Abstract
BACKGROUND
Muscle protein synthesis is stimulated in the elderly when amino acid availability is increased.
OBJECTIVE
To determine which mode of delivery of amino acids (intravenous vs. oral ingestion) is more effective in stimulating the rate of muscle protein synthesis in elderly subjects.
DESIGN
Fourteen elderly subjects were assigned to one of two groups. Following insertion of femoral arterial and venous catheters, subjects were infused with a primed, continuous infusion of L-[ring-2H5] phenylalanine. Blood samples and muscle biopsies were obtained to measure muscle protein fractional synthesis rate (FSR) with the precursor-product model, phenylalanine kinetics across the leg with the three-pool model, and whole body phenylalanine kinetics. Protein metabolism parameters were measured in the basal period, and during the administration of oral amino acids (n=8) or a similar amount of intravenous amino acids (n=6).
RESULTS
Enteral and parenteral amino acid administration increased amino acid arterial concentrations and delivery to the leg to a similar extent in both groups. Muscle protein synthesis as measured by both FSR, and the three-pool model, increased during amino acid administration (P < 0.05 vs. basal) in both groups with no differences between groups. Whole body proteolysis did not change with the oral amino acids whereas it increased slightly during parenteral amino acid administration.
CONCLUSIONS
Increased amino acid availability stimulates the rate of muscle protein synthesis independent of the route of administration (enteral vs. parenteral).
Keywords: amino acids, aging, muscle protein synthesis, stable isotopes
Introduction
The loss of muscle mass associated with aging is termed sarcopenia (1). The loss of muscle as one ages is significant because the concomitant decreases in muscle function and strength increase the risk for falls and serious injury (such as bone fractures). In addition, a reduction in muscle function and strength can lead to a reduction in overall physical activity levels in the elderly with the possible and subsequent metabolic alterations such as obesity, insulin resistance and a reduction in bone density. Thus, finding a way to attenuate sarcopenia in the elderly is greatly needed.
One valid way to improve muscle functioning and strength in the elderly is to enroll the elderly in a progressive resistance training program. Several studies have shown that the elderly respond to such a training program with increases in muscle strength, function, mass and increased rates of protein synthesis (2–5). However, strength training cannot be universally prescribed to older people due to pre-existing health problems, and compliance with the exercise schedule may be a problem.
Another means to counteract sarcopenia includes proper nutrition. Our group has shown that increasing amino acid availability (via intravenous infusion or oral ingestion) increases muscle protein synthesis in the young at rest (6), following resistance exercise (6;7), and in the elderly at rest (8;9). In addition, the response of muscle protein synthesis to the increased amino acid availability is similar in the elderly and in the young (8;9), indicating that the response of muscle protein synthesis to the nutritional stimulus is preserved with aging. Under a practical standpoint it is important to know whether muscle protein synthesis is better stimulated in the elderly by enteral or parenteral amino acid administration. This is an important question that needs to be addressed since both approaches are available in certain conditions, such as in hospital and nursing home patients. If enteral nutrition can be identified as a means to counteract the loss of muscle in the elderly, at least as effectively as parenteral nutrition, then it would be much more convenient for elderly subjects to increase their amino acid availability via oral ingestion rather than intravenous infusion. The answer to this question may significantly impact the clinical approach to the elderly at risk of loss of muscle mass due to acute inactivity (i.e. bed rest due to elective surgery or acute illness), and the frail elderly.
Thus, the purpose of the present study was to determine if oral ingestion of amino acids is at least as effective as intravenous infusion in stimulating muscle protein synthesis in the elderly.
Methods
Subjects
The Institutional Review Board of the University of Texas Medical Branch at Galveston approved the study and all subjects gave written, informed consent before participation in the study. Fourteen healthy elderly volunteers (age 71 ± 1 yr, weight 78 ± 4 kg, and body mass index-BMI 27 ± 1 kg/m2, 2 females and 12 males) were recruited through the Center on Aging Volunteers Registry of the University of Texas Medical Branch. The eligibility of the volunteers was assessed by a physical examination, electrocardiogram, blood count, plasma electrolytes, blood glucose concentration, liver and renal function tests. Exclusion criteria were heart disease, diabetes, obesity, hypertension, coagulation disorders, peripheral arterial or venous diseases, cancer, acute or chronic pulmonary diseases, infectious diseases, and allergy to iodides. All subjects were active, although not exercise trained, and living on their own with no limitation in ambulation or history of falls.
