Abstract
Background
Nutritional supplementation may be used to treat muscle loss with aging (sarcopenia). However, if physical activity does not increase, the elderly tend to compensate for the increased energy delivered by the supplements with reduced food intake, which results in a calorie substitution rather than supplementation. Thus, an effective supplement should stimulate muscle anabolism more efficiently than food or common protein supplements. We have shown that balanced amino acids stimulate muscle protein anabolism in the elderly, but it is unknown whether all amino acids are necessary to achieve this effect.
Objective
We assessed whether nonessential amino acids are required in a nutritional supplement to stimulate muscle protein anabolism in the elderly.
Design
We compared the response of muscle protein metabolism to either 18 g essential amino acids (EAA group: n = 6, age 69 ± 2 y; x̄ ± SD) or 40 g balanced amino acids (18 g essential amino acids + 22 g nonessential amino acids, BAA group; n = 8, age 71 ± 2 y) given orally in small boluses every 10 min for 3 h to healthy elderly volunteers. Muscle protein metabolism was measured in the basal state and during amino acid administration via l-[ring-2H5]phenylalanine infusion, femoral arterial and venous catheterization, and muscle biopsies.
Results
Phenylalanine net balance (in nmol · min−1 · 100 mL leg volume−1) increased from the basal state (P < 0.01), with no differences between groups (BAA: from −16 ± 5 to 16 ± 4; EAA: from −18 ± 5 to 14 ± 13) because of an increase (P < 0.01) in muscle protein synthesis and no change in breakdown.
Conclusion
Essential amino acids are primarily responsible for the amino acid–induced stimulation of muscle protein anabolism in the elderly.
Keywords: Sarcopenia, aging, protein synthesis, proteolysis, nutritional supplements
INTRODUCTION
Sarcopenia is a multifactorial problem of the elderly and is characterized by muscle loss and reduced strength, which can lead to functional dependence (1, 2). Undernutrition has been identified as one of the contributing factors that might be prevented by increased protein or energy intakes, or both (3-5). However, attempts to increase muscle mass, strength, and protein synthesis with commercial nutritional supplements or high-protein diets have been unsuccessful (6-8).
There are at least 2 possible reasons that can explain the inability of nutritional supplements or increased protein intake to enhance muscle growth and strength. First, there is evidence that the presence of carbohydrate in a nutritional supplement for the elderly is not beneficial (9) and may in fact impair the anabolic response of muscle proteins to the positive effect of amino acids alone (10, 11). The fact that both the commercial supplements and the high-protein diet tested in elderly adults contained carbohydrate might explain the ineffectiveness of the nutritional interventions tested in the previous studies (6-8). Second, it has been reported that elderly subjects who took supplements but did not increase their physical activity decreased their dietary intake accordingly; therefore, their total daily energy intake remained unchanged (6). This suggests that a nutritional supplement for the elderly should best be considered as more of a dietary substitute than a supplement. Thus, if the nutrient composition of the supplement is similar to that of the normal diet, it is likely that supplementation will be ineffective.
Hence, a nutritional supplement for the prevention or treatment of sarcopenia should stimulate muscle anabolism more efficiently than does food or common protein supplements in order to achieve the highest protein anabolic efficiency per energy unit. We previously reported that a balanced amino acid mixture stimulated muscle protein anabolism in elderly subjects and in younger adults (11). Data obtained in young subjects suggest that essential amino acids are mostly responsible for the amino acid stimulation of muscle protein synthesis (12, 13), whereas nonessential amino acids are apparently ineffective even at very high doses (13). Nonessential amino acids comprise a significant portion of dietary proteins, including the high-quality proteins (eg, whey and egg) that are typically used to supplement protein-poor diets. If nonessential amino acids are unnecessary for the stimulation of muscle anabolism in elderly people, even high-quality proteins could be inadequate for the effective treatment of sarcopenia, because they contain a significant amount of calories in the form of nonessential amino acids. In this case, the elimination of nonessential amino acids should maximize the anabolic efficiency of a supplement for the elderly by decreasing the total energy content while maintaining the stimulatory effect on muscle protein synthesis.
Therefore, to test whether nonessential amino acids are required for the amino acid–stimulated increase in muscle protein synthesis and anabolism in the elderly, we measured the response of muscle protein synthesis, breakdown, and net balance in healthy elderly subjects to either an essential amino acid supplement or a balanced amino acid supplement. The balanced amino acid supplement was designed by simply adding nonessential amino acids to the essential amino acid supplement. Thus, the essential amino acid content of both supplements was identical, whereas the total energy and nitrogen content of the balanced amino acid supplement was more than double that of the essential amino acid supplement.
SUBJECTS AND METHODS
Subjects
The subjects were recruited through the Sealy Center on Aging of the University of Texas Medical Branch. After giving written informed consent to the study protocol, which was approved by the University of Texas Medical Branch Institutional Review Board, the subjects underwent screening, which included a clinical history, a physical examination, and an electrocardiogram; had blood drawn for a complete blood count, for the measurement of blood glucose concentrations, and to test for hepatitis, HIV, and liver and kidney function; and had a urinalysis. Exclusion criteria were heart disease, coagulation disorders, vascular disease, hypertension, diabetes, obesity, cancer, acute or chronic pulmonary diseases, infectious diseases, and severe allergies. Subjects who had been taking anabolic steroids or corticosteroids long term were excluded. A careful clinical assessment of the amount of daily physical activity was performed to exclude any subjects engaged in regular aerobic or resistance-exercise training, with a history of falls (≥ 2/y), or with any impairment in their activities of daily living. The subjects performed their regular activities and maintained their usual diet during the week preceding the study. This was preferred to an early admission to the General Clinical Research Center or to the administration of a standardized diet for several days preceding the study, because these manipulations might affect basal muscle protein turnover (14). Six healthy elderly subjects were assigned to the essential amino acid protocol and were compared with a similar group of 8 healthy elderly subjects who were assigned to the balanced amino acid protocol (11) (Table 1). The 2 groups were studied sequentially.
TABLE 1.
