed a much wider extent of manipulation-related activation within this WM network, with significantly increased activity relative to younger adults found within bilateral PFC. The results suggest that activation and age-differences in lateral PFC engagement during WM manipulation conditions may reflect strategy use and controlled processing demands rather than reflect the act of manipulation per se.
Keywords: Working memory, Maintenance, Manipulation, Aging
Introduction
The domain of working memory (WM) – which refers to temporary, on-line storage of information in a form that can be used to support on-going processing – has been an object of intense investigation across the disciplines of psychology and neuroscience, and has received particular focus in studies of cognitive aging. A long-standing assumption in the cognitive aging literature is that performance on serial recall tasks (referred to here as simple maintenance tasks; e.g., digit or letter span) is relatively unaffected by aging, whereas tasks that require the rearrangement of items prior to recall (referred to here as manipulation tasks; e.g., backward digit span, alphabet span) are more age-sensitive. This is presumed to be because manipulation tasks require greater prefrontal involvement than maintenance tasks, a view supported by current neuroimaging evidence (see Wager and Smith, 2003, for a review). That is, because normal aging is widely thought to be accompanied by changes in prefrontal cortex structure and function, age-related cognitive changes are most strongly observed in the tasks that are most strongly sensitive to frontal function (i.e., the so-called “frontal cortex theory of aging”, e.g., West, 1996), such as working memory tasks requiring manipulation more so than those requiring simple maintenance.
The behavioral evidence for older adults being particularly poor at manipulating items in working memory, however, is somewhat equivocal. For example, some studies indicate a larger age difference in backward recall than in forward (serial) recall (Babcock and Salthouse, 1990; Bopp and Verhaeghen, 2005), but other studies find equivalent age differences in forward and backward recall (Gregoire and Van der Linden, 1997; Myerson et al., 2003; Wilde et al., 2004). More complex manipulations of items in working memory, such as that required by alphabetical re-ordering or letter–number sequencing, often show larger age differences than simple serial or backward recall (Craik, 1986; Myerson et al., 2003), but this may be due to more general age-related memory deficits that are exacerbated by the manipulation requirement (Belleville et al., 1998; Emery et al., 2007).
The interpretation of the behavioral results is further complicated by recent neuroimaging studies of cognitive aging, which generally find that older adults show more activation than young adults do during the performance of many cognitive tasks. In studies of working memory, older adults often show more prefrontal activation than young adults in the hemisphere contralateral to the typical prefrontal activation location. That is, older adults show more right prefrontal activation than do young adults during the performance of verbal maintenance tasks (Reuter-Lorenz et al., 2000), and more left prefrontal activation than do young adults during the performance of nonverbal maintenance tasks (Grady et al., 1998; Reuter-Lorenz et al., 2000). To date, however, almost all of the published studies of working memory and aging have compared older and younger adults only on maintenance tasks (e.g., Grady et al., 1998; Reuter-Lorenz et al., 2000; Rypma et al., 2001), with no manipulation task for comparison. Therefore, although there are several pieces of evidence about how age differences in working memory maintenance are manifest in the brain, very little evidence exists regarding age-related differences in brain activation that are unique to manipulation tasks.
One exception is a recent study by Sun et al. (2005), in which older and younger adults were imaged while performing versions of the forward and backward digit span test. An Age Group × Recall Order interaction was found in right inferior frontal cortex (BA 44/45), such that older adults showed more activation in this area than did young adults, but only during backward digit span. In addition, the volume activated in this area was significantly correlated with backward digit span performance in the older adults but not in the younger adults. This would suggest that even though behavioral studies often show equivalent decline of forward and backward digit span, older and younger adults may be arriving at their backward digit span performance in different ways.
The current study expands on the imaging research conducted by Sun et al. using a more complex manipulation task with some unique properties. In the current study, we use a modified version of the WMS-III Letter–number sequencing task, an item-manipulation task that is in wide use in neuropsychological testing and often shows larger age differences than either forward or backward digit span (Myerson et al., 2003). In the paradigm used here, participants are shown a series of alternating letters and numbers presented one at a time on a computer screen. Before the items are presented, participants are told either that they will have to recall the items in the order they appeared (simple maintenance condition) or will recall the digits first, in ascending order, followed by the letters in alphabetical order (manipulation condition). In order to encourage participants to manipulate the items as they are being presented, the items are presented at a relatively slow rate. Under these conditions, previous research has shown a somewhat unexpected and striking phenomenon: both older and younger adults are able to remember at least as many or more items in the manipulation condition than in the maintenance condition (Emery et al., 2007; Robertson et al., 2006). This is presumably because, in the manipulation condition, when participants group and reorder the items as the items are being presented the resulting arrangement is more memorable. That is, remembering “257BKT” is easier than remembering “T2B7K5”, even though it takes some work to change the order from the latter order to the former. Thus, the current paradigm presents a unique opportunity to investigate a manipulation task in which performance is often equal to or better than its maintenance counterpart, testing the generalizability of previous results showing that manipulation produces greater prefrontal cortex activation than does maintenance.
In addition, the current study used a unique method of matching the performance of older and younger adults that improves upon previous methods of performance matching. One perennial problem in testing for age group differences in specific cognitive processes is the presence of differences in baseline performance. Because of these baseline performance differences, it can be difficult to rule out the possibility that different patterns of brain activity in older adults during working memory performance are due to differences in the subjective difficulty of the task (that is, are older adults just “working harder” than young adults?). The typical solution to this problem is to match young and older adults on observed performance, by testing older adults in a lower load condition than younger adults. Matching observable performance at one pair of load levels, however, does not necessarily eliminate the possibility that older adults are “working harder” to maintain that (smaller number) of items than younger adults are to maintain their (larger number) of items. This is particularly the case if, as described below, performance is near ceiling for both groups.