Study Design
Eight of the subjects (2 F, 6 M) participated in the oral amino acid (OAA) group and the remaining six males were assigned to the intravenous infusion (IV) group. Data from these fourteen subjects have been published elsewhere (8;9). All volunteers were instructed to eat their normal diets for the week prior to participation in the study. The evening before the study the subjects were admitted to the General Clinical Research Center of the University of Texas Medical Branch. They were given a light dinner at 1900, after which they were allowed only water ad libitum. The experimental protocol was identical in both groups with the only difference between groups being the mode of administration of amino acids. At 0600, polyethylene catheters were inserted into a forearm vein for infusion of labeled phenylalanine, in the wrist vein of the opposite hand for arterialized blood sampling, and into the femoral artery and vein of one leg for blood sampling. The catheter in the femoral artery was also used for the infusion of indocyanine green (ICG).
After an initial blood sample was obtained for the measurement of background phenylalanine enrichment and ICG concentration, a primed (2 µmol/kg), continuous infusion of L-[ring-2H5] phenylalanine (0.05 µmol·kg−1·min−1) was started (time = 0).
At 120 min, the first muscle biopsy (~80–100 mg) was taken from the lateral portion of the vastus lateralis muscle of the leg with the femoral catheters inserted, ~20 cm above the knee, using a 4-mm Bergstrom biopsy needle. The tissue was immediately frozen in liquid nitrogen and stored at −80°C until analyzed.
Between 240 and 270 min, ICG dye (0.5 mg·ml−1) was infused into the femoral artery and four blood samples were obtained from the femoral vein and the peripheral vein to measure leg blood flow. In addition, four blood samples were obtained from the femoral artery and vein to measure plasma free phenylalanine concentrations and enrichments. At 240 and 300 min, additional blood samples were drawn from the femoral artery for measurement of insulin concentrations. At 300 min, a second muscle biopsy was taken.
Immediately after the biopsy was taken, the oral administration or intravenous infusion of an amino acid mixture was started and continued until the end of the study (480 min). The IV group was infused with a commercial amino acid mixture (10% Travasol, total amino acids 100 mg·ml−1; Clintec Nutrition Co., Deerfield, IL) and a freshly prepared glutamine solution (30 mg·ml−1; Kyowa, Tokyo, Japan). The total amino acid infusion was 149 mg·kg−1·h−1. Approximately 35% of the amino acid infusate was essential amino acids with 3.3 grams of arginine. The OAA group was given a mixture of amino acids orally. The mixture was based on the known amino acid composition of beef, and consisted of 40 grams of amino acids and a sugar-free flavoring (Crystal Light, Kraft Foods, White Plains, NY) dissolved in 540 ml of water. The mixture was given as small boluses (30 ml) every 10 min to maintain plasma phenylalanine enrichments and concentrations at steady state. The total amount of amino acid ingested was 184 ± 8 mg·kg−1·h−1 with 45% of the solution being essential amino acids and 2.3 grams of arginine. Table 1 shows the amino acid composition of the intravenous solution and the oral amino acid mixture. The two solutions contained similar amounts of all essential amino acids except lysine that was lower in the intravenous solution. In addition, L-[ring-13C6]phenylalanine (18 µmol·kg−1 body weight) was added to the solution in order to measure phenylalanine first-pass splanchnic extraction.
Table 1.