Characteristics of the 2 groups of healthy elderly subjects supplemented with either 18 g essential amino acids or 40 g balanced amino acids1
| 18 g Essential amino acids (n = 1 F, 5 M) | 40 g Balanced amino acids (n = 2 F, 6 M) | |
|---|---|---|
| Weight (kg) | 86 ± 5 | 74 ± 5 |
| Height (cm) | 174 ± 5 | 170 ± 3 |
| BMI (kg/m2) | 28 ± 1 | 26 ± 1 |
| Leg volume (L) | 10.91 ± 0.82 | 9.16 ± 0.44 |
x̄ ± SE. There were no significant differences between groups by two-tailed t test.
Experimental protocol
Each subject was studied on one occasion after fasting overnight. On the evening before the study, the subjects were admitted to the General Clinical Research Center of the University of Texas Medical Branch. After 2200, the subjects were allowed only water ad libitum. At 0600, polyethylene catheters were inserted into a forearm vein for infusion of labeled phenylalanine, in a vein of the opposite hand for arterialized blood sampling, and into the femoral artery and vein of one leg for blood sampling. The femoral arterial catheter was also used for the infusion of indocyanine green (ICG; Akorn, Buffalo Grove, IL).
After a blood sample was obtained for the measurement of background phenylalanine enrichment and the ICG concentration, a primed (2 μmol/kg) continuous infusion of l-[ring-2H5]phenylalanine (0.05 μmol · kg−1 · min−1) was started (time 0) and maintained until the end of the experiment (480 min). A muscle-biopsy sample (≈80–100 mg tissue) was taken 120 min after the start of the infusion from the lateral portion of the vastus lateralis of the leg with the femoral catheters, ≈20 cm above the knee, with the use of a Bergström biopsy cannula (Micrins, Lake Forest, IL). The tissue was immediately frozen in liquid nitrogen and stored at −80 °C until analyzed. At 230 min the continuous infusion of ICG was started in the femoral artery (0.5 mg/min) and continued until 270 min. During the infusion, blood samples were taken 4 times (every 10 min) from the femoral vein and the hand vein to measure ICG concentrations. After the ICG infusion ended, between 270 and 300 min, blood samples were taken 4 times (every 10 min) from the femoral artery and vein to measure arterial and venous phenylalanine concentrations and enrichments and the arterial total amino acid concentration. At 300 min, a second muscle-biopsy sample was taken.
Immediately after the second biopsy sample was taken, the oral administration of either an essential amino acid or a balanced amino acid supplement was started and continued for 3 h until the end of the study. The amino acid content of the 2 mixtures is reported in Table 2. The crystalline amino acids (Sigma-Aldrich, St Louis) were dissolved in 540 mL water containing a sugar-free flavor (Crystal Light; Kraft Foods Inc, White Plains, NY). The amino acids were given in small boluses (30 mL) every 10 min to maintain plasma phenylalanine enrichments and concentrations at steady state. l-[ring-13C6]phenylalanine or l-[15N]phenylalanine (18 μmol/kg body wt) was added to measure the first-pass splanchnic extraction of phenylalanine. An intravenous priming dose of the oral tracer (4 μmol/kg) was also administered at the time of the first oral bolus to rapidly achieve a steady state enrichment. Between 420 and 480 min, leg blood flow was measured again, and blood samples were taken as described for the basal period. At 480 min, before the tracer infusion and amino acid administration ended, a third muscle biopsy sample was taken.
TABLE 2.
Amino acids content of the 2 amino acid supplements1
| 18 g Essential amino acids (n = 6) | 40 g Balanced amino acids (n = 8) | |
|---|---|---|
| Alanine (g) | — | 2.5 ± 0.02 |
| Arginine (g) | — | 2.8 ± 0.02 |
| Asparagine (g) | — | 3.7 ± 0.02 |
| Cysteine (g) | — | 0.5 ± 0.02 |
| Glutamine (g) | — | 5.8 ± 0.02 |
| Glycine (g) | — | 1.9 ± 0.02 |
| Histidine (g) | 2.0 ± 0.0 | 2.0 ± 0.0 |
| Isoleucine (g) | 1.9 ± 0.0 | 1.9 ± 0.0 |
| Leucine (g) | 3.2 ± 0.0 | 3.2 ± 0.0 |
| Lysine (g) | 3.9 ± 0.0 | 3.9 ± 0.0 |
| Methionine (g) | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Phenylalanine (g) | 1.7 ± 0.0 | 1.6 ± 0.0 |
| Proline (g) | — | 1.9 ± 0.02 |
| Serine (g) | — | 1.7 ± 0.02 |
| Threonine (g) | 1.9 ± 0.0 | 2.0 ± 0.0 |
| Tryptophan (g) | 0.5 ± 0.0 | 0.5 ± 0.0 |
| Tyrosine (g) | — | 1.5 ± 0.02 |
| Valine (g) | 2.0 ± 0.4 | 2.2 ± 0.0 |
| Total amino acids (g) | 18.2 ± 0.4 | 40.7 ± 0.12 |
| Total energy | ||
| (kJ) | 309 ± 6 | 693 ± 22 |
| (kcal) | 74 ± 1 | 166 ± 0 |
x̄ ± SE. The supplements were made of crystalline amino acids and dissolved in 540 mL of water containing a sugar-free flavor.
Significantly different from 18 g essential amino acids, P < 0.0001.
Analytic methods
ICG concentrations in the infusate and serum were measured spectrophotometrically at λ = 805 nm (15). ICG concentrations in the serum samples were measured directly in the spectrophotometer after separation of the serum from the remainder of the blood components (eg, cells and fibrin).
The arterial concentration of all amino acids was measured in plasma EDTA samples from 5 subjects per group with the use of HPLC (Waters Corp, Milford, MA) after extraction by ultrafiltration and orthophthalaldehyde derivatization.
The enrichments and concentrations of phenylalanine in arterial and venous blood samples were determined after the addition of an internal standard, deproteinization with sulfosalicylic acid, extraction with cation-exchange chromatography, and derivatization to tert-butyldimethylsilyl with gas chromatography–mass spectrometry in electron impact mode (GC HP 5890 and MSD HP 5989; Hewlett-Packard, Palo Alto, CA) (16).