In the current study, participants' performance was instead matched across a range of load levels, with older adults' memory being tested from 3–6 items, and younger adults from 4–7 items. Pilot testing (conducted in both younger and older adults) indicated that, using these ranges, the two age groups were performance-matched across the entire range in both the maintenance and manipulation tasks. Although this does not completely eliminate the problem of “subjective difficulty”, it significantly improves upon previous types of matching for the following reason. In a typical memory span task, participants are asked to remember and recall several series of items that increase in series length. Performance typically remains very high when participants have a small number of items to remember, but drops precipitously when participants reach their “span”. A participant (or group of participants) may maintain high performance over many series lengths before reaching the point at which performance begins to fall. If groups are only matched at one series length (when participants in both groups are maintaining high performance), it is possible that participants in one group may be very near to their point of rapid performance drop, whereas the other group is still several items from that point. Thus, the former group is undoubtedly working harder to maintain the items (and experiencing greater subjective difficulty) than the latter, even though objective performance is equivalent. By matching observable performance across a range of series lengths, our aim was to span the point at which performance drops for both groups. As a result, it is possible to have greater confidence that each group was working equally “hard”.
The analyses were developed to answer two questions about brain activation as participants engaged in WM manipulation within the letter–number sequencing task: (1) is performance of the manipulation condition associated with increased activity in brain regions that are a part of the canonical WM manipulation network? And (2) are there subsets of these regions that show significant age differences in the effect of manipulation? To answer the first question, we conducted separate analyses in the younger and older adults, using an ROI-based approach. To answer the second question, we determined whether any of the identified brain regions showing manipulation effects further exhibited an Age×Task interaction.
Materials and methods
Participants
Participants were 10 healthy young adults (Age: M = 21.9 years; SD = 2.6; Range 18 to 27); and 11 healthy older adults (Age: M = 71.2 years; SD = 6.2; Range 65 to 82) recruited from subject pools maintained by the Department of Psychology at Washington University in St. Louis. All participants were right-handed. The groups did not differ in gender breakdown, c2 = 1.2, df = 1, N = 21, p = .275. Participants were screened for diagnosed medical disorders (including treated or untreated hypertension, diabetes and thyroid problems), neurological disorders (including past head injuries involving loss of consciousness for 5 or more minutes or a documented concussion), psychiatric disorders, or medication histories that could influence cognitive performance as well as for any contraindication for MR scanning (e.g., sleep medications, psychotropic medications). Older adults were administered the Blessed Orientation-Memory-Concentration Test (Katzman et al., 1983) over the telephone. Individuals making five or more errors were excluded. Participants were paid $25 per hour remuneration for their participation. All methods were approved by the Washington University Medical School IRB, and participants signed an informed consent document before participating in the neuroimaging study.
Behavioral tasks
Maintenance task
In this task, participants saw the warning sign, “Ready-Forward,” signaling the beginning of the task. For each subsequent trial, participants saw a series of alternating numbers and letters (i.e., 9, K, 2, T) presented one item at a time on the computer screen. Each item was presented in the center of the computer screen for 1500 ms, with a 500 ms pause between the items. After a series was presented, there was a 3000 ms pause, followed by the word “Recall”. Participants then recalled the items aloud in the order in which they were presented. Participants indicated when they were finished with recall with a button-press response, which then initiated the start of the next trial. Button-press responses were made using a hand-held button box with a fiber optic interface. Verbal responses were digitally recorded, and coded for accuracy off-line using Cool Edit audio editing software.
Manipulation task
The procedure for the manipulation task was identical to the maintenance task with the following exceptions. First, participants saw the warning sign, “Ready-Sequence,” to prepare them for the task to begin. When the cue “RECALL” was presented, the participant was instructed to recall the numbers first, in ascending order, followed by the letters in alphabetical order (e.g., 2, 9, K, T).
Functional imaging
Images were acquired on a Siemens 1.5 Tesla Vision System (Erlangen, Germany) with a standard circularly-polarized head coil. A mask was used to minimize head movement, and headphones were used to dampen scanner noise and to communicate with the participants. Both structural and functional images were acquired at each scan. High-resolution (1.25 mm × 1 mm × 1 mm) structural images were acquired using a sagittal MP-RAGE 3D T1-weighted sequence (TR = 9.7 ms, TE = 4 ms, flip = 12, TI = 300 ms) (Mugler and Brookeman, 1990). Functional images were acquired using an asymmetric spin-echo echo-planar sequence (TR = 2500 ms, TE = 50 ms, flip = 90°). Each image consisted of 16 contiguous, 8 mm thick axial slices acquired parallel to the anterior–posterior commissure plane (3.75×3.75 mm in-plane), allowing complete brain coverage at a high signal-to-noise ratio (Conturo et al., 1996).
A blocked design was used in which all of the trials in a scanning run were of the same series length and recall order (“forward” or “sequenced”); this resulted in a total of 8 scanning runs per participant (4 levels of load × 2 levels of recall order). The order of these 8 conditions were randomly determined for each participant. Each of the 8 scanning runs consisted of alternating cycles of fixation (3 per run) and task (2 per run) blocks. Task blocks were 6 trials in duration, for a total of 12 trials (2 blocks × 6 trials) per run. Because the task was self-paced, the precise duration of the task blocks varied between runs. The fixation blocks (denoted by a centrally presented crosshair) were 37.5 s in duration. Finally, the first four images in each scanning run allowed the scanner to reach steady state, and hence were discarded.
Visual stimuli were presented using PsyScope software (Cohen, MacWhinney, Flatt, and Provost, 1993) running on an Apple PowerMac G4. Stimuli were projected to subjects with an AmPro LCD projector (model 150) onto a screen positioned at the head end of the bore. Participants viewed the screen through a mirror attached to the head coil. A fiber-optic, light-sensitive key press interfaced with the PsyScope Button Box was used to record subjects' behavioral performance.