Amino acid composition of the intravenous and oral solutions
| Amino Acid | Intravenous (grams) | Oral (grams) |
|---|---|---|
| Alanine | 7.0±0.8 | 2.4* |
| Arginine | 3.9±0.4 | 2.8 |
| Asparagine | - | 3.7* |
| Cysteine | - | 0.5* |
| Glutamine | 3.4±0.4 | 5.8 |
| Glycine | 3.5±0.4 | 1.8* |
| Histidine | 1.6±0.2 | 2.0 |
| Isoleucine | 2.0±0.2 | 1.9 |
| Leucine | 2.5±0.3 | 3.2 |
| Lysine | 2.0±0.2 | 3.9* |
| Methionine | 1.3±0.1 | 1.0 |
| Phenylalanine | 1.9±0.2 | 1.6 |
| Proline | 2.3±0.2 | 1.9 |
| Serine | 1.7±0.2 | 1.6 |
| Threonine | 1.4±0.2 | 1.9 |
| Tryptophan | 0.6±0.1 | 0.5 |
| Tyrosine | 0.1±0.1 | 1.4* |
| Valine | 2.0±0.2 | 2.2 |
| Total | 37.1±4.0 | 40.1 |
P < 0.05 vs. Intravenous Amino Acid
In both groups, between 420 and 480 min the measurement of leg blood flow was repeated and blood samples were taken as described for the basal period. At 480 min, before the tracer infusion was terminated, a third muscle biopsy was obtained.
Analytical methods
The blood samples for the measurement of plasma phenylalanine concentration and enrichment were collected in tubes containing 15% sulfosalicylic acid. An internal standard (50 µmol/l of L-[ring-13C1]phenylalanine or L-[ring-13C6]phenylalanine) of 200 µl/ml of blood was added for measurement of phenylalanine concentrations. Amino acids were separated with cation-exchange chromatography as previously described (10). The enrichment and the concentrations of phenylalanine in the arterial and venous blood samples were determined with gas chromatography-mass spectrometry (Hewlett-Packard, Palo Alto, CA).
Muscle samples were weighed and the proteins precipitated with 450 µl of 10% SSA. An internal standard (2 µl/mg wet tissue) containing 3 µmol/l of L-[ring-13C1]phenylalanine or L-[ring-13C6]phenylalanine was added to measure the intracellular concentration of phenylalanine. The tissue was homogenized, centrifuged and the supernatant collected. This procedure was repeated three times. The pellet containing mixed muscle proteins was washed, dried, and the proteins hydrolyzed in 6 N HCl at 110°C for 24 h. The amino acids in the hydrolysate was then extracted with cation-exchange chromatography, dried, and the enrichment of free tissue phenylalanine were determined by gas chromatography-mass spectrometry (GC 8000 series, MD 800, Fisons Instruments, Manchester, UK) using the standard curve approach as described by Calder et al. (11). Intracellular enrichment and concentration of phenylalanine was measured in the supernatant as described above for blood phenylalanine enrichment and concentration.
The concentration of plasma insulin was measured with a commercial RIA kit (Incstar, Stillwater, MN). The serum concentration of ICG was measured by means of a spectrophotometer set at λ = 805 nm.
Calculations
The protocol was designed to assess the effect of enteral vs. parenteral amino acid intake on muscle protein fractional synthesis rate (FSR) as measured by the precursor-product model in the elderly. In addition, the response of intracellular phenylalanine kinetics in skeletal muscle and whole body phenylalanine kinetics were evaluated.
Muscle protein FSR was calculated by dividing the increment in enrichment in the product, that is the increment in protein bound phenylalanine tracer/tracee ratio (ΔEP), by the enrichment in the precursor (free intracellular phenylalanine tracer/tracee ratio), in the basal period (2nd-1st biopsy ΔEP) and during amino acid administration (3rd-2nd biopsy ΔEP).
Use of the three-pool model allowed us to determine the rate of utilization of phenylalanine for muscle protein synthesis and appearance from breakdown since phenylalanine is neither oxidized nor synthesized in muscle. Net balance was determined by multiplying the difference between arterial and venous phenylalanine concentration by the blood flow. The three-pool model calculations for the rates phenylalanine delivery to the leg, release from the leg, inward and outward transport, shunting from artery to vein, intracellular appearance (protein breakdown), and utilization (protein synthesis) have been described in detail elsewhere (12).