Muscle samples were weighed and the proteins were precipitated with 450 μL of 10% sulfosalicylic acid. An appropriate internal standard solution was added to measure the intracellular phenylalanine concentration. The tissue was homogenized and centrifuged (2400 × g, 4 °C, 10 min), and the supernatant fluid was collected. This procedure was repeated 3 times. The enrichment and concentration of free tissue phenylalanine was determined on its tert-butyldimethylsilyl derivative (16) as described above. The intracellular concentration of phenylalanine was then calculated from the tissue value, accounting for the ratio of intracellular to extracellular water (17). The pellet containing mixed muscle proteins was washed and dried, and the proteins were hydrolyzed in a 6 mol HCl/L solution at 110 °C for 24 h. The amino acids in the hydrolysate were extracted and derivatized as described for the plasma samples, and phenylalanine enrichment was measured by gas chromatography–mass spectrometry (GC 8000 series and MD 800; Fisons Instruments, Manchester, United Kingdom) with selective ion monitoring at mass-to-charge ratios of 237 and 239 and the use of the standard curve approach as described by Calder et al (18).
Calculations
The muscle phenylalanine kinetic parameters were calculated by using 2 arteriovenous (AV) balance methods: a 2-pool model (19) and a 3-pool model, which was described and validated previously (17). The 2-pool model relies on the net amino acid balance and measurement of phenylalanine enrichments and concentrations in the femoral artery and vein to estimate muscle protein synthesis and breakdown. These parameters are based on the extraction of labeled phenylalanine from the femoral artery, the appearance of unlabeled phenylalanine from the muscle in the femoral vein, and the net AV difference in phenylalanine concentrations (19). The 3-pool model is an expansion of the 2-pool model and relies not only on the measurement of phenylalanine enrichments and concentrations in the femoral artery and vein but also on the direct measurement of phenylalanine enrichment in the free tissue water. This allows for the direct measurement of phenylalanine intracellular utilization for protein synthesis and release from protein breakdown. In addition, it is possible to calculate the rate of phenylalanine transport from the artery into the muscle tissue and from the muscle tissue into the venous blood.
Phenylalanine is used with both methods because it is an essential amino acid and is not oxidized in the muscle tissue. Thus, phenylalanine utilization in the muscle is a direct index of muscle protein synthesis, and its release from the muscle is a measure of muscle protein breakdown.
The 2-pool and the 3-pool models share the following parameters:
| (1) |
| (2) |
| (3) |
where CA and CV are plasma phenylalanine concentrations in the femoral artery and vein, respectively; BF is leg blood flow; and NB is net balance. The remaining parameters of phenylalanine kinetics with the 2-pool model can be calculated by using the following equations:
| (4) |
| (5) |
| (6) |
where Ra is the rate of appearance; EA and EV are phenylalanine enrichments (tracer-tracee ratio) in the femoral artery and vein, respectively; and Rd is the rate of disappearance. Data are presented per 100 mL leg volume. In these calculations it is assumed that enrichment in the vein is the most similar to that of the intracellular pool. Leg Rd is a measure of the rate of incorporation of blood amino acids into muscle proteins, and leg Ra is the sum of the amino acids released from protein breakdown that appear in the blood plus the amino acid influx. Protein synthesis from amino acids derived from protein breakdown is not included.
The specific parameters of the 3-pool model were calculated as follows:
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
where EM is the phenylalanine enrichment (tracer-tracee ratio) in the muscle. Data are presented per 100 mL leg volume (17).
Intracellular amino acid availability is given by the sum of transport into the muscle (FM,A) and the muscle protein breakdown rate (FM,0). Thus, it is possible to calculate protein synthesis efficiency as follows:
| (12) |
Leg plasma flow was calculated at dye dilution steady state as previously described (15, 20). Leg blood flow was calculated by correcting the plasma flow by the hematocrit.
The fractional synthesis rate (FSR) of mixed muscle proteins was calculated from the incorporation rate of l-[ring-2H5]phenylalanine into the proteins and the free-tissue phenylalanine enrichment by using the precursor-product model (21):
| (13) |
where ΔEP is the increment of protein-bound phenylalanine enrichment between 2 sequential biopsies, t is the time interval between the 2 sequential biopsies, and EM(1) and EM(2) are the phenylalanine enrichments (tracer-tracee ratio) in the free muscle pool in 2 subsequent biopsies. The results are presented as %/h.
The whole-body phenylalanine Ra (in μmol · kg−1 · min−1) was calculated by using the single-pool model:
| (14) |
where I(IV) is the intravenous tracer infusion rate (μmol · kg−1 · min−1) and EA(IV) is the arterial phenylalanine enrichment with the intravenous tracer. The whole-body total Ra represents a measure of whole-body endogenous proteolysis in the basal period, whereas in the supplement period it includes not only endogenous proteolysis but also the Ra in the systemic circulation of the unlabeled phenylalanine derived from the amino acid supplement. Thus, to calculate the whole-body endogenous proteolysis in the supplement period, it is necessary to subtract the contribution of dietary phenylalanine from the total phenylalanine Ra. Part of the dietary phenylalanine should not be considered in this calculation because it is taken up by the splanchnic tissues at first pass. Thus, we measured the first-pass splanchnic extraction of phenylalanine with the use of the double-tracer technique as previously described (22):
| (15) |
where EA(oral) is the arterial enrichment and I(oral) is the infusion rate of the oral tracer.
The first-pass splanchnic extraction was used to correct the oral phenylalanine intake values to calculate the phenylalanine Ra from endogenous proteolysis during amino acid supplementation:
| (16) |
Statistical analysis
The primary endpoints were measures of muscle protein synthesis, breakdown, and phenylalanine net balance across the leg. The two-tailed t test was used to assess the group effect on the variables with 2 levels only: subjects’ characteristics, oral tracer enrichment, oral intake of unlabeled phenylalanine, and first-pass splanchnic extraction of phenylalanine. The group and supplement effects on the other response variables were analyzed by using analysis of variance (ANOVA) with correction for repeated measures. Post hoc multiple comparisons were carried out when appropriate with the use of Tukey’s test. Analysis of covariance was performed on the pre-post differences when appropriate by using the basal values as the covariates.