Image analysis
Functional imaging data were pre-processed and statistically analyzed using in-house software. Pre-processing involved temporal alignment of volume slices (to correct for asynchronous slice acquisition), normalization within each scanning run to a fixed image intensity value, and then correction for motion using a rigid-body rotation and translation algorithm (Friston, Williams, Howard, Frackowiak, and Turner, 1996; Snyder, 1996). Anatomical images were transformed into standardized atlas space (Talairach and Tournoux, 1988), using a 12-dimensional affine transformation (Woods, Cherry, and Mazziotta, 1992; Woods, Grafton, Watson, Sicotte, and Mazziotta, 1998). The functional data were then resampled into 3 mm cubic voxels, registered to the subject's anatomical images, and spatially smoothed with a 9 mm FWHM Gaussian kernel. Statistical analysis was based on a general linear model (GLM) that estimated, for each participant, task-related activation in each voxel using a boxcar function convolved with a canonical hemodynamic response with separate estimates for the maintenance and manipulation conditions at each series length. Parameter estimates from each participant's GLM were submitted to second level tests treating participant as a random factor.
We first identified brain regions showing effects of manipulation separately in the younger and older adult groups. An ROI-based approach was used in which analyses were restricted to brain regions belonging to the canonical network engaged in WM manipulation tasks. This was done by creating a mask of a priori regions of interest using anatomical coordinates described in two meta-analyses of WM manipulation tasks (Owen, McMillan, Laird, and Bullmore, 2005, Table 2 — Verbal stimuli; Wager and Smith, 2003, Table 4). Based on these seed point coordinates, spherical ROIs were generated with 10 mm radius. This mask was then used as a means of constraining analyses in a theoretically-motivated manner to only those voxels that are most reliably thought to be part of the canonical WM manipulation network (e.g., dorsolateral, ventrolateral and posterior PFC, inferior and superior parietal cortex, cerebellum, etc.) We have generated this WM manipulation ROI mask for use in two recent studies (Fales et al., in press; Locke and Braver, 2008), and utilized the identical approach for the current work. From this mask, we identified clusters of 7 or more voxels that showed both manipulation > fixation and manipulation > maintenance effects (p<.05, uncorrected).
The regions identified in each age group were then subjected to a second-stage analysis to test for potential age-differences. This analysis was conducted by averaging signal across all voxels in the region, and extracting percent signal change estimates in each task condition for each participant. Mixed model ANOVAs were then carried out on each region (using SPSS software), testing for main effects of Age or Age × Task (manipulation vs. maintenance) interactions.
We supplemented this primary ROI-based approach with an exploratory whole-brain analysis, in order to identify regions outside the canonical WM manipulation network that showed manipulation-specific activity in one age group but not the other. In this analysis, we identified voxels that showed manipulation > fixation in either the younger adult or older adult groups, plus a significant age × task (manipulation vs. maintenance) interaction, with both tests set at a significance threshold of p<.001. Additionally, regions were only considered to be significant if they contained a cluster of 7 or more voxels all meeting the above criteria.
Results
Behavioral data
To ensure that our performance matching of age groups was successful, we analyzed performance across the four levels of load that each age group completed. That is, load levels 1–4 refer to series lengths 4–7 for young adults, and series lengths 3–6 for the older adults.
Accuracy
The primary behavioral measure was response accuracy, measured as the percentage of trials answered correctly. A 2 (Age: young vs. old)×2 (Task: maintenance vs. manipulation)×4 (Load: Level 1 vs. Level 2 vs. Level 3 vs. Level 4) ANOVA indicated significant main effects of task, F(1,19) = 7.75, p<.05, and load, F(3, 57) = 30.33, p<.001, and a significant task by load interaction, F(3, 57) = 3.34, p<.05. As may be seen in Fig. 1A, participants in both groups made more errors in the maintenance condition than the manipulation condition. Linear contrasts indicated that the main effect of load had both linear F(1,19) = 48.78, p<.05, and quadratic F(1,19) = 11.43, p<.05, components, such that performance decrements accelerated at higher load lengths. The contrast for the load × condition interaction only showed a significant linear component, F(1,19) = 12.21, p<.05, indicating that the linear decrease in performance was greater for the maintenance than the manipulation condition. Importantly, there was no main effect or interactions with age (all Fs<1.0), supporting the assumption that the age groups were matched well on performance.
Fig. 1.
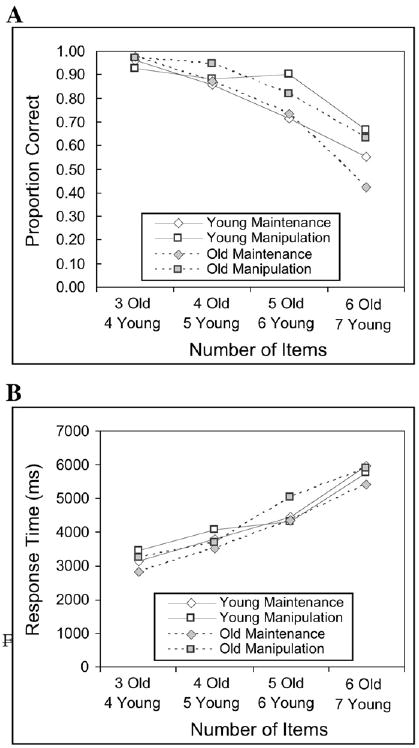
Behavioral accuracy (A) and response times (B) in younger and older adults in the maintenance and manipulation tasks.
Response times
For each person, any response times that were more than 3 standard deviations from that person's mean were discarded from analysis, and only RTs for correct responses were analyzed. A 2 (Age: Young vs. Old)×2 (Task: maintenance vs. manipulation)×4 (Load: Level 1 vs. Level 2 vs. Level 3 vs. Level 4) ANOVA showed only a significant main effect of Load, F(2,38) = 49.52, p<.001; no other effects were significant. The main effect of load is to be expected, as participants were reporting more items at each load level.
It should be noted that participants were equally fast at responding in the manipulation condition as they were in the maintenance condition. This is consistent with our supposition that, in the manipulation condition, participants would rearrange the items as they were being presented rather than maintaining the items in order and rearranging them at recall. Some caution in the interpretation of the response times is warranted, however, as response speed was not emphasized to the participants in the instructions.