Whole body phenylalanine kinetics was calculated using the single pool model. Phenylalanine rate of appearance (Ra) was calculated by dividing the infusion rate of labeled phenylalanine (I) by the arterial enrichment of phenylalanine (Ea). During amino acid administration the endogenous phenylalanine Ra, an index of whole-body protein breakdown, was calculated by subtracting the unlabeled phenylalanine infusion rate from the total Ra in the IV group. In the OAA group, endogenous Ra was calculated by subtracting from the total phenylalanine Ra the rate of ingested phenylalanine corrected by the splanchnic first pass extraction rate. The first-pass splanchnic extraction of oral phenylalanine was calculated with the double-tracer technique as previously described (13): first pass splanchnic extraction = 1 – [(EA oral/Ioral)/(EA i,v/Iiv)] where EA oral is the arterial enrichment, Ioral is the infusion rate of the oral tracer, EA i,v is the arterial enrichment, and Iiv is the infusion rate of the intravenous tracer.
Statistics
The comparisons between the OAA and IV groups were carried out using ANOVA. Differences were considered significant at P < 0.05. Data are reported as mean±SE.
Results
There were no differences between the IV and OAA groups for age (71 ± 2 vs. 71 ± 2 yr) and BMI (26 ± 1 vs. 29 ± 3 kg/m2) respectively.
The basal values of insulin were not different between the OAA and IV (6 ± 3 vs. 5 ± 1 µIU/ml), and increased in both the OAA and IV groups (10 ± 4 vs. 9 ±1 µIU/ml), respectively. There were no differences between groups in their insulin response to the amino acids (P = NS).
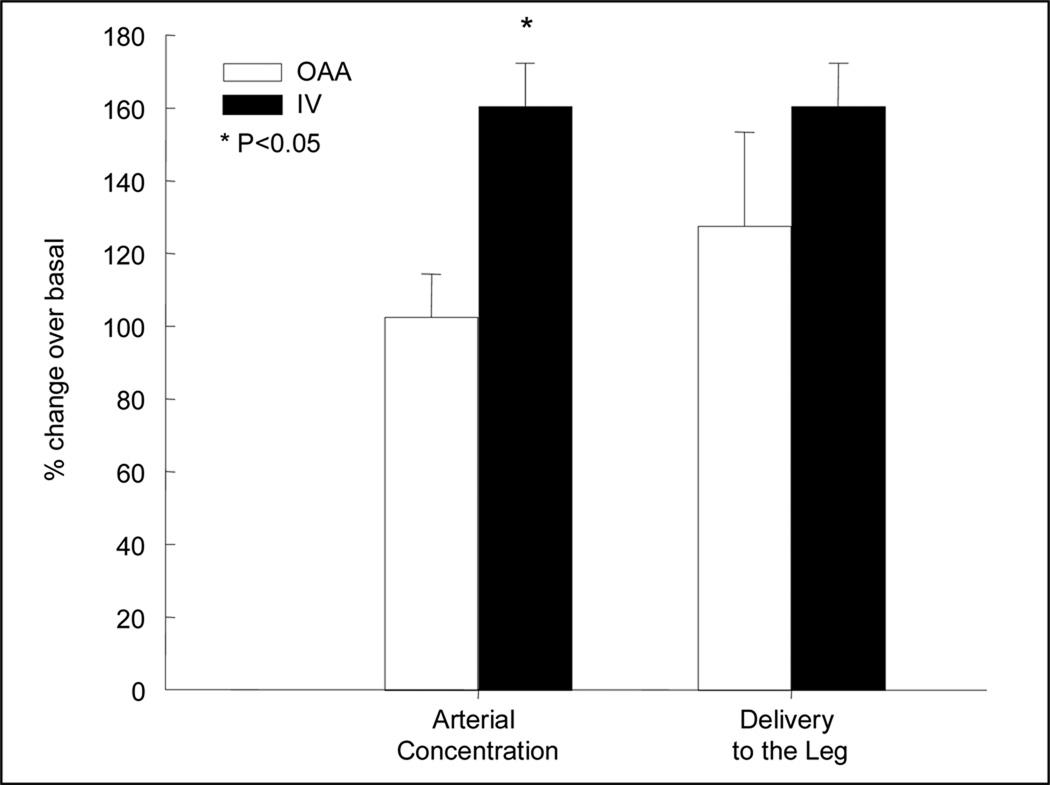
In the last hour of the basal period (240–300 min) and during the last hour of the IV and/or OAA period (420–480 min), blood amino acid concentrations and enrichments of phenylalanine in the femoral artery and vein were at steady state in both groups (see references (8;9)). In addition, the average concentration of phenylalanine in the femoral artery and vein increased significantly with amino acid administration in both groups, although the increase was slightly but significantly higher in the IV group. Nevertheless, phenylalanine delivery to the leg increased to the same extent in both groups, with no differences between IV and OAA (Figure 1).