Sample size and power calculations were performed by using the 2-sample Bonferroni-corrected t test because it is more conservative than is ANOVA, ie, if we had enough subjects with the 2-sample t test analysis then we would have enough subjects for the ANOVA model. We considered that a difference between treatments is physiologically significant if it leads to a measurable muscle gain over a relatively short chronic daily treatment (6–12 mo). Consequently, the most important parameter is phenylalanine net balance, because it is the direct measure of the net anabolic or catabolic effect of the treatments on muscle proteins. The smallest physiologically significant difference for this parameter is ≈30 nmol · min−1 · 100 mL leg volume−1, because it corresponds to a protein gain of ≈2–3 g/leg per single treatment administration. Assuming that the acute response does not change over time, such a difference would result in a gain of ≈400–500 g muscle mass per leg (measurable by dual-energy X-ray absorptiometry or magnetic resonance imaging) over a 6-mo chronic daily treatment. For the sample size calculation, we used the highest variability (13 nmol · min−1 · 100 mL leg volume−1) measured in a previous study (11). We found that we needed 5 subjects per group to detect a difference of 30 nmol · min−1 · 100 mL leg volume−1 with α = 0.05/2 and β = 0.8 or 6 subjects per group for a β = 0.9. We divided the type 1 error by 2 because we were comparing 2 differences. Statistical analysis was performed by using JMP statistical software (version 4.0.2; SAS Institute Inc, Cary, NC). Data are reported as means ± SEs. Differences were considered significant at P ≤ 0.05.
RESULTS
Subject characteristics
The characteristics of the subjects were not significantly different between the essential amino acid (EAA) and balanced amino acid (BAA) groups (Table 1).
Leg blood flow
Leg blood flow was not different between the 2 groups (group effect: P = 0.94) and did not change significantly from basal values during administration of the supplement (supplement effect: P = 0.31) in either group: EAA group (from 2.84 ± 0.37 to 3.24 ± 0.77 mL · min−1 · 100 mL leg volume−1; n = 6) and BAA group (from 2.98 ± 0.49 to 3.23 ± 0.49 mL · min−1 · 100 mL leg volume−1; n = 8). The treatment-by-group interaction was not significant (P = 0.81).
Concentrations and enrichments
Plasma insulin concentrations were not significantly different in the basal state. Insulin concentrations increased slightly but significantly from basal values only during balanced amino acid consumption (from 4.6 ± 0.9 to 8.8 ± 1.3 μU/mL; n = 8), with no significant change during essential amino acid consumption (from 2.9 ± 1.2 to 4.1 ± 0.9 μU/mL; n = 5). The group effect was not significant (P = 0.06), whereas the supplement effect (P = 0.0002) and the treatment-by-group interaction (P = 0.011) were significant.
Arterial amino acid concentrations in 5 subjects per group are reported in Table 3. The concentrations of all essential amino acids increased significantly in both groups, except for methionine, which increased significantly only in the BAA group. The concentrations of alanine, glutamate plus glutamine, and tyrosine increased significantly in both groups, whereas the concentrations of arginine, aspartate plus asparagine, glycine, and serine increased significantly only in the BAA group.
TABLE 3.
Arterial amino acid concentrations in the 2 groups of healthy elderly subjects in the basal state and during supplementation with either 18 g essential amino acids or 40 g balanced amino acids1
| 18 g Essential amino acids (n = 5)
|
40 g Balanced amino acids (n = 5)
|
P (ANOVA)
|
|||||
|---|---|---|---|---|---|---|---|
| Basal state | Supplementation | Basal state | Supplementation | Group effect | Supplement effect | Interaction | |
| μmol/L | μmol/L | ||||||
| Essential amino acids | |||||||
| Histidine | 57 ± 7 | 80 ± 15 | 56 ± 4 | 95 ± 10 | 0.58 | 0.0027 | 0.29 |
| Isoleucine | 41 ± 2 | 130 ± 32 | 50 ± 4 | 132 ± 5 | 0.77 | 0.0008 | 0.81 |
| Leucine | 166 ± 14 | 556 ± 54 | 159 ± 14 | 439 ± 24 | 0.10 | < 0.0001 | 0.09 |
| Lysine | 137 ± 22 | 255 ± 56 | 126 ± 10 | 224 ± 20 | 0.58 | 0.0032 | 0.70 |
| Methionine | 26 ± 4 | 27 ± 4 | 18 ± 3 | 36 ± 62 | 0.91 | 0.0014 | 0.0024 |
| Phenylalanine | 66 ± 4 | 151 ± 18 | 59 ± 3 | 111 ± 10 | 0.06 | 0.0001 | 0.14 |
| Threonine | 91 ± 9 | 152 ± 39 | 91 ± 7 | 170 ± 5 | 0.68 | 0.0063 | 0.67 |
| Valine | 187 ± 20 | 340 ± 66 | 170 ± 13 | 376 ± 46 | 0.85 | 0.0006 | 0.44 |
| Total | 770 ± 56 | 1691 ± 195 | 729 ± 46 | 1583 ± 34 | 0.53 | < 0.0001 | 0.74 |
| Nonessential amino acids | |||||||
| Alanine | 333 ± 49 | 430 ± 52 | 255 ± 39 | 311 ± 28 | 0.12 | 0.0061 | 0.37 |
| Arginine | 88 ± 10 | 88 ± 14 | 82 ± 7 | 153 ± 63 | 0.12 | <0.0001 | < 0.0001 |
| Aspartate and asparagine | 43 ± 9 | 41 ± 6 | 56 ± 2 | 124 ± 43 | 0.0003 | < 0.0001 | < 0.0001 |
| Glutamate and glutamine | 851 ± 142 | 953 ± 131 | 988 ± 66 | 1077 ± 70 | 0.41 | 0.0172 | 0.85 |
| Glycine | 131 ± 22 | 113 ± 19 | 181 ± 19 | 222 ± 243 | 0.0289 | 0.0375 | 0.0002 |
| Serine | 122 ± 27 | 128 ± 22 | 120 ± 10 | 182 ± 11 | 0.33 | 0.0021 | 0.0063 |
| Tyrosine | 52 ± 6 | 64 ± 9 | 46 ± 7 | 60 ± 5 | 0.58 | 0.0169 | 0.91 |
| Total | 1620 ± 246 | 1827 ± 231 | 1727 ± 98 | 2128 ± 79 | 0.43 | 0.0008 | 0.13 |
x̄ ± SE.