Imaging data
Manipulation effects
The regions found to show significant manipulation-related activity in each age group are presented in Fig. 2 and Table 1. The top portion of Table 1 lists the areas that showed manipulation-sensitive activation in the older adults; these areas are represented by blue and green in Fig. 2. The middle portion of Table 1 lists the areas that showed manipulation-sensitive activation in the younger adults; these areas are represented by red in Fig. 2. Regions that show manipulation-related activity in both older and younger adults are shown in purple in Fig. 2.1
Fig. 2.
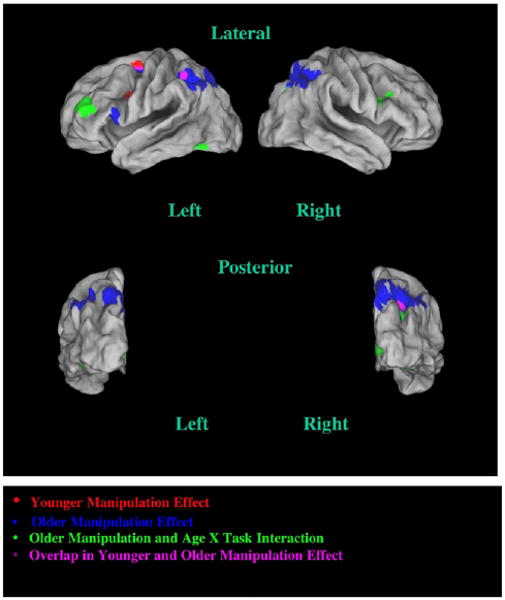
Cortical surface rendering indicating brain regions showing increased activity associated with manipulation, plus regions for which there were significant age differences in the manipulation effect. Regions identified in younger adults appear in red (manipulation). Regions identified in older adults appear in blue (manipulation) and green (age × task interaction). Regions overlapping in both age groups appear in purple (manipulation).
Table 1.
WM manipulation effects and interactions
| Region of interest | Brodmann area | x | y | z | Volume (mm3) | Test statistic | ||
|---|---|---|---|---|---|---|---|---|
| Task (t) | Age (t) | Task × Age (F) | ||||||
| Manipulation effects: older adults | ||||||||
| Left dorsolateral prefrontal cortex | 46/10 | −33 | 39 | 17 | 1701 | 2.72 | 0.14 | 4.83a,b |
| Right dorsolateral prefrontal cortex | 46/9 | 35 | 24 | 27 | 243 | 2.21 | 0.10 | 4.81a,b |
| Left ventrolateral prefrontal cortex | 44/45 | −53 | 15 | 7 | 486 | 2.19 | 0.26 | 2.97 |
| Right inferior frontal junction | 44/6 | 44 | 7 | 24 | 351 | 2.46 | 1.00 | 9.42a |
| Left lateral premotor cortex | 6 | −24 | −1 | 50 | 810 | 2.60 | 2.24a | 0.00 |
| Left inferior parietal lobule | 40 | −35 | −50 | 39 | 6480 | 3.86 | 4.13a | 3.29 |
| Right superior parietal cortex | 7 | 20 | −58 | 42 | 17739 | 2.88 | 4.30a | 4.25 |
| Medial extrastriate cortex | 17/18 | 3 | −80 | 0 | 297 | 2.33 | 1.34 | 11.36a |
| Right lateral cerebellum | – | 32 | −56 | −17 | 216 | 2.62 | 4.07a | 6.16a,b |
| Left lateral cerebellum | – | −36 | −60 | −16 | 648 | 4.39 | 2.97a | 27.78a |
| Medial Cerebellum | – | 1 | −64 | −18 | 1134 | 2.14 | 2.77a | 1.95 |
| Manipulation effects: younger adults | ||||||||
| Left inferior frontal junction | 44/6 | −42 | 1 | 28 | 189 | 2.11 | 0.81 | 2.94 |
| Left premotor cortex | 6 | −26 | −4 | 56 | 621 | 2.46 | 1.73 | 1.73 |
| Left inferior parietal lobule | 40 | −33 | −46 | 31 | 324 | 2.31 | 3.89a | 0.48 |
| Left inferior parietal lobule | 40 | −38 | −46 | 45 | 189 | 2.17 | 2.01 | 0.51 |
| Right inferior parietal lobule | 40 | 27 | −59 | 35 | 216 | 1.94 | 2.91a | 3.63 |
| Additional regions showing Age × Task interactions | ||||||||
| Right parieto-occipital cortex | 19/40 | 27 | −70 | 30 | 297 | – | 3.16a | 14.27a |
| Right extrastriate cortex | 19 | 22 | −54 | −3 | 405 | – | 2.75a | 28.30a |
| Medial extrastriate cortex | 17/18 | 4 | −76 | −5 | 513 | – | 1.77 | 22.21a |
| Medial cerebellum | – | −1 | −44 | −10 | 243 | – | 1.04 | 14.75a |
| Left lateral cerebellum | – | −32 | −61 | −17 | 243 | – | 1.76 | 17.47a |
Note: Regions in bold and italic indicate significant Age × Task interactions. Reported Task effect is computed within each age group with a one-tailed t-test.
Effect significant at p<.05.
Effect does not survive FDR correction.
As may be seen in Table 1, there was a large asymmetry in the extent of manipulation-related activation between older and younger adults. In older adults, eleven regions were identified (total volume: 30,105 mm3) that formed a large subset of the canonical WM manipulation network, including dorsolateral, ventrolateral and posterior PFC, along with inferior and superior parietal cortex and cerebellum. In younger adults, only five small regions were identified within the WM manipulation network (total volume: 1539 mm3), though these also included both lateral PFC and parietal regions. In fact, the parietal (as well as premotor) regions activated in the younger adults represented a small, but fully overlapping subset region of the parietal activation observed in the older adults. In these overlapping regions, as expected, the effect of manipulation was statistically identical in both age groups. However, in the parietal regions there was also a significant main effect of age, such that the older adults had greater activation than younger adults in both maintenance and manipulation conditions. Fig. 3 shows a representative pattern for the left inferior parietal cortex.