Figure 1.
Percent change from basal of phenylalanine arterial concentration and delivery to the leg. Open bars, oral ingestion of amino acids (OAA); closed bars, intravenous infusion of amino acids (IV).
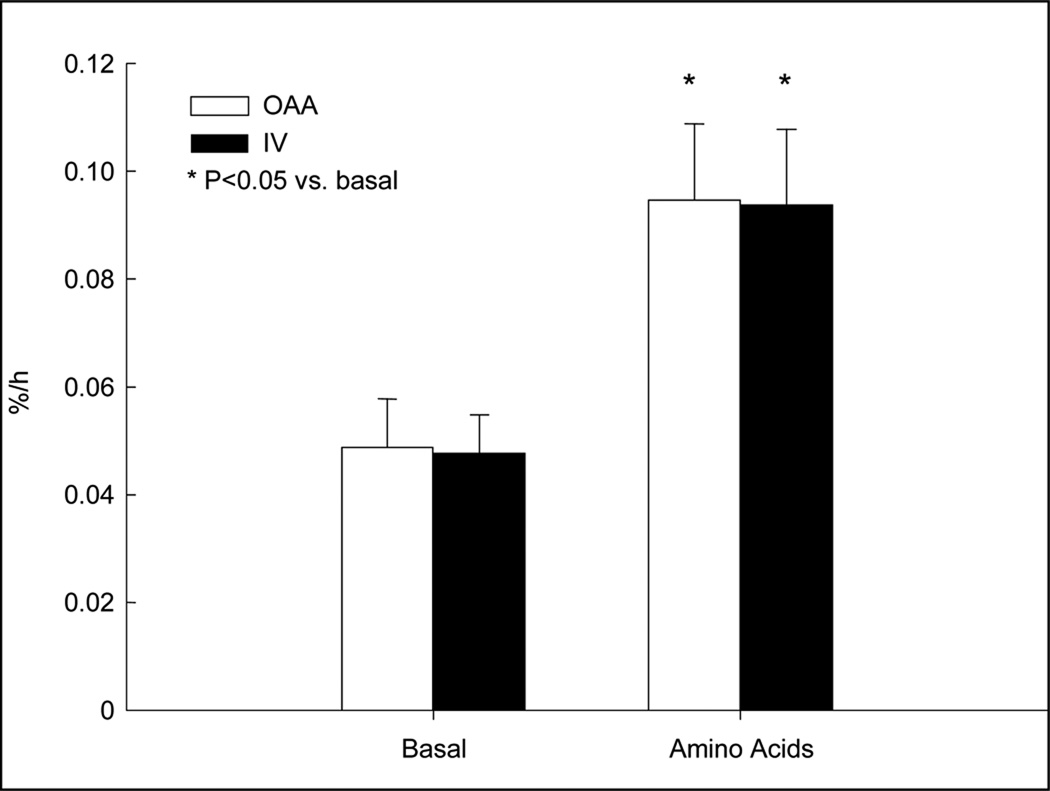
There were no differences between groups for basal FSR (OAA: 0.0495 ± 0.0088 %·h−1, IV: 0.0483 ± 0.0066 %·h−1, P = NS). FSR increased by ~120% from basal values in both groups during the amino acid period (OAA: 0.0953 ± 0.0144 %·h−1, IV: 0.0940 ± 0.0143 %·h−1, P < 0.01 vs. basal), with no differences between groups (Figure 2).
Figure 2.
Muscle protein fractional synthetic rate (FSR) as measured by the precursor-product method. Open bars, oral ingestion of amino acids; closed bars, intravenous infusion of amino acids.