Significantly different from the basal state (within group), P < 0.05.
Significantly different from the essential amino acids group (within study period), P < 0.05.
Free phenylalanine concentrations in femoral artery, femoral vein, and muscle tissue increased significantly from the basal state during amino acid supplementation (Table 4). Although there was a significant group effect for all 3 variables, there was no significant treatment-by-group interaction. Phenylalanine enrichments in femoral artery, femoral vein, and muscle tissue decreased significantly from the basal state during amino acid supplementation; however, no significant differences between the 2 groups or a significant treatment-by-group interaction were found.
TABLE 4.
Free phenylalanine concentrations and enrichments in the femoral artery and vein and in the muscle tissue of the 2 groups of healthy elderly subjects during supplementation with either 18 g essential amino acids or 40 g balanced amino acids1
| 18 g Essential amino acids (n = 6)
|
40 g Balanced amino acids (n = 8)
|
P (ANOVA)
|
|||||
|---|---|---|---|---|---|---|---|
| Basal state | Supplementation | Basal state | Supplementation | Group effect | Supplement effect | Interaction | |
| Concentration (μmol/L) | |||||||
| Artery | 68 ± 4 | 148 ± 15 | 59 ± 2 | 120 ± 8 | 0.0455 | < 0.0001 | 0.26 |
| Vein | 74 ± 4 | 140 ± 12 | 65 ± 3 | 115 ± 8 | 0.0414 | < 0.0001 | 0.32 |
| Muscle | 96 ± 9 | 149 ± 10 | 130 ± 12 | 195 ± 16 | 0.0433 | < 0.0001 | 0.51 |
| Enrichment (tracer-tracee ratio × 100) | |||||||
| Intravenous tracer | |||||||
| Artery | 8.21 ± 0.21 | 4.73 ± 0.28 | 7.91 ± 0.60 | 4.93 ± 0.27 | 0.92 | < 0.0001 | 0.41 |
| Vein | 5.99 ± 0.27 | 4.19 ± 0.23 | 6.22 ± 0.55 | 4.41 ± 0.30 | 0.66 | < 0.0001 | 0.98 |
| Muscle | 5.04 ± 0.27 | 4.03 ± 0.18 | 4.82 ± 0.69 | 3.82 ± 0.32 | 0.72 | 0.0055 | 0.98 |
| Oral tracer | |||||||
| Artery | — | 5.30 ± 0.29 | — | 5.17 ± 0.13 | 0.89 | — | — |
x̄ ± SE.
Whole-body phenylalanine kinetics
Whole-body phenylalanine kinetic parameters are reported in Table 5. Whole-body total phenylalanine rate of appearance increased significantly with amino acid supplementation in both groups, but no significant differences between the groups and no significant treatment-by-group interaction were found. The endogenous phenylalanine rate of appearance, an index of whole-body protein breakdown, was not significantly different between the 2 groups and did not change significantly during amino acid supplementation; no significant treatment-by-group interaction was found. The first-pass splanchnic extraction of the orally administered phenylalanine was not significantly different between the 2 groups.
TABLE 5.
Whole-body phenylalanine kinetics for the 2 groups of healthy elderly subjects in the basal state and during supplementation with either 18 g essential amino acids or 40 g balanced amino acids1
| 18 g Essential amino acids (n = 6)
|
40 g Balanced amino acids (n = 8)
|
P (ANOVA)
|
|||||
|---|---|---|---|---|---|---|---|
| Basal state | Supplementation | Basal state | Supplementation | Group effect | Supplement effect | Interaction | |
| Whole-body total Ra (μmol · kg−1 · min−1) | 0.61 ± 0.02 | 1.08 ± 0.07 | 0.67 ± 0.05 | 1.05 ± 0.06 | 0.89 | <0.0001 | 0.36 |
| Oral phenylalanine intake (μmol · kg−1 · min−1) | — | 0.68 ± 0.08 | — | 0.76 ± 0.04 | 0.32 | — | — |
| Whole-body endogenous Ra (μmol · kg−1 · min−1) | 0.61 ± 0.02 | 0.70 ± 0.02 | 0.67 ± 0.05 | 0.65 ± 0.04 | 0.97 | 0.36 | 0.15 |
| First-pass splanchnic extraction (%) | — | 44 | — | 47 | 0.53 | — | — |
x̄ ± SE. Ra, rate of appearance.
Leg phenylalanine kinetics
The parameters of phenylalanine kinetics across the leg are reported in Table 6. Phenylalanine delivery to the leg and release from the leg increased significantly from the basal state in both groups during amino acid supplementation; no significant differences between groups and no significant treatment-by-group interaction were found. Phenylalanine net balance across the leg was negative in the basal state in both groups, indicating net muscle protein catabolism, and became positive during amino acid supplementation in both groups, indicating net muscle protein anabolism; no significant differences between groups or a significant treatment-by-group interaction were found. The between-group difference in the pre-post response of net balance was < 1 nmol · min−1 · 100 mL leg volume−1, which is much lower than the difference that we consider to be physiologically significant (30 nmol · min−1 · 100 mL leg volume−1 (see Statistical analysis).
TABLE 6.
