Abstract
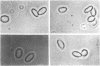
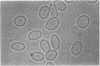
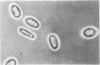
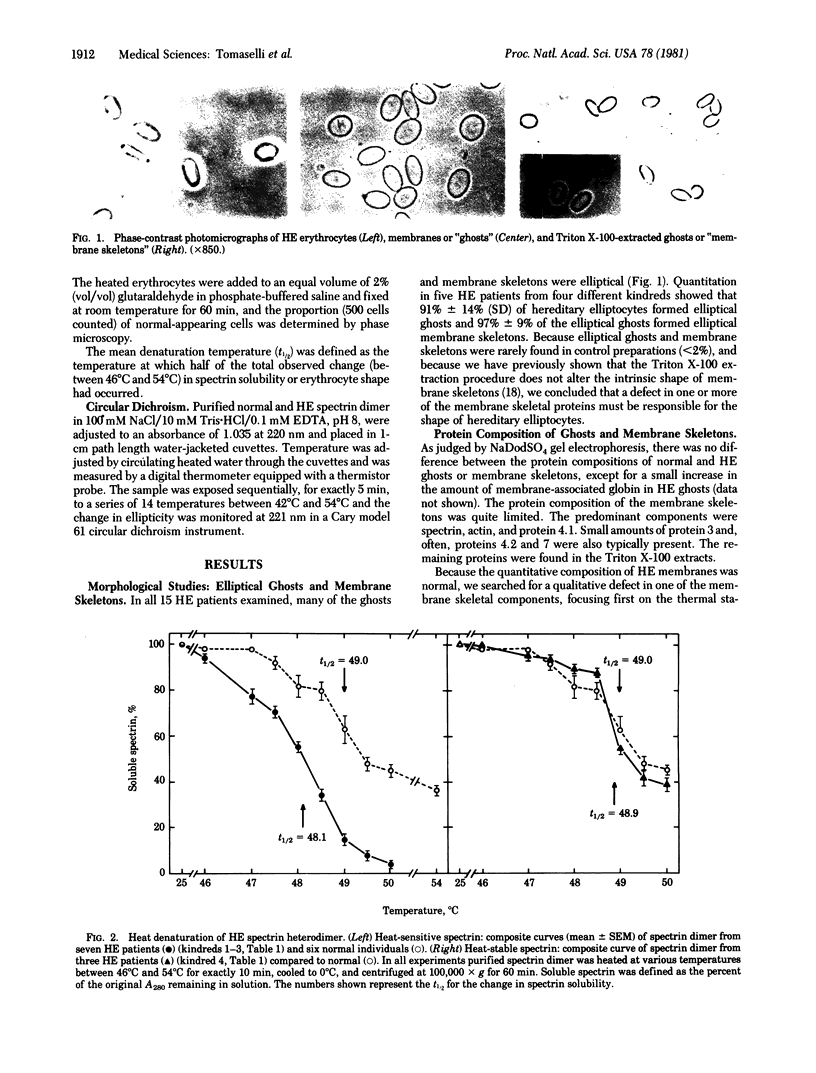
Erythrocyte membranes (ghosts) and membrane skeletons (submembranous reticula of spectrin, actin, and protein 4.1 prepared by extracting ghosts with Triton X-100) from 15 patients with hereditary elliptocytosis (HE) were elliptical, which indicates that the primary defect responsible for the abnormal shape of these cells resides in the skeleton. The protein composition of HE skeletons was normal, but in three kindreds purified spectrin heterodimer from 7/7 HE patients was heat sensitive and denatured at 48.0 +/- 0.1 degrees C instead of 49.0 +/- 0.3 degrees C (P less than 0.0005). Heat sensitivity was detected by precipitation and, in the spectrin from one patient, by changes in circular dichroism. In one other kindred spectrin dimer from 3/3 patients denatured at the normal temperature. In two of the three kindreds with heat-sensitive spectrin, intact erythrocytes exhibited budding and fragmentation at the temperature at which spectrin denatured. In the third kindred spectrin was heat sensitive, but erythrocytes were not. The symptoms in the latter kindred were clinically more severe (hemolytic HE with spherocytosis) than in the other three (mild HE). We conclude that defects in the erythrocyte membrane skeleton may be a common feature of HE. As judged by heat denaturation of erythrocytes and purified spectrin dimer, three phenotypically distinct forms of HE exist, two of which are characterized by defective, heat-sensitive spectrin. It remains to be determined whether the molecular defect in spectrin responsible for heat sensitivity is the primary genetic defect responsible for HE.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979 Apr 10;254(7):2533–2541. [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. Spectrin/actin complex isolated from sheep erythrocytes accelerates actin polymerization by simple nucleation. Evidence for oligomeric actin in the erythrocyte cytoskeleton. J Biol Chem. 1980 Feb 25;255(4):1670–1676. [PubMed] [Google Scholar]
- Cook P. J., Noades J. E., Newton M. S., De Mey R. On the orientation of the Rh: El1 linkage group. Ann Hum Genet. 1977 Oct;41(2):157–162. doi: 10.1111/j.1469-1809.1977.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Avruch J., Dino J. E., Patel V. P. Phosphorylation and dephosphorylation of spectrin. J Supramol Struct. 1978;9(1):97–112. doi: 10.1002/jss.400090110. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fowler V., Taylor D. L. Spectrin plus band 4.1 cross-link actin. Regulation by micromolar calcium. J Cell Biol. 1980 May;85(2):361–376. doi: 10.1083/jcb.85.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenquist A. C., Shohet S. B., Bernstein S. E. Marked reduction of spectrinin hereditary spherocytosis in the common house mouse. Blood. 1978 Jun;51(6):1149–1155. [PubMed] [Google Scholar]