Similarly, muscle protein synthesis measured using the three-pool model increased by 92 ± 24% in the OAA group during the amino acid period and by 72 ± 19% in the IV group (8;9). No difference was detected between the two groups (P = NS). Muscle protein breakdown measured using the three-pool model decreased by 4 ± 16% in the OAA group during the amino acid period and by 25 ± 16% in the IV group. No differences between groups were detected (P = NS). The rate of delivery of phenylalanine to the leg increased significantly (P<0.05 vs. basal) (OAA: 128 ± 26%; IV: 161 ± 12%; P = NS), and the rate of inward muscle transmembrane transport increased significantly (155 ± 47% vs. 221 ± 60%, P < 0.05) in the OAA and IV groups, respectively. There were no differences between groups (P = NS). The net balance of phenylalanine across the leg, an indicator of overall muscle anabolism, increased significantly from basal values (246 ± 40% vs. 226 ± 31%, P < 0.05) in the OAA and IV groups, respectively. No differences were detected between the OAA and IV groups (P = NS).
The basal whole body phenylalanine Ra, an index of whole body protein breakdown, was similar both in the OAA and IV groups (0.67 ± 0.05 vs. 0.69 ± 0.03 µmol·kg−1·min−1, P = NS). During amino acid administration, the total whole body phenylalanine Ra, which includes both endogenous proteolysis and phenylalanine appearance from the exogenous sources (oral or i.v.), increased significantly in both groups (OAA: 1.05 ± 0.06; IV: 1.49 ± 0.10 µmol·kg−1·min−1, P < 0.05 vs. basal), but the increase was significantly blunted in the OAA group due to a 47 ± 3 % extraction of the orally ingested amino acids (percent increase from basal values OAA: 61 ± 10 %; IV: 116 ± 19 %, P < 0.01). Endogenous whole body phenylalanine Ra during amino acid administration (an index of protein breakdown) did not change significantly from basal values (OAA: 0.65 ± 0.04; IV: 0.73 ± 0.10 µmol·kg−1·min−1, P = NS). However, when the percent change over the basal values was compared between the two groups we found that the IV group had a slight but significant increase in endogenous whole body phenylalanine Ra (OAA: 0 ± 8%; IV: 5 ± 16 %, P < 0.01).
Discussion
The most important finding of this study is that in healthy older adults muscle protein synthesis is stimulated to a similar extent when amino acid availability is increased via either intravenous infusion or oral ingestion of amino acids. In addition, the amino acid administration route does not appear to play a critical role in the response of the other muscle metabolism parameters, protein breakdown and amino acid transport. On the other hand, we found that the whole body endogenous phenylalanine Ra, an index of endogenous proteolysis, showed a slight but significantly different response to amino acids according to the administration mode. The percent change in endogenous proteolysis was in fact slightly higher in the group receiving parenteral amino acids compared to the group given enteral amino acids, suggesting that at the whole body level oral amino acids may be more anabolic than intravenous amino acids. However, the amino acid compositions of the IV and OAA solutions were slightly different and may account for some of the differences in the response of endogenous proteolysis.
When interpreting our results one need to consider that the stimulation of muscle protein synthesis obtained in our subjects was achieved using quite a large amount of mixed amino acids (approximately 35–40g over three hours), representing almost half of the recommended dietary allowance for protein in normal adults (14). To our knowledge no dose-response study has been performed on the effects of graded amounts of amino acids on muscle protein synthesis in the elderly. However, we can reasonably assume that the dose of amino acids we administered to our subjects represented a maximal or quasi-maximal stimulus for muscle protein synthesis. Thus, we are unable to assess whether differences between the enteral and the parenteral administration route are present when lower doses of amino acids are administered to elderly subjects. Another issue to consider is that the present study tested only the acute effects of amino acids on muscle protein synthesis. Thus, from our data we cannot exclude that the positive effect of amino acids may dissipate if daily (i.e. chronic) amino acid supplementation is used in elderly subjects to combat sarcopenia. Further investigations are warranted.