Phenylalanine kinetics across the leg in the 2 groups of healthy elderly subjects in the basal state and during supplementation with either 18 g essential amino acids or 40 g balanced amino acids1
| 18 g Essential amino acids (n = 6)
|
40 g Balanced amino acids (n = 8)
|
P (ANOVA)
|
|||||
|---|---|---|---|---|---|---|---|
| Basal state | Supplementation | Basal state | Supplementation | Group effect | Supplement effect | Interaction | |
| nmol · min−1 · 100 mL leg volume−1 | nmol · min−1 · 100 mL leg volume−1 | ||||||
| Common parameters | |||||||
| Delivery to the leg | 190 ± 22 | 433 ± 47 | 174 ± 28 | 396 ± 75 | 0.68 | < 0.0001 | 0.78 |
| Release from the leg | 208 ± 27 | 419 ± 59 | 190 ± 30 | 380 ± 72 | 0.67 | 0.0002 | 0.79 |
| Net balance across the leg | −18 ± 5 | 14 ± 13 | −16 ± 5 | 16 ± 4 | 0.81 | 0.0001 | 0.99 |
| 2-Pool model | |||||||
| Leg Ra | 261 ± 28 | 491 ± 62 | 223 ± 35 | 437 ± 77 | 0.53 | 0.0001 | 0.85 |
| Release in the blood from proteolysis | 70 ± 8 | 58 ± 17 | 49 ± 10 | 41 ± 9 | 0.16 | 0.27 | 0.78 |
| Leg Rd | 52 ± 5 | 72 ± 9 | 33 ± 8 | 57 ± 9 | 0.14 | 0.0003 | 0.56 |
| 3-Pool model | |||||||
| Transport into the muscle2 | 129 ± 16 | 343 ± 42 | 98 ± 24 | 195 ± 42 | 0.0423 | 0.0001 | 0.06 |
| Transport from the muscle3 | 147 ± 19 | 329 ± 55 | 114 ± 25 | 179 ± 40 | 0.0477 | 0.0022 | 0.09 |
| AV shunting | 61 ± 18 | 90 ± 35 | 76 ± 16 | 201 ± 55 | 0.15 | 0.0412 | 0.18 |
| Release from proteolysis | 80 ± 9 | 61 ± 17 | 58 ± 13 | 51 ± 11 | 0.35 | 0.52 | 0.17 |
| Utilization for protein synthesis | 62 ± 6 | 75 ± 10 | 43 ± 11 | 67 ± 11 | 0.34 | 0.0019 | 0.23 |
x̄ ± SE. Ra, rate of appearance; Rd, rate of disappearance; AV, arteriovenous.
Analysis of covariance on pre-post differences (eg, between basal state and supplementation) between groups with the use of basal values as covariates: 2 P = 0.30, 3 P = 0.41.
Amino acid supplementation significantly increased the total phenylalanine leg Ra, did not change the release of phenylalanine in the blood from proteolysis, and significantly increased the utilization of blood phenylalanine for muscle protein synthesis (leg Rd); no significant differences between groups or a significant treatment-by-group interaction were found.
The transport of phenylalanine from the femoral artery into the muscle and from the muscle into the femoral vein increased significantly from the basal state during amino acid supplementation; the group effect was significant, but no significant treatment-by-group interaction was found. AV shunting of phenylalanine slightly but significantly increased during amino acid supplementation, but no significant group effect or treatment-by-group interaction was found. The release of phenylalanine from muscle proteolysis did not change significantly in either group, and no significant differences between groups or a significant treatment-by-group interaction were found.
The utilization of phenylalanine for muscle protein synthesis increased significantly in both groups, but no significant difference between groups or treatment-by-group interaction was found. Muscle protein synthesis efficiency was not significantly different between the groups in the basal state (EAA group: 30 ± 3%; BAA group: 28 ± 4%) and did not change significantly during amino acid intake in either group (EAA group: 20 ± 4%; BAA group: 30 ± 4%)
Fractional synthesis rates in muscle protein
The FSR of mixed muscle proteins increased significantly in both groups, but no significant difference between groups or treatment-by-group interaction was found (Figure 1).
FIGURE 1.
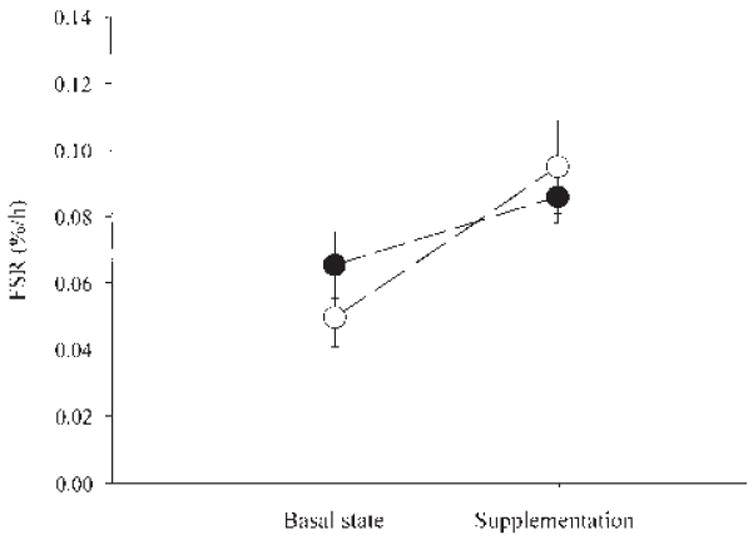
Mean (± SE) fractional synthesis rates (FSRs) in mixed muscle protein from 2 groups of healthy elderly subjects in the basal state and during supplementation with 18 g of an essential amino acid supplement (●; n = 6) or 40 g of a balanced amino acid supplement that contained 18 g essential amino acids (○; n = 8). Group effect: P = 0.77. Supplement effect: P = 0.0136. Treatment-by-group interaction: P = 0.29.
DISCUSSION
The results of our experiment show that essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in elderly persons, whereas nonessential amino acids did not provide any additional significant stimulation of muscle anabolism above that reached with essential amino acids alone. Specifically, the phenylalanine net balance across the leg, which was negative in the basal state (indicating net muscle protein catabolism), shifted to a positive value during the intake of 18 g essential amino acids alone or in combination with 22 g nonessential amino acids (balanced mixture), which indicated net muscle protein anabolism. The magnitude of the anabolic effect of both supplements was not different between groups (32 nmol · min−1 · 100 mL leg volume−1).