Fig. 3.
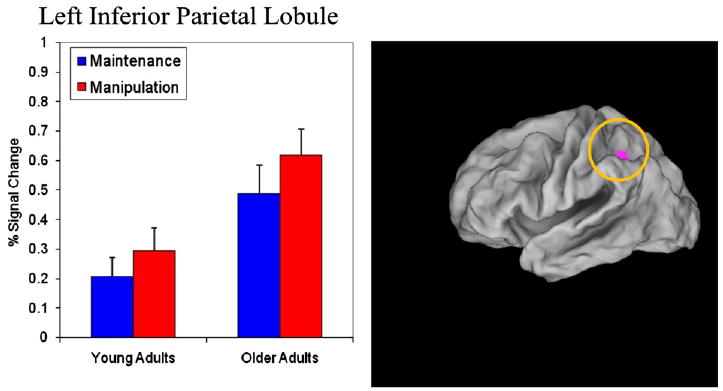
Percent change in signal intensity for the left inferior parietal lobule (BA 40; −33, −46, 31). Note that the size of regions was enhanced in these figures for descriptive purposes; because of its location deep in the sulcus, the region is not visible in a lateral surface rendering without such enhancement (which is why it does not show up in Fig. 2).
Age × Task interaction
We conducted a second analysis on the ROIs identified to show manipulation-sensitive activity, which tested for age differences in this effect via a significant Age × Task interaction. A number of regions were identified for which older adults showed a significantly greater effect of WM manipulation than did younger adults, including dorsolateral and posterior PFC regions, as well as the cerebellum and extrastriate cortex. These regions are presented in bold and italics in Table 1, and are presented in green in Fig. 2. No regions were identified for which the opposite pattern was found, of greater manipulation-related activity in younger adults compared to older adults. However, as discussed further below, within the left posterior PFC, a non-significant trend (p = .10) in this direction was observed.
The successful matching of older and younger adults on performance in the maintenance and manipulation conditions suggests that the identified age differences in activation are not due to possible performance-related confounds. However, to directly verify this assumption, we re-analyzed all of the identified ROIs after statistically controlling for performance in each task condition (via Analysis of Covariance). All of the previously identified regions remained significant at p<.05. Finally, as a more conservative test of the Age × Task interaction effect, we corrected for multiple comparisons using a False Discovery Rate (FDR) correction (q = .05). Three of these regions (right inferior frontal junction, medial striate cortex, and the left lateral cerebellum) survived FDR correction, but the remaining three (right dorsolateral PFC, left dorsolateral PFC, and right lateral cerebellum) did not (see Table 1).
The ROI-based analyses were complemented by a whole-brain exploratory analysis that directly identified voxel clusters showing a significant Age × Task interaction (p<.001). This analysis tested for the possibility of additional regions, outside the canonical WM manipulation network that showed manipulation effects. This analysis identified an additional five regions located posteriorly, within parietal and occipital cortex, and cerebellum; these regions are listed in the bottom portion of Table 1. As in the primary analyses, all of these regions also showed the pattern of a greater manipulation-related increase in older adults compared to younger adults.
Interestingly, two distinct patterns of activation emerged in the identified Age × Task regions (both from the ROI and whole-brain analyses). Within the cerebellar regions, the Age × Task interaction was superimposed on a main effect of age: older adults showed more activation than young adults in both conditions, and older adults showed a further increase in activation in the manipulation condition. Conversely, within the frontal and extrastriate regions, activation was similar among the two age groups in the simple maintenance condition. However, in the manipulation condition activation significantly increased in the older adults but tended to actually show a decrease among younger adults. Fig. 4A shows the pattern of activation in the right posterior PFC region, that appeared to be anatomically located within right inferior frontal junction (IFJ; Derrfuss et al, 2004).
Fig. 4.
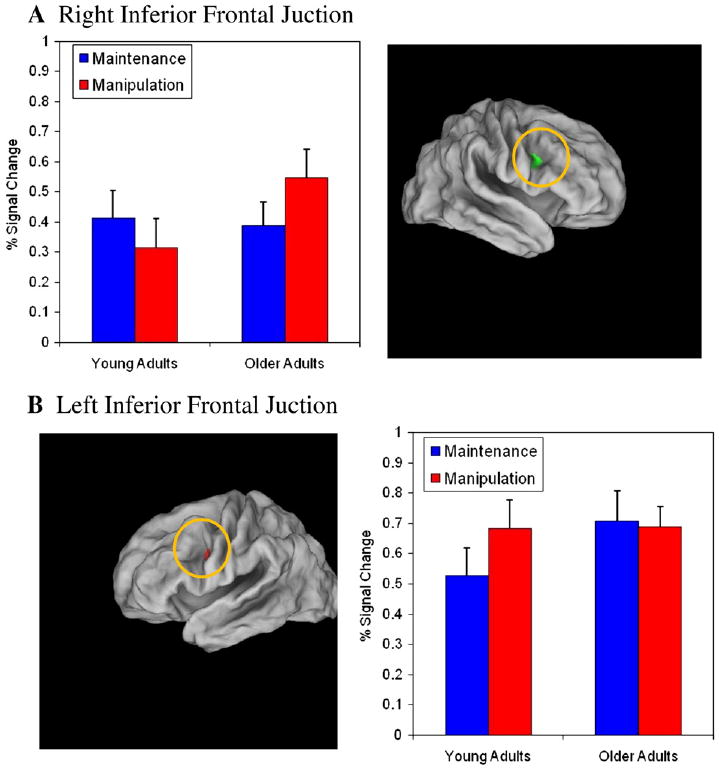
Percent change in signal intensity in the inferior frontal junction. A. Right inferior frontal junction (BA 44/6, 44, 7, 24). B. Left inferior frontal junction (BA 44/6, −42, 1, 28). Note that size of regions was enhanced in this figure for descriptive purposes.