- Haest C. W., Plasa G., Kamp D., Deuticke B. Spectrin as a stabilizer of the phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta. 1978 May 4;509(1):21–32. doi: 10.1016/0005-2736(78)90004-4. [DOI] [PubMed] [Google Scholar]
- Luna E. J., Kidd G. H., Branton D. Identification by peptide analysis of the spectrin-binding protein in human erythrocytes. J Biol Chem. 1979 Apr 10;254(7):2526–2532. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Karnovsky M. J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J Clin Invest. 1976 Oct;58(4):955–963. doi: 10.1172/JCI108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON N. E. The detection and estimation of linkage between the genes for elliptocytosis and the Rh blood type. Am J Hum Genet. 1956 Jun;8(2):80–96. [PMC free article] [PubMed] [Google Scholar]
- Rebuck J. W., Van Slyck E. J. An unsuspected ultrastructural fault in human elliptocytes. Am J Clin Pathol. 1968 Jan;49(1):19–25. doi: 10.1093/ajcp/49.1.19. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P. Integral membrane protein interaction with Triton cytoskeletons of erythrocytes. Biochim Biophys Acta. 1979 Oct 19;557(1):122–134. doi: 10.1016/0005-2736(79)90095-6. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Reinhardt B. N., Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem. 1980 Jul 25;255(14):7034–7039. [PubMed] [Google Scholar]
- Tökés Z. A., Chambers S. M. Proteolytic activity associated with human erythrocyte membranes. Self-digestion of isolated human erythrocyte membranes. Biochim Biophys Acta. 1975 May 6;389(2):325–338. doi: 10.1016/0005-2736(75)90325-9. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979 Aug 30;280(5725):811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- WOLMAN I. J., OZGE A. Studies on elliptocytosis. I. Hereditary elliptocytosis in the pediatric age period; a review of recent literature. Am J Med Sci. 1957 Dec;234(6):702–712. doi: 10.1097/00000441-195712000-00009. [DOI] [PubMed] [Google Scholar]
- Wolfe L. C., Lux S. E. Membrane protein phosphorylation of intact normal and hereditary spherocytic erythrocytes. J Biol Chem. 1978 May 10;253(9):3336–3342. [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]
- Yu J., Goodman S. R. Syndeins: the spectrin-binding protein(s) of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 May;76(5):2340–2344. doi: 10.1073/pnas.76.5.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkowsky H. S. Heat-induced erythrocyte fragmentation in neonatal elliptocytosis. Br J Haematol. 1979 Apr;41(4):515–518. doi: 10.1111/j.1365-2141.1979.tb05889.x. [DOI] [PubMed] [Google Scholar]
- Zarkowsky H. S., Mohandas N., Speaker C. B., Shohet S. B. A congenital haemolytic anaemia with thermal sensitivity of the erythrocyte membrane. Br J Haematol. 1975 Apr;29(4):537–543. doi: 10.1111/j.1365-2141.1975.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Ziparo E., Lemay A., Marchesi V. T. The distribution of spectrin along the membranes of normal and echinocytic human erythrocytes. J Cell Sci. 1978 Dec;34:91–101. doi: 10.1242/jcs.34.1.91. [DOI] [PubMed] [Google Scholar]