The stimulation of muscle protein anabolism by both the essential and the balanced amino acid supplements was due to similar increases in muscle protein synthesis, as measured by the precursor-product approach (FSR) and the AV balance method with both the 2- and the 3-pool models. Muscle protein breakdown did not change significantly from the basal state with either amino acid supplement, regardless of the method used to measure it (2- or 3-pool model). This finding confirmed our previous observations in young (11, 23) and elderly (10, 11) subjects. Consistently, whole-body proteolysis remained unchanged during the ingestion of the supplements.
Our experiment was designed so that the essential amino acid content and composition of both supplements was identical (18 g). Therefore, the balanced amino acid supplement (18 g essential amino acids plus 22 g nonessential amino acids) delivered more than twice as much energy and amino nitrogen as did the essential amino acid supplement. Despite such a higher dose of amino nitrogen, the balanced amino acid supplement failed to improve any of the indexes of muscle protein anabolism as compared with the essential amino acid supplement. Thus, the anabolic efficiency (anabolic effect per unit of amino nitrogen and energy) of the essential amino acid supplement was double that of the balanced amino acid supplement. In addition, the results support the notion that the stimulation of muscle protein synthesis by amino acids is not simply driven by a generalized increase in substrate availability but is regulated by the specific effect of some or all of the essential amino acids. Several authors have shown that branched-chain amino acids (24, 25), particularly leucine (26-28), stimulate muscle protein synthesis in rats by enhancing messenger RNA translation via increased formation of the eukaryotic initiation factor 4F complex, activation of ribosomal protein S6, and some other mechanism that has not yet been elucidated (27, 28). Furthermore, it was shown in young human subjects that a flooding dose of a single essential amino acid (leucine, phenylalanine, threonine, or valine) doubled the synthesis rates of skeletal muscle proteins, whereas flooding doses of single nonessential amino acids (arginine, glycine, and serine) had no effect on muscle protein synthesis (12, 13, 29).
Nevertheless, we cannot completely exclude the possibility that the lack of an additional benefit of the balanced amino acid supplement over the essential amino acid supplement might have been due to the achievement of a maximal muscle protein synthesis response to the essential amino acid supplement (18 g over 3 h). In fact, there are no dose-repose data regarding the stimulation of muscle protein synthesis by amino acids or proteins in elderly people. However, we believe that this is not the case. The subjects in both groups received ≈0.2 g essential amino acids/kg, and the subjects in the BAA group received a total of 0.4 g balanced amino acids/kg. These values correspond roughly to one-fourth and one-half, respectively, of the recommended dietary allowance for protein (0.8 g · kg−1 · d−1) (30). In other words, we tested the effects of the amino acids that are normally contained in a main meal. Nonetheless, further dose-response studies are warranted.
It was recently shown that a pulse-feeding pattern improves protein retention in elderly women more so than does a spread pattern (31), whereas it does not affect protein retention in younger women (32). Because we used a continuous feeding pattern to measure muscle protein turnover under steady state conditions, this design could have reduced our ability to detect a difference between the essential amino acid and the balanced amino acid supplements. However, if such a problem had occurred, we should have at least found a trend for muscle protein anabolism to be increased with the balanced amino acid supplement. Yet, the magnitude of the anabolic response obtained with both supplements in our 2 groups of elderly subjects was virtually identical, as indicated by the phenylalanine net balance across the leg values and the results of the power calculation that we had previously performed. Thus, it is unlikely that the feeding pattern affected our ability to detect any potential differences between supplements.
Phenylalanine first-pass splanchnic extraction, arterial concentrations, and delivery to the leg increased similarly from the basal state during essential amino acid and balanced amino acid supplementation. Nonetheless, we found that its transport from the arterial blood into the muscle and from the muscle into the femoral vein was significantly higher during essential amino acid supplementation than during balanced amino acid supplementation. We hypothesize that the slower phenylalanine transport rate during balanced amino acid supplementation was due to competition of some nonessential amino acids deriving from the supplement for the system L, which is the only transmembrane transporter for phenylalanine (33, 34). This speculation has yet to be tested. On the other hand, it is more likely that the slower transport rates in the BAA group were simply due to basal differences. Analysis of covariance, with the basal values as covariates, suggests that this may be the case, because we found no significant group differences in the transport rates.
Finally, we found that the administration of balanced amino acids approximately doubled the basal insulin concentration, whereas the essential amino acid supplement did not induce any significant changes, possibly because of the lack of arginine. Insulin is an important regulator of muscle protein turnover (35). It is possible that the higher insulin concentrations in the BAA group might have been responsible for the slightly lower phenylalanine transport rates in the basal state and during supplementation. However, this is unlikely because it was shown in vitro and in vivo that insulin stimulates only the amino acid transport system A, whereas phenylalanine is transported by amino acid transport system L (33, 34, 36). Regardless, we do not think that these small differences in insulin concentrations had a significant effect on the overall results of our study. The lack of significant differences in muscle protein breakdown and synthesis, the 2 parameters most likely to be affected by insulin, suggests that such a small difference in insulin concentrations was insufficient to affect muscle protein turnover. Nevertheless, specific dose-response studies of insulin action on protein turnover are warranted.
In conclusion, our data support the notion that essential amino acids are primarily responsible for the amino acid–induced muscle protein anabolism in elderly subjects. Although we cannot exclude the possibility that nonessential amino acids per se may somewhat affect muscle protein anabolism, we nonetheless conclude from our data that nonessential amino acids do not provide any additional benefit when given together with essential amino acids at the doses used in our study. This is particularly important if nutritional supplementation alone is to be used to treat muscle loss with aging, because elderly persons who take nutritional supplements tend to decrease their food intake in a calorie-for-calorie fashion (6). Thus, for nutritional supplementation to be beneficial, a supplement has to be more effective in stimulating muscle protein growth than is regular food or conventional protein supplements. The results of the present study and those of our previous study, which indicate that the addition of carbohydrates to a supplement for the elderly may impair the anabolic response of muscle proteins (9), suggest that an effective supplement should contain essential amino acids and no carbohydrates. This composition would dramatically increase the anabolic efficiency of a nutritional supplement for the treatment of sarcopenia in the elderly. Further studies are required to assess whether specific essential amino acids are more beneficial than others and to test whether the long-term treatment with essential amino acid supplements can effectively increase muscle mass in elderly persons.