Hemispheric dissociations in PFC activity
The right hemisphere IFJ region that showed increased manipulation-related activity in older adults was homotopic to the left hemisphere region that showed manipulation-related activity in younger adults. Moreover, as mentioned above, the left hemisphere IFJ region also showed a trend towards an Age × Task interaction (see Fig. 4B). Consequently, we tested whether these qualitative distinctions in the two IFJ regions were statistically reliable with a 2 (Age: young vs old)×2 (Task: maintenance vs. manipulation)×2 (Hemisphere: right vs. left) ANOVA. The three-way interaction was found to be significant, F(1,19) = 11.47, p = .003. In right IFJ, older adults show a significant increase in activation from the maintenance to the manipulation task, whereas young adults show no difference in activation between conditions. Conversely, in left IFJ young adults showed an increase in activation from maintenance to the manipulation task, whereas older adults showed no difference between conditions. Note that in right IFJ, older adults are showing more activation than young adults in the manipulation task, but in left IFJ older and younger adults are showing equal activation in the manipulation task condition. In other words, in left IFJ performing the manipulation task increases activation in the young adults to a level comparable to that found in the older adults in both maintenance and manipulation conditions.
The finding of manipulation-related activity bilaterally in PFC within older adults, but exclusively in the left hemisphere in younger adults, is generally consistent with previous studies of WM in aging (Cabeza, 2002). This right-hemisphere PFC activation in older adults is sometimes thought to be due to “compensatory” efforts to maintain successful WM performance (e.g., Cabeza et al., 2002). Such a view would suggest that the older adults showing the greatest right PFC activation should be better at performing the manipulation tasks. To determine if this is the case, we correlated performance on the manipulation task with right DLPFC and inferior frontal activation in the manipulation task in both older and younger adults. In the older adults, however, performance and activation were uncorrelated (DLPFC: r = −.06, p = .86; IFJ: r = .05, p = .88). Somewhat surprisingly, the younger adults showed a large negative correlation between performance and right prefrontal activation, though the correlation only reached significance in the right IFJ region (DLPFC: r = −.35, p = .36; IFJ: r = −.75, p = .01). We performed the same correlation analysis on the left IFJ, the region which showed significant manipulation-related activity only in the young. Again, increased activation in the young adults was associated with poorer performance of the task, although the result failed to reach statistical significance with this small sample size (r = −.55, p = .10). Conversely, in the older adults performance and activation were positively, but again not significantly, correlated (r = .50, p = .12). This apparent age difference in the nature of the brain-behavior relationship in left IFJ was confirmed in the form of a statistically-reliable interaction through a multiple regression analysis (Age × Activation effect predicting performance, F(1,17) = 6.24, p = .02). However, the interaction did not reach statistical significance for the right IFJ (F(1,17) = 2.33, p = .15).
Discussion
This study posed two questions for investigation: does the letter-number sequencing task lead to increased activity in the canonical brain network for WM manipulation, and are there age differences in the activation of these regions? To answer these questions, we performed a series of analyses that first searched for manipulation effects separately in each age group, using a set of ROIs that have been identified through meta-analysis to be the core neural components of WM manipulation. Within this set of ROIs, we then looked for significant differences between the two groups in the activation of these regions by testing for Age × Task interactions. To be sure that there were not additional regions showing Age × Task interactions that were missed by our original set of ROIs, we performed an additional whole-brain analysis.
We found that within each age group there were several regions that showed significant manipulation-specific activity. In the older adults, the manipulation conditions produced significantly increased activation within most of the core WM manipulation circuitry, including dorsolateral, ventrolateral and posterior (i.e., IFJ) regions of PFC, along with bilateral parietal cortex and cerebellum. In younger adults, however, manipulation in WM was associated with a much more minimal and focal pattern of activation, but also involving both lateral PFC and inferior parietal regions. Indeed, the parietal regions fully overlapped with the manipulation-specific regions identified in the older adults.
The second stage of analysis showed that, within the regions identified as showing significant manipulation-effects in either age group, several regions further showed a significant Age × Task interaction, including bilateral PFC. Most interestingly, the PFC regions showed significant Age × Task interactions in the absence of a significant main effect of age. Moreover, Age × Task interactions in brain activity were found despite utilizing task conditions for which behavioral performance was equivalent in the older and younger adults. Finally, within the IFJ we observed qualitative age differences in the nature of the relationship between manipulation-related activation and behavioral performance. Specifically, we found: a) differential engagement of right vs. left IFJ across the two age groups; and b) manipulation-related increases in left IFJ activity were negatively related to performance in younger adults, whereas a positive relationship was observed in older adults.
Overall, these results suggest two conclusions about both the cortical structures underlying the manipulation of items in working memory and the age differences that may occur in the performance of these tasks. First, because bilateral posterior parietal cortex showed both significant manipulation-related activity and a similar pattern of activation in each age groups, these regions are likely responsible for the act of manipulating items in working memory. Previous research has suggested that the superior parietal cortex plays a role in the shifting of attentional focus (Shomstein and Yantis, 2006; Wager, Jonides, and Reading, 2004) and in WM for order information (Marshuetz and Smith, 2006); these processes may be particularly tapped by manipulation requirements, as participants selectively attend to each item in as they place it in the correct order amongst the other items. Second, because other regions, such as those in right and left IFJ, showed distinct patterns of manipulation-specific activity and brain-behavior relationships in younger and older adults, these regions may instead be tied to differences in strategy use or engagement of specific control processes that vary across different age groups during performance of letter–number sequencing. This distinction between regions involved in the act of manipulating items and regions that are involved in control processes associated with manipulating items is consistent with the results of a recent study by Champod and Petrides (2007). Likewise, the finding of qualitative differences between younger and older adults in the relationship between brain activation and behavioral performance is also consistent with prior neuroimaging studies of WM and aging (e.g., Rypma and D'Esposito, 2000). Together, these results suggest that when examining age differences on working memory tasks, an examination of the general control processes and strategies engaged may be more useful than simply varying the presence of a manipulation requirement. Below, we discuss possible reasons for the specific pattern of activation found in the older and younger adults.