Parenteral nutrition is usually reserved for acutely ill patients where the use of the enteral route is contraindicated, or for patients with gastro-intestinal problems. The subjects of our study were healthy, independent older adults with no major diseases. Therefore, extrapolations to the clinical setting need to be weighed in light of these limitations. Nevertheless, out data can be considered as a starting point for future studies in selected patient populations. In addition, the information that this study provides can be used in cases in which otherwise healthy older subjects are exposed to the risk of severe muscle loss, such as during the recovery from a lower extremity fracture when other means of muscle preservation or recovery (e.g. exercise) are not applicable.
Overall, our results provide evidence that both enteral and parenteral amino acid administration can effectively stimulate muscle protein synthesis in the elderly. On the basis of these results, the physiological enteral route appears to be more appealing than the parenteral mode of delivery for amino acid supplementation in older people. The results presented here should provide valuable information for clinicians treating and counseling the elderly suffering from sarcopenia. In general, nutritional supplementation should be used as one of several strategies to counteract sarcopenia in the elderly. Resistance exercise increases muscle protein synthesis in young and elderly (2–4;15;16). Increasing availability of amino acids following resistance exercise appears to have an additive effect on muscle protein synthesis in young adults (6;7). The fact that a study has shown that a high protein diet in combination with exercise does not increase muscle protein synthesis in the elderly when compared to a normal diet with exercise (17) should not discourage clinicians from prescribing increased protein/amino acid intake. In fact, recent data suggest that such negative results might be due to the type of protein supplementation used: increased amino acid intake may not have any effect on muscle protein synthesis in the healthy elderly in certain circumstances, specifically when amino acids are ingested in combination with carbohydrates, due to a negative interaction between these two categories of nutrients in older people (18). Thus, preventive and curative strategies need to be developed in order to better utilize the ability of amino acids and other means, such as exercise, to reduce, reverse, and possibly prevent muscle loss with aging.
Acknowledgments
We are indebted to Marilyn Brodwick, MPH, Susan L. Minello, RN, MSN, ANP, and James S. Goodwin, MD, University of Texas Medical Branch, for their assistance in recruiting the volunteers for the study, and with Zhi Ping Dong, MS, and Guy Jones, Shriners Hospital, for their technical assistance. Supported by grants from the National Institutes of Aging # R01 AG15780 (to RRW), # R01 AG18311 (to EV), and the UTMB Claude Pepper Older Americans Independent Center # P60 AG17231 (to J.S. Goodwin). Conducted at the General Clinical Research Center of the University of Texas Medical Branch at Galveston, TX, funded by the Grant # M01 RR00073 from the National Center for Research Resources, NIH, USPHS. Dr Volpi is a Brookdale National Fellow.
References
- 1.Dutta C, Hadley EC. The significance of sarcopenia in old age. J Gerontol 1995, A Biol Sci Med Sci. 50(Spec No):1–4. doi: 10.1093/gerona/50a.special_issue.1. [DOI] [PubMed] [Google Scholar]
- 2.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol Endocrinol Metab. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- 3.Yarasheski KE, Pak-Loduca J, Hasten DL, et al. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/=76 yr old. Am J Physiol Endocrinol Metab. 1999;277:E118–E125. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]
- 4.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- 5.Fiatarone MA, O'Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 6.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 8.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first pass splanchnic extraction. Am J Physiol Endocrinol.Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe RR. Appendix A: Laboratory Methods. In: Wolfe RR, editor. Radioactive and stable isotope tracers in biomedicine. Principles and practice of kinetic analysis. New York: Wiley-Liss; 1992. pp. 417–438. [Google Scholar]
- 11.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Sp. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 12.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab. 1995;268:E75–E84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol Endocrinol Metab. 1993;264:E109–E118. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council. Recommended Dietary Allowances. Washington, DC: National Academy of Science Press; 1989. Food and Nutrition Board. [Google Scholar]
- 15.Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol Endocrinol Metab. 1990;259:E470–E476. doi: 10.1152/ajpendo.1990.259.4.E470. [DOI] [PubMed] [Google Scholar]
- 16.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 17.Welle S, Thornton CA. High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62- to 75-yr-old men and women. Am J Physiol. 1998;274:E677–E683. doi: 10.1152/ajpendo.1998.274.4.E677. [DOI] [PubMed] [Google Scholar]
- 18.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2001;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]