Acknowledgments
We thank James S Goodwin and Sue Minello (University of Texas Medical Branch) for their assistance in recruiting the volunteers; Guy Jones, Julie Vargas, and Zhi Ping Dong (Shriners Hospital) for technical assistance; and the nurses and personnel of the General Clinical Research Center (University of Texas Medical Branch) for assistance with the infusion studies.
Footnotes
EV contributed to the study design, data collection, data analysis, and writing of the manuscript; HK contributed to the data collection and data analysis; MS-M contributed to the data collection and writing of the manuscript; BM contributed to the data collection and the writing of the manuscript; RRW contributed to the study design, data analysis, and writing of the manuscript. No author had any financial or personal interest in any organization sponsoring the research.
Supported by NIH/NIA grant R01 AG15780 (to RRW); NIH/NIA Claude D Pepper Older Americans Independence Center grant P60 AG17231; NIH/NIA grant R01 AG18311 (to EV); NIH/NCRR, USPHS, General Clinical Research Center grant M01 RR00073; the Brookdale National Fellowship (to EV); and the European Society of Parenteral and Enteral Nutrition Research Fellowship (to EV).
References
- 1.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50:64–7. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–84. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 3.Roberts SB. Effects of aging on energy requirements and the control of food intake in men. J Gerontol A Biol Sci Med Sci. 1995;50:101–6. doi: 10.1093/gerona/50a.special_issue.101. [DOI] [PubMed] [Google Scholar]
- 4.Evans WJ. Exercise, nutrition and aging. J Nutr. 1992;22(suppl):796–801. doi: 10.1093/jn/122.suppl_3.796. [DOI] [PubMed] [Google Scholar]
- 5.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–80. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 6.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WW, Crim MC, Young VR, Joseph LJ, Evans WJ. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol. 1995;268:E1143–53. doi: 10.1152/ajpendo.1995.268.6.E1143. [DOI] [PubMed] [Google Scholar]
- 8.Welle S, Thornton CA. High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62- to 75-yr-old men and women. Am J Physiol. 1998;274:E677–83. doi: 10.1152/ajpendo.1998.274.4.E677. [DOI] [PubMed] [Google Scholar]
- 9.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–7. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first pass splanchnic extraction. Am J Physiol. 1999;277:E513–20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 12.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with [1-13C]leucine stimulates human muscle protein incorporation of continuously infused l-[1-13C]valine. Am J Physiol. 1992;262:E372–6. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- 13.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–8. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- 14.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–73. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe RR. Appendix A: laboratory methods. In: Wolfe RR, editor. Radioactive and stable isotope tracers in biomedicine Principles and practice of kinetic analysis. New York: Wiley-Liss; 1992. pp. 417–38. [Google Scholar]
- 17.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol. 1995;268:E75–84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 18.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–4. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe RR. Selection of tracer infusion and sampling sites. In: Wolfe RR, editor. Radioactive and stable isotope tracers in biomedicine Principles and practice of kinetic analysis. New York: Wiley-Liss; 1992. pp. 167–88. [Google Scholar]
- 20.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism. 1978;27:97–106. doi: 10.1016/0026-0495(78)90128-2. [DOI] [PubMed] [Google Scholar]
- 21.Chinkes DL, Rosenblatt J, Wolfe RR. Assessment of the mathematical issues involved in measuring the fractional synthesis rate of protein using the flooding dose technique. Clin Sci. 1993;84:177–83. doi: 10.1042/cs0840177. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol. 1993;264:E109–18. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- 23.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–9. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 24.Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta. 1978;544:351–9. doi: 10.1016/0304-4165(78)90103-4. [DOI] [PubMed] [Google Scholar]
- 25.Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. 1988;254:579–84. doi: 10.1042/bj2540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buse MG, Reid SS. Leucine: a possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–61. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–9. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 28.Anthony JC, Reiter AK, Anthony TG, et al. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 2002;51:928–36. doi: 10.2337/diabetes.51.4.928. [DOI] [PubMed] [Google Scholar]
- 29.Smith K, Essen P, McNurlan MA, Rennie MJ, Garlick PJ, Werneman J. A multi-tracer investigation of the effect of a flooding dose administered during the constant infusion of tracer amino acid on the rate of tracer incorporation into human muscle protein. Proc Nutr Soc. 1992;51:109A. abstr. [Google Scholar]
- 30.National Research Council, Food and Nutrition Board. Recommended dietary allowances. Washington, DC: National Academy of Science Press; 1989. [Google Scholar]
- 31.Arnal MA, Mosoni L, Boirie Y, et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69:1202–8. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- 32.Arnal MA, Mosoni L, Boirie Y, et al. Protein feeding pattern does not affect protein retention in young women. J Nutr. 2000;130:1700–4. doi: 10.1093/jn/130.7.1700. [DOI] [PubMed] [Google Scholar]
- 33.Guidotti GG, Gazzola GC. Amino acid transporters: systematic approach and principles of controls. In: Kilberg MS, Haüssinger D, editors. Mammalian amino acid transport. New York: Plenum Press; 1992. pp. 3–29. [Google Scholar]
- 34.Shotwell MA, Kilberg MS, Oxender DL. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta. 1983;737:267–84. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe RR, Volpi E. Insulin and protein metabolism. In: Jefferson LS, Cherrington AD, editors. Handbook of physiology: a critical comprehensive presentation of physiologic knowledge and concepts: Section 7: the endocrine system. Vol 2 The endocrine pancreas and regulation of metabolism. New York: Oxford University Press; 2000. pp. 733–55. [Google Scholar]
- 36.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95:811–9. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]