WM manipulation in older adults
In the right DLPFC and right IFJ, the older adults showed significant increases in activation in the manipulation condition relative to the maintenance condition. In contrast, young adults showed a tendency toward decreased activation in the manipulation condition in these areas. Interestingly, this pattern of manipulation-specific activation is consistent with that found by Sun et al. (2005) in right lateral PFC, although the specific regions showing age differences in manipulation-related activity were different across the two studies (ventrolateral PFC in Sun et al., dorsolateral PFC and IFJ in the current study). This discrepancy in location may be due to the different task demands of letter-number sequencing compared to backward digit span. For example, in backward digit span (particularly as implemented in the Sun et al. study), participants must wait until the entire sequence is presented before reordering the items. In contrast, letter-number sequencing (particularly as implemented in the current study), participants have the option of manipulating the items as they are being presented.
One possibility for the increased right PFC activation in the older adults during letter-number sequencing performance is that older adults have more difficulty keeping pace with the reordering process as the items are presented, and lag slightly behind the young adults in rearranging the items. As discussed in the introduction and behavioral results, both young and older adults appear to rearrange items in the letter-number sequencing task as they are being presented, resulting in equivalent response times and better memory performance relative to the serial recall condition. Previous behavioral research, however, has suggested that young adults may perform this rearrangement more quickly and efficiently than do the older adults (Emery et al., 2007). If this is the case, the reordering may place a greater temporary attentional control burden on the older adults, as they must encode the next item while simultaneously completing the rearrangement of the previous item. These types of divided attention effects are the hallmark of complex WM span tasks, and are well-established to engage DLPFC regions (e.g., DiPisapia et al., 2007; Iidaka, Anderson, Kapur, Cabeza, and Craik, 2000; Smith et al., 2001). Older adults may thus have experienced such divided attention demands more acutely in the manipulation condition, which would be the source of the age-specific increase in DLPFC activity.
Functional dissociation within IFJ
Older adults showed increased manipulation-related activity in the right IFJ, whereas younger adults showed manipulation-related effects within left IFJ. These distinct hemispheric patterns across the two age groups were statistically reliable. The right and left IFJ patterns were not merely mirror-images of each other, however. In left IFJ, the manipulation condition increased the level of activation in the young adults to the same level found in the older adults in both the maintenance and manipulation conditions. Conversely, in right IFJ, the older adults showed significantly greater activation than younger adults in the manipulation condition (but not the maintenance condition). However, in both regions, manipulation-related IFJ activation was negatively related to performance in younger adults, but tended to show a positive, or lack of relationship in older adults.
Previous research has suggested that the IFJ is involved in cue-based retrieval (or reactivation) of task representations (Brass, Derrfuss, Forstmann, and von Cramon, 2005). One possibility for the pattern of results in the current study is that, for young adults, the manipulation task requires greater maintenance of task goals than does the simple maintenance task. That is, maintaining the items in their presented order may be the “default” goal and require little active retrieval/reactivation of the goal representation. When sequencing is required, reactivation of task goals is needed – especially for poorer performing younger adults – in order appropriately engage in the reordering process rather than default storage and encoding. For older adults, an impaired ability to maintain task goals – i.e., a tendency towards “goal neglect” (Braver and West, 2007) – may lead to a need to retrieve and refresh these goals in the maintenance condition as well as manipulation. The left IFJ might have thus been equivalently engaged in both task conditions, whereas in manipulation, the right IFJ was additionally recruited.
WM manipulation in younger adults
The young adult findings also provide a somewhat different interpretation of WM manipulation effects than found in previous studies. In particular, several previous studies in young adults have tended to find that WM manipulation conditions were associated with a substantial increase in activation of lateral PFC regions, particularly dorsolateral PFC (D'Esposito et al., 1999; Postle et al., 1999; Veltman et al., 2003). One obvious difference between the current study and these previous studies is in the choice of manipulation task used. Most notably, the WM manipulation tasks used in previous studies require participants to wait until the end of a presented sequence to manipulate items. This may have increased the demands to monitor items during the manipulation phase, in order to minimize interference (between the original and transformed WM contents) or memory decay. Likewise, in the N-back task (Veltman et al., 2003), manipulation demands occur as a result of the continuous updating of items kept in WM, which might also engage additional monitoring processes. In contrast, the current letter–number sequencing task encourages participants to manipulate the items as they are presented rather than waiting until the end of a sequence. This may have minimized monitoring demands, at least for the young adults, if they were able to keep pace in WM manipulation with the presentation of each item. If instead the task demands required participants to wait until the end of a sequence to manipulate items, a reasonable prediction to make is that the pattern of manipulation-related activity in younger adults would be much closer in similarity to that observed in older adults.
Limitations of performance matching
One unique aspect of the current study is the attempt to control for differences in difficulty, both between age groups and between task types. Despite the fact that we included a range of performance over which older and younger adults performed similarly in both tasks, we still found age differences in manipulation-related activation. Moreover, these age differences remained intact even after we conducted a follow-up analysis that statistically controlled for task performance directly. In addition, although the manipulation task resulted in better performance than the maintenance task for both age groups, the older adults showed much more manipulation-related activity than did younger adults. Taken together, these results permit stronger inferences that age-differences in neural activity during WM manipulation are not purely a result of behavioral performance-related (or other psychometric) confounds.
One caveat to this finding is that we operationalized “difficulty” as “observed performance”, but the two may not necessarily be equivalent. That is, participants may be performing similarly even though one group is working harder to achieve that performance level. As described in the introduction, we believe that our use of a range of series lengths for each age group greatly reduces this possibility. Nevertheless, because we have no direct measure of “perceived difficulty”, it remains possible that older adults are showing more manipulation-related activity than young adults because they are simply “working harder” than young adults in that condition. This is in line with the general notion of compensatory activation in the cognitive aging literature, although this description may better fit the pattern observed within parietal cortex (in which increased overall activity was observed in older adults but a similar pattern of manipulation-related effects), rather than lateral PFC (since in these regions older adults showed more activation than young adults exclusively in the manipulation condition).
Conclusions
The current study adds to the literature on age-related changes in WM function by demonstrating that older and younger adults show different patterns of manipulation-related neural activity during WM tasks, even when the manipulation task yields better performance than the maintenance task and age differences in performance are nonexistent. In particular, the results suggest that activation and age-differences in lateral PFC engagement during WM manipulation conditions may reflect strategy use and controlled processing demands rather than reflect the act of manipulation per se. As such, the results highlight the utility of providing greater psychometric control over task performance, in terms of the use of multiple load levels and performance-matching, to provide a clearer window into the neural circuitry of WM and how changes with advancing age.
Footnotes
It might be questioned why the analyses of manipulation effects are collapsed over load. We initially searched for regions that showed an effect of load, conditionalized on a parametric change in activation as load increased. These initial analyses, however, revealed an unexpected phenomenon: rather than linear or quadratic increases or decreases in activation with load as we expected, both older and younger adults showed more activation when an even number of items was presented. Although a specific explanation of this phenomenon is beyond the scope of this paper, we suspect that this is related to the natural grouping that occurs as a result of having two different categories of memory items (letters and numbers). The analysis of load is further complicated by the fact that, because of our method of performance matching, older adults were always remembering an odd number of items when young adults were remembering an even number of items. Thus, in addition to the absence of a parametric effect of load on activation, the main effect of load was always accompanied by an Age × Load interaction that was not specifically linked to performance level. Importantly for our analyses of manipulation effects, however, load did not interact with condition, nor were there three-way Load × Condition × Age interactions, in any of the regions discussed. Thus, these effects of load do not impact the reported results.
References
- Babcock RL, Salthouse TA. Effects of increased processing demands on age differences in working memory. Psychol Aging. 1990;5:421–428. doi: 10.1037//0882-7974.5.3.421. [DOI] [PubMed] [Google Scholar]
- Belleville S, Rouleau N, Caza N. Effect of normal aging on the manipulation of information in working memory. Mem & Cog. 1998;26:572–583. doi: 10.3758/bf03201163. [DOI] [PubMed] [Google Scholar]
- Bopp KL, Verhaeghen P. Aging and verbal memory span: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2005;60(5):P223–P233. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9(7):314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, West R. Working memory, executive control and aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Third. Psychology Press; 2007. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. PNAS. 2007;104(37):14837–14842. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. PsyScope: an interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behav Res Meth Instrum Comput. 1993;25(2):257–271. [Google Scholar]
- Conturo TE, McKinstry RC, Akbudak E, Snyder AZ, Yang T, Raichle ME. Sensitivity optimization and experimental design in functional magnetic resonance imaging. Soc Neurosci Abstr. 1996;26:7. [Google Scholar]
- Craik FIM. A functional account of age differences in memory. In: Klix F, Hagendorf H, editors. Human memory and cognitive capabilities. Elsevier; Amsterdam: 1986. pp. 409–420. [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23(2):604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- DiPisapia N, Slomski JA, Braver TS. Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cereb Cortex. 2007;17:993–1006. doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- Emery L, Myerson J, Hale S. Age differences in item-manipulation span: the case of letter–number sequencing. Psychol Aging. 2007;22:75–83. doi: 10.1037/0882-7974.22.1.75. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, Braver TS. Anxiety and cognitive efficiency: Differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. doi: 10.3758/cabn.8.3.239. in press. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age-related changes in regional cerebral blood flow during working memory for faces. NeuroImage. 1998;8:409–425. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- Gregoire J, Van der Linden M. Effects of age on forward and backward digit spans. Aging Neuropsychol Cogn. 1997;4:140–149. [Google Scholar]
- Iidaka T, Anderson ND, Kapur S, Cabeza R, Craik FI. The effect of divided attention on encoding and retrieval in episodic memory revealed by positron emission tomography. J Cogn Neurosci. 2000;12:267–280. doi: 10.1162/089892900562093. [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cogn Affect Behav Neurosci. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE. Working memory for order information: multiple cognitive and neural mechanisms. Neuroscience. 2006;139(1):195–200. doi: 10.1016/j.neuroscience.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Mugler JPI, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP-RAGE) Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- Myerson J, Emery L, White DA, Hale S. Effects of age, domain, and processing demands on memory span: evidence for differential decline. Aging Neuropsychol Cogn. 2003;10:20–27. [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D'Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci. 1999;96:12959–12964. doi: 10.1073/pnas.96.22.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C. Age differences in the frontal lateralization of verbal and satial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memroy. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Gabrieli JD. Age differences in prefrontal cortical activity in working memory. Psychol Aging. 2001;16:371–384. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. J Neurosci. 2006;26(2):435–439. doi: 10.1523/JNEUROSCI.4408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: Effects of performance and aging. Proc Natl Acad Sci. 2001;98(4):2095–2100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AZ. Difference image versus ratio image error function forms in PET–PET realignment. In: Bailer D, Jones T, editors. Quantification of Brain Function Using PET. Academic Press; San Diego: 1996. pp. 131–137. [Google Scholar]
- Sun X, Zhang X, Chen X, Zhang P, Bao M, Zhang D, et al. Age-dependent brain activation during forward and backward digit recall revealed by fMRI. Neuroimage. 2005;26(1):36–47. doi: 10.1016/j.neuroimage.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003;18(2):247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wilde NJ, Strauss E, Tulsky DS. Memory span on the Wechsler Scales. J Clin Exp Neuropsychol. 2004;26:539–549. doi: 10.1080/13803390490496605. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JDG, Sicotte NL, Mazziotta JC. Automated image registration: II. intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22(1):153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